Abstract
The aims of this study were to verify the prevalence of Cryptosporidium spp. and Giardia spp. in animal feces and drinking water on dairy farms and to identify a possible relation between the exposure factors and the presence of these parasites. Fecal samples from cattle and humans and water samples were collected on dairy farms in Paraná, Brazil. Analysis of (oo)cysts in the feces was performed by the modified Ziehl-Neelsen staining and centrifugal flotation in zinc sulfate. Test-positive samples were subjected to nested PCR amplification of the 18SSU ribosomal RNA gene for identification of Cryptosporidium and Giardia and of the gp60 gene for subtyping of Cryptosporidium. Microbiological analysis of water was carried out by the multiple-tube method and by means of a chromogenic substrate, and parasitological analysis was performed on 31 samples by direct immunofluorescence and nested PCR of the genes mentioned above. Identification of the species of Cryptosporidium was performed by sequencing and PCR with analysis of restriction fragment length polymorphisms. The prevalence of Giardia and Cryptosporidium was higher in calves than in adults. Among the samples of cattle feces, Cryptosporidium parvum was identified in 41 (64%), C. ryanae in eight (12.5%), C. bovis in four (6.3%), C. andersoni in five (7.8%), and a mixed infection in 20 samples (31.3%). These parasites were not identified in the samples of human feces. Thermotolerant coliform bacteria were identified in 25 samples of water (45.5%). Giardia duodenalis and C. parvum were identified in three water samples. The gp60 gene analysis of C. parvum isolates revealed the presence of two strains (IIaA20G1R1 and IIaA17G2R2) in the fecal samples and one (IIaA17G2R1) in the water samples. The presence of coliforms was associated with the water source, structure and degradation of springs, rain, and turbidity. The prevalence of protozoa was higher in calves up to six months of age. C. parvum and G. duodenalis were identified in the water of dairy farms, as were thermotolerant coliforms; these findings point to the need for guidance on handling of animals, preservation of water sources, and water treatment.
Introduction
Water is an important vehicle for pathogens responsible for diarrhea worldwide [1]. Despite the recognition that the best quality of water (for human consumption) reduces the incidence of diseases, in Brazil, the water supply of small towns and rural areas differs from that in large urban centers [2]. In rural areas, ~70% of households consume water from alternative sources, with unmonitored potability [3].
Bacteria and protozoa are often primarily responsible for waterborne-illness outbreaks, but Giardia spp. and Cryptosporidium spp. have been the main cause of this problem in recent decades [4,5,6,7]. These protozoans are gastrointestinal parasites of vertebrates, including humans, and the life cycle is completed in a single host with the production of cysts and oocysts: environmental stages excreted with feces [8,9,10]. Thus, the fecal-oral transmission route facilitates the infection of humans by different vehicles contaminated by animal and human feces, especially water sources [11,12]. Developed countries have efficient systems for reporting and investigating of outbreaks of waterborne diseases, which is not the case in developing countries, where this information is obtained from research results reported in the scientific literature [4,7].
Cattle, especially calves, are an important source of infection for humans; the calves are reservoirs of Cryptosporidium parvum, a species with the zoonotic potential [8,9,13]. By analyzing the gp60 gene of C. parvum, researchers have identified zoonotic strains in cattle feces and demonstrated the importance of these animals as sources of environmental contamination and of human infections [14,15,16]. Cattle are also reservoirs of Giardia duodenalis, and although they are most commonly infected with assemblage E of G. duodenalis, cattle have also been reported to be infected with zoonotic assemblage A and, occasionally, B [17,18,19,20]. Calves play an important role in environmental contamination, by excreting large quantities of (oo)cysts into the environment; these (oo)cysts can contaminate water sources used for human and animal consumption [4,21,22]. The contamination of water sources by these protozoa is of great importance in public health, since conventional methods of water treatment reduce these parasites, but do not completely eliminate them [23].
Due to the need for better understanding of the importance of cattle in the contamination of water sources on farms, the aim of this study was to determine the prevalence and to identify the species of Cryptosporidium and Giardia in fecal samples and in the water for human and animal consumption on dairy farms. Another aim was to find possible associations of exposure factors with the presence of these parasites.
Materials and methods
Study location and population
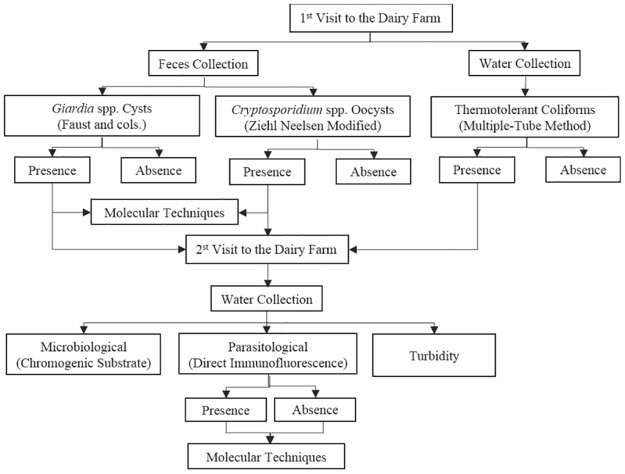
This study was conducted in the cities of Campo Mourao and Araruna, western central Paraná, Brazil. The inclusion criteria were as follows: small family-own dairy farms assisted by the Paraná Institute of Technical Assistance and Rural Extension (EMATER) of Campo Mourao, totaling 55 farms: 20 in Araruna and 35 in Campo Mourao. The flowchart of the steps for retrieval and analysis of the stool and water samples in shown in Fig 1.
Fig 1. A flowchart of retrieval and analysis of fecal and water samples from 55 dairy farms in Paraná, Brazil, during 2012–2014.
Collection and initial analysis of the fecal samples
Collection of the samples
This study was approved by the Ethics Committee on Animal Experimentation (CEEA) of the Integrado College of Campo Mourao (approval # 242/2011) and by the Ethics Committee on Research Involving Humans (CEP-UEL) of the State University of Londrina (approval # 277/2011). The collections of water and feces from the animals were performed after written consent of the owners, through a science and authorization term approved by the CEEA. The collections of informations and human feces were performed after written consent of the participants, or their legal guardians (in the case of minors under 18 years of age) through a free and informed consent term approved by the CEP.
We collected fecal samples from all cattle from 0 to 24 months and, randomly, from a half of the herd of dairy cows. The human feces were collected from residents of the dairy farms who agreed to participate in the parasitological survey. The fecal samples of animals and humans were kept refrigerated until the analysis (performed within 24 h).
Analysis by optical microscopy
The fecal samples were diluted in distilled water, filtered and centrifuged, and the precipitate was used to find cysts and oocysts of protozoans. The presence of Cryptosporidium oocysts and Giardia cysts was verified by the modified Ziehl-Neelsen staining method [24] and centrifugal flotation in a 33% zinc sulfate solution [25], respectively. All test-positive fecal samples were stored in 2.5% potassium dichromate (final concentration) at 4°C for further molecular analysis.
Collection and initial analysis of water samples
Collection of samples
Water samples were collected from human or animal sources in two visits. During the first visit, the collection of water was carried out at two points of each water source: one in the place of origin of the water and the other from the faucet. Approximately 250 mL of water was collected into a sterile bottle [26] in order to verify the presence of coliforms. During the second visit, new water collection was performed only on farms that showed contamination with fecal coliforms in water and/or the presence of (oo)cysts of Cryptosporidium and/or Giardia in fecal samples of cattle or humans obtained during the first visit (Fig 1). At this second stage, we performed new collection of water, for analysis of coliforms, and 10 L were collected into plastic containers for identification of Giardia and Cryptosporidium.
Microbiological analyses and turbidity
The water samples obtained during the first and second visits were subjected to microbiological analysis by the multiple-tube method and the chromogenic substrate method, respectively [27]. Samples from the second visit were also subjected to turbidity analysis on a HACH 2100Q turbidimeter (HACH®, Loveland, CO, EUA).
Concentration of water and the IFA
For concentration of the water samples, 3 L of each 10 L sample obtained during the second visit was filtered through cellulose ester membranes with 47 mm diameter and porosity 1.2 μm (Millipore®, Merck, Darmstadt, Germany), in a vacuum pump system [28]. In samples with high turbidity, where it was not possible to perform membrane filtration, the flocculation method of calcium carbonate was employed for water concentration [29]. We used 10 μL aliquots of each sample to perform a direct immunofluorescence assay (IFA) using the Merifluor Cryptosporidium/Giardia commercial kit (Merifluor®, Meridian Bioscience, Cincinnati, OH, USA). The criteria for identification and the enumeration of the (oo)cysts present in the water samples were described by our colleagues [30,31].
Molecular analyses in feces and water samples
Nested PCR analysis for Giardia spp. and Cryptosporidium spp
All the samples were subjected to DNA extraction using the commercial kit (NucleoSpin® Tissue, Macherey-Nagel, Düren, Germany).
