Abstract
VEGFA is an angiogenic factor secreted by tumors, in particular those with VEGFA amplification, as well as by macrophages and lymphocytes in the tumor microenvironment. Here we sought to define the presence of M1/M2 macrophages, PD-1-positive lymphocytes and PD-L1 tumoral and stromal expression in colorectal cancers harboring VEGFA amplification or chromosome 6 polysomy. 38 CRCs of which 13 harbored VEGFA amplification, 6 with Chr6 polysomy and 19 with neutral VEGFA copy number were assessed by immunohistochemistry for CD68 (marker for M1/M2 macrophages), CD163 (M2 macrophages), programmed death 1(PD-1)- tumor infiltrating and stromal lymphocytes as well as tumoral and stromal PD-1 ligand (PD-L1) expression. CRCs with VEGFA amplification or Chr6 polysomy were associated with decreased M1/M2 macrophages, reduced PD-1-expressing lymphocyte infiltration, as well as reduced stromal expression of PD-L1 at the tumor front. Compared to intermediate-grade CRCs, high-grade CRCs were associated with increased M1/M2 macrophages and increased tumoral expression of PD-L1. Our results suggest that VEGFA amplification or Chr6 polysomy is associated with an altered tumor immune microenvironment.
Introduction
The complex interactions between tumor cells and non-tumoral cells within the tumor microenvironment contribute to the hallmarks of cancer cells [1]. The tumor microenvironment is composed of many different cell types including endothelial cells, pericytes, fibroblasts and immune cells [1]. The recent promising results of PD-1/PD-L1 blockade as an immunotherapy check-point in different cancer entities [2–5] have underscored the essential role of the immune system in the control of tumor growth.
Tumor-associated macrophages (TAMs) are found within tumors as well as in the surrounding non-malignant tissues [6] and can be either pro- or anti-tumorigenic in response to environmental changes [7–9]. Macrophages are broadly classified into two major groups, M1 and M2. M1 macrophages are involved in inflammatory response, pathogen clearance and antitumor immunity through the expression of pro-inflammatory cytokines such as IL-1β, IL-6, IL-12, IL-23, TNFα and nitric oxide synthase 2 (iNOS) [6,10–13]. By contrast, M2 macrophages are known to promote tissue remodeling and repair, angiogenesis and tumor progression [14,15]. M2 macrophages release anti-inflammatory cytokines such as IL-10 and transforming growth factor β (TGFβ) and are characterized by an upregulation of mannose receptors (e.g. CD206) and arginase-1, and a downregulation of iNOS production [10,16,17]. The prognostic implication of the extent of macrophage infiltration is uncertain in colorectal carcinomas (CRCs) with reports variably showing associations with favorable prognosis [18] and with adverse prognosis [19] but is generally associated with poor prognosis in other cancer types [20,21]. The contradictory results may be associated with the type and localization of macrophages in the tumor and/or with macrophage infiltration at the tumor front [18].
Activated T-cells and other immune cells typically show upregulation of programmed cell death-1 (PD-1), which plays an immune-suppressive role when bound to its ligand PD-L1 [2]. PD-L1 is expressed by T and B cells, dendritic cells, macrophages, endothelial, muscle and pancreatic cells [22] and its upregulation in cancer cells has been implicated in shutting down immune response in cancer cells [22]. The interaction between PD-1 and PD-L1 results in the downregulation of lymphocyte proliferation and cytokine production [23]. Tumor infiltrating PD-1-positive T-cells and tumoral expression of PD-L1 have been associated with poor prognosis in several tumors, including esophageal, pancreatic, gastric, hepatocellular, urothelial and renal cell carcinomas, follicular lymphoma, melanoma as well as soft tissue sarcomas [23–33]. However, the role of PD-1/PD-L1 in CRC is controversial [22,34]. The contradictory results may be caused by technical limitations, as well as by the heterogeneity and variability of these markers which are strongly affected by temporal and spatial factors [34], leading to different interpretation when detected in different sections of the same tumor.
