Abstract
ExoU, a potent patatin-like phospholipase, causes rapid cell death following its injection into host cells by the Pseudomonas aeruginosa type III secretion system. To better define regions of ExoU required for cytotoxicity, transposon-based linker insertion mutagenesis followed by site-directed mutagenesis of individual residues was employed by using a Saccharomyces cerevisiae model system. Random insertion of five amino acids identified multiple regions within ExoU that are required for cell killing. Five regions were chosen for further characterization: three corresponded to the oxyanion hole, hydrolase motif, and catalytic aspartate motif of the patatin-like domain within the N-terminal half of ExoU; one corresponded to an uncharacterized part of the patatin-like domain; and one corresponded to a region near the C terminus. Specific individual amino acid substitutions in each of the four N-terminal regions prevented killing of yeast and significantly reduced phospholipase activity. Whereas five amino acid insertions in the fifth region near the C terminus markedly reduced cytotoxicity and phospholipase activity, substitution of individual amino acids did not abolish either activity. To determine whether each of the five identified regions of ExoU was also essential for cytotoxicity in human cells, representative mutant forms of ExoU fused to green fluorescent protein were expressed in HeLa cells. These variants of ExoU were readily visualized and caused minimal cytotoxicity to HeLa cells, while wild-type ExoU fused to green fluorescent protein induced significant cell lysis and no detectable fluorescence. Thus, a minimum of five regions, including one which is well removed from the patatin-like domain, are required for the cytotoxicity and phospholipase activity of ExoU.
Pseudomonas aeruginosa is responsible for a significant proportion of nosocomial infections (2). It causes acute infections such as pneumonia, urinary tract infections, wound infections, and keratitis in hospitalized patients and is also a common cause of chronic respiratory infections in individuals with cystic fibrosis (36). Given that P. aeruginosa is naturally resistant to many antibiotics and readily acquires resistance to others, there is a pressing need for novel therapies. A better understanding of P. aeruginosa pathogenesis will assist efforts to develop new treatments for these infections.
The type III secretion system is a major virulence determinant of P. aeruginosa and, as such, is implicated in disease pathogenesis (13). Four known effector proteins are secreted by this system: ExoS, ExoT, ExoU, and ExoY. Evidence for a significant role in virulence is particularly compelling with ExoU. This toxin is cytolytic to many mammalian cell types including macrophages, neutrophils, epithelial cells, and fibroblasts (5-7, 10-12, 16, 17, 26, 37). Cytotoxicity, in turn, is thought to contribute to the increased severity of infections associated with strains that secrete ExoU (31). In animal models of acute pneumonia, disruption of the exoU gene resulted in decreased virulence (11, 17), whereas transformation with an exoU-expressing plasmid increased the virulence of strains that did not naturally secrete ExoU (1). Although only 30% of isolates from acute infections harbor the gene that encodes this protein (9), animals or humans infected with ExoU-secreting strains appear to have more severe illness than those infected with strains that do not secrete this toxin. For example, patients with hospital-acquired pneumonia who were infected with ExoU-secreting isolates had poorer outcomes than patients infected with isolates that did not secrete type III proteins (15), suggesting that this toxin contributes to P. aeruginosa pathogenesis in humans.
Recently, it has been shown that the N-terminal half of ExoU contains a patatin-like phospholipase domain and that ExoU is indeed a phospholipase (24, 29). Patatin is the major storage glycoprotein found in potatoes (14, 38) but also has phospholipase A2 (PLA2) activity used for protection under conditions of stress or infection (18, 35). Proteins containing patatin-like domains are more commonly encoded by bacterial pathogens and symbionts than by nonpathogenic bacteria, suggesting that these domains play important roles in bacterium-host interactions (3). Patatin contains three conserved motifs: (i) an oxyanion hole (G-G-X-R/K), which stabilizes the negative charge that develops upon nucleophilic attack by the catalytic serine during substrate cleavage (8); (ii) a hydrolase motif (G-X-S-X-G), which contains a catalytic serine (18, 28); and (iii) a catalytic aspartate motif (D-X-G/A) (18, 28). These same motifs are present in ExoU (Fig. 1A), and mutation of the putative catalytic serine (S142) or catalytic aspartate residues (D344) resulted in a loss of in vitro and in vivo phospholipase activity as well as cytotoxicity (24, 29). Interestingly, phospholipase activity was detected in vitro only in the presence of eukaryotic cell extracts (24, 29), indicating that a eukaryotic factor(s) is required for ExoU enzymatic activity. The identity and binding site of this cofactor are currently unknown.
FIG. 1.
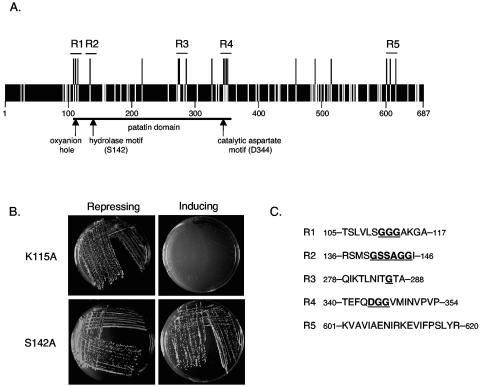
Multiple regions of ExoU are required for cytotoxicity in yeast. A. Transposon-based linker insertion mutagenesis was employed to randomly insert five amino acids throughout ExoU. The location of each insertion within the 687-amino-acid ExoU protein is depicted by a white bar. Insertions that abolished cytotoxicity in yeast are depicted as black bars extending above the protein. Five regions, represented as R1, R2, R3, R4, and R5, were chosen for further characterization. B. The effects of representative single-amino-acid substitutions in ExoU on yeast viability are shown. As was the case for wild-type ExoU, a K115A substitution did not affect the growth of yeast under conditions that repress expression of ExoU (SC-Glc agar) but prevented growth under conditions that induced expression of ExoU (SC-Gal agar). In contrast, a S142A substitution prevented ExoU-mediated killing during growth on inducing SC-Gal agar. C. Amino acids within regions 1 to 5 that resulted in a loss of cytotoxicity when changed are shown in boldface type and are underlined. All other listed amino acids did not abolish cytotoxicity when changed.
Limited structure-function studies have identified several large regions of ExoU that are necessary for cytotoxicity (27, 29). Expression of ExoU in Chinese hamster ovary (CHO) cells or in a Saccharomyces cerevisiae model system yielded similar results and demonstrated that deletion of amino acids 83 to 119, 301 to 342, or 624 to 687 of this 687-amino-acid toxin abolished cytotoxicity (27, 29). Subsequent studies found that deletion of as few as 20 amino acids (668 to 687) from the C terminus of ExoU was sufficient to prevent ExoU-mediated cell killing (24). In contrast, deletion of amino acids 1 to 82 did not affect cytotoxicity (27, 29). These studies indicated that multiple regions of ExoU, including portions of the N terminus, the C terminus, and internal regions, are required for cytotoxicity and that S. cerevisiae is an appropriate model system for studying ExoU-mediated cell killing.
In this study, we performed transposon-based linker insertion mutagenesis to further define the regions of ExoU that were required for cytotoxicity. We found that at least five regions of ExoU are necessary for the killing of yeast and human cells and that these regions are also required for phospholipase activity. Within these regions, specific amino acid residues essential for ExoU-mediated killing were identified. These data indicate that phospholipase activity and cytotoxicity are directly correlated and that, importantly, regions beyond the patatin-like domain are required for phospholipase activity and cytotoxicity.