To detect the presence of Giardia spp., fragments of the 18S ribosomal RNA (rRNA) were amplified using nPCR [32,33]. The samples were processed in triplicate; each sample contained 1× PCR Buffer, 200 μM each dNTP, 1.5 mM MgCl2, 400 nM each primer, 5% of dimethyl sulfoxide, 1.25 U of Platinum Taq DNA Polymerase, 1.5 μL of the extracted DNA, and ultrapure water. The amplification conditions were as follows: 5 min at 95°C; followed by 35 cycles of 45 s at 94°C, 45 s at 58°C in first reaction and 45 s at 55°C in the second reaction, and 60 s at 72°C; with 5 min at 72°C as the final extension step.
To detect Cryptosporidium spp., fragments of the 18S rRNA gene were amplified using nPCR [34]. The samples were processed in triplicate, and each reaction mixture contained 1× PCR Buffer, 200 μM dNTP, 2.5 mM MgCl2, 400 nM each primer, 1.25 U of Platinum Taq DNA Polymerase, 2.0 μL of the DNA extracted from each sample, and ultrapure water. The material obtained in the first reaction was diluted with 50 mL before use in the second reaction. The amplification conditions for both the first and second reaction were as follows: 5 min at 95°C; followed by 35 cycles of 45 s at 94°C, 45 s at 55°C, and 60 s at 72°C; with the final extension step 5 min at 72°C.
The PCR products were subjected to electrophoresis in a 1.5% agarose gel (UltraPure™ Agarose, Invitrogen, Waltham, MA, USA) stained with DNA gel stain (SYBR® Safe, Invitrogen, Waltham, MA, USA) and visualized with ultraviolet light.
DNA sequencing
This procedure was performed on all water samples positive for Giardia spp. and all fecal and water samples positive for Cryptosporidium. Sequencing was performed using an ABI3500 sequencer genetic analyzer (Applied Biosystems, Life Technologies™, Carlsbad, CA, USA). The resulting nucleotide sequences were compared with the standard Cryptosporidium and Giardia sequences in GenBank by means of the Basic Local Alignment and Search Tool (BLAST) and by manual alignment in the BioEdit software (Biological Sequence Alignment Editor).
PCR with restriction fragment length polymorphism analysis of Cryptosporidium
Samples positive for Cryptosporidium (in the nPCR analysis) that yielded more than one gene fragment in the amplified sequence were subjected to genetic characterization by means of Restriction Fragment Length Polymorphism Analysis (RFLP). To identify the species in a sample, the products obtained in the second reaction of the nPCR were cleaved with restriction enzymes SspI, AseI, MboII, and DdeI [35,36]. The reaction was performed with 5 μL of DNA, 2 μL of a restriction buffer, 3 IU of the enzyme (New England Biolabs, Ipswich, MA, USA), and ultrapure water. The digestion was performed at 37°C for 1 h, and the products were subjected to electrophoresis in a 2.5% agarose gel stained with SYBR® Safe.
The gp60 gene of C. parvum
For subtyping of C. parvum isolates, we performed nPCR on one fragment (832 bp) of the gp60 gene [37]. The first reaction was composed of 1× PCR Buffer, 200 μM dNTP, 2.5 mM MgCl2, 400 nM each primer, 1.25 U of Platinum Taq DNA Polymerase, 2.0 μL of the extracted DNA, and ultrapure water. In the second reaction, the same concentrations were used, but 1.0 μL of the material obtained in the first reaction was used instead of the DNA sample. The amplification conditions for both the first and the second reaction were the following: 3 min at 95°C; followed by 35 cycles of 45 s at 94°C, 45 s at 50°C, and 1 min at 72°C; with final extension for 10 min at 72°. The amplicons were subjected to electrophoresis, purified, and sequenced as described above.
Rainfall
To compare the results from the samples to seasonal variables, the water precipitation data 24 and 48 h before collection were obtained from the Paraná Meteorological System (SIMEPAR).
Epidemiological and statistical analysis
The farmers were interviewed using an epidemiological questionnaire containing variables for the type of production, rural sanitation, and health and individual characteristics of the animals and humans; these variables were analyzed for association with the presence/absence of fecal coliforms in the water and Cryptosporidium spp. and Giardia spp. in feces and water. The comparison between the frequency data from the analysis of feces and water and the variables of the epidemiological survey was performed using the chi-squared test or Fisher’s exact test. The magnitude of the association was determined by calculating the odds ratio (OR). The calculations were performed in the EpiInfo™ software, version 3.5.2 (CDC, Atlanta, GA, USA), with the level of statistical significance at 5%.
Results
Analyses of cattle fecal samples
We collected fecal samples from 937 heads of cattle: 558 in Campo Mourao and 379 in Araruna. According to optical microscopy (OM), 71 (7.6%) stool samples were positive for Giardia spp. cysts from 27 (49.1%) farms and, among the positive samples according to OM, 48 also positive in the nPCR for the 18S rRNA gene. Cryptosporidium spp. were identified in 96 (10.2%) samples from 37 (67.3%) farms, when analyzed by OM, and among these, 64 samples were also positive for the 18S rRNA gene (Table 1). The prevalence was higher among calves up to 6 months of age, and among them, was higher in the age group 0–2 months old for both Giardia spp. and Cryptosporidium spp. (Table 1).
Table 1. Prevalence of Giardia spp and Cryptosporidium spp in feces of cattle from 55 dairy farms in Paraná, Brazil, from 2012 to 2014.
| Age Group (months) | Number of samples | Giardia spp. | Cryptosporidium spp. | ||
|---|---|---|---|---|---|
| OM (%) | nPCR 18S (%)a | OM (%) | nPCR 18S (%)a | ||
| 0–2 | 146 | 25 (17.1) | 15 (60) | 37 (25.3) | 34 (91.9) |
| 2–4 | 133 | 15 (11.3) | 13 (86.7) | 9 (6.8) | 7 (77.8) |
| 4–6 | 99 | 6 (6.1) | 5 (83.3) | 8 (8.1) | 6 (75) |
| Total (0 a 6) | 378 | 46 (12.2) | 33 (71.7) | 54 (14.3) | 47 (87) |
| 6–12 | 81 | 6 (7.4) | 6 (100) | 5 (6.2) | 3 (60) |
| 12–24 | 54 | 0 | 0 | 6 (11.1) | 1 (16.7) |
| >24 | 424 | 19 (4.5) | 9 (47.4) | 30 (7.1) | 13 (43.3) |
| Total (> 6 months) | 559 | 25 (4.8) | 15 (2.7) | 41 (7.3) | 17 (3.0) |
| Total (All) | 937 | 71 (7.6) | 48 (67.6) | 96 (10.2) | 64 (66.7) |
a Percentage calculated using the number of positive data from optical microscopy (OM). nPCR: nested PCR, 18S: 18S rRNA gene.
Sequence analysis of the 18S rRNA gene was successful in 34 of the 64 samples positive for Cryptosporidium according to nPCR. Among them, 27 (42.2%) showed 100% similarity to C. parvum, five (7,7%) to C. ryanae and two (3,1%) to C. bovis (GenBank: KX929930 to KX929963).
In 20 (31.1%) sequenced samples, there were overlapping gene fragments in the electropherogram. The species that were identified by genetic sequences in various age groups of the cattle are shown in Table 2.
Table 2. Distribution of Cryptosporidium species by age groups according to genetic sequencing of fecal samples from 64 heads of cattle and PCR-RFLP of fecal samples from 15 heads of cattle from 28 dairy farms in Paraná, Brazil, from 2012 to 2014.
| Age Group (months) | DNA sequencing (%) | PCR-RFLPb Cryptosporidium spp. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive Samples (nPCRa) | C. parvum | C. ryanae | C. bovis | ND c | Fragments of overlap | C. parvum/C. bovis | C. parvum/C. ryanae | C. parvum/C. andersoni | Single species | ND | |
| 0–2 | 33 | 16 | 0 | 0 | 4 | 13 | 2 | 2 | 0 | 5d | 4 |
| 2–4 | 8 | 4 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 0 |
| 4–6 | 8 | 4 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6–12 | 3 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1e | 0 |
| 12–24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| >24 | 12 | 2 | 3 | 1 | 3 | 3 | 0 | 0 | 3 | 0 | 0 |
| Total | 64 | 27 (42.2) | 5 (7.7) | 2 (3.1) | 10 (15.6) | 20 (31.3) | 2 (3.1) | 3 (4.7) | 5 (7.8) | 6 (9.4) | 4 (6,3) |
anPCR: nested PCR,
bPCR-RFLP: restriction fragment length polymorphism,
c ND: species not determined (ND) due to illegible sequence (sequencing data) or absence of DNA (PCR-RFLP),
dC. parvum,
eC. bovis.
The PCR-RFLP analysis of 20 samples with overlapping fragments allowed us to identify two samples positive for C. parvum and C. bovis, three for C. parvum and C. ryanae, five for C. parvum and C. andersoni, five only for C. parvum, and one only for C. bovis. Four cattle samples could not be analyzed by PCR-RFLP due to the absence of DNA (Table 2). The samples of cattle with a likely mixed infection belonged to 16 farms, 15 of which kept animals of different ages in the same environment.
Among the 42 samples that tested positive for C. parvum, 20 underwent nPCR amplification of gp60 gene, all calves. The sequencing analysis in 16 of these samples was successful. Among the samples analyzed, we identified two subtypes that showed 100% of identity with subtypes IIaA17G2R1 and IIaA20G1R1 (GenBank: KY073279 to KY073281).
Among the variables analyzed, the production system, the number of cows greater than 40, feeding of cows from the trough, frequent diarrhea on the farm, diarrheal stool samples, and age up to 6 months were significantly associated with the presence of Giardia cysts in the fecal samples. The European breed, diarrheal stool samples, and age up to 6 months were associated with the presence of Cryptosporidium oocysts in feces (Table 3).
Table 3. Variables with a statistically significant association with the presence of cysts of Giardia spp. and/or oocysts of Cryptosporidium spp. in fecal samples from 937 heads of dairy cattle in Paraná, Brazil, from 2012 to 2014.
| Exposure variables | Cysts of Giardia spp. | ORb(95% CIc) | p d |
| Positive samples/Total (%) | |||
| Production system | |||
| Business | 10/63 (15.9) | 2.51a(1.08–5.31) | 0.0318 |
| Family | 61/874 (7.0) | ||
| Lactating Cows | |||
| 1 to 40 | 53/801 (6.6) | 0.46 (0.26–0.82) | 0.0117 |
| >40 | 18/136 (13.2) | ||
| Feeding of Cows | |||
| Only trough | 10/44 (22.7) | 4.02a(1.68–8.78) | 0.002 |
| Trough and pasture | 61/893 (6.8) | ||
| Diarrhea frequency | |||
| High | 41/346 (11.8) | 2.51(1.54–4.11) | 0.0003 |
| Low | 30/591 (5.1) | ||
| Characteristics of feces (calves up to 2 months old) | |||
| Liquid/pasty | 11/35 (31.4) | 3.18 (1.28–7.87) | 0.0203 |
| Firm | 14/111 (12.6) | ||
| Age Group (All) | |||
| 0–6 months | 46/377 (12.2) | 2.97 (1.79–4.93) | 0.0001 |
| >6 months | 25/560 (4.5) | ||
| Age Group (up to 6 months) | |||
| 0–2 months | 25/146 (17.1) | 2.08 (1.12–3.87) | 0.0296 |
| 2–6 months | 21/232 (9.1) | ||
| Exposure variables | Oocysts of Cryptosporidium spp. | OR (95% CIc) | p d |
| Positive samples/Total (%) | |||
| Breed | |||
| European | 78/646 (12.1) | 2.21 (1.29–3.81) | 0.005 |
| Zebu or Cross-bred Zebu | 17/291 (5.8) | ||
| Characteristics of feces | |||
| Liquid/pasty | 23/98 (23.5) | 3.31 (1.96–5.61) | 0.0001 |
| Firm | 72/839 (8.6) | ||
| Age Group (All) | |||
| 0–6 months | 54/95 (56.8) | 2.12 (1.38–3.25) | 0.0007 |
| >6 months | 41/559 (7.3) | ||
| Age Group (up to 6 months) | |||
| 0–2 months | 37/146 (25.3) | 4.29 (2.31–7.97) | 0.0001 |
| 2–6 months | 17/232 (7.3) | ||
aFisher's exact test;
bOR: odds ratio;
cCI: confidence interval;
dp: probability.
Analyses of the human fecal samples
We collected fecal samples from 83 humans, among them, 15 (18.3%) were positive for at least one parasite. Endolimax nana was identified in six (40%) samples, Entamoeba coli in four (26.7%), Iodamoeba bütschlii in three (20%), and Entamoeba histolytica in two (13.3%). The age group with the highest prevalence of parasitism was 0–12 years: six positive samples (35.3%). No samples were positive for Cryptosporidium spp. and Giardia spp.
Analyses of the water samples
Among the 55 farms visited, in 48 (87.3%), the water sources for human and animal consumption were the same, and 27 (49.1%) were springs.
During the first visit to the farms, we collected 124 samples from 62 sources. Among these, 50 samples (40.3%) and 27 sources (43.5%) were positive for thermotolerant coliforms on 25 (45.5%) farms. During the second visit, a water sample was collected at sources totaling 31 samples; in 15 of them (48.4%), thermotolerant coliforms were detected (Table 4).
Table 4. Prevalence of thermotolerant coliforms in 155 water samples obtained during the first visit and second visit to dairy farms in Paraná, Brazil, from 2012 to 2014.
| Water sources | First Visit | Second Visit | ||||
|---|---|---|---|---|---|---|
| Number of sources | Number of samples | Positive Samples, TTCa (%) | Number of sources | Number of samples | Positive Samples, TTCb (%) | |
| Spring | 31 | 62 | 35 (56.5) | 24 | 24 | 12 (50) |
| Artesian well | 20 | 40 | 3 (7.5) | 2 | 2 | 0 |
| Shallow well | 8 | 16 | 6 (37.5) | 2 | 2 | 0 |
| River | 3 | 6 | 6 (100) | 3 | 3 | 3 (100) |
| Total | 62 | 124 | 50 (40.3) | 31 | 31 | 15 (48.4) |
aThermotolerant coliforms according to the multiple-tube method;
baccording to the chromogenic substrate method.
The four test-positive samples showed high turbidity and presence of thermotolerant coliforms and were collected after rainy days (Table 5). All samples positive for Giardia spp. and/or Cryptosporidium spp. in the IFA were also test-positive in the nPCR analysis. Among the test negative samples in the IFA, only one farm (number 27) yielded amplified DNA of Cryptosporidium during nPCR (Table 5).
Table 5. Springhead, turbidity, bacteriological parameters of water samples positive for Cryptosporidium or Giardia species identified by genetic sequencing, and rainfall data 24 and 48 h prior to collection on four dairy farms in Paraná, Brazil, in 2014.
| Property | Source of water | Giardia spp. | Cryptosporidium spp. | Turbidity (NTU)h | TTCi (MPNj/100mL) | Rainfall prior to collection (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFAb (Cy/L)d | nPCRf | Sequencing | Positive Cattle | IFAb (Oo/Lc) | nPCRf | Sequencing/PCR-RFLPg | Positive Cattle | 24 h | 48 h | ||||
| 26 | Spring | 0 | (-) | - | Yes | 1.3 | (+) | C. parvum | Sim | 36.4 | 1046 | 83 | 76 |
| 27 a | River | 12 | (+) | NDe | No | 0 | (+) | C. parvum/C. andersoni | Não | 98.5 | 2419.5 | 44 | 0 |
| 34 | Spring | 0.34 | (+) | G. duodenalis | Yes | 0.34 | (+) | C. parvum/C. andersoni | Sim | 25.6 | 24.5 | 43 | 0 |
| 44 | Spring | 2.1 | (+) | G.duodenalis | Yes | 0 | (-) | - | Sim | 64.47 | 2419.6 | 182.4 | 0 |
aStrongly concentrated by the flocculation method of calcium carbonate [29];
bIFA: direct immunofluorescence assay;
cOo/L: oocysts/liter of water;
dCy/L: cysts/liter of water;
eND: Not determined due to illegible sequence;
fnPCR: nested PCR;
gPCR-RFLP: restriction fragment length polymorphism;
hNTU: nephelometric turbidity unit;
iTTC: thermotolerant coliforms;
jMPN: most probable number.
Sequencing analysis of the 16S rRNA gene of the three water samples positive for Giardia was successful in two, which showed 100% identity with G. duodenalis (accession # KJ867494.1). The sequencing analysis of the three water samples positive for Cryptosporidium spp. according to nPCR was successful in one sample, which showed 100% identity with C. parvum (GenBank: KX929941). The other two samples showed an overlap of the sequencing fragments and were subjected to PCR-RFLP, which revealed the presence of C. parvum and C. andersoni in both samples (Table 5).
Sequencing analysis of the gp60 gene in three water samples positive for C. parvum was successful in two, where the identified subtype was IIaA17G2R1 (GenBank: KY073279 e KY073280). One of these samples belongs to farm 26, where we identified the same subtype in two calves younger than 30 d.
Among the variables analyzed, open sources like rivers and springs, collection of samples at the source, the absence of a cover and side protection at the source, rain in the last 48 h, and the absence of riparian vegetation near the springs were significantly associated with the presence of thermotolerant coliforms in water (Table 6).
Table 6. Variables with a statistically significant association with the presence of thermotolerant coliforms in 124 water samples from 55 dairy farms in Paraná, Brazil, from 2012 to 2014.
| Variables | Thermotolerant Coliforms | ORa (95% CIb) | pc |
|---|---|---|---|
| Positive samples/Total (%) | |||
| Source of Water | |||
| River | 6/6 (100) | - | 0.0001 |
| Spring | 35/62 (56.45) | 15.99 (4.45–57.45) | 0.0001 |
| Shallow well | 9/60 (15) | 7.4 (1.57–34.93) | 0.01294 |
| Artesian well | 3/40 (7.5) | 1 | |
| Collection site | |||
| Source | 22/37 (59.46) | 3.09 (1.4–6.85) | 0.0084 |
| Water tank/Tap | 28/87 (32.2) | ||
| Slapping source | |||
| Yes | 36/103 (34.95) | 0.27 (0.08–0.8) | 0.0074 |
| No | 14/21 (66.7) | ||
| Rainfall prior to collection (up to 48 h) | |||
| Yes | 25/46 (54.3) | 2.52 (1.2–5.34) | 0.0241 |
| No | 25/78 (32.1) | ||
| Spring with side protection | |||
| Yes | 18/40 (45) | 0.24 (0.074–0.78) | 0.02892 |
| No | 17/22 (77.3) | ||
| Spring with riparian forest | |||
| Yes | 19/42 (45.2) | 0.21 (0.06–0.72) | 0.02107 |
| No | 16/20 (80) | ||
aOR: odds ratio;
bCI: confidence interval;
cp: probability.
Among the water samples obtained during the second visit to farms, we identified a significant association between turbidity above 5 UT and the presence of thermotolerant coliforms (p = 0.049; OR = 0.17; 95% confidence interval [CI] 0.035–0.79). The association of the samples positive for Giardia spp. and/or Cryptosporidium spp. with exposure variables evaluated in this study was not statistically significant, but the presence of these parasites was found in water samples with thermotolerant coliforms at >1000 most probable number (MPN) per 100 mL, turbidity above 5 UT, rainfall above 43 mm 24 h before the sample collection, the absence of riparian vegetation and of external protection, and closeness to pasture.
Discussion
Cryptosporidium spp. are economically important enteropathogens that cause diarrhea in calves and can compromise development of the animal or kill it [38]. The prevalence of infections with Cryptosporidium spp. among cattle varies widely due, among other factors, to age differences among the animals. In studies that showed high prevalence, most of the sampled animals were younger than 6 months, and in the studies that showed lower prevalence, more than 50% of the sample consisted of animals older than six months, as is the case in our study [39,40,41,42,43]. When analyzed separately, the prevalence of Cryptosporidium spp. was significantly higher among calves up to 2 months old (25.3%; p = 0.0001) and up to 6 months old (14.3%; p = 0.0007) when compared with the calves older than 2 or 6 months, respectively. In a longitudinal study conducted in the USA [44], prevalence of cryptosporidiosis among 30 heads of cattle from birth to 2 years of age was inversely proportional to the age of the animals, findings consistent with the results of our study.
The prevalence of Giardia cysts in the feces of cattle was higher among younger calves in agreement with previous study [45]. Nonetheless, higher prevalence of G. duodenalis is commonly observed among postweaned calves when compared to preweaned ones or animals older than 12 months, owing to sudden changes in the management of these animals [18,45,46,47,48,49,50]. In our study, factors such as the type of establishment, the number of animals sampled, management conditions, and the environment may have interfered with the analysis, so that the highest prevalence was observed in the age group of preweaned calves.
The variation in the prevalence of bovine cryptosporidiosis and giardiasis reported in different studies within the same age group can also be caused by factors related to the management and characteristics of the animals. In our study, we demonstrated a significant association between the feeding of cows exclusively from the trough and the number of lactating animals greater than 40 with the presence of Giardia spp. in feces. Research groups have reported higher prevalence on farms with breeding of cows and calves in a closed collective environment and with more animals [43,51]. Closed collective environments are favorable for the maintenance of these parasites because of shading and humidity, keeping the infectivity for longer periods; furthermore, the higher the animal density in an environment, the greater the contamination, and consequently, the greater the ingestion of (oo)cysts [52]. In our study, there was greater prevalence of cryptosporidiosis in European-bred animals when compared to Zebu animals or animals cross-bred with Zebu. Other studies have shown that prevalence of cryptosporidiosis is similar in Zebu and European breeds, indicating that management practices in the livestock influence the parasitism more than a difference in breeds [41,53]. In our study, although most farms were characterized as a family business, those who possessed higher levels of technologies with higher stocking density, were breeding Holstein cows, whereas farms with lower stocking density worked with animals cross-bred with the Zebu breed. These characteristics may have influenced the association between cryptosporidiosis and the European breed, as demonstrated by other researchers [39].
Among the animals that had diarrhea, 23.5% also excreted oocysts of Cryptosporidium spp., with a significant association, but this rate increased when we considered only calves of 0 to 2 months of age (71.4%). Other studies have shown the high prevalence of this parasite in the feces of calves with diarrhea [50,54,55]. The immune system is immature in neonates; thus, changes in the intestinal mucosa are more pronounced than in older calves; this situation increases the morbidity of cryptosporidiosis [50]. In addition, the environment where the newborns remain promotes greater contact with feces, and consequently, higher intake of oocysts because cows are asymptomatic carriers of the parasite and excrete larger amounts of oocysts with feces in the farrowing period, thereby increasing environmental pollution and the risk of infection among neonates [52,56]. In extensive farming systems, the calves are moved to the pasture with age; thus, the above contact is reduced [38]. When we analyzed the association between diarrhea and excretion of Giardia spp. in all the animals, there was no statistically significant relation, and only 11 (12.6%) of the 97 animals with diarrhea also excreted cysts. Nevertheless, this frequency increased to 31.4% if we considered only the population of calves of 0 to 2 months of age, with a statistically significant association. Despite the uncertainty regarding the role of Giardia as a primary pathogen in cattle diarrhea, some studies have shown this possibility, in which were identified identified cysts of Giardia spp. in 36% of 669 fecal samples [57] and in 36.7% of 690 fecal samples [50] from cattle, and a significant association was observed between the presence of the parasite and diarrhea; this result is consistent with our findings. As is the case with other parasites (such as Cryptosporidium spp.), Giardia diarrhea is determined by several factors such as virulence of the parasite, the host immune response, and the infectious dose, the latter being strongly dependent on the management system, with the greatest influence in intensive farming systems [22].
In our study, there were differences in the amount of positive samples in optical microscopy and nested PCR. This may have occurred due to the method used to preserve the parasites, in potassium dichromate. The potassium dichromate are PCR inhibitor and extensive washes are required to remove it, wich may lead to a loss of cysts/oocysts thus reducing PCR sensitivity, especially in samples with small amount. Some samples collected had a small number of cysts / oocysts in the microscopic analysis, even after the concentration of these samples [58].
Judging by the results of sequencing and PCR-RFLP, C. parvum was the most frequent species in our study (64%), most notably among calves up to 6 months of age. In several studies around the world, C. parvum has been identified with higher prevalence among calves up to 2 months of age, with a considerable decrease in frequency above this age [44,59,60,61,62,63]. In the present study, the high prevalence of this species among calves older than 2 months may be a consequence of the handling of these animals on the farm because a large number of producers did not separate calves of different ages into different environments. A few samples were identified as C. ryanae and C. bovis, in line with other reports [36,61,64,65,66], but many researchers have detected these species more often when compared to C. parvum among cattle older than 2 months [44,53,59,66]. In others studies was suggested that infection with C. bovis or C. ryanae in animals (parasitized with greater severity by C. parvum) may be latent because PCR analysis of fragments of 18SSU rRNA identifies the predominant species [36,67,68]. C. andersoni was also identified in a few samples, mostly in animals older than 24 months. This species is mainly identified among heifers and adult animals [44,63,69], but some investigators in Brazil identified C. andersoni at a high frequency in young calves too [66,70]. In our study, the prevalence of C. parvum among the adult cattle was also higher than that of the other species; such data are scarce in the literature [71,72]. The high prevalence of this species among adult animals can contribute to infection of newborn calves, and consequently, increase environmental contamination [38].
The gp60 gene has a high degree of sequence polymorphism in C. hominis, C. meleagridis and C. parvum isolates, making it possible to identify these species in subtypes groups [68]. Groups IIa and IId of C. parvum have been identified in humans and ruminants, but the IIa family is the most commonly identified in cattle and humans worldwide [13]. Analysis of the gp60 gene of C. parvum is a useful tool for identification of subtypes and zoonotic strains not widely reported in Brazil. In our study, we identified two strains of the IIa family in 16 stool samples: strains IIaA20G1R1 and IIaA17G2R1, which not been reported in studies in this same country. The most common strain in our study, IIaA20G1R1, has been detected in cattle in some regions of the world: Serbia and Montenegro, Sweden, and Argentina [73,74,75,76], but there are no reports of this strain in humans. The second most common strain identified in stool samples, IIaA17G2R1, has been detected in other studies on cattle [77,78] and on humans [79,80]. After an outbreak of human cryptosporidiosis at a camp in North Carolina, USA, the Centers for Disease Control and Prevention (CDC) have demonstrated that ~60% of the confirmed cases were caused by C. parvum strain IIaA17G2R1, and the same strain was present in the animal feces at the camp, suggesting that zoonotic transmission had taken place [80]. Calves infected with Cryptosporidium and Giardia spp. may excrete ~106–107 (oo)cysts per gram of feces, with higher excretion peaks among young animals, becoming intermittent with the development of adaptive immunity [38,81]. Therefore, the presence of calves infected with a zoonotic strain of C. parvum and/or G. duodenalis favors greater contamination of the immediate environment, and consequently, of the water; this state of affairs can increase the risk of human cryptosporidiosis and giardiasis [38].
On the visited farms, the main sources of drinking water for humans were underground sources, where the presence of fecal coliforms is relevant, especially in springs and shallow wells. The absence of riparian vegetation and of spring protective structures, such as raised edges and a cover, was significantly associated with the presence of thermotolerant coliforms in the water. A study conducted in the state of Goias, Brazil, revealed poor quality of water owing to high environmental degradation caused by a shortage of vegetation near the springs, animal trampling, and proximity to the areas of pasture and crops [82]. The vegetation around springs serves as a paperlike physical barrier between the ground and water, thereby reducing runoff and erosion, and thus the contamination of water [83]. Therefore, it is not surprising that higher turbidity (p = 0.0490) and precipitation preceding the collection of water samples (p = 0.0366) showed a significant association with the presence of thermotolerant coliforms in our study. This presence in six of the 16 samples collected from shallow wells did not show an association with the type of well structure because all had adequate protective caps, an outer wall above the ground, internal coating, and paving around the well. These characteristics have been identified as protective factors against contamination of shallow wells with thermotolerant coliforms in a study conducted in Mato Grosso do Sul, Brazil [84]. The risk of contamination of groundwater via the passage of microorganisms through the ground exists; this is because although layers of soil and rock decrease this risk, they are not capable of preventing contamination, particularly in agricultural areas with substantial soil degradation and dumping of animal feces [85].
The presence of Cryptosporidium spp. and/or Giardia spp. was demonstrated in four of the 31 water samples analyzed here, three of which came from springs and a river. The presence of protozoa in springs underscores the importance of protecting these water sources because these three sources had no riparian vegetation or adequate protective structures and were situated on the lower ground and close to grazing areas. Contamination of springs by these protozoa and its relation to protective structures and the water source environment have been demonstrated in other studies. In Campos do Jordão, SP, Brazil, was reported the presence of at least one of these protozoa in two springs in an urban area and in one spring in a rural area and identified a relation with poor plumbing, inadequate tanks, soil porosity, the presence of sewage, localization in lower parts of the land, and proximity to pastures [86]. In indigenous lands, Paraná, Brazil was reported the presence of these protozoa in 42.8%, 14.3%, and 14.3% of water samples collected from a river, springs, and artesian wells, respectively, and attributed their presence to contamination by human and animal feces [87]. The number of cysts (0.34–12/L) and oocysts (0.34–1.3/L) identified in the water samples is highly relevant to public health because a small dose of (oo)cysts is sufficient to cause a clinical infection [4]. Furthermore, the three contaminated sources identified in the present study were used for human consumption. Water samples positive for protozoa were collected after precipitation, and the concentration of (oo)cysts in the samples increased with turbidity. Although there was no significant association between these parameters and the presence of protozoa, some studies have established a positive correlation. In Australia was reported a positive and significant correlation between turbidity and the flow of water in samples positive for Cryptosporidium spp. collected in a river of multiple use [88]. In Viçosa, Minas Gerais, Brazil, was identified a high concentration of oocysts in drinking-water sources that were characterized by unprotected basins with intensive human settlement and agricultural activities; those authors inferred the influence of rainfall on the presence of these protozoa (not statistically significant) [89]. This correlation between turbidity and the presence of parasites also demonstrates the importance of the use of adequate techniques for the filtration of samples with high turbidity rates to monitor the contamination of water sources. In our study, in one of the samples, it was not possible to perform the concentration by filtration in membranes, and it was necessary to use another technique, in this case the flocculation method, which proved efficient but laborious. Other studies have demonstrated efficient methodologies to recuperate (oo)cysts in water samples with high turbidity, as the combination of polyester microbibre filters (ARAD) and a loop-mediated isothermal amplification (LAMP) to detect few (oo)cyst in large quantity of water with high turbidity [90]
Thermotolerant coliforms are key indicators of fecal contamination of water, and their presence is suggestive of the presence of other fecal microorganisms. In this study, the water sources positive for Cryptosporidium and/or Giardia spp. also showed high bacterial counts: three of them above 1000 MPN per 100 mL. Ordinance 2914/11 [91] establishes the necessity of monitoring of these protozoa at points of water capture when the annual geometric mean of Escherichia coli identified in the surface raw water is ≥1000 cells per 100 mL. Even though the high bacterial count identified in the samples of the present study represents a one-off result, it is important to recognize that strong fecal contamination of these sources poses a risk to human and animal health on these farms. In addition, ~70% of Brazilian rural municipalities use alternative sources of water supply, without treatment and monitoring of potability established by a relevant ordinance [3].
The relation between the presence of Giardia spp. in the samples and the presence of these protozoa in animal feces on these farms was impossible because this association was not detected by DNA sequencing of cattle fecal samples owing to low sensitivity in terms of identification of assemblages of the 18SSU gene used in this study [92]. G. duodenalis was identified in water samples this study, but markers for identification of assemblages were not used; therefore, it was not possible to establish the source of contamination [93,94]. All water samples where we detected cysts of Giardia spp. were from farms with animals infected by this parasite. Although assemblage E (not zoonotic) is most commonly identified in cattle, some studies have shown the prevalence of zoonotic-infection assemblages of 3–43% among animals in various categories; these data point to the importance of these animals for environmental contamination by zoonotic assemblages of parasites [18,20,46,46–48,95].
Our analysis of gene sequencing and PCR-RFLP analysis of Cryptosporidium isolates from water samples revealed the presence of C. parvum on three farms and C. andersoni on two. The species most commonly associated with diseases in humans are C. hominis (exclusively anthroponotic) and C. parvum (a zoonotic species most often found in rural areas) [16,96,97]. Other studies have shown an association of a high frequency of infection with C. parvum among humans with the presence of infected cattle near water catchment areas [72,98]. C. parvum has different hosts; therefore, its presence in water may be the result of contamination by feces of domestic and/or wild animals or by human sewage [43,99]. On the other hand, the presence of C. andersoni and C. parvum strain IIaA17G2R1 in water and feces of cattle on these farms allows us to conclude that these animals are the main contaminators of the nearby environment. In this study, we did not detect Cryptosporidium in the feces of human residents of these farms; this result may be due to various factors such as the small sample size of the population of susceptible humans (children and elderly), collection of human feces at time points that are different from those for collection of water, and the possibility that parasites identified in water were not capable of a clinical infection. Nevertheless, the identified species and strains and epidemiological characteristics observed in these environments pose a serious risk of contamination that may affect the population in question.
Conclusions
In conclusion, the cattle of dairy farms in the region under study are infected with Cryptosporidium spp. and Giardia spp., with higher prevalence among neonates: calves up to 2 months of age. The presence of Giardia spp. in water samples may not be related to the excretion of cysts by the cattle because we did not identify assemblages of this parasite. Nevertheless, a zoonotic C. parvum strain was identified in water samples and feces from the animals of the same farm. Because fecal contamination was found in most water sources used for human and animal consumption on these farms, there is a need for a) assistance to the rural population regarding the management measures to reduce infection rates among the animals, b) preservation of water sources, and c) water treatment before consumption. The evidence seems to be sufficient to demonstrate the existing drawbacks in the water supply system of rural populations and the imminent risk to public and animal health.
Supporting information
(PDF)
aPercentage calculated using the number of positive data from optical microscopy (OM). nPCR: nested PCR, 18S: 18S rRNA gene.
(PDF)
anPCR: nested PCR, bPCR-RFLP: restriction fragment length polymorphism, c ND: species not determined (ND) due to illegible sequence (sequencing data) or absence of DNA (PCR-RFLP), dC. parvum, eC. bovis.
(PDF)
aFisher's exact test; bOR: odds ratio; cCI: confidence interval; dp: probability.
(PDF)
aThermotolerant coliforms according to the multiple-tube method; baccording to the chromogenic substrate method.
(PDF)
aStrongly concentrated by the flocculation method of calcium carbonate [29]; bIFA: direct immunofluorescence assay; cOo/L: oocysts/liter of water; dCy/L: cysts/liter of water; eND: Not determined due to illegible sequence; fnPCR: nested PCR; gPCR-RFLP: restriction fragment length polymorphism; hNTU: nephelometric turbidity unit; iTTC: thermotolerant coliforms; jMPN: most probable number.
(PDF)
aOR: odds ratio; bCI: confidence interval; cp: probability.
(PDF)
Acknowledgments
We thank the veterinarian of the Institute of Technical Assistance and Rural Extension (EMATER) of Campo Mourao, Iole Elsa Canali, for facilitating access to farmers.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the Araucária Foundation to support the scientific and technological development of Paraná, after approval of funding in the call for projects 24/2012 of the Universal Program of Basic and Applied Research. The agreement was established under number 196/2014. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO—WORLD HEALTH ORGANIZATION. Diarrhea: Why children are still dying and what can be done. Geneva: WHO; 2009. [Google Scholar]
- 2.Silva CV, Heller L, Carneiro M. Cisternas para armazenamento de água de chuva e efeito na diarreia infantil: um estudo na área rural do semiárido de Minas Gerais. Eng Sanit Ambient. 2012;17:293–400 [Google Scholar]
- 3.Brasil, Ministério do Planejamento, Orçamento e Gestão. Instituto Brasileiro de Geografia e Estatística—IBGE. Pesquisa nacional por amostras de domicílio—PNAD, 2012. Rio de Janeiro: Diretoria de Pesquisa e Coordenação de Trabalho e Rendimento; 2012. [Google Scholar]
- 4.Franco RMB. Protozoários de veiculação hídrica: relevância em saúde pública. Rev Panam Infectol. 2007;9:36–3. [Google Scholar]
- 5.Karanis P, Kourent C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5:1–38 [DOI] [PubMed] [Google Scholar]
- 6.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks—An update 2004 e 2010. Water Res. 2011;45:6603–14 10.1016/j.watres.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 7.Efstratiou A, Ongerth JE, Karanis P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2011–2016. Water Res. 2017;114:14–22 10.1016/j.watres.2017.01.036 [DOI] [PubMed] [Google Scholar]
- 8.Fayer R. Cryptosporidium: a water-borne zoonotic parasite. Vet Parasitol. 2004;126: 37–56 10.1016/j.vetpar.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 9.Thompson RCA. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. 2004;126:15–35 10.1016/j.vetpar.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Thompson RCA, Monis PT. Variation in Giardia: implications for taxonomy and epidemiology. Adv Parasitol. 2004;58:69–137 10.1016/S0065-308X(04)58002-8 [DOI] [PubMed] [Google Scholar]
- 11.Karanis P, Opiela K, Renoth S, Seitz HM. Possible implication of muskrats to contaminate surface waters supplies with Giardia sp. cysts. Int J Med Microbiol. 1996;284:302–306. [DOI] [PubMed] [Google Scholar]
- 12.Plutzer J, Karanis P. Neglected waterborne parasitic protozoa and their detection in water. Water Res. 2016;101:318–32 10.1016/j.watres.2016.05.085 [DOI] [PubMed] [Google Scholar]
- 13.Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–9 10.1016/j.exppara.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 14.Feltus DC, Giddings CW, Schneck BL, Monson T, Warshauer D, McEvoy JM. Evidence supporting zoonotic transmission of Cryptosporidium in Wisconsin. J Clin Microbiol. 2006;44(12):4303–08 10.1128/JCM.01067-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trotz-Williams LA, Martin DS, Gatei W, Cama V, Peregrine AS, Martin SW, et al. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol Res. 2006;99(4):346–52 10.1007/s00436-006-0157-4 [DOI] [PubMed] [Google Scholar]
- 16.Chalmers RM, Smith R, Elwin K, Clifton-Hadley FA, Giles M. Epidemiology of anthroponotic and zoonotic human cryptosporidiosis in England and Wales, 2004–2006. Epidemiol Infect. 2011;139(5):700–12. 10.1017/S0950268810001688 [DOI] [PubMed] [Google Scholar]
- 17.O’Handley RM, Olson M E, Fraser D, Adams P, Thompson RC. Prevalence and genotypic characterization of Giardia in dairy calves from Western Australia and Western Canada. Vet Parasitol. 2000;90(3):193–200 [DOI] [PubMed] [Google Scholar]
- 18.Trout JM, Santín M, Greiner E, Fayer R. Prevalence of Giardia duodenalis genotypes in pre-weaned dairy calves. Vet Parasitol. 2004;124(3–4):179–86 10.1016/j.vetpar.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Coklin T, Farber J, Parrington L, Dixon B. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet Parasitol. 2007;150(4):297–305 10.1016/j.vetpar.2007.09.014 [DOI] [PubMed] [Google Scholar]
- 20.Karanis P, Ey PL. Characterization of axenic isolates of Giardia intestinalis established from humans and animals in Germany. Parasitol Res. 1998;84(6):442–9 [DOI] [PubMed] [Google Scholar]
- 21.Karanis P, Schoenen D, Seitz HM. Giardia and Cryptosporidium in backwash water from rapid sand filters used for drinking water production. Int J Med Microbiol. 1996;28(1):107–114 [DOI] [PubMed] [Google Scholar]
- 22.Geurden T, Vercruysse J, Claerebout E. Is Giardia a significant pathogen in production animals? Exp Parasitol. 2010;124(1):98–106. 10.1016/j.exppara.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 23.Karanis P, Schoenen D, Seitz HM. Distribution and removal of Giardia and Cryptosporidium in water supplies in Germany. Wat Sci Tech. 1998;37(2):9–18. [Google Scholar]
- 24.Henriksen SA, Pohlenz JFL. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22(3–4):594–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faust EC, Sawitz W, Tobie J, Odom V, Peres C, Lincicome DR. Comparative efficiency of various techinics for de diagnosis of protozoa and helminth in feces. J Parasitol. 1939;25:241–62. [Google Scholar]
- 26.Brasil, Ministério da Saúde. Fundação Nacional de Saúde (FUNASA). Manual prático de análise de água. 4st ed Brasília: FUNASA; 2013. [Google Scholar]
- 27.APHA (2005) Standard Methods for the Examination of Water and Wastewater. 21st ed Washington, DC. [Google Scholar]
- 28.Branco N, Leal DAG, FRANCO RMB. A Parasitological Survey of Natural Water Springs and Inhabitants of a Tourist City in Southeastern Brazil. Vector Borne Zoonotic Dis. 2012;12(5):410–7. 10.1089/vbz.2011.0679 [DOI] [PubMed] [Google Scholar]
- 29.Vesey G, Slade JS, Byrne M, Shepherd K, Dennis PJL, Fricker CR. A new method for the concetration of Cryptosporidium oocysts from water. J Appl Bacteriol. 1993;75(1):82–6 [DOI] [PubMed] [Google Scholar]
- 30.Redlinger T, Corella-Barud V, Graham J, Galindo A, Avitia R, Cárdenas V. Hyperendemic Cryptosporidium and Giardia in households lacking municipal sewer and water on the United States-México border. Am J Trop Med Hyg. 2002; 66:794–98 [DOI] [PubMed] [Google Scholar]
- 31.Cantusio-Neto R, Franco RMB. Ocorrência de oocistos de Cryptosporidium spp. e cistos de Giardia spp. em diferentes pontos do processo de tratamento de água em Campinas, São Paulo, Brasil. Higiene Alimentar. 2004;18;52–9. [Google Scholar]
- 32.Hopkins RM, Meloni BP, Groth DM, Wetherall JD, Reynoldson JA, Thompson RC. Ribosomal RNA sequencing reveals differences between the genotypes of Giardia isolates recovered from humans and dogs living in the same locality. J Parasitol. 1997;83(1):44–1 [PubMed] [Google Scholar]
- 33.Appelbee AJ, Frederick LM, Heitman TL, Olson ME. Prevalence and genotyping of Giardia duodenalis from beefcalves in Alberta, Canada. Vet Parasitol. 2003;112(4):289–94 [DOI] [PubMed] [Google Scholar]
- 34.Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, Shulaw W, Thompson RC, Fayer R, Lal AA. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol. 1999;65(8):3386–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao L, Singh A, Limor J, Graczyk TK, Gradus S, Lal A. Molecular Characterization of Cryptosporidium Oocysts in Samples of Raw Surface Water and Wastewater. Appl Environ Microbiol. 2001;67:1097–101. 10.1128/AEM.67.3.1097-1101.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y, Ortega Y, He G, Das P, Xu M, Zhang X, et al. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet Parasitol. 2007;144(1–2):1–9 10.1016/j.vetpar.2006.10.001 [DOI] [PubMed] [Google Scholar]
- 37.Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antune F. Subgenotype Analysis of Cryptosporidium Isolates from Humans, Cattle, and Zoo Ruminants in Portugal. J Clin Microbiol. 2003;41(6):2744–47 10.1128/JCM.41.6.2744-2747.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins-Vieira MBC, Brito LAL, Heller L. Oocistos de Cryptosporidium parvum em fezes de bezerro infectado experimentalmente. Arq Bras Med Vet Zootec. 2009; 61(6):1454–58. [Google Scholar]
- 39.Feitosa FLF, Shimamura GM, Roberto T, Meireles MV, Nunes CM, Ciarlini PC, Borges AS. Prevalência de criptosporidiose em bezerros na região de Araçatuba, Estado de São Paulo. Ciênc Rural. 2004;34(1):189–3 [Google Scholar]
- 40.Langoni H, Linhares AC, De Ávila FA, Silva AV, Elias AO. Contribuição ao estudo da etiologia das diarréias em bezerros de aptidão leiteira no Estado de São Paulo, Brasil. Braz J of Vet Res An Sci. 2004;41(5):313–9 [Google Scholar]
- 41.Oliveira Filho JP, Silva DPG, Pacheco MD, Mascarini LM, Ribeiro MG, Alfieri AA, et al. Diarreia em bezerros da raça Nelore criados extensivamente: estudo clínico e etiológico. Pesq Vet Bras. 2007;27(10):419–24 [Google Scholar]
- 42.Cardoso JMS, Silveira FL, Araújo AJUS, De Carvalho JCC, Kanamura HY. Ocorrência de Cryptosporidium spp. em um rebanho bovino leiteiro no município de Caçapava, estado de São Paulo, Brasil. Rev Bras Parasitol Vet. 2008;17:239–42. [PubMed] [Google Scholar]
- 43.Budu-Amoako E, Greenwood SJ, Dixon BR, Barkema HW, McClure JT. Giardia and Cryptosporidium on dairy farms and the role these farms may play in contaminating water sources in Prince Edward Island, Canada. J Vet Intern Med. 2012;26(3):668–73 10.1111/j.1939-1676.2012.00930.x [DOI] [PubMed] [Google Scholar]
- 44.Santín M, Trout JM, Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet Parasitol. 2008;155(1–2):15–23 10.1016/j.vetpar.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 45.Santín M, Trout JM, Fayer R. A longitudinal study of Giardia duodenalis genotypes in dairy cows from birth to 2 years of age. Vet Parasitol. 2009;162:40–5 10.1016/j.vetpar.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 46.Trout JM, Santín M, Greiner E, Fayer R. Prevalence and genotypes of Giardia duodenalis in post-weaned dairy calves. Vet Parasitol. 2005;130(3–4):177–83. 10.1016/j.vetpar.2005.03.032 [DOI] [PubMed] [Google Scholar]
- 47.Trout JM, Santín M, Greiner E, Fayer R. Prevalence and genotypes of Giardia duodenalis in 1–2 year old dairy cattle. Vet Parasitol. 2006; 140(3–4):217–22 10.1016/j.vetpar.2006.03.025 [DOI] [PubMed] [Google Scholar]
- 48.Trout JM, Santín M, Fayer R. Prevalence of Giardia duodenalis genotypes in adult dairy cows.Vet Parasitol. 2007; 147(3–4):205–9 10.1016/j.vetpar.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 49.Paz e Silva FM, Lopes RS, Araújo Júnior RJP. Genetic characterization of Giardia duodenalis in dairy cattle in Brazil. Folia Parasitol. 2012;59(1):15–20. [PubMed] [Google Scholar]
- 50.Ouchene N, Ouchene-Khelifi NA, Zeroual F, Benakhla A, Adjou K. Study of Giardia spp., Cryptosporidium spp. e Eimeria spp. infetions in dairy cattle in Algeria. J Parasitol Vector Biol. 2014;6(4):61–5 [Google Scholar]
- 51.Ortolani EL, Soares PC. Aspectos epidemiológicos de la criptosporidiosis em becerros de rebaños lecheros. Parasitol Latinoam. 2003;58:122–7 [Google Scholar]
- 52.Vargas Júniro FS, Marcolongo-Pereira C, Adrien NML, Fiss L, Molarinho KR, Soares MP, et al. Surto de criptosporidiose em bezerros no sul do Rio Grande do Sul. Pesq Vet Bras. 2014;34(8):749–52 [Google Scholar]
- 53.Lima RCA, Aquino MCC, Inácio SVI, Viol MA, Zucatto AS, Neto LS. Caracterização molecular de Cryptosporidium spp. em bezerros (Bos taurus e Bos indicus) no município de Formiga, Minas Gerais—Brasil. Semin Ciên Agrár. 2013; 34(6):3747–54 [Google Scholar]
- 54.Huetink REC.; Vander Giessen JWB, Noordhuizen JPTM. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a dairy farm. Vet Parasitol. 2001. December; 102(1–2):53–67 [DOI] [PubMed] [Google Scholar]
- 55.Silverlas C, Bosaeus-reineck H, Naslund K, Björkman C. Is there a need for improved Cryptosporidium diagnostics in Swedish calves? Int J Parasitol. 2013;43(2):155–61. 10.1016/j.ijpara.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muniz Neta ES, Sampaio DC, Galvão GS, Munhoz AD. Comparação das técnicas de Ziehl-Neelsen modificada e contraste de fase na detecção de oocistos do gênero Cryptosporidium Tyzzer, 1907 (Apicomplexa: Cryptosporidiidae) em bovinos assintomáticos. Rev Bras Med Vet. 2010;32(4):201–4. [Google Scholar]
- 57.McAllister TA, Olson ME, Fletch A, Wetzstein M, Entz T. Prevalence of Giardia and Cryptosporidium in beef cows in southern Ontario and in beef calves in southern British Columbia. Can Vet J. 2005;46:47–5 PMC1082856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lalonde LF, Gajadhar AA. Effect of storage media, temperature and time on preservation of Cryptosporidium parvum oocysts for PCR analysis. Vet Parasitol. 2009; 160:185–89 10.1016/j.vetpar.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 59.Geurden T, Berkvens D, Martens C, Casaert S, Vercruysse J, Claerebout E. Molecular epidemiology with subtype analysis of Cryptosporidium in calves in Belgium. Parasitology. 2007;134(Pt.14):1981–7 10.1017/S0031182007003460 [DOI] [PubMed] [Google Scholar]
- 60.Plutzer J, Karanis P. Genotype and subtype analyses of Cryptosporidium isolates from cattle in Hungary. Vet Parasitol. 2007;146(3–4):357–62 10.1016/j.vetpar.2007.02.030 [DOI] [PubMed] [Google Scholar]
- 61.Thompson RCA, Palmer CS, Handley RO. The public health and significance of Giardia and Cryptosporidium in domestic animals. Vet J. 2008;177(1):18–25. 10.1016/j.tvjl.2007.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Follet J, Guyot K, Leruste H, Folletdumonulin A, Hammouma-Ghelboun O, Certad G, Dei-Cas E, Halama P. Cryptosporidium infection in a veal calf cohort in France: molecular characterization of species in a longitudinal study. Vet Res. 2011; 42:116 10.1186/1297-9716-42-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meireles MV, De Oliveira FP, Teixeira WF, Coelho WM, Mendes LC. Molecular characterization of Cryptosporidium spp. in dairy calves from the state of São Paulo, Brazil. Parasitol Res. 2011; 109(3):949–51 10.1007/s00436-011-2336-1 [DOI] [PubMed] [Google Scholar]
- 64.Santín M, Trout JM, Xiao L, Zhou L, Greiner E, Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol. 2004;122(2):103–17 10.1016/j.vetpar.2004.03.020 [DOI] [PubMed] [Google Scholar]
- 65.Fayer R, Santín M, Xiao L. Cryptosporidium bovis n. sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus). J Parasitol. 2005;91(3):624–9 10.1645/GE-3435 [DOI] [PubMed] [Google Scholar]
- 66.Paz e Silva FM, Lopes RS, Araújo-Júnior JP. Identification of Cryptosporidium species and genotypes in dairy cattle in Brazil. Rev Bras Parasitol Vet. 2013;22(1):22–8 [DOI] [PubMed] [Google Scholar]
- 67.Cama V, Gilman RH, Vivar A, Ticona E, Ortega Y, Bern C, Xiao L. Mixed Cryptosporidium infections and HIV. Emerg Infect Dis. 2006;12(6):1025–8. 10.3201/eid1206.060015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plutzer J, Karanis P. Genetic polymorphism in Cryptosporidium species:na update. Vet Parasitol. 2009;165:187–99 10.1016/j.vetpar.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 69.Fayer R, Santín M, Trout JM. Prevalence of Cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Vet Parasitol. 2007;145(3–4):260–6 10.1016/j.vetpar.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 70.Sevá AP, Funada MR, Souza SO, Nava A, Richtzenhain LJ, Soares RM. Occurrence and molecular characterization of Cryptosporidium spp. isolated from domestic animals in a rural area surrounding Atlantic dry forest fragments in Teodoro Sampaio municipality, State of São Paulo, Brazil. Rev Bras Parasitol Vet. 2010;19(4):249–253 [DOI] [PubMed] [Google Scholar]
- 71.Smith RP, Clifton-Hadley FA, Cheney T, Giles M. Prevalence and molecular typing of Cryptosporidium in dairy cattle in England and Wales and examination of potential on-farm transmission routes. Vet Parasitol. 2014;204(3–4):111–9 10.1016/j.vetpar.2014.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wells B, Shaw H, Hotchkiss E, Gilray J, Ayton R, Green J, et al. Prevalence, species identification and genotyping Cryptosporidium from livestock and deer in a catchment in the Cairngorms with a history of a contaminated public water supply. Parasit Vectors. 2015; 8: 66–79 10.1186/s13071-015-0684-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Misic Z, Abe N. Subtype analysis of Cryptosporidium parvum isolates from calves on farms around Belgrade, Serbia and Montenegro, using the 60 kDa glycoprotein gene sequences. Parasitology. 2007;134(Pt 3):351–8. 10.1017/S0031182006001508 [DOI] [PubMed] [Google Scholar]
- 74.Siverlas C, Bosaeus-Reineck H, Näslund K, Björkaman C. Is there a need for improved Cryptosporidium diagnostics in Swedish calves. Int J Parasitol. 2013;43(2):155–61. 10.1016/j.ijpara.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomazic ML, Maidana J, Dominguez M, Uriarte EL, Galarza R, Garro C, et al. Molecular characterization of Cryptosporidium isolates from calves in Argentina. Vet. Parasitol. 2013;198: 382–6. 10.1016/j.vetpar.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 76.Del Coco VF, Córdoba MA, Bilbao G, Castro APA, Basualdo JA, Fayer R, et al. Cryptosporidium parvum GP60 subtypes in dairy cattle from Buenos Aires, Argentina. Res Vet Sci. 2014;96(2):311–4. 10.1016/j.rvsc.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 77.Kaupke A, Rzeżutka A. Emergence of novel subtype of Cryptosporidium parvum in calves in Poland. Parasitol Res. 2015;114(12):4709–16 10.1007/s00436-015-4719-1 [DOI] [PubMed] [Google Scholar]
- 78.Nolan MJ, Jex AR, Mansell PD, Browning GF, Gasser RB. Genetic characterization of Cryptosporidium parvum from calves by mutation scanning and targeted sequencing-zoonotic implications. Electrophoresis. 2009;30(15):2640–7. 10.1002/elps.200900071 [DOI] [PubMed] [Google Scholar]
- 79.Koehler AV, Bradbury RS, Stevens MA, Haydon SR, Jex AR, Gasser RB. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning-coupled approach. Electrophoresis. 2013;34(12):1720–8 10.1002/elps.201300100 [DOI] [PubMed] [Google Scholar]
- 80.CDC—Centers for Disease Control and Prevention. Cryptosporidiosis Outbreak at a Summer Camp, North Carolina, 2009. CDC. 2011;60(27):918–922. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6027a3.htm. [PubMed] [Google Scholar]
- 81.O’Handley RM, Ceri H, Anette C, Olson ME. Passive immunity and serological immune response in dairy calves associated with natural Giardia duodenalis infections. Vet Parasitol. 2003;113(2):89–98 [DOI] [PubMed] [Google Scholar]
- 82.Vaz L, Orlando PHK. Importância das matas ciliares para manutenção da qualidade das águas de nascentes: diagnóstico do ribeirão Vai-Vem de Ipameri—GO. In: XXI Encontro Nacional de Geografia Agrária, 21., 2012, Uberlândia. Anais…Uberlândia: UFU, 2012. p. 1–20.
- 83.Andrade J, Sanquetta CR, Ugaya C. Identificação de áreas prioritárias para recuperação da mata ciliar na UHE Salto Caxias. Espaço Energia. 2005; 3: 6–13. [Google Scholar]
- 84.Capp N, Ayach LR, Santos TMB, Guimarães SSTL. Qualidade da água e fatores de contaminação de poços rasos na área urbana de Anastácio (MS). Geografia Ensino e Pesquisa. 2012;16(3):77–91. [Google Scholar]
- 85.Taylor R, Cronin A, Pedley S, Barker J, Atkinson T. The implicatins of grounwater velocity variations on microbiol transport na wellhead protection—review of field evidence. FEMS Microbiology Ecology. 2004;49:17–7. 10.1016/j.femsec.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 86.BRANCO N; LEAL DAG; FRANCO RMB. A Parasitological Survey of Natural Water Springs and Inhabitants of a Tourist City in Southeastern Brazil. Vector Borne Zoonotic Dis. 2011;12(5):410–7. [DOI] [PubMed] [Google Scholar]
- 87.Nishi L, Bergamasco R, Toledo MJO, Falavigna DLM, Gome ML, Mota LT et al. Giardia spp. and Cryptosporidium spp. in the Ivaí Indigenous Land, Brazil. Vector Borne Zoonotic Dis. 2009;9(5):543–7. 10.1089/vbz.2008.0021 [DOI] [PubMed] [Google Scholar]
- 88.Swaffer BA, Vial HM, King BJ, Daly R, Frizenschaf J, Monis PT. Investigating sourcewater Cryptosporidium concentration, species and infectivity rates during rainfall-runoff in a mutil-use catchment. Water Res. 2014; 67:310–20. 10.1016/j.watres.2014.08.055 [DOI] [PubMed] [Google Scholar]
- 89.Heller L, Bastos RKX, Vieira MBCM, Bevilacqua PD, De Brito LLA, Mota SMM, et al. Oocistos de Cryptosporidiume cistos de Giardia: circulação no ambiente e riscos à saúde humana. Epidemiol Serv Saúde. 2004;13:79–92 [Google Scholar]
- 90.Plutzer J, Torokne A, Karanis P. Combination of ARAD micro-fiber filtration and LAMP methodology for simple, rapid and cost-effective detection of human pathogenic Giardia duodenalis and Cryptosporidium spp. in drinking water. Lett Appl Microbiol. 2010;50:82–88 10.1111/j.1472-765X.2009.02758.x [DOI] [PubMed] [Google Scholar]
- 91.Brasil MS (2011) Portaria No 2,914, 12 December 2011. http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html. Accessed 20 March 2012.
- 92.Ryan U, Cacciò SM. Zoonotic Potential of Giardia. Int J Parasitol. 2013;43(12–13):943–56. 10.1016/j.ijpara.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 93.Cacciò SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. 2008;160(2):75–80 10.1016/j.molbiopara.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 94.Cacciò SM, Sprong H. Giardia duodenalis: genetic recombination and its implications for taxonomy and molecular epidemiology. Exp Parasitol. 2010;124(1):107–12. 10.1016/j.exppara.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 95.Geurden T, Vanderstichel R, Pohle H, Ehsan A, Von Samson-Himmelstjerna G, Morgan ER, et al. A multicentre prevalence study in Europe on Giardia duodenalis in calves, with molecular identification and risk factor analysis. Vet Parasitol. 2012;190(3–4):383–90 10.1016/j.vetpar.2012.06.039 [DOI] [PubMed] [Google Scholar]
- 96.Zintl A, Proctor AF, Read C, Dewaal T, Shanaghy N, Fanning S, Mulcahy G. The prevalence of Cryptosporidium species and subtypes in human faecal samples in Ireland. Epidemiol Infect. 2009;137(2):270–7 10.1017/S0950268808000769 [DOI] [PubMed] [Google Scholar]
- 97.Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141(13):1667–85. 10.1017/S0031182014001085 [DOI] [PubMed] [Google Scholar]
- 98.Wilkes G, Ruecker N J, Neumann NF, Gannon VP, Jokinen C, Sunohara M, et al. Spatio temporal analysis of Cryptosporidium species/genotypes and relationships with other zoonotic pathogens in surface water from mixed-use watersheds. Appl Environ Microbiol. 2013;79(2): 434–48 10.1128/AEM.01924-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao L, Fayer R, Ryan U, Upton SJ. Cryptosporidium taxonomy: Recent advances and implications for public health. Clin Microbiol Rev. 2004;17(1):72–97 10.1128/CMR.17.1.72-97.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
aPercentage calculated using the number of positive data from optical microscopy (OM). nPCR: nested PCR, 18S: 18S rRNA gene.
(PDF)
anPCR: nested PCR, bPCR-RFLP: restriction fragment length polymorphism, c ND: species not determined (ND) due to illegible sequence (sequencing data) or absence of DNA (PCR-RFLP), dC. parvum, eC. bovis.
(PDF)
aFisher's exact test; bOR: odds ratio; cCI: confidence interval; dp: probability.
(PDF)
aThermotolerant coliforms according to the multiple-tube method; baccording to the chromogenic substrate method.
(PDF)
aStrongly concentrated by the flocculation method of calcium carbonate [29]; bIFA: direct immunofluorescence assay; cOo/L: oocysts/liter of water; dCy/L: cysts/liter of water; eND: Not determined due to illegible sequence; fnPCR: nested PCR; gPCR-RFLP: restriction fragment length polymorphism; hNTU: nephelometric turbidity unit; iTTC: thermotolerant coliforms; jMPN: most probable number.
(PDF)
aOR: odds ratio; bCI: confidence interval; cp: probability.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.