Recently, we showed that a subgroup (~7%) of highly aggressive CRCs harbor copy number amplification of vascular endothelial growth factor A (VEGFA), a member of the vascular endothelial growth factor (VEGF) family, which also includes VEGFB, VEGFC, VEGFD and placental growth factor (PlGF) [35]. VEGFs have been shown to play multi-faceted roles in stimulating neo-angiogenesis and tumor growth [36] and among the VEGFs, VEGFA, in particular, has been shown to mediate angiogenesis, a critical step in both tumor growth and metastasis formation [2,37]. In fact, VEGFA is a key regulator of proliferation, survival, migration and permeability of blood endothelial cells in both physiological and pathological angiogenesis [2,37]. Consistent with these results, copy number amplification and overexpression of VEGFA have been associated with poor prognosis in various cancer types [38–40]. In addition to its well-documented angiogenic roles, VEGFA has also been shown to have immunosuppressive properties, including the inhibition of dendritic cell maturation and T-cell production [41,42]. In fact, a recent study demonstrated that VEGFA produced in the tumor microenvironment directly increases PD-1 expression on intratumoral CD8+ T-cells and combined anti-PD-1 and anti-VEGFA blockade showed a synergistic effect in tumors with high levels of VEGFA [43]. It is also important to note that in addition to tumor cells, macrophages and, to a lesser extent, tumor infiltrating lymphocytes (TIL), represent major sources of VEGFA, and macrophage-produced VEGFA has been shown to promote tumor angiogenesis and invasion [44,45]. Thus the interactions between tumors with VEGFA amplification, macrophages and PD-1-expressing lymphocytes are likely to be intricate.
To address the question whether there is any association of VEGFA copy number status and alterations of the immune microenvironment in CRCs, we performed an immunohistochemical study to define the presence of M1/M2 macrophage (using CD68 and CD163 as markers for M1/M2 and M2 macrophages, respectively), the presence of PD-1-positive tumor infiltrating and stromal lymphocytes and the distribution of PD-L1 expression in the tumor and the stroma. We found that VEGFA gene copy number amplification/polysomy was associated with reduced macrophages, PD-1-positive tumor infiltrating lymphocytes and PD-L1 stromal expression.
Materials and methods
Ethics
Samples were anonymized prior to analysis and the study has been approved by the Institutional Review Board of the Institute of Pathology, University Hospital Basel (USB), Switzerland, and the Ethics Committee of Nordwest/Central Switzerland (EKNZ). Participants in the USB underwent a written, informed consent process at enrolment.
Tissue samples
The biobank at the Institute of Pathology, University Hospital Basel, Switzerland, was searched for CRCs diagnosed between 2007 and 2013. In total, formalin-fixed paraffin-embedded (FFPE) samples of 150 CRCs and 45 adjacent non-malignant tissue samples were retrieved. Additionally, whole sections of 8 CRCs previously found to harbor VEGFA copy number amplification (n = 2) or chromosome 6 polysomy (n = 6) [35] were retrieved from the Institutes of Pathology of the Cantonal Hospitals of Aarau and St. Gallen, Switzerland.
Tissue microarray (TMA) construction
All FFPE samples had sufficient material for TMA construction. Hematoxylin and eosin-stained sections were obtained from each FFPE block to define representative tumor tissue regions. TMAs were constructed by punching the regions of interest using core cylinders of 1 mm diameter using TMA-GM® (Sysmex AG, Switzerland). Four-μm-thick slides of the resulting TMAs were cut using Microtome (Thermo Fisher Scientific Inc., USA).