MATERIALS AND METHODS
Bacterial and yeast strains, media, and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strain XL1-Blue was grown in Luria-Bertani broth. P. aeruginosa strain PA103 was grown on Luria-Bertani agar or in MINS medium (23) at 37°C. Antibiotics were used when necessary at the following concentrations: 50 μg of ampicillin/ml and 5 μg of tetracycline/ml for E. coli and 500 μg of carbenicillin/ml and 100 μg of tetracycline/ml for P. aeruginosa. S. cerevisiae strain INVSc1 was grown in yeast-peptone-dextrose medium or in synthetic complete (SC) medium containing 2% glucose (SC-Glc) or 2% galactose (SC-Gal) and lacking uracil to maintain plasmids (27). HeLa cells were grown in Eagle's minimal essential medium (American Type Culture Collection, Manassas, Va.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) in 5% CO2 at 37°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PA103 | Secretes ExoT and ExoU; laboratory isolate | 21 |
| PA103ΔU | Secretes only ExoT | This work |
| PA103ΔUT | Secretes no known effectors; makes functional secretion apparatus | This work |
| PA103ΔUT/G112W | Secretes ExoU-G112W | This work |
| PA103ΔUT/S142A | Secretes ExoU-S142A | This work |
| PA103ΔUT/G286W | Secretes ExoU-G286W | This work |
| PA103ΔUT/D344A | Secretes ExoU-D344A | This work |
| PA103ΔUT/LS608 | Secretes ExoU-LS608 (MFKHE inserted before residue N608) | This work |
| PA103ΔUT/ExoU | Secretes wild-type ExoU | This work |
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZ ΔM15 Tn10 (Tetr)] | Stratagene |
| XL1-Blue MRF′ Kan | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZ ΔM15 Tn5 (Kanr)] | Stratagene |
| S17.1 | thi pro hsdR recA RP4-2 (Tet::Mu) (Km::Tn7) | 34 |
| S. cerevisiae INVSc1 | his3Δ1/his3Δ1 leu2/leu2 trp1-289/trp1-289 ura3-52/ura3-52 | Invitrogen |
| Plasmids | ||
| Mini-CTX1 | Plasmid for gene integration into the chromosomal attB locus; Tetr | 19 |
| Mini-CTX1-ExoU | exoU gene ligated into mini-CTX1; Tetr | This work |
| pEX100T | Plasmid for allelic replacement; Apr (Cbr)a | 32 |
| pAH806 | exoU gene in pBR322; Apr | 17 |
| pAH808 | pAH806 with deleted 1.5-kb BsmI-BsmI fragment 5′ of the exoU gene; Apr | This work |
| pGS012 | ΔexoUb in pBR322; Apr | This work |
| pGS013 | ΔexoU in pEX100T; Apr (Cbr) | This work |
| pCM104 | ΔexoT in pEX100T; Apr (Cbr)c | 33 |
| pYC2/NT A (pVector) | Plasmid for galactose-inducible expression in yeast; Apr (Ura+)d | Invitrogen |
| pExoU | exoU in pVector; Apr (Ura+) | 28 |
| pExoU-G112W | exoU-G112W in pVector; Apr (Ura+) | This work |
| pExoU-S142A | exoU-S142A in pVector; Apr (Ura+) | This work |
| pExoU-G286W | exoU-G286W in pVector; Apr (Ura+) | This work |
| pExoU-D344A | exoU-D344A in pVector; Apr (Ura+) | This work |
| pExoU-LS608 | exoU-LS608 (MFKHE inserted before residue N608) in pVector; Apr (Ura+) | This work |
| pCDNA 3.1 NT-GFP (pGFP) | Plasmid for GFP expression in mammalian cells; Apr | Invitrogen |
| pGFP-ExoU | exoU in pGFP encoding ExoU containing N-terminal GFP tag; Apr | This work |
| pGFP-ExoU-G112W | exoU-G112W in pGFP encoding ExoU-G112W with N-terminal GFP tag; Apr | This work |
| pGFP-ExoU-S142A | exoU-S142A in pGFP encoding ExoU-S142A with N-terminal GFP tag; Apr | This work |
| pGFP-ExoU-G286W | exoU-G286W in pGFP encoding ExoU-G286W with N-terminal GFP tag; Apr | This work |
| pGFP-ExoU-D344A | exoU-D344A in pGFP encoding ExoU-D344A with N-terminal GFP tag; Apr | This work |
| pGFP-ExoU-LS608 | exoU-LS608 in pGFP encoding ExoU-LS608 with N-terminal GFP tag; Apr | This work |
Apr in E. coli, Cbr in P. aeruginosa.
ΔexoU refers to an allele of exoU containing an in-frame deletion.
ΔexoT refers to an allele of exoT containing an in-frame deletion.
Apr in E. coli, Ura+ in S. cerevisiae.
Yeast cytotoxicity assay.
The exoU gene was expressed from an inducible GAL1 promoter in pYC2/NT A (hereafter referred to as pVector) such that toxin production was repressed in the presence of glucose and induced in the presence of galactose. pVector containing the wild-type exoU gene (pExoU) (27) or a mutated exoU allele was transformed into INVSc1 by using the Frozen-EZ Yeast Transformation II method (ZymoResearch, Orange, Calif.) as described by the manufacturer. Yeast-containing plasmids were grown on SC-Glc agar, and individual colonies were then streaked onto both SC-Glc agar and SC-Gal agar. Following growth at 30°C for 3 to 5 days, plates were visually inspected for yeast colonies.
Transposon-based linker insertion mutagenesis.
The GPS-LS Linker Scanning method (New England Biolabs, Beverly, Mass.) was used to randomly insert Tn7-based transposons throughout pExoU, and the resulting constructs were transformed into XL1-Blue cells. Plasmid DNA from transformants was purified by using a Qiaprep Spin Miniprep approach (QIAGEN, Valencia, Calif.), digested with BamHI and NotI (New England Biolabs), and electrophoresed through a 0.8% (wt/vol) agarose gel. The size of the exoU-containing DNA fragment was estimated to determine whether a transposon had inserted within the gene or within the vector backbone. The exact location and frame of each insertion within the exoU gene were determined by sequencing the DNA adjacent to the transposon insertion site. Nucleotide sequencing was performed on the corresponding plasmid with a primer (5′-CATAACAAAAGTCCAGTATG-3′) complementary to the 3′ region of the transposon. Plasmids in which the transposon insertion resulted in the creation of a stop codon, an event that occurred in one-third of insertions, were not analyzed further. The remaining plasmids were digested with PmeI and religated to remove all of the transposon sequence except a residual 15-bp segment that encoded a five-amino-acid insertion in the ExoU protein.
Site-directed mutagenesis of the exoU gene.
Individual amino acids were changed to the corresponding least favorable amino acid (4) by using the QuikChange II site-directed mutagenesis approach (Stratagene, La Jolla, Calif.). Briefly, 50 ng of pExoU DNA was combined with 1.5 μl of forward primer (10 μM), 1.5 μl of reverse primer (10 μM), 1 μl of deoxynucleoside triphosphates (supplied by manufacturer), and 1 μl of 2.5 U of Pfu Ultra polymerase/ml (see Table S1 in the supplemental material [http://bugs.mimnet.northwestern.edu/hauser/labsite/research.htm] for primer sequences). Amplification was performed by using the following parameters: 95°C for 3 min and then 16 cycles of 95°C for 30 s, 55 to 58°C for 1 min, and 72°C for 8.5 min, followed by 72°C for 10 min. The amplified products were digested with DpnI to degrade the parental DNA and transformed into XL1-Blue cells (22). DNA was isolated by using the Qiaprep Spin Miniprep method, and all mutated exoU alleles were verified by nucleotide sequencing.
PLA2 assay.
PLA2 activity was measured by using the Cayman Chemicals (Ann Arbor, Mich.) cPLA2 assay as described by the manufacturer. Briefly, yeast transformants containing plasmids expressing wild-type or mutated alleles of the exoU gene were grown at 30°C with shaking in SC-Glc medium. After 18 h, yeast cells were collected by centrifugation at 2,300 × g, resuspended, diluted in SC-Gal medium to an optical density at 600 nm of 0.4, and grown for 30 min at 30°C to induce expression of the toxin. Yeast cells from 75 ml of culture were then collected by centrifugation at 2,300 × g for 5 min, washed once with 500 μl of H2O, and resuspended in 60 μl of 0.02 M Tris-HCl (pH 8.0)-0.05 M ammonium acetate supplemented with a Complete Mini EDTA-free protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, Ind.). Following the addition of acid-washed glass beads (≤100-μm diameter; Sigma, St. Louis, Mo.), cells were vortexed at 4°C for 10 min and allowed to settle on ice. Supernatants were cleared of debris by centrifugation at 14,000 × g for 10 min at 4°C, and 15 μl of each supernatant was added to 200 μl of 1-O-hexadecyl-2-deoxy-2-thio-R-arachidonoyl-sn-glyceryl-3-phosphorylcholine substrate (provided by the manufacturer) in a 96-well tray. After incubation at room temperature for 1 h, 10 μl of Ellman's reagent [5,5′-dithio-bis(2-dinitrobenzoic acid)] with EGTA (provided by manufacturer) was added to each well, and the absorbance at 490 nm (A490) was measured. Bee venom (300 pg; provided by the manufacturer) was used as a positive control.
Generation of GFP-tagged ExoU.
Fusion proteins consisting of ExoU (wild type or mutant) and green fluorescent protein (GFP) were generated as follows. An AgeI restriction endonuclease site was introduced 5′ and a KpnI site was introduced 3′ of the exoU gene by PCR amplification of pExoU by using upstream primer 5′-AAAAAAACCGGTAATGCATATCCAATCG-3′ (the AgeI site is in italics) and downstream primer 5′-AAAAAAGGTACCTCATGTGAACTCCTTATTCC-3′ (the KpnI site is in italics). The DNA was amplified by using the following parameters: 95°C for 2 min and then 35 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 90 s, followed by 72°C for 10 min. The amplified products and the mammalian transfection vector pCDNA 3.1 NT-GFP (hereafter called pGFP) were digested with AgeI and KpnI (New England Biolabs) and purified by electrophoresis through a 0.8% (wt/vol) agarose gel. The two DNA fragments were ligated together and transformed into XL1-Blue competent cells. Transformants were checked for correct insertion by restriction endonuclease digestion with AgeI and KpnI, and the exoU gene in each construct was verified by nucleotide sequencing.
Mammalian cell transfections.
One day prior to transfection, 5 × 104 HeLa cells were seeded into each well of a 24-well tissue culture plate. Transient transfections were performed by adding 25 μl of prewarmed serum-free medium, 0.75 μl of Fugene 6 (Roche Applied Science), and 0.25 μg of DNA to each well of cells, followed by incubation at 37°C in 5% CO2 for 24 h.
Microscopic detection of ExoU in transfected cells.
To quantify the percentage of cells expressing GFP-tagged forms of ExoU, transfected HeLa cells were viewed at a magnification of ×100 by using a Leica Eclipse fluorescent microscope with a fluorescein isothiocyanate filter. Fluorescent cells and total cells were counted in three randomly chosen fields from each of three wells per transfection, and the percentage of fluorescent cells was calculated.
Human cell cytotoxicity assays.
ExoU-mediated cell killing of transfected HeLa cells was quantified by measuring lactate dehydrogenase (LDH) release using the CytoTox 96 Non-Radioactive cytotoxicity method (Promega, Madison, Wis.). Briefly, cells grown for 24 h posttransfection in 24-well plates were centrifuged at 180 × g for 5 min, and 50 μl of the overlying medium was added to 50 μl of substrate (provided by manufacturer). The mixture was incubated at room temperature in the dark for 30 min, after which 50 μl of stop solution (provided by the manufacturer) was added to each well. The A490 was then measured. Triton X-100 (0.9% [vol/vol]) was added to control wells to achieve 100% lysis of HeLa cells. The percent lysis of cells in sample wells was calculated as follows: 100 × (A490 sample − A490 uninfected cells)/(A490 cells and Triton X-100 − A490 uninfected cells).
Construction of PA103ΔUT.
In-frame deletions in the endogenous exoU and exoT genes of P. aeruginosa strain PA103 were created to generate strain PA103ΔUT, which did not secrete any known effector proteins but had an intact type III secretion system. To accomplish this, the exoU gene of PA103 was first replaced with an in-frame deletion allele by using the method of Schweizer and Hoang (32). The exoU gene was subcloned from the plasmid pAH806 by digestion with BsmI and self-ligation to remove a 1.5-kb DNA fragment 5′ of the exoU gene. The resulting plasmid, designated pAH808, was digested with XmaI and SalI, blunted by incubation with T4 DNA polymerase, and self-ligated to remove a 1.6-kb internal DNA fragment encoding amino acids 121 to 655 of ExoU, creating pGS012. In-frame religation was confirmed by nucleic acid sequencing. A 1.7-kb MscI-NruI DNA fragment of pGS012 containing the deletion exoU allele was blunted with T4 DNA polymerase and ligated into the SmaI site of the suicide vector pEX100T to create pGS013. pGS013 was transformed into E. coli strain S17.1 which was then mated with PA103 as described previously (33). Replacement of the wild-type exoU allele with the deleted allele was verified by using PCR amplification with the following primers: 5′-AGCGTTAGTGACGTGCG-3′ and 5′-GCAGCCTATCGTGCAAG-3′. This strain was designated PA103ΔU. PA103ΔUT was constructed by mating PA103ΔU with S17.1 carrying pCM104, a plasmid containing an in-frame deletion allele of the exoT gene, as previously described (33). Replacement of the endogenous exoT gene with the in-frame deletion was verified by using primers exoT-CM-2 and exoT5 (33). The absence of ExoU and ExoT secretion by PA103ΔUT was confirmed with immunoblot analysis with ExoU and ExoT antisera.
Expression of ExoU variants in P. aeruginosa.
ExoU variants were expressed in P. aeruginosa by integrating a copy of the mutated exoU allele with its endogenous promoter into the chromosome of strain PA103ΔUT. First, mutated exoU alleles were constructed by digesting pAH806, which contains the exoU gene, with BamHI and AgeI. The 3.1-kb exoU-containing fragment was purified by gel electrophoresis and ligated into mini-CTX1 that had been previously digested with BamHI and XmaI to create mini-CTX1-ExoU. Specific mutations were then introduced into mini-CTX1-ExoU by using the QuikChange II XL site-directed mutagenesis approach (Stratagene) with appropriate primers and transformed into XL1-Blue MRF′ Kan competent cells. (See Table S1 in the supplemental material [http://bugs.mimnet.northwestern.edu/hauser/labsite/research.htm] for primer sequences.) Each of these constructs was individually conjugated into the P. aeruginosa strain PA103ΔUT and integrated into the chromosomal attB site according to the method of Hoang et al. (19). Thus, mutant forms of ExoU were expressed under the control of the endogenous exoU promoter from a single-copy chromosomal insertion.
Immunoblot analyses.
Expressed variants of ExoU were detected in yeast by immunoblot analysis using polyclonal ExoU antiserum as previously described (27). The secretion of variant forms of ExoU by P. aeruginosa was tested by performing immunoblot analysis on the culture supernatants of bacteria grown in secretion-inducing MINS medium as previously described (31). To detect ExoU within HeLa cells, transfected cells were grown for 24 h and then washed twice with phosphate-buffered saline (PBS) without CaCl2 and MgCl2 (Gibco-Invitrogen, Carlsbad, Calif.). Cells in 3 wells of a 24-well plate were lysed and combined in a total of 500 μl of 1% (vol/vol) Triton X-100 (Fisher Scientific, Pittsburgh, Pa.) in phosphate-buffered saline supplemented with a Complete Mini EDTA-free protease inhibitor cocktail tablet (Roche Applied Science). Cell lysates were centrifuged at 10,000 × g, and 60 μl of the supernatant was mixed with 60 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Each sample was electrophoresed through a sodium dodecyl sulfate-10% polyacrylamide gel and transferred to a membrane. Immunoblot analysis was then performed as previously described by using polyclonal ExoU antiserum (17).
RESULTS
Identification of regions of ExoU required for cytotoxicity in yeast.
Previous studies had demonstrated that S. cerevisiae is a useful model system for the study of ExoU-mediated cytotoxicity (27, 29). Expression of wild-type ExoU in yeast resulted in the absence of growth, whereas expression of certain ExoU mutants did not affect viability. Production of even small amounts of wild-type ExoU was sufficient to cause such rapid cell death that it was not possible to detect this toxin within cells by immunoblot analysis (27). These characteristics and the genetic tractability of S. cerevisiae make it an ideal host for the performance of structure-function analyses of ExoU.
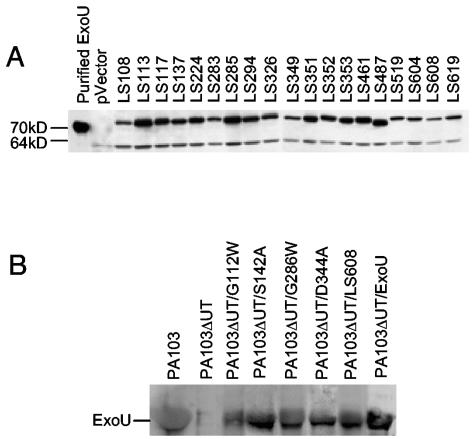
Transposon-based linker insertion mutagenesis was employed to define regions of ExoU necessary for cytotoxicity in yeast. This technique was used to randomly insert 15 nucleotides throughout pExoU, a plasmid containing the exoU gene under control of the GAL1 inducible promoter. A total of 115 constructs that contained insertions within the 2,064-bp exoU gene were identified (an average of one insertion every 18 nucleotides). Nucleotide sequencing was used to determine the location and nature of each insertion. Forty-two insertions that resulted in the creation of premature stop codons were identified. Since previous reports had shown that the C-terminal 20 amino acids of ExoU were essential for cytotoxicity (24), all insertions that resulted in truncation were not studied further. The remaining 73 constructs were transformed into yeast, and cytotoxicity was assessed by growth on SC-Glc agar, which repressed expression of the mutated exoU genes, and SC-Gal agar, which induced expression. As expected, yeast containing each of the 73 constructs grew on SC-Glc agar (data not shown). However, 19 of the constructs failed to prevent growth of yeast on inducing SC-Gal agar (Fig. 1A), suggesting that an essential region of the encoded ExoU protein had been altered in each construct. Insertions associated with a loss of cytotoxicity are shown in Table 2. (See Table S2 in the supplemental material [http://bugs.mimnet.northwestern.edu/hauser/labsite/research.htm] for insertions that did not abolish cytotoxicity.) Immunoblot analysis using polyclonal ExoU antiserum confirmed that each of these ExoU variants was stably expressed in yeast (Fig. 2A).
TABLE 2.
Noncytotoxic linker insertion variants of ExoUa
| Designation | Amino acid insertion |
|---|---|
| LS108 | VFKHL-V |
| LS113 | CLNSG-G |
| LS117 | VFKQG-A |
| LS137 | MFKHR-S |
| LS224 | VFKHL-E |
| LS283 | CLNTL-N |
| LS285 | CLNNI-T |
| LS294 | LFKQR-P |
| LS325 | MFKHQ-K |
| LS349 | MFKHM-I |
| LS351 | VFKHN-V |
| LS352 | CLNNV-P |
| LS353 | VFKQP-V |
| LS461 | CLNKE-R |
| LS487 | VFKQL-A |
| LS519 | CLNTF-T |
| LS604 | VFKQA-V |
| LS608 | MFKHE-N |
| LS619 | CLNTL-Y |
Amino acid insertions are represented by the five inserted amino acids followed by the residue (after the hyphen) immediately after the insertion. For example, LS108 contains VFKHL inserted before residue V108.
FIG. 2.
Noncytotoxic variant forms of ExoU are stably expressed in yeast and secreted by P. aeruginosa. A. Immunoblot analysis of cell lysates of yeast expressing noncytotoxic variants of ExoU. pVector refers to yeast containing empty vector. See Table 2 for an explanation of mutant ExoU designations. The lower band represents a yeast protein that is recognized by the anti-ExoU antiserum. B. Immunoblot analysis to detect wild-type and mutant ExoU secreted into supernatants of P. aeruginosa cultures.
The majority of insertions that resulted in a loss of cytotoxicity were clustered into four regions of ExoU comprised of residues 105 to 117, 278 to 288, 340 to 354, and 601 to 620 (Fig. 1A and C). These regions were chosen for further analysis. During the course of these studies, a patatin-like phospholipase domain was identified within the N-terminal half of ExoU (24, 29) (Fig. 1A). The putative oxyanion hole of this patatin-like domain was encompassed by the first of these regions (residues 105 to 117), and the catalytic aspartate motif was within another region (residues 340 to 354). The hydrolase motif containing the putative catalytic serine (S142) of this patatin-like domain was near a single insertion at residue 137 identified in our screen as abolishing cytotoxicity (Table 2 and Fig. 1A), suggesting that this region was also important in ExoU-mediated killing. Therefore, this region (residues 136 to 146) was also chosen for further analysis (Fig. 1A and C). Together, these regions were designated regions 1 to 5 (Fig. 1A and C). Regions 1 to 4 were within the patatin-like phospholipase domain of ExoU, whereas region 5 was close to the C terminus. Note that in addition to these clusters of insertions, single insertions associated with a loss of cytotoxicity were located at residues 224, 326, 461, 487, and 519 (Fig. 1A and Table 2). These findings confirmed that the patatin-like phospholipase domain of ExoU is required for cytotoxicity but also indicate that at least one region outside this domain is required for ExoU-mediated killing.
Identification of individual amino acids within regions 1 to 5 of ExoU required for cytotoxicity in yeast.
To identify individual amino acids necessary for ExoU-mediated cytotoxicity, site-directed mutagenesis was employed to change each residue within regions 1 to 5. An amino acid substitution resulting in a nonconservative change was made at each residue within these regions (Table 3). Constructs containing the mutated exoU alleles were transformed into yeast cells that were then grown on SC-Glc-repressing agar or SC-Gal-inducing agar. Yeast viability was assessed after 5 days of growth (Fig. 1B). Within region 1, cytotoxicity was abolished following changes to G111, G112, and G113, but the 4 to 6 amino acids on each side of these glycines were not necessary for killing (Table 3 and Fig. 1C). In region 2, six contiguous amino acids (140-G-S-S-G-G-G-145) but not adjacent residues were each required for killing. A single amino acid in region 3, G286, was required for yeast cytotoxicity. Three contiguous amino acids (344-D-G-G-346) within region 4 were each necessary for cell death. Finally, although multiple five-amino-acid insertions within region 5 resulted in a loss of cytotoxicity, no individual amino acids in this region were essential for killing. Each noncytotoxic single-amino-acid mutant of ExoU was stably expressed in yeast (see Fig. S1 in the supplemental material [http://bugs.mimnet.northwestern.edu/hauser/labsite/research.htm]).
TABLE 3.
Site-directed mutagenesis of individual residues within regions 1 to 5a
| Region 1 | Region 2 | Region 3 | Region 4 | Region 5 |
|---|---|---|---|---|
| T105G | R136L | Q278W | T340G | K601F |
| S106L | S137L | I279G | E341L | V602G |
| L107D | M138D | K280F | F342G | A603W |
| V108W | S139L | T281W | Q343W | V604W |
| L109D | G140W | L282D | D344A | I605G |
| S110L | S141L | N283F | G345W | A606W |
| G111W | S142A | I284G | G346W | E607W |
| G112W | S142L | T285W | V347G | N608F |
| G113W | A143W | G286W | M348G | I609G |
| A114W | G144W | T287W | I349G | R610F |
| K115A | G145W | A288W | N350F | K611F |
| K115F | I146G | V351G | E612W | |
| G116W | P352W | V613G | ||
| A117W | V353G | I614G | ||
| F615G | ||||
| P616W | ||||
| S617L | ||||
| L618G | ||||
| Y619G | ||||
| R620L |
Each mutation is designated as the wild-type amino acid, the location of the amino acid, and the amino acid to which it was changed. For example, T105G represents a change of the threonine at residue 105 to a glycine. Mutations resulting in a loss of cytotoxicity are shown in boldface type.
Analysis of PLA2 activity of ExoU mutants.
Since the phospholipase activity of ExoU is essential for cytotoxicity and PLA2 inhibitors prevent ExoU-mediated killing (24, 29), we wished to examine whether regions 1 to 5 of ExoU were required for PLA2 activity. ExoU variants containing the following amino acid substitutions were selected as representative mutants from regions 1 to 4: G112W, S142A, G286W, and D344A. These variants of ExoU were referred to as ExoU-G112W, ExoU-S142A, ExoU-G286W, and ExoU-D344A, respectively. Since no single-amino-acid change in region 5 abolished cytotoxicity, ExoU containing the five-amino-acid insertion at residue 608 (hereafter called ExoU-LS608) was chosen as a representative mutant for this region. As a control, the cytotoxic variant of ExoU containing an E607W substitution (referred to as ExoU-E607W) was also included in the analysis.
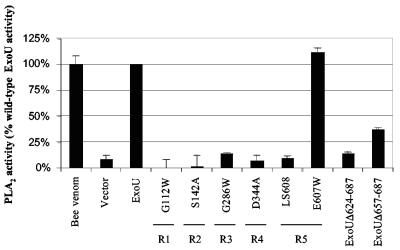
The PLA2 activities of the representative ExoU proteins with mutations in regions 1 to 5 were measured. After being induced to express ExoU, yeast cells were lysed and incubated with a synthetic arachidonoyl-thio-glycerophospholipid substrate. Cleavage of the sn-2 fatty acid in this substrate resulted in a free thiol group that reacted with Ellman's reagent to yield a chromogenic product measurable by spectrophotometry. The expression of wild-type ExoU in yeast for 30 min yielded a 10-fold increase in PLA2 activity relative to vector alone (Fig. 3). Similarly, the cytotoxic mutant ExoU-E607W was associated with wild-type levels of PLA2 activity. In contrast, ExoU-G112W, ExoU-S142A, ExoU-G286W, and ExoU-D344A were associated with levels of PLA2 activity similar to those of vector only, indicating that regions 1 to 4 of the patatin-like domain were each required for PLA2 activity. Likewise, ExoU-LS608, which contained a five-amino-acid insertion in region 5, also was associated with minimal PLA2 activity (Fig. 3). These data indicate that the loss of cytotoxicity observed with mutations in regions 1 to 4 of the patatin-like domain was associated with a reduction in PLA2 activity. Interestingly, region 5, which is well removed from the patatin-like domain, was also necessary for PLA2 activity.
FIG. 3.
Regions 1 to 5 of ExoU are required for PLA2 activity. PLA2 activity is expressed as a percentage of the activity observed with wild-type ExoU. Bee venom, which has potent PLA2 activity, was used as a positive control. Vector refers to yeast containing empty vector, and ExoU refers to yeast expressing wild-type ExoU. The PLA2 activities of ExoU with representative mutations in each of regions 1 to 5 are shown. E607W is an amino acid substitution mutant that retained cytotoxicity and is therefore used as a control. ExoUΔ624-687 is a noncytotoxic deletion mutant of ExoU lacking the C-terminal 64 amino acids, and ExoUΔ657-687 is a partially noncytotoxic deletion mutant lacking the C-terminal 31 amino acids. R1 to R5 refer to regions 1 to 5, respectively. Each assay was performed in triplicate. Error bars represent standard errors of the means.
Since the requirement of regions outside the patatin-like domain for phospholipase activity was unexpected, the correlation between cytotoxicity and PLA2 activity in the C-terminal half of ExoU was examined further. For these experiments, two previously constructed C-terminal truncated variants of ExoU were utilized. The first truncation was a noncytotoxic form of ExoU in which the C-terminal 64 amino acids were deleted (referred to as ExoUΔ624-687) (27). The second construct encoded a partially cytotoxic variant of ExoU lacking the C-terminal 31 amino acids (referred to as ExoUΔ657-687). Unlike the noncytotoxic ExoUΔ624-687, expression of ExoUΔ657-687 resulted in only a threefold reduction in cytotoxicity (27). PLA2 activity assays were performed on yeast expressing these two variants of ExoU. Whereas ExoUΔ624-687 was associated with minimal PLA2 activity, ExoUΔ657-687 was associated with only a threefold reduction in PLA2 activity compared to wild-type ExoU (Fig. 3). Together, these results show that PLA2 activity correlates with ExoU-mediated cytotoxicity and indicate that regions beyond the patatin-like phospholipase domain of ExoU are essential for PLA2 activity.
Cytotoxicity of mutant forms of ExoU in HeLa cells.
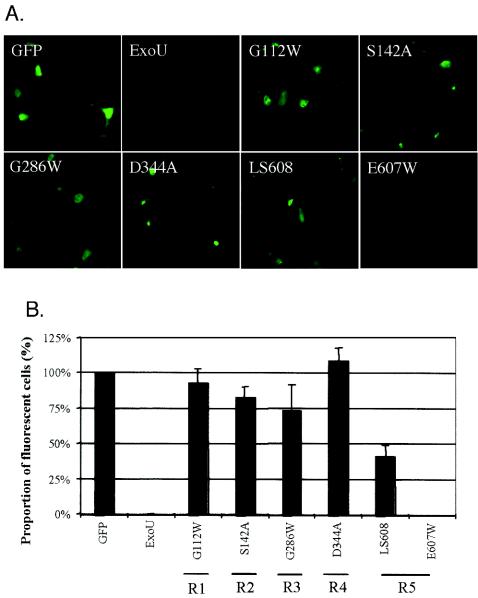
Having shown that regions 1 to 5 were essential for the killing of yeast, we next wished to determine whether these regions were also required for cytotoxicity in human cells. The exoU alleles encoding ExoU-G112W, ExoU-S142A, ExoU-G286W, ExoU-D344A, and ExoU-LS608 were ligated into the mammalian expression vector pCDNA3.1 NT-GFP (hereafter called pGFP), which resulted in the expression of ExoU fusion proteins with N-terminal GFP tags (referred to as GFP-ExoU-G112W, etc.). These constructs were transfected into HeLa cells, and their effects were examined microscopically. No fluorescence was visualized in HeLa cells transfected with a construct encoding wild-type ExoU fused to GFP, confirming that expression of ExoU was lethal to mammalian cells (10, 24) (Fig. 4A). Note that due to its potency, wild-type ExoU has not been detectable within other eukaryotic cell types (10, 27). Likewise, no GFP fluorescence was detected after transfection of the construct encoding the region 5 control GFP-ExoU-E607W, indicating that, as was seen in yeast, this mutant form of ExoU was cytotoxic to HeLa cells. However, fluorescence was observed in cells transfected with constructs encoding each of the other ExoU mutants fused to GFP (Fig. 4A), indicating that these fusion proteins were expressed within HeLa cells and were not as cytotoxic as wild-type ExoU. The proportion of cells expressing each of the GFP-tagged ExoU mutants was quantified by manually counting the percentage of fluorescent cells in representative fields. Whereas expression of wild-type ExoU fused to GFP and the point mutant GFP-ExoU-E607W, both of which were cytotoxic in yeast, resulted in no observed fluorescence, expression of the regions 1 to 5 representative mutant forms of ExoU tagged with GFP was associated with fluorescence of significant numbers of cells (Fig. 4B). Immunoblot analyses confirmed these findings. Wild-type ExoU fused to GFP was not detectable by immunoblot analysis of concentrated cell lysates from transfected cells using polyclonal ExoU antiserum, suggesting that expression of even small amounts of this protein was toxic to HeLa cells (see Fig. S2 in the supplemental material [http://bugs.mimnet.northwestern.edu/hauser/labsite/research.htm]). In contrast, each of the fusion proteins comprised of mutant forms of ExoU and GFP was detected in transfected HeLa cell lysates, indicating that these proteins were appropriately expressed and were not as cytotoxic as wild-type ExoU. Together, these results indicate that each variant of ExoU was expressed in HeLa cells, that wild-type ExoU was sufficient for the killing of these cells, and that regions 1 to 5 were essential for the killing process. In addition, these findings suggest that the lack of cytotoxicity associated with the ExoU mutants of regions 1 to 5 was not due simply to misfolding, since the observed fluorescence indicated that the GFP portion of these proteins was folded correctly, a finding that has been shown to correlate with proper folding of the entire protein (39, 40). Also consistent with appropriate folding was that each ExoU mutant (without a GFP tag) was secreted through the type III secretion apparatus into the culture medium when expressed in P. aeruginosa (Fig. 2B).
FIG. 4.
Mutations in regions 1 to 5 result in GFP-ExoU fusion proteins that are detectable in HeLa cells, indicating that they abolish or reduce cytotoxicity. A. Representative fields showing fluorescence of cells transfected with constructs encoding GFP or GFP-ExoU fusion proteins. B. Percentage of transiently transfected cells that fluoresced. Fluorescent and total cells were counted in three randomly chosen fields from each of three wells for each transfection to calculate the percentage of cells that fluoresced. R1 to R5 refer to regions 1 to 5, respectively, and indicate the region in which each representative mutation is located. Error bars represent standard errors of the means. The transfection efficiency was approximately 7%.
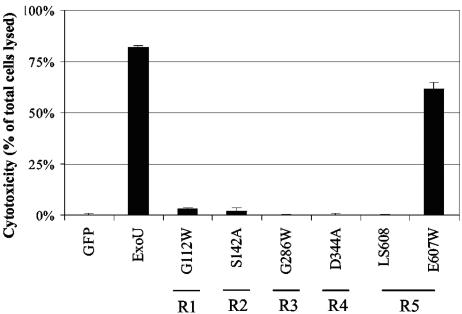
Cytotoxicity of each ExoU variant in HeLa cells was directly assessed by measuring release of LDH, a cytosolic enzyme of mammalian cells that escapes into the cell culture medium upon loss of membrane integrity (Fig. 5). Expression of the pGFP vector control was associated with minimal cytotoxicity in HeLa cells, while wild-type ExoU fused to GFP resulted in the death of approximately 80% of cells, confirming that wild-type ExoU was killing significant numbers of HeLa cells following transfection. Similarly, the control mutant GFP-ExoU-E607W, which was cytotoxic in yeast, was associated with high levels of killing in HeLa cells. In contrast, expression of GFP-ExoU-G112W, GFP-ExoU-S142A, GFP-ExoU-G286W, GFP-ExoU-D344A, or GFP-ExoU-LS608 caused very low levels of cell killing, comparable to the expression of GFP alone, indicating that regions 1 to 5 of ExoU were necessary for the killing of human cells. Thus, all regions identified as being essential for the killing of yeast were also necessary for cytotoxicity in human cells, confirming that S. cerevisiae is a useful model system for the study of ExoU-mediated killing.
FIG. 5.
Regions 1 to 5 of ExoU are required for cytotoxicity in HeLa cells. HeLa cells were transiently transfected with constructs encoding GFP or GFP-ExoU fusion proteins. Cytotoxicity was assessed by measuring LDH release into the culture medium 24 h after transfection and is expressed as the percentage of cells lysed. R1 to R5 refer to regions 1 to 5, respectively, and indicate the region in which each representative mutation is located. Error bars represent standard errors of the means.
DISCUSSION
Prior to the initiation of this work, several broad regions of ExoU had been identified as essential for cytotoxicity. Here, we used transposon-based linker insertion mutagenesis followed by site-directed mutagenesis to further define the key regions and residues that are necessary for ExoU-induced cell death. Five regions were characterized and shown to be essential for cytotoxicity in both yeast and HeLa cells. Four of these regions were located in the N-terminal half of ExoU, and a fifth region was near the C terminus of ExoU. Interestingly, each of these regions was also essential for PLA2 activity. These results emphasize that multiple regions of ExoU are required for phospholipase activity and cytotoxicity.
Since the commencement of this study, several advances that enhance the interpretation of our findings have been reported. A patatin-like phospholipase domain was identified in the N-terminal half of ExoU (24, 29), ExoU was shown to have phospholipase activity (24, 29), and the crystal structure of patatin was solved (28). Typical of patatin-like proteins, ExoU contains three motifs thought to be important in phospholipase activity: an oxyanion hole, a hydrolase motif, and a catalytic aspartate motif (24, 29). Superimposition of the patatin-like domain of ExoU onto the crystal structure of patatin isozyme Pat17 (28) allows prediction of the location of these motifs within the structure of ExoU (Fig. 6). An appreciation of these motifs, the essential role of phospholipase activity in cytotoxicity, and the putative structure of ExoU places regions 1 to 4 of our study in the appropriate context and helps explain their essential nature in ExoU-mediated cell killing.
FIG. 6.
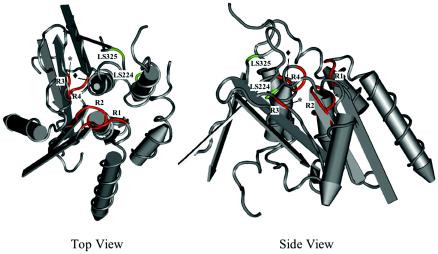
Regions 1 to 4 within the patatin-like domain of ExoU are predicted to border the active site binding channel. The patatin-like domain of ExoU was superimposed upon the crystal structure of patatin isozyme Pat17 (28) to map the location of regions identified as essential for cytotoxicity. Regions 1 to 4 are shown in red. The asterisks denote G286, and the diamonds denote D344. The insertion sites of LS224 and LS325, which also resulted in a loss of cytotoxicity, are shown in green. In the top view, the active site is facing the reader. In the side view, the structure is rotated 90° so that the active site is facing upward. Views were generated with Cn3D (41).
Region 1 of ExoU contains the putative oxyanion hole (112-G-G-A-K-115) (Fig. 1A), which forms part of the active binding channel in the putative crystal structure of ExoU (Fig. 6). Upon nucleophilic attack by the catalytic serine, the oxyanion hole stabilizes the negative charge that develops during substrate cleavage (8). Individual amino acid substitutions within this region confirmed that the two consecutive glycine residues that comprise the oxyanion hole (G112 and G113) of ExoU were required for cytotoxicity, as was the adjacent glycine G111. Surprisingly, K115, also a part of the oxyanion hole motif, was not required for cytotoxicity. This residue is highly conserved among the patatin-like domains of bacterial proteins, nearly always being a lysine or an arginine (3), and is thought to interact with the phosphate moiety of the phospholipid substrate (25). Previously published reports differ as to whether the corresponding residue in patatin (K17 in patatin B2 and R40 in patatin Pat17) is required for enzymatic activity (18, 28).
Region 2 (residues 136 to 146) is also predicted to form part of the active site binding channel of ExoU (Fig. 6) and encompasses the hydrolase motif G-X-S-X-G containing the putative catalytic serine S142 (24, 29). In lipases, the hydrolase motif lies in a tight turn between a β strand and an α helix, exposing the catalytic serine at the tip of a “nucleophilic elbow” (30). The small side chains of the glycines in this motif allow for this arrangement (30). In support of this model, G140, S141, A143, and G144 of region 2 were each essential for ExoU-mediated cytotoxicity. The glycine residue immediately adjacent to the hydrolase motif, G145, was also required for killing. This position may likewise require an amino acid with a small side chain, since it is nearly always occupied by a serine, alanine, or glycine in bacterial patatin-like proteins (3). Additionally, our results confirmed previous reports showing that the catalytic serine S142 was itself required for cytotoxicity (24, 29).
Although region 3 (residues 278 to 288) did not correspond to any previously described motifs in patatin, a single amino acid within this region, G286, was found to be essential for cytotoxicity. G286 is not predicted to lie in the active site binding channel of ExoU but rather immediately behind the catalytic aspartate motif (Fig. 6). Therefore, the substitution of a bulky tryptophan residue for a glycine at this position may have affected phospholipase activity by altering the position or orientation of the catalytic aspartate (D344).
Region 4 (residues 340 to 354) contains the catalytic aspartate motif, which also forms part of the putative active site binding channel of ExoU (Fig. 6). Individual amino acid substitutions within this region confirmed previous reports showing that the putative catalytic aspartate D344 was indeed required for cytotoxicity (24, 29). The adjacent two glycine residues in this motif, G345 and G346, were also essential for ExoU-mediated killing.
Of note, two other linker insertions within the patatin-like domain, LS325 and LS224, also abolished cytotoxicity (Fig. 1A and Table 2). Although these insertions were not chosen for further characterization, their locations were still informative. LS325 maps near the putative active site binding channel of ExoU (Fig. 6). This insertion lies immediately adjacent to a proline residue (P320 in ExoU) identified by Banerji and Flieger as being conserved in all bacterial patatin-like proteins (3). The importance of this region is consistent with the previous reports showing that the deletion of amino acids 301 to 342 of ExoU resulted in a loss of cytotoxicity (10, 27). LS224 maps to an α helix near the active site binding channel (Fig. 6). Substitutions of alanines for the native amino acids in nearby positions in patatin resulted in a 50 to 70% reduction in PLA2 activity (28). Thus, additional residues within the patatin-like domain of ExoU besides those in regions 1 to 4 are likely to be required for PLA2 activity and cytotoxicity.
Although the N terminus of ExoU contains the patatin-like phospholipase domain, the C terminus appears to be equally important for cytotoxicity. Because this portion of ExoU does not share homology with other characterized proteins, a crystal structure is not available for modeling purposes. Previously, it had been shown that a C-terminal deletion of as few as 20 amino acids from ExoU abolished cytotoxicity (10, 24, 27). In this study, linker insertions between amino acid residues 603 and 619 prevented ExoU-induced cell death. Together, these results suggest that residues 600 to 687 are essential for ExoU-mediated killing. However, linker insertions at amino acids 641, 658, 659, and 660 and individual amino acid substitutions of residues 601 to 620 did not prevent killing (Fig. 1C) (see Table S2 in the supplemental material [http://bugs.mimnet.northwestern.edu/hauser/labsite/research.htm]). This finding indicates that there is functional redundancy in this region of ExoU or that spacing but not amino acid composition is of critical importance. The C terminus may modify the lipase activity of the N terminus, perhaps by providing a binding site for the required eukaryotic cofactor. Prior studies have demonstrated that ExoU's phospholipase activity is apparent only in the presence of an unidentified host cofactor, although it is unclear how this cofactor interacts with ExoU. The C terminus may function as a binding site for such a factor or as a site for other kinds of posttranslational modifications essential for expression of phospholipase activity. In support of this hypothesis, a representative noncytotoxic variant of ExoU with a linker insertion in region 5 (ExoU-LS608) was also deficient in PLA2 activity. Alternatively, lipase activity alone may not be sufficient for cell death. For example, the C terminus may play an essential role in localizing the patatin-like phospholipase domain to a particular cellular compartment or may itself have a second activity required for killing.
These studies clearly show that regions 1 to 5 of ExoU are necessary for cytotoxicity but do not delineate their role in this process. These regions may participate in essential interactions with substrates, cofactors, or binding partners or may simply be required for proper folding of the ExoU protein. However, a number of observations suggested that the noncytotoxic variants of ExoU were correctly folded. Representatives of each class of mutants were stable when expressed in yeast and HeLa cells, fluoresced when expressed in HeLa cells as fusion proteins with GFP, and were appropriately secreted by the P. aeruginosa type III secretion machinery. Nonetheless, we cannot conclusively exclude the possibility that misfolding accounted for the loss of activity observed with these mutants.
All linker insertions in the N-terminal 102 amino acids of ExoU did not prevent cytotoxicity. This result is in agreement with previous reports showing that the deletion of the N-terminal 44, 51, or 82 amino acids of ExoU had no effect on killing but that the deletion of the N-terminal 99 or 119 amino acids abolished cytotoxicity when the toxin was introduced into yeast or CHO cells (10, 24, 27). Thus, the first approximately 80 amino acids of this protein are dispensable for cell killing but, as is the case with other type III effector proteins (20), may instead carry information that targets ExoU to the type III secretion and translocation apparatus.
The identification of regions of ExoU required for cytotoxicity has important implications both for understanding the mechanism by which this toxin kills eukaryotic cells and for preventing this killing. The use of the mutants derived in this study may allow for a better understanding of the role of each region of ExoU in killing. Furthermore, these regions constitute potential targets for therapeutic interventions, such as dominant-negative peptides, aimed at blocking this process. Neutralization of ExoU toxicity during infection would likely provide considerable clinical benefit, given the significant role this toxin plays in disease pathogenesis.
Acknowledgments
We thank Everett Roark, Parwez Nawabi, Sarah Hart, Amanda Silva, Ruth Emrick, Sarah Antinone, Grant Schulert, and Ciara Shaver for helpful technical suggestions and assistance. We also thank Herbert Schweizer for generously providing the pEX100T and mini-CTX1 vectors, Anjen Chenn for use of his microscope, and Richard Longnecker, Ciara Shaver, and Andrew Rabin for critical reading of the manuscript.
This work was supported by the National Institutes of Health (AI053674 [A.R.H.]).
Editor: J. B. Bliska
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 1998. National Nosocomial Infections Surveillance (NNIS) System report, data summary from October 1986-April 1998, issued June 1998. Am. J. Infect. Control 26:522-533. [DOI] [PubMed] [Google Scholar]
- 3.Banerji, S., and A. Flieger. 2004. Patatin-like proteins: a new family of lipolytic enzymes present in bacteria? Microbiology 150:522-525. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. J., and R. B. Russell. 2003. Amino acid properties and consequences of substitutions. .In M. R. Barnes and I. C. Gray (ed.), Bioinformatics for geneticists. Wiley, Hoboken, N.J.
- 5.Coburn, J., and D. Frank. 1999. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect. Immun. 67:3151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosson, P., L. Zulianello, O. Join-Lambert, F. Faurisson, L. Gebbie, M. Benghezal, C. van Delden, L. K. Curty, and T. Köhler. 2002. Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system. J. Bacteriol. 184:3027-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dessen, A., J. Tang, H. Schmidt, M. Stahl, J. D. Clark, J. Seehra, and W. S. Somers. 1999. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell 97:349-360. [DOI] [PubMed] [Google Scholar]
- 9.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 10.Finck-Barbançon, V., and D. W. Frank. 2001. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 183:4330-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck-Barbançon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. J. Fleiszig, C. Wu, L. Mende-Mueller, and D. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig, S. M., D. J. Evans, N. Do, V. Vallas, S. Shin, and K. E. Mostov. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank, D. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 14.Ganal, M. W., M. W. Bonierbale, M. S. Roeder, W. D. Park, and S. D. Tanksley. 1991. Genetic and physical mapping of the patatin genes in potato and tomato. Mol. Gen. Genet. 225:501-509. [DOI] [PubMed] [Google Scholar]
- 15.Hauser, A. R., E. Cobb, M. Bodí, D. Mariscal, J. Vallés, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 16.Hauser, A. R., and J. N. Engel. 1999. Pseudomonas aeruginosa induces type III secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser, A. R., P. J. Kang, and J. Engel. 1998. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 18.Hirschberg, H. J., J. W. Simons, N. Dekker, and M. R. Egmond. 2001. Cloning, expression, purification and characterization of patatin, a novel phospholipase A. Eur. J. Biochem. 268:5037-5044. [DOI] [PubMed] [Google Scholar]
- 19.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 20.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:279-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J. Infect. Dis. 116:112-116. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Nicas, T. I., and B. H. Iglewski. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 25.Pickard, R. T., X. G. Chiou, B. A. Strifler, M. R. DeFelippis, P. A. Hyslop, A. L. Tebbe, Y. K. Yee, L. J. Reynolds, E. A. Dennis, R. M. Kramer, and J. D. Sharp. 1996. Identification of essential residues for the catalytic function of 85-kDa cytosolic phospholipase A2. Probing the role of histidine, aspartic acid, cysteine, and arginine. J. Biol. Chem. 271:19225-19231. [DOI] [PubMed] [Google Scholar]
- 26.Pukatzki, S., R. H. Kessin, and J. J. Mekalanos. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 99:3159-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabin, S. D. P., and A. R. Hauser. 2003. Pseudomonas aeruginosa ExoU, a toxin transported by the type III secretion system, kills Saccharomyces cerevisiae. Infect. Immun. 71:4144-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rydel, T. J., J. M. Williams, E. Krieger, F. Moshiri, W. C. Stallings, S. M. Brown, J. C. Pershing, J. P. Purcell, and M. F. Alibhai. 2003. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry 42:6696-6708. [DOI] [PubMed] [Google Scholar]
- 29.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrag, J. D., and M. Cygler. 1997. Lipases and alpha/beta hydrolase fold. Methods Enzymol. 284:85-107. [DOI] [PubMed] [Google Scholar]
- 31.Schulert, G. S., H. Feltman, S. D. P. Rabin, C. G. Martin, S. E. Battle, J. Rello, and A. R. Hauser. 2003. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J. Infect. Dis. 188:1695-1706. [DOI] [PubMed] [Google Scholar]
- 32.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 33.Shaver, C. M., and A. R. Hauser. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed]
- 34.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Strickland, J. A., G. L. Orr, and T. A. Walsh. 1995. Inhibition of Diabrotica larval growth by patatin, the lipid acyl hydrolase from potato tubers. Plant Physiol. 109:667-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stryjewski, M. E., and D. J. Sexton. 2003. Pseudomonas aeruginosa infections in specific types of patients and clinical settings, p. 1-15. In A. R. Hauser and J. Rello (ed.), Severe infections caused by Pseudomonas aeruginosa, vol. 7. Kluwer Academic Publishers, Boston, Mass.
- 37.Vallis, A. J., V. Finck-Barbançon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vancanneyt, G., U. Sonnewald, R. Hofgen, and L. Willmitzer. 1989. Expression of a patatin-like protein in the anthers of potato and sweet pepper flowers. Plant Cell 1:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldo, G. S., B. M. Standish, J. Berendzen, and T. C. Terwilliger. 1999. Rapid protein-folding assay using green fluorescent protein. Nat. Biotechnol. 17:691-695. [DOI] [PubMed] [Google Scholar]
- 40.Wang, H., and S. Chong. 2003. Visualization of coupled protein folding and binding in bacteria and purification of the heterodimeric complex. Proc. Natl. Acad. Sci. USA 100:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, Y., L. Y. Geer, C. Chappey, J. A. Kans, and S. H. Bryant. 2000. Cn3D: sequence and structure views for Entrez. Trends Biochem. Sci. 25:300-302. [DOI] [PubMed] [Google Scholar]