Abstract
For more than 50 years ecological niches have been defined as combinations of multidimensional environmental conditions permitting a species to survive and reproduce. A fundamental niche (NF) is defined as the set of conditions within which a species can live in the absence of competitors, and a realized niche (NR) is a NF hypothetically reduced by competitive interactions (and some other limiting factors). This definition implies that NF is “larger” than NR, something that has been nearly universally accepted by ecologists. However, there have been few attempts at empirical tests. Here, we present a novel quantitative test using one-dimensional estimates of NF for 105 species of reptiles and amphibians, and estimates of NR obtained from ~1.4 x 104 field observations. To specify our test, we operationalize the original classification of niche types. Our results predominantly support the hypothesis that NF ‘is larger’ than NR and we highlight the theoretical and practical importance of quantifying niches.
Introduction
The fundamental niche (NF) of a species is determined by its physiological range of tolerance to environmental factors in the absence of biotic interactions [1–3], and the regions of the planet with environments in NF would represent some sort of potential area of distribution for the species. However, the presence of competitors and predators [4], and dispersal limitations of the species [5] reduce the occupied geographic range from its full potential, suggesting a partial occupation of available NF. Furthermore, limited climate availability [6] is also expected to reduce NF to a smaller realized niche (NR). On the other hand, mutualistic interactions should have the opposite effect [3, 7–10].
Hutchinson [1] first proposed the idea that NF should in some sense be larger than NR. Although old and superficially obvious, Hutchinson’s idea has seldom been tested, partially because the concept of “niche” originally combined many types of variables that caused not only terminological imprecision [11] but also serious complications at measuring the fundamental niche, mostly when the variables used to define it are resources consumed by the species in question [11]. Here, we use the Grinnellian niche concept [10, 12] in which niche space is defined using non-interactive conditions (i.e., scenopoetic variables [13]); specifically, we use one climatic variable (average monthly temperature) measured at every cell of a discrete geographic grid of the planet (G). Although we restrict our example to a niche defined by a single scenopoetic variable, all the computations can be performed using more variables, as we show in S1 File, Section 2: Estimation of niches in two dimensions.
Data for niches defined for climatic variables are substantially more available [7] than those based on interactive variables, like resources [12, 14, 15], and permit establishing straightforward relationships between metrics in environmental and geographic spaces [16–18]. For climatic variables, Hutchinson’s idea can be interpreted as a set inequality: NF ⊇ NR, which predicts that climates in localities where a species has been observed (NR) should be located inside the geometric shape defining its NF. Hutchinson (1957) chose as shapes simple rectangles. This can be empirically true or false, since the environments where a species is observed may be outside the shape defining its fundamental niche, for a variety of reasons discussed below. We stress that the inequality is interpreted as points being inside a shape.
Although Hutchinson [1] briefly mentioned that not every environment in a fundamental niche may actually exist at a given time and space, this very important insight was essentially ignored until Jackson and Overpeck [10] rediscovered it in the context of paleoclimatic effects on vegetation. The “existing niche”, denoted by N*(t, G), is the intersection (in the sense of points being inside shapes) of the fundamental niche, which is modeled as some geometric shape, with the discrete set of environments available at time t in region G, (including the points where observations of the species has taken place) henceforth denoted by E(t, G). This intersection generates the subset of the existing environmental space that is suitable for a species. E(t, G), a set of points, can be modelled continuously using smooth kernels (see S1 File, Fig A1). Then, the existing niche can be added as an additional term to Hutchinson’s inequality as follows [19]:
| (1) |
There is a correspondence between points in environmental space and the cells in the grid G called the Hutchinson’s Duality [7, 18]. The correspondence is not necessarily one to one [16] but it can be made to be using enough independent variables and good precision. This correspondence allows a spatial interpretation of Eq (1). First notice that although NF per se cannot be mapped geographically (it is a physiological feature of a species), both N*(t, G) and NR(t, G) can, since they are sets of points in environmental space corresponding to geographic localities [2, 7, 18]. Therefore, the inequalities Eq (1) have implications for both niches and distributional areas: (i) the physiology of a species determines the potential geographical limits of its distribution (at a given time), by defining the regions with climates within its limits of tolerance. However, the actual occupied area is the result of other factors that probably constrain this potential. NR(t, G) thus corresponds to the climates in the actual species distribution [7]. (ii) The inequality Eq (1) sets the limits of niche modeling because the output of presence-only correlative distribution models can be interpreted to be approximately within the potential and occupied areas [20]; therefore, a niche modeled using correlative methods is probably intermediate between N*(t, G) and NR(t, G). (iii) Because the data used in niche modeling algorithms are samples of NR(t, G), the inequality highlights that algorithms that very faithfully model such samples may be poor at estimating NF. (iv) In niche evolution, NF is the unit upon which evolution acts [21]; however, NR(t, G), which is estimated by correlative methods may change in position or shape due to a combination of evolutionary, ecological, and climate-availability variables mentioned above [19]. In particular, the fundamental niche is being “distorted” all the time by the shape of the existing climate in a particular region. Therefore, the inequalities Eq (1) implies that observing niche differences among regions should be possible even in the absence of niche evolution in a strict sense [22]. And finally, (vi) when applied to economically important species (invasive, forestry, vectors of diseases), the inequalities suggest that regions with environments suitable for potential introduction of such species may be substantially larger than what correlative models, based on NR(t, G) data, may predict [23]. The above factors indicate the importance of testing empirically Hutchinson’s inequalities, and it is surprising that so few attempts have been made to do it [15, 23, 24].
Notice also that the inequalities Eq (1), which describe sets, suggest inequalities on the sizes of such sets. Using vertical bars to denote a measure on the size of the sets we can hypothesize:
| (2) |
And since in principle it is possible for the realized niche to be outside the fundamental, inequalities Eqs (1) and (2) are to a degree independent of each other.
One of the reasons why few attempts have been made to test Hutchinson’s inequalities is that information on the multivariate fundamental niche is extremely rare. To test the inequalities one needs data on the physiologically defined limits of tolerance of species to extreme values of relevant niche variables. These are mostly available for one variable: temperature. In this work we will use as proxies for NF (actually, a projection of it in one dimension), data on the lower critical temperature for reptiles and amphibians, and the upper lethal temperature for amphibians and upper critical for reptiles (see Methods), compiled by Sunday et al. [25]. In the supporting information, and for the purpose of illustration, we present calculations based on two dimensional ranges of tolerance, calculations that can be generalized to more dimensions, if data were available (S1 File, Section 2).
Using a single variable to model the fundamental niche is a serious assumption when testing Eq (1), because a multidimensional cloud of points is projected into a line, and only one inequality is tested. The observed points may be outside the inequalities in variables not included, but inside the inequality used. This is a very important caveat, but we cannot see a way out unless experimental information about NF in higher dimensional spaces become available in the future.
To estimate NR(t, G), records of observed field occurrences were obtained from the Global Biodiversity Information Facility (GBIF) [www.gbif.org]. Because environments in these points are instances of the realized niche, Hutchinson’s inequality predicts that the environments at the occurrence points of a species should be inside its NF range. To estimate E(t, G) for the world at present, and thus N*(t, G), we used WorldClim data (see Methods). To test Eq (2) we used numerical integration on smooth kernels calculated around the data points representing N*(t, G) and NR(t, G). The analysis clarifies the meaning of Hutchinson’s inequalities and provides unequivocal support for them.
Comparing macroclimatic data to critical temperatures obtained in the laboratory has a number of problems, since the temperature that individuals experience in the field may correlate poorly with macroclimate measurements [26]. One way of dealing with the problems is by using soil, wind, cloud cover, micro topography and habitat information to parameterize macro-to-micro-climate models [27]. Unfortunately, as of today, lack of data for most regions prevents the method from being generally applicable. On the other hand, many macroecological (i.e., coarse spatial resolution) patterns are assumed to be the result of interactions between macroclimate and physiology [28, 29], and for the problem of understanding how physiological limits affect geographic distributions, long-term climatic averages may matter more [30]. Indeed, many recent studies combine macroclimatic and physiological data without modeling microclimate [31–33]. Finally, since the most likely effect of microclimatic variation would be to add favorable spots to spatial units that appear climatically unfavorable at low resolutions [34], by ignoring microclimatic complications we will be erring on the conservative side, because cells unfavorable at the macroclimate level but with documented occurrences due to favorable microclimates will be misclassified.
Methods
To obtain occurrence climates, the emerged Earth’s surface was divided into a 104 km2 per-cell grid using the Behrmann equal area projection [35]. The resulting surface (excluding Antarctica) was represented by 16,712 cells. Present data of mean temperature were obtained for each cell by applying the ‘extract’ function in the ‘raster’ R package [36] to climates in WorldClim [37] at 10’ of resolution (~3.4 x 102 km2 near the equator), to extract the variable Bio1 (mean monthly temperature X 10). Using the function SmoothKernelDistribution of Mathematica™, a smooth kernel kE(T) was fitted to the present E-space (Bio1/10) (Fig A1 in S1 File).
Using the ‘gbif’ function contained in the dismo R package [38], and the ‘thin’ function in the spThin package, and scripts developed in R, we queried the Global Biodiversity Information Facility (GBIF, www.gbif.org), obtaining ~76,000 coordinates for 105 names, out of the 158 originally in the Sunday et al. [25] database. We kept only 14,051 non-redundant (at 10 km) [39, 40] georeferenced records from this database (S1 File, Fig A2). Obvious georeferencing problems were detected by checking the presence of GBIF records within the recorded borders of countries (Environmental Systems Research Institute; http://www.esri.com/) and excluding them from the database. The ‘extract’ function in the ‘raster’ R package [36] was applied to coordinates in the GBIF occurrences, to obtain values of variable Bio1 in WorldClim to be divided by10.
We defined the fundamental niches of each species as the set of temperatures contained in the interval between the limits of physiological critical and/or lethal temperatures for 151 species of reptiles and amphibians, obtained from Sunday et al. [25], who give details of these definitions. The measure of NFj for species j is simply its range . To have a common measure for comparison, the extracted values of Bio1/10, per species, were standardized to a common scale of fundamental niches using , where is the standardized i-th observation of temperature, for species j, and Tmin j and Tmax j are the lethal minimum and maximum temperatures, respectively, for species j.
To obtain a null model for the distribution of NF volumes, we randomly generated 10,000 left and right limits within the range of values of Bio1 in the current climate and calculated the range value for pairs with positive ΔT = (Bio1Max−Bio1Min)/10.
On a species per species basis, we counted the number of times that the temperature at species occurrences (GBIF) was inside their NFj. Significance testing required estimating the probability that random points from the E cloud will be located inside an NFj range. The limits of the critical/lethal temperatures of each species (the fundamental niches) were used to integrate numerically the kernel of climate, yielding the proportion of the kernel inside every NF, which is a proportional measure of the existing niche: . This is an estimate of the proportion of E(t, G) located inside a particular NF, or . Assuming random placement of points in the E-space, for species j, the probability of having k(j) points inside the j-th NF, out of n(j) occurrences, is binomial with probability . Thus, for each of the 105 species, we tested the significance of the number of observed points inside NF assuming random placing. A sequential 0.01 level of significance Bonferroni correction [41] was used.
It is known that climatic variables at coarse resolution can underestimate the amount of favorable environments in habitats protected by topographic or vegetation features. In our analysis, this will overestimate the number of occurrences that are actually outside the fundamental niche, and thus underestimate the probability of a random occurrence having favorable climate. Therefore, the performed binomial test errs on the conservative side (more violations to Hutchinson’s predictions that actually taking place).
The estimates of the realized niches were obtained by fitting smooth kernels to the observed temperatures. Smooth kernels are simple niche models [42], and we decided to fit them only for species with at least 5 points (92 species). For each species the intersection of its smooth kernel with E(t, G) inside the corresponding NF was calculated integrating numerically the minimum of the species kernel and the climatic kernel, and multiplying by the observed range of temperatures, per species [43]. This is an estimate of how much the occurrences distribution actually overlap with the available niche space. All processing was performed in R and Mathematica™.
Results
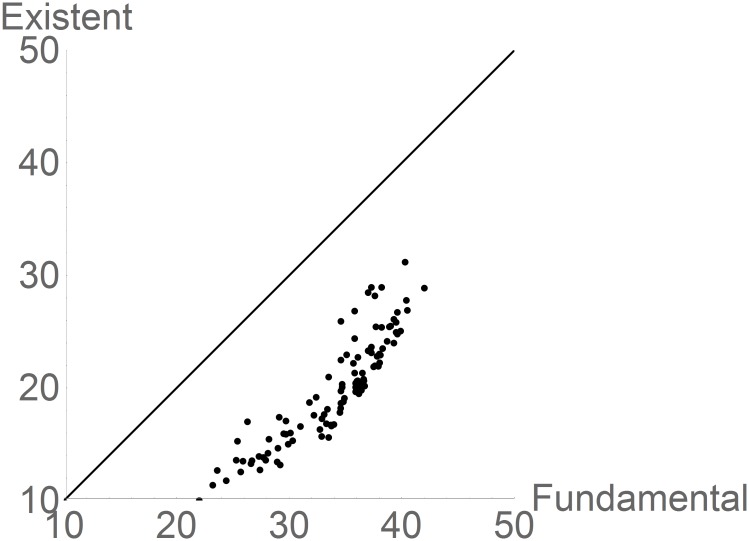
Eq (1) states that NF ⊇ N*(t, G). This is true by definition. However, a measure of how much larger NF is than N*(t, G), for a specific time and region, is a measure of how much “unused” fundamental niche exists for a species, at a given time, and therefore how much geographic area would be left to be occupied, given the correspondence previously mentioned between metrics in niche and geographic space. This is shown in Fig 1. Roughly speaking, species occupy available favorably climates in proportion to their capacity, since N*(t; G) is proportional to NF (N* = -11.5 + 0.925 NF, p << 0.01, r2 = 0.799). However, there is a gap between the identity line and the existing niches. This gap is a measure of how much the existing and favorable temperatures in the world are not used by a species, and this can also be interpreted as a measure of the potential to evolve wider temperature tolerances, given the actual temperature distributions. Hutchinson’s duality allows looking at the results from a geographic perspective: the number of grid cells with temperatures inside the existing niches, N*(t; G) is a measure of the size of the potential distribution and constitutes an upper bound to the size (number of pixels of 104 km2 in geographic space) of the actual distribution of a species [12]. The range of the number of geographic cells with climates inside the critical/lethal ranges of the species in the database was [395, 2215], which implies that with the current climate, actual ranges larger than ~2.1 x 107 km2 should not exist.
Fig 1. Scattergram (decimal logarithm) of the measure of the existing (N*(t, G)) versus the fundamental (NF) niches for 105 species.
The units for NF and N*(t, G) are in °C. The straight line is the line of Existing = Fundamental. The units of the existing niche can be transformed to units of area potentially suitable for each species. The distance between a point and the line is a measure of how much potential for evolving wider tolerance ranges a species has (see text).
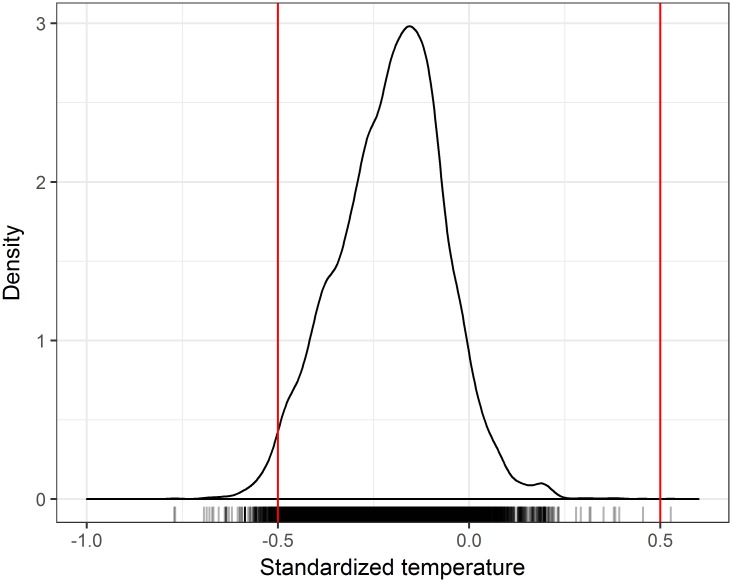
Eq (1) also states that climates in NR(t, G) should be inside of NF (the original Hutchinson’s prediction). In total, 85.86% of the ~14000 observation records of all species had climates inside their corresponding NF boxes, a result highly unlikely assuming random data on a uniform distribution (Fig 2). However, since every fundamental niche is different, and temperature is not uniformly distributed, for each species we estimated the probability that random sampling from E(t, G) would give the observed or a greater number of points inside the corresponding niche, with a 0.01 significance sequential Bonferroni correction for multiple comparisons (see Methods). When using species with at least five occurrences, 73 out of 92 species have more observations inside the limits of their NF than expected by chance. Although 29 species have at least one observation outside its NF, at this level (0.05/151) no species has more observations outside its limits than expected by chance (Table A in S1 File).
Fig 2. Proportion of GBIF points inside the NF for a common scale of fundamental niches (bars) and smooth histogram of the distribution of observed standardized temperatures.
The red bars show the limits of the 105 NF in the common scale.
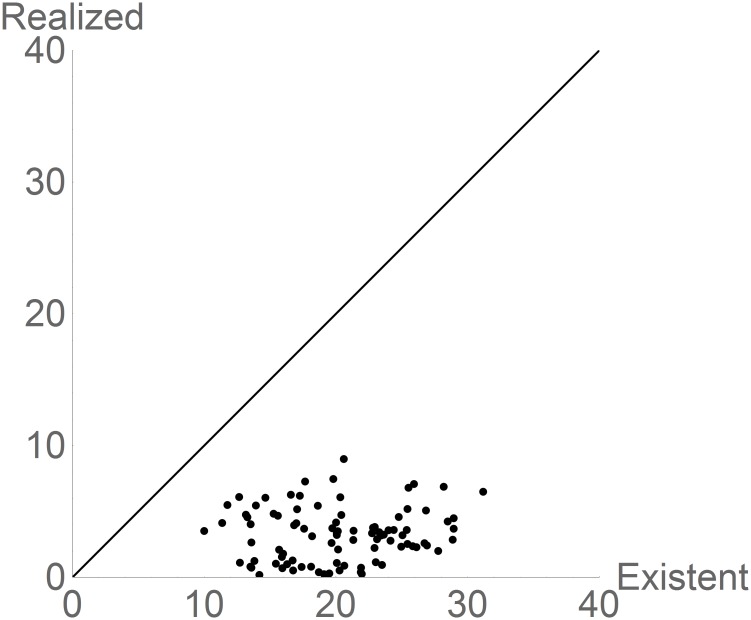
The inequalities in Eq (2) predict that for every species, and at a given time t and region G, the measure of the NR(t, G) should be smaller than the measure of the corresponding N*(t, G). These two variables are displayed in Fig 3 showing that for most species the amount of realized niche space actually used, as estimated from the occurrences data, is almost constant, at about 2–10 degrees of temperature, but there are many species for which there is a very substantial amount of existing niche space that is not used. This is a measure of invasibility potential.
Fig 3. Scattergram (decimal logarithm) of the measure of NR(t, G) vs. N*(t, G) for 92 species with more than five occurrences.
The units for NR(t, G) and N*(t, G) are in °C. The straight line is the line of Realized = Existing. The distance between a point and the line is a measure of how much favorable space is not being used by a species, maybe due to interactions, dispersal limitations or other such factors (see text).
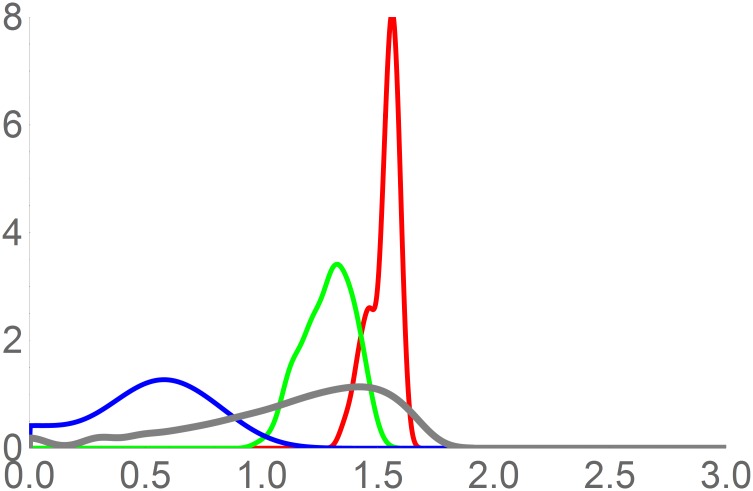
In Fig 4 we present smooth histograms of all the niches described above, and of a null model, showing that the data of ranges of temperature of fundamental niches for reptiles and amphibians gives a much narrower and significantly different distribution (Kolmogorv-Smirnov, p < 10−16) than a random distribution of volumes.
Fig 4. Smooth histograms of the decimal logarithm of values of a null model of fundamental niche (grey), the 105 values of fundamental (red), 105 existing (green) and 92 realized (blue).
Discussion
Strictly speaking, only physiologically-obtained ranges of conditions suitable for species, should be used as measures of fundamental niches. Today, for more than one dimension, such measures are scarce, and our test then was performed on a limited number of species using a single niche variable. Although we used a single variable dataset, all the operations we described can be performed in two or more dimensions, assuming that information on multiple physiological limits is available. In S1 File, Section 2, we present an illustration of how to do this for two dimensional niches.
For the one-dimensional dataset we used, the results are very clear: both Hutchinson’s equations (sets and their measures) are valid for niches of reptiles and amphibians, based on temperature as niche variable, and GBIF occurrences. In order to get this result, we needed definitions of fundamental, existing and realized niches that are precise (mathematical) and operational, i.e., capable of being calculated with actual data. A disadvantage of this is that such definitions narrow the meanings of the terms used, but in return relationships and predictions become clearer. It is also important to realize that the original ideas of Hutchinson can be interpreted both as set inequalities, predicting “containment” (Eq 1), and magnitude inequalities, predicting size relationships (Eq 2) and these are independent in regards to the realized niche: the realized niche may not be a part of the fundamental and still its magnitude may be smaller than that of the realized. This is not the case in any of the species we analyzed, but it is possible in principle.
Figs 1 and 3 display the sizes of the inequalities Eq (2). By construction NF ⊇ N*(t, G) is true (see Methods), although N*(t, G) ⊇ NR(t, G) can be empirically false. However the size of the difference in the measures of NF, N*(t, G) and NR(t, G) is very interesting. In the current climate, for all species there is a gap of approximately 11 degrees between their range of tolerances (NF) and the actual available range of temperatures, suggesting the possibility of the existence of selective pressures that could lead to evolution of the fundamental niches. On the other hand, most realized niches are well below their existing niche potential which suggests that for the species in this analysis there are large areas of the world, currently not reported as occupied in the GBIF database, but with the favorable temperatures. This is an index of invasibility potential.
It is possible for NR(t, G) to have parts outside its NF, in violation of Eq (1), although none of our species violate Eq (1) significantly. This has interesting implications for correlative niche modeling, since it suggests that for some species a correlative ENM will be a very poor estimate of the size of the existing niche, and confirming that niche estimates based on species occurrence should not, in general, be regarded as estimates of fundamental niches. The only situation in which this is the case is when the species has had access to all possible environmental conditions, unrestricted by barriers, dispersal disequilibrium, or negative interactions [44], a highly hypothetical situation. An obvious morale of this is that the output of correlative niche models, like Maxent, Bioclim or others should not be used to perform evolutionary analysis unless there is proof that confounding ecological, dispersal or climatic factors can be disregarded, or the evolutionary hypothesis is stated in a way that takes into account the preceding caveats. For instance, Hof et al. [45] explored whether there was a phylogenetic signal in the realized niches of the amphibians of the world, which they found to be the case. Their hypothesis was carefully designed to take into account the complications introduced by use of the realized niche, unfortunately, this is not always the case.
The data we used to estimate realized niches come from GBIF observations, which (assuming random sampling) can be regarded as data representing true realized niches. Even correcting for spatial autocorrelation by thinning data, the climate of most occurrences is located inside their corresponding NF. However, most points are grouped towards the cool side of the graph (Fig 2), both those inside or out of the NF box. It is known that distributions of temperature requirements [46] commonly have sharp cut-offs at high temperatures and longer tails at low temperatures. Also, a recent analysis of the temperature tolerance of more than 2,500 plants and of endothermic and ectothermic animals [32] reported significantly higher variability in tolerance to lower than to upper thermal limits. Therefore, an asymmetric physiological tolerance (their NF) could explain the distribution of occurrences in Fig 2. However, the proportion of environmental space available on different regions around the NF of the species is not uniform. The simplest explanation for the skewed distribution of the environments of the occurrence points then would be simply that the realized niches of the species we use are biased to the left of their fundamental niches because environmental space is lacking on the hotter parts (S1 File, Fig A1). This is an explanation based on the combination of the shape of E(t, G) and the location of the species’ NF, as Eq (1) would suggest it should be done, thus highlighting the importance of the existing niche, a seldom remembered concept. In fact, the hypothesis that larger range sizes correspond to larger “niche breadth,” [47], without making the distinctions among niche types that become obvious by using symbols and operational definitions, misses the critical component of the availability of niche variables, a point made long ago [9].
We did not document species with a significant number of occurrences outside their NF, however, this can indeed happen. Leaving aside the possibility that the databases we use may contain poor estimates of ranges, taxonomy mistakes, or biased or non-obvious georeferencing errors, there are four ways that we see for Hutchinson’s inequality Eq (1) to fail. (i) Some facilitating mechanisms, natural or anthropogenic [48] are operating. This could result in points occurring outside purely physiological limits due to interactions, an interesting theoretical possibility that has not been explored empirically, but it is unlikely for the species we use. Also, favorable microhabitats or microclimatic spots may be found inside cells with unfavorable climate, or by behavioural responses. Maybe some of the points outside NF left limit in Fig 2 are examples of this possibility, something we cannot test. (ii) Niche evolution sensu stricto occurred and populations shifted their physiological limits relative to the Sunday et al. [25] baselines. This possibility can be checked experimentally, documenting variability in tolerance ranges over geographic space [49, 50]. (iii) Because a single NF range summarizes just the mortality part of the life history of a species, differences in requirements across the life history were ignored, which could lead to misleading results if, for example, different stages in the life history have different physiological requirements [51]. In other words, points outside a respective NF may be true sink populations [52] and not errors of the prediction. Finally, (iv) the NF may not be a convex set but instead may contain “holes” [42, 53], creating unsuitable regions inside the extremes of reported tolerances. This possibility could arise if physiological tolerances are not mono-modal, which is unlikely, or if there is marked niche differentiation in subpopulations [49]. These factors would render the use of intervals, boxes, ellipsoids or other convex sets unsuitable for representing NF. Testing these hypotheses is outside the scope of this communication, but stating them highlight the fundamental interest of Hutchinson’s inequalities.
Notice that evolution of the fundamental niche may occur in its size, shape and position. In a recent work, Gouveia et al. [31] showed that the larval critical thermal maximum, a physiological proxy for the NF, correlates better with the realized niche position than with measures of niche size. The fact that the authors were able to show a highly significant explanation of NR position as predicted by their NF proxy is suggestive that inequality Eq (1) is also fulfilled in their system.
Generally speaking, the problem of estimating fundamental niches from basic physiology or experimental data is still a neglected field, restricted mostly to temperature as niche variable [25, 29, 31, 54, 55]. Although a few experimental multivariate attempts to assess NF exist, [56, 57], an interesting alternative is to measure fundamental niches using the physiological models of Carbon and Nitrogen allocation in plants [58, 59] that would allow calculation of strict fundamental niches. Higgins and Richardson [60] fitted such models to observed occurrences of Acacia and Eucalyptus in Australia, and show that independent GBIF observation records are very well predicted by extrapolation from the physiological models, which is consistent with the predictions of Eq (1). Unfortunately, by fitting the physiological model to observed occurrences Higgins and Richardson [60] did not provide a strict test of Eq (1), since their fit was biased by processes other than physiological that affect observed records. On the other hand, Higgins and Richardson [60] also applied their fitted models to a hypothetical world with equally common environmental zones. This means a uniform distribution of variable values in E space, which by Eq (1) would imply they removed the reducing effects of anisotropic environmental space yielding values of N*(t, G) much larger than for the real world, a result that indeed is shown in their Fig 2 but is not elaborated in their discussion.
Our tests of niche inequalities mostly support their validity but required a restricted definition of niche based on a specific type of variable (scenopoetic climate). This definition is operational and enables the use of large and existing datasets, but most importantly, multivariate climate niches allow definitions to be based on straightforward set operations with direct relationships to area, which is a major advantage. Less restrictive definitions can be used to try to understand relations between niches and areas of distribution [47], but many complications may thus remain hidden. For instance, inequalities Eq (1) reveals the critical importance of considering the existing niche space as an integral part of niche analysis [9, 61]. The fundamental niche is expressed in specific environments which are dynamic. Our analysis shows how apparent patterns in distributions of realized niches may have as a parsimonious explanation the bias in the location of fundamental niches in the anisotropic environmental space.
Although our test of Hutchinson’s inequality was essentially successful, it relies on reducing the scope of meaning of terms, using only certain types of variables for the multivariate space, resorting to some mostly ignored concepts (existing niche), and stating explicitly a number of assumptions about fundamental niches (convexity, inequalities). These are steps in the development of a rigorous theory of Grinnellian niches, one that we think is much needed not only to provide a conceptual scaffolding to the burgeoning field of niche modeling, but also because it may end clarifying and illuminating many question in biogeography and macroecology.
Supporting information
(DOCX)
Acknowledgments
We are grateful to Luis Osorio for his help writing R scripts, to T. Peterson for valuable advice on an early version of the MS, to A. Diniz-Filho for his insightful comments and to an anonymous reviewer for helpful suggestions. We are also grateful to the innumerable sources of GBIF data, primarily museums and herbaria worldwide. This work was partially supported by NSF’s ARBOR 1208472, and BIOTAPHY 1458640 grants to JS and by a Consejo Mexiquense de Ciencia y Tecnología—Consejo Nacional de Ciencia y Tecnología 215842 scholarship for studies abroad (Mexico) for BA. JS would also like to acknowledge the support of Blitzi Soberón.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partially supported by NSF’s ARBOR 1208472, and BIOTAPHY 1458640 grants to JS and by a Consejo Mexiquense de Ciencia y Tecnología - Consejo Nacional de Ciencia y Tecnología 215842 scholarship for studies abroad (Mexico) for BA. JS would also like to acknowledge the support of Blitzy Soberón.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hutchinson GE. Concluding remarks. Cold Spring Harbor Symp Quant Biol. 1957; 22:415–27. [Google Scholar]
- 2.Kearney M, Porter WP. Mapping the fundamental niche: physiology, climate and the distribution of a nocturnal lizard. Ecology. 2004; 85(11):3119–31. [Google Scholar]
- 3.Austin M. A silent clash of paradigms: some inconsistencies in community ecology. Oikos. 1999;86:170–8. [Google Scholar]
- 4.Leathwick J. Are New Zealand's Nothofagus species in equilibrium with their environment? J Veg Sci. 1998; 9(5):719–32. [Google Scholar]
- 5.Svenning J-C, Skov F. Limited filling of the potential range in European tree species. Ecol Lett. 2004; 7(7):565–73. [Google Scholar]
- 6.Soberón J, Nakamura M. Niches and distributional areas: concepts, methods, and assumptions. Proceedings of the National Academy of Sciences. 2009; 106(Supplement 2):19644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson AT, Soberón J, Pearson RG, Anderson R, Martínez-Meyer E, Nakamura M, et al. Ecological Niches and Geographic Distributions. Levine SA, Horn HS, editors. Princeton: Princeton University Press; 2011. 336 p. [Google Scholar]
- 8.Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. Glob Ecol Biogeogr. 2006; 33:1677–88. [Google Scholar]
- 9.Colwell RK, Futuyma D. On the measurement of niche breadth and overlap. Ecology. 1971; 52(4):567–76. [DOI] [PubMed] [Google Scholar]
- 10.Jackson ST, Overpeck JT. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology. 2000; 26 (Supplement)(4):194–220. [Google Scholar]
- 11.Chase JM, Leibold M. Ecological Niches: Linking Classical and Contemporary Approaches. Chicago and London: University of Chicago Press; 2003. 212 p. [Google Scholar]
- 12.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007; 10(12):1115–23. 10.1111/j.1461-0248.2007.01107.x [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson GE. An Introduction to Population Ecology. New Haven: Yale University Press; 1978. 260 p. [Google Scholar]
- 14.Chase JM, Leibold MA. Spatial scale dictates the productivity-biodiversity relationship. Nature. 2002; 416:427–30. 10.1038/416427a [DOI] [PubMed] [Google Scholar]
- 15.Cooper VS. Long-term experimental evolution in Escherichia coli. X. Quantifying the fundamental and realized niche. BMC Evol Biol. 2002;2:12 10.1186/1471-2148-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aspinall R, Lees BG. Sampling and analysis of spatial environmental data. In: Waugh TC, Healey RG, editors. Proceedings of the Sixth International Symposium on Spatial Data Handling. 2. Edinburgh: University of Edinburgh; 1994. p. 1066–97.
- 17.Lindenmayer DB, Nix HA, McMahon JP, Hutchinson MF, Tanton T. The conservation of Leadbeater's possum, Gymnobelideus leadbeateri (McCoy): a case study of bioclimatic modelling. J Biogeogr. 1991; 18:371–83. [Google Scholar]
- 18.Colwell RK, Rangel TF. Hutchinson's duality: the once and future niche. Proc Natl Acad Sci USA. 2009; 106(suppl. 2):19644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soberón J, Peterson AT. Ecological shifts and environmental space anisotropy: a cautionary note. Rev Mex Biodiv. 2011; 82:1348–55. [Google Scholar]
- 20.Jiménez-Valverde A, Lobo JM, Hortal J. Not as good as they seem: the importance of concept in species distribution modelling. Diversity Distrib. 2008; 14(6):885–90. [Google Scholar]
- 21.Holt RD, Gomulkiewicz R. The evolution of species niches: a populations dynamic perspective In: Othmer HG, Adler FR, Lewis MA, Dillon J, editors. Case Studies in Mathematical Modelling: Ecology, Physiology and Cell Biology. Englewood Cliffs, NJ: Prentice-Hall; 1997. p. 25–50. [Google Scholar]
- 22.Guisan A, Petitpierre B, Broennimann O, Daehler C, Kueffer C. Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol. 2014; 29(5):260–9. 10.1016/j.tree.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 23.Malanson GP, Westman WE, Yan Y-L. Realized versus fundamental niche functions in a model of chaparral response to climatic change. Ecol Model. 1992; 64:261–77. [Google Scholar]
- 24.Malanson GP. Simulated responses to hypothetical fundamental niches. J Veg Sci. 1997; 8(2):307–16. [Google Scholar]
- 25.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society of London B: Biological Sciences. 2011; 278(1713):1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy A. Bridging the gap between general circulation models (GCM) output and biological microenvironments. International Journal of Biometeorology. 1997; 40:119–22. [Google Scholar]
- 27.Kearney MR, Shamakhy A, Tingley R, Karoly DJ, Hoffmann AA, Briggs PR, et al. Microclimate modelling at macro scales: a test of a general microclimate model integrated with gridded continental-scale soil and weather data. Methods in Ecology and Evolution. 2014; 5(3):273–86. [Google Scholar]
- 28.Chown S, Gaston K, Robinson D. Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Functional Ecology. 2004; 18(2):159–67. [Google Scholar]
- 29.Bozinovic F, Calosi P, Spicer JI. Physiological correlates of geographic range in animals. Annual Review of Ecology, Evolution and Systematics. 2011; 42:155–79. [Google Scholar]
- 30.Cain SA. Foundations of Plant Geography. New York and London: Harper & Brothers; 1944. 556 p. [Google Scholar]
- 31.Gouveia SF, Hortal J, Tejedo M, Duarte H, Cassemiro FA, Navas CA, et al. Climatic niche at physiological and macroecological scales: the thermal tolerance–geographical range interface and niche dimensionality. Glob Ecol Biogeogr. 2014; 23(4):446–56. [Google Scholar]
- 32.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. Heat freezes niche evolution. Ecol Lett. 2013; 16(9):1206–19. 10.1111/ele.12155 [DOI] [PubMed] [Google Scholar]
- 33.Barve N, Martin C, Brunsell NA, Peterson AT. The role of physiological optima in shaping the geographic distribution of Spanish moss. Glob Ecol Biogeogr. 2014; 23(6):633–45. [Google Scholar]
- 34.De Frenne P, Rodríguez-Sánchez F, Coomes DA, Baeten L, Verstraeten G, Vellend M, et al. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences. 2013; 110(46):18561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceballos G, Ehrlich PR, Soberón J, Salazar I, Fay JP. Global mammal conservation: what must we manage? Science. 2005; 309:603–7. 10.1126/science.1114015 [DOI] [PubMed] [Google Scholar]
- 36.Hijmans RJ, Van Etten J. Raster: geographic data analysis and modeling. R package version 2.1–49. See http://CRAN.R-project.org/package=raster; 2013.
- 37.Hijmans RJ, Cameron S, Parra J, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climat. 2005; 25:1965–78. [Google Scholar]
- 38.Hijmans RJ, Phillips S, Leathwick J, Elith J. Package dismo: Species distribution modeling. 2011.
- 39.Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography. 2015; 38(5):541–5. [Google Scholar]
- 40.Dormann CF, McPherson J, Araújo M, Bivand R, Bolliger J, Carl G, et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography. 2007; 30:609–28. [Google Scholar]
- 41.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. 537 p. [Google Scholar]
- 42.Blonder B, Lamanna C, Violle C, Enquist BJ. The n-dimensional hypervolume. Glob Ecol Biogeogr. 2014; 23(5):595–609. [Google Scholar]
- 43.Stine RA, Heyse JF. Non-parametric estimates of overlap. Statistics in medicine. 2001; 20(2):215–36. [DOI] [PubMed] [Google Scholar]
- 44.Soberón J, Peterson AT. Interpretation of models of fundamental ecological niches and species' distributional areas. Biodiv Info. 2005; 2:1–10. [Google Scholar]
- 45.Hof C, Rahbek C, Araújo MB. Phylogenetic signals in the climatic niches of the world's amphibians. Ecography. 2010; 33(2):242–50. [Google Scholar]
- 46.Angilletta M. Thermal Adaptation A Theoretical and Empirical Synthesis. Oxford: Oxford University Press; 2009. 289 p. [Google Scholar]
- 47.Slatyer RA, Hirst M, Sexton JP. Niche breadth predicts geographical range size: a general ecological pattern. Ecol Lett. 2013; 16(8):1104–14. 10.1111/ele.12140 [DOI] [PubMed] [Google Scholar]
- 48.Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends Ecol Evol. 2003; 18(3):119–25. [Google Scholar]
- 49.Kelly MW, Sanford E, Grosberg RK. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proceedings of the Royal Society of London B: Biological Sciences. 2012; 279(1727):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angert AL, Schemske DW. The evolution of species distributions: reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution. 2005; 59(8):1671–84. [PubMed] [Google Scholar]
- 51.Grubb PJ. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol Rev. 1977; 52:107–45. [Google Scholar]
- 52.Pulliam R. On the relationship between niche and distribution. Ecol Lett. 2000; 3(4):349–61. [Google Scholar]
- 53.Drake JM. Range bagging: a new method for ecological niche modelling from presence-only data. Journal of The Royal Society Interface. 2015; 12(107):20150086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheth SN, Angert AL. The evolution of environmental tolerance and range size: a comparison of geographically restricted and widespread Mimulus. Evolution. 2014; 68(10):2917–31. 10.1111/evo.12494 [DOI] [PubMed] [Google Scholar]
- 55.Calosi P, Bilton DT, Spicer JI, Votier SC, Atfield A. What determines a species’ geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). J Anim Ecol. 2010; 79(1):194–204. 10.1111/j.1365-2656.2009.01611.x [DOI] [PubMed] [Google Scholar]
- 56.Gaston KJ, Spicer JI. The relationship between range size and niche breadth: a test using five species of Gammarus (Amphipoda). Glob Ecol Biogeogr. 2001; 10(2):179–88. [Google Scholar]
- 57.Birch LC. Experimental background to the study of the distribution and abundance of insects: I. The influence of temperature, moisture and food on the innate capacity for increase of three grain beetles. Ecology. 1953; 34(4):698–711. [Google Scholar]
- 58.Kleidon A, Mooney HA. A global distribution of biodiversity inferred from climatic constraints: results from a process-based modelling study. Global Change Biology. 2000; 6(5):507–23. [Google Scholar]
- 59.Higgins SI, O’Hara RB, Bykova O, Cramer MD, Chuine I, Gerstner E-M, et al. A physiological analogy of the niche for projecting the potential distribution of plants. J Biogeogr. 2012; 39(12):2132–45. [Google Scholar]
- 60.Higgins SI, Richardson DM. Invasive plants have broader physiological niches. Proceedings of the National Academy of Sciences. 2014; 111(29):10610–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soberón J, Peterson AT. Ecological niche shifts and environmental space anysotropy: a cautionary note. Rev Mex Biodiv. 2011; 82(4):1348–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.