Fluorescence In Situ Hybridization (FISH)
FISH for VEGFA gene copy number status was performed using validated protocols established at the Institute of Pathology at the University Hospital Basel as described previously [35,46]. VEGFA-amplified cases were defined as a VEGFA/Chr6 ratio of <2.0 and an average VEGFA copy number of ≥6.0 signals per cell or a VEGFA/Chr6 ratio ≥2.0 with an average VEGFA copy number of ≥4.0 signals per cell. Samples with a VEGFA/Chr6 ratio of <2.0 and an average VEGFA copy number <4.0 signals per cell were classified as not amplified. Samples with a VEGFA/Chr6 ratio <2.0 and an average VEGFA copy number ≥ 4.0 and <6.0 signals per cell were classified as equivocal. For Chr6 polysomy status, low polysomy 6 was defined as an average between 2.26 and 3.75 Chr6 copy number and high polysomy 6 was defined as an average higher than 3.75 Chr6 copy number [46]. For TMA punches that were positive or equivocal for VEGFA amplification or Chr6 polysomy, FISH was performed on a whole slide from the corresponding FFPE block to confirm the positive results or to resolve the equivocal cases [35,46].
Immunohistochemistry
Whole FFPE sections were pre-treated with CC1 (Ventana Medical Systems, Tucson, Arizona, USA) as previously described [47] and incubated with primary antibodies against CD68 (IR613, Dako, Denmark, pre-diluted), CD163 (Cat. No. 760–4437, Ventana Medical Systems Inc., USA, pre-diluted), PD-1 (Cat. No. 760–4895, Ventana Medical Systems Inc., USA) and PD-L1 (Cat. No. 13684, Cell Signaling Technology, USA). Positive and negative controls were included in each experiment. Immunohistochemistry for each marker was evaluated twice by the same observer (KB) using the BX43 light microscope (Olympus, Japan). Discordant cases were reviewed by two pathologists with a special interest in gastrointestinal pathology (LT and LMT) to reach a consensus. Representative micrographs were acquired using the cellSens Dimension software (Olympus, Japan) and the DP73 Camera (Olympus, Japan) installed on the BX43 light microscope.
For CD68 (M1/M2 macrophage marker) and CD163 (M2 macrophage marker), IHC scoring was performed for 4 randomly selected fields at the tumor front for each case. The number of macrophages was counted for each marker on a total field of 2.2 mm2 using the ImageJ program (version 1.46r). Semi-quantitative/ categorical comparisons were also performed using the following thresholds. For CD68-positive macrophages, fewer than 100 macrophages was considered low infiltration, between 100 and 130 moderate infiltration and more than 130 high infiltration. For CD163-positive macrophages, fewer than 60 macrophages was defined as low infiltration, between 60 and 90 macrophages as moderate infiltration and more than 90 macrophages as high infiltration.
For PD-1, 4 random spots at the tumor front of each case were selected and photographed with a 40X objective and a 10X ocular with a total magnification of 400X. A total of 152 pictures in 38 tumors were evaluated. PD-1 expression in tumor infiltrating and stromal lymphocytes were evaluated separately. The number of PD-1-positive cells were counted on a total field of 2.2 mm2 using the ImageJ program (version 1.46r). For categorical analyses, the infiltration of positive PD-1 cells per 2.2mm2 was scored as no infiltration (0 to 8 cells), low infiltration (9 to 39 cells) and high infiltration (more than 39 cells). PD-1 expression in tumor infiltrating and stromal lymphocytes were evaluable in 36/38 samples.
PD-L1 expression was evaluated using the scoring system as described by Kim et al. [23] to evaluate the intensity and the area of staining, separately in tumor and stromal areas at the tumor front. Staining intensity was graded semi-quantitatively as: 0 for negative staining, 1 for weakly positive staining, 2 for moderately positive staining and 3 for strongly positive staining. Area of staining was scored as 0 for 0–10% stained cells, 1 for 11–33% stained cells, 2 for 34–66% stained cells and 3 for 67–100% stained cells. A combined PD-L1 score was defined as the sum of the intensity score and the area of staining score, with a minimum score of zero and a maximum combined score of six. For categorical analyses, total scores were divided into three groups: 0 for no expression, 1 to 2 for low expression, and 3 to 6 for high expression. PD-L1 expression in tumoral and stromal areas were evaluable in 38/38 and 37/38 samples, respectively.
Statistical analysis
Statistical analyses for categorical and non-categorical variables were performed using Chi-Square/ Fisher’s Exact and Mann-Whitney U/ Student’s t tests as described in the manuscript or figure legends. All tests were two-sided. p-values <0.05 were considered statistically significant. All analyses were performed using Graphpad Prism 6.0 (Graphpad Software, Inc., La Jolla, CA) or R x64 Version 3.2.1 (http://www.R-project.org).
Results
VEGFA copy number assessment and sample selection
To identify cases of CRC with VEGFA gene amplification, we screened a TMA consisting of 150 CRCs by FISH for VEGFA gene amplification and/or chromosome 6 (Chr6) polysomy. Of the 124 evaluable samples, we identified 11 samples (9%) with VEGFA amplification and none that displayed Chr6 polysomy. The remaining 113 samples (91%) were VEGFA copy number neutral. In addition, we retrieved the whole sections from eight cases previously found to harbor VEGFA amplification (n = 2) or Chr6 polysomy (n = 6) [35]. In total, we selected the 19 CRCs with VEGFA amplification (n = 13) or Chr6 polysomy (n = 6) and the same number of VEGFA copy number neutral CRCs (n = 19) as control (Fig 1 and S1 Table). Most the included CRCs were of intermediate tumor grade (i.e. grade 2, n = 29) and the remaining were of high grade (i.e. grade 3, n = 9, S1 Table). The VEGFA status stratified on the histologic grade revealed that in the intermediate tumor grade CRCs VEGFA was amplified, polysomic or diploid in 38% (11/29), 14% (4/29) and 48% (14/29) respectively. In the CRCs of high tumor grade VEGFA was amplified, polysomic or diploid in 22% (2/9), 22% (2/9) and 56% (5/29) respectively. Further analysis revealed that there were no differences in sex, T/N/M stage, tumor grade, lymphatic and venous invasion between the VEGFA amplified/polysomic and the control group (p>0.05, Chi-square tests).
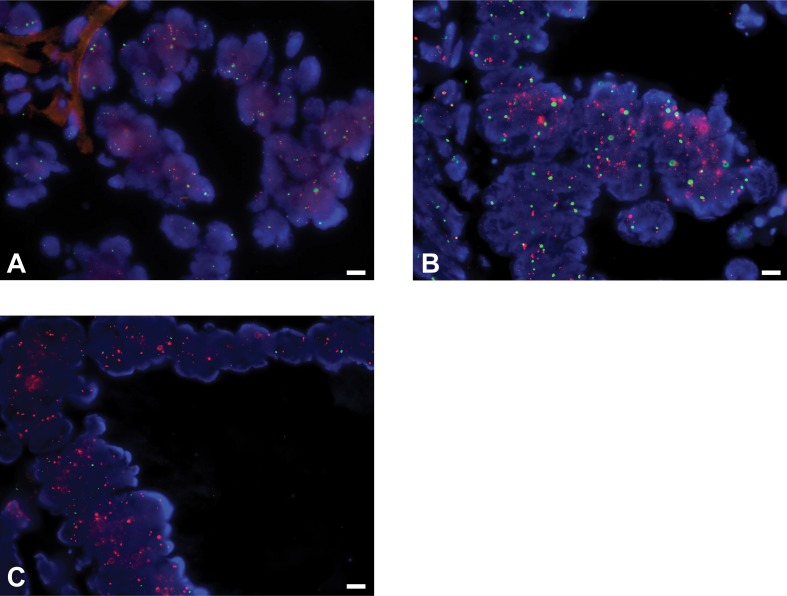
Fig 1. VEGFA copy number status in colorectal cancers measured by fluorescent in situ hybridization.
Representative micrographs of (A) diploid, (B) polysomic and (C) amplified VEGFA using fluorescent in situ hybridization (FISH). FISH analysis was performed using two-color probes for VEGFA (red) and internal control (green). Scale bar 20 μm.
VEGFA amplification/polysomy is associated with reduced M1 and M2 macrophages
To determine the distribution of macrophages in CRCs, we performed immunohistochemical analysis using CD68 as a marker for both M1 and M2 macrophages and CD163 as a marker for M2 macrophages [6]. In the 38 CRCs included in our cohort, both CD68-positive cells and CD163-positive cells were almost exclusively located in the tissue surrounding the tumors, especially along invasive tumor front (Fig 2). Semi-quantitative evaluation of CD68 and CD163 expression at the tumor front revealed that 16 (42%), 13 (34%) and 9 (24%) CRCs had low, moderate and high infiltration of CD68-positive cells, respectively, and 33 (87%), 3 (8%) and 2 (5%) CRCs had low, moderate and high infiltration of CD-163-positive cells, respectively (S2 Table).
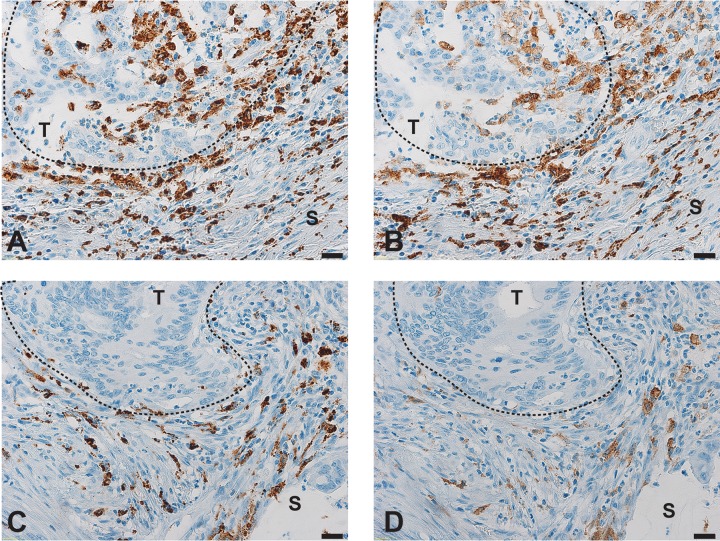
Fig 2. Distribution of macrophages in colorectal cancers, using CD68 and CD163 markers.
Representative micrographs of (A, C) CD68+ cells and (B, D) CD163+ cells (A, B) at the invasive tumor front (T) and (C, D) in the surrounding tumor tissue (S). Magnification 40X. Scale bar 20 μm.
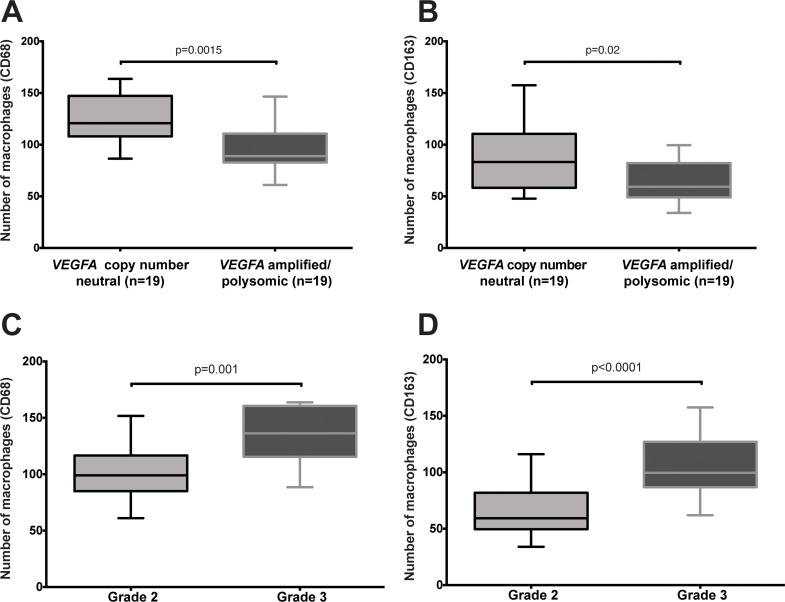
Statistical analysis between the number of CD68- and CD163-positive cells with and without VEGFA copy number amplification/ polysomy revealed reduced CD68-positive cells in CRCs with VEGFA gene amplification or Chr6 polysomy compared to those that were VEGFA copy number neutral (p = 0.0015, Mann–Whitney U test; Fig 3A). When we categorized the number of CD68-positive cells into low, moderate and high infiltration, a predominantly low infiltration (p = 0.0139, Chi-square test, S2 Table) was found in CRCs harboring VEGFA gene amplification or Chr6 polysomy. Similarly, we observed fewer CD163-positive cells in CRCs with VEGFA gene amplification/ Chr6 polysomy than VEGFA copy number neutral CRCs (p = 0.02, Mann–Whitney U test; Fig 3B). However, there was no statistical difference we categorized the number of CD163-positive cells into low, moderate and high infiltration (p = 0.218, Chi-square test, S2 Table). We further observed that both CD68 and CD163 infiltration were increased in high-grade tumors (grade 3) compared to intermediate-grade tumors (grade 2, p = 0.001 and p<0.0001, respectively, Mann–Whitney U tests; Fig 3C and 3D and p = 0.002 and p<0.0001, respectively, Chi-square test, S2 Table). The increased CD-163 positive cells are M2 macrophages. The fraction of M1 macrophages was on the contrary reduced in high-grade cases.
Fig 3. Number of macrophages in colorectal cancers, using CD68 and CD163 markers.
Boxplots depict the number of (A, C) CD68+ and (B, D) CD163+ cells (A, B) in CRCs with and without VEGFA amplification/polysomy and (C, D) in CRCs of intermediate and high grade.
Taken together, these results suggest that CRCs with VEGFA gene amplification or Chr6 polysomy are associated with reduced M1/M2 (CD68-positive) and M2 (CD163-positive) macrophage infiltration, whilst high-grade CRCs are associated with increased M2 and reduced M1 macrophage infiltration.
VEGFA amplification/polysomy is associated with reduced PD-1-positive tumor lymphocytes and PD-L1 stromal expression
Next we investigated the association of VEGFA gene copy number status with the presence of PD-1-positive tumor infiltrating and stromal lymphocytes and PD-L1 tumoral and stromal expression at the tumor front. In this cohort, we observed no PD-1-expressing tumor infiltrating lymphocytes and PD-L1 tumoral expression in 50% and 66% of the CRCs analyzed. By contrast, PD-1 positive lymphocytes were present and PD-L1 was expressed in the stroma in 89% and 92% of cases respectively (S2 Table).
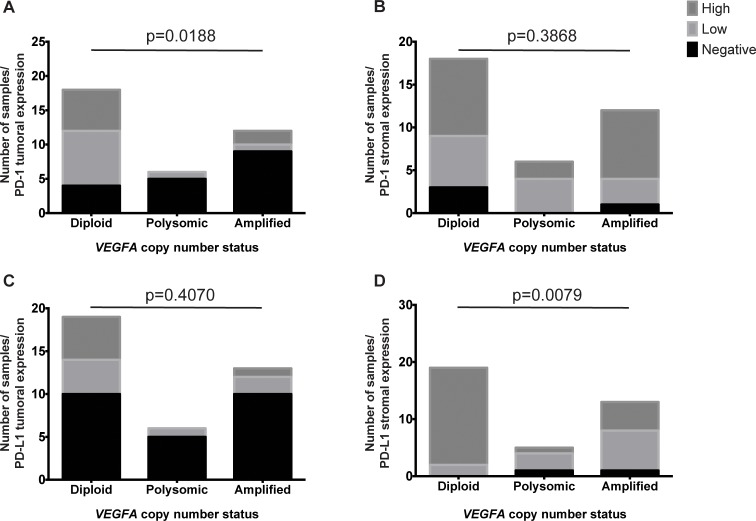
Similar to the reduction in M1 and M2 macrophages, CRCs with VEGFA amplification or Chr6 polysomy were preferentially associated with the absence of or the reduction in PD-1-positive tumor infiltrating lymphocytes than in VEGFA copy number neutral CRCs (p = 0.0188, Chi-square test; Fig 4A), but were not different from VEGFA copy number neutral CRCs in terms of the number of PD-1-positive stromal lymphocytes (p = 0.3868, Chi-square test; Fig 4B). We further observed no difference in PD-L1-tumoral expression between CRCs with VEGFA amplification or Chr6 polysomy and VEGFA copy number neutral CRCs (p = 0.407, Chi-square test; Fig 4C). However, we observed that CRCs with VEGFA amplification or Chr6 polysomy had lower PD-L1 expression in the stroma than in VEGFA copy number neutral CRCs (p = 0.0079, Chi-square test; Fig 4D). Neither PD-1-positive tumor infiltrating nor stromal lymphocytes was associated with tumor grade (p = 0.4355 and p = 0.839, respectively, Chi-square tests; S2 Table). However, increased tumoral but not stromal PD-L1 expression was associated with high-grade (grade 3) compared to intermediate-grade tumors (grade 2, p = 0.0173 and p = 0.4743, respectively, Chi-square test; S2 Table).
Fig 4. PD-1-positive tumor infiltrating and stromal lymphocytes and PD-L1 tumoral and stromal expression in colorectal cancers.
Barplots depict the number of samples with high, low and negative expression of (A, B) PD-1 and (C, D) PD-L1 in (A, C) tumoral and (B, D) stromal areas of CRCs.
Taken together, these results suggest that CRCs with VEGFA gene amplification or Chr6 polysomy are associated with reduced PD-1-positive tumor infiltrating lymphocytes and PD-L1 stromal expression.
Discussion
To understand the immune microenvironment of CRCs with VEGFA gene copy number amplification or Chr6 polysomy, we performed a hypothesis-generating immunohistochemical analysis and found an association between VEGFA gene copy number amplification or Chr6 polysomy and reduced number of M1 and M2 macrophages, reduced PD-1-expressing lymphocyte infiltration, as well as reduced stromal expression of PD-L1 at the tumor front. We further observed a higher number of M2 macrophages and increased PD-L1 tumoral expression in high-grade tumor compared with intermediate-grade CRCs.
In accordance with Forssell et al. [18], we observed that most CD68+ and/or CD163+ macrophages were found in the stroma along the tumor front. This suggests that macrophages are attracted to or recruited to the invasive front. In high-grade tumors these were mostly CD163+, M2 macrophages. These results are in keeping with similar data recently obtained in other neoplastic entities as gastric and lung carcinoma [48,49]. In addition, M2 macrophage polarization has been reported to be significantly associated with advanced histopathologic stage and the presence of metastasis, probably mediated by Caspase recruitment domain-containing protein 9 (CARD9) through activation of the nuclear factor-kappa B signaling pathway [50]. Moreover, we further found that VEGFA gene copy number amplification or Chr6 polysomy, a feature that characterizes a subset of aggressive CRCs, was associated with reduced TAMs. These results are in agreement with the previous report linking high CD68+ macrophage infiltration at the tumor front of CRC to improved survival of patients [18]. By contrast, this subset of aggressive CRCs is also associated with lower levels of PD-1-positive tumor infiltrating lymphocytes but high levels of PD-1-positive tumor infiltrating lymphocytes have been robustly associated with poor survival in many cancers [23,26,30–32]. Unfortunately due to the limited number of cases, we were unable to perform survival analysis for this cohort.
VEGFA has previously been associated with immunosuppression in tumors [41,42]. In addition to being produced by a substantial proportion of tumors and being over-expressed when amplified [38–40], VEGFA is also secreted by various cell types, including macrophages and lymphocytes, in the tumor microenvironment. Our observations led to the hypothesis that VEGFA amplification may suppress the attraction of macrophages and lymphocytes towards the tumor front. We speculate that pre-angiogenic tumor tissues, which do not harbor the VEGFA gene copy number amplification and therefore express low levels of VEGFA, send signals to the bone marrow and/or the blood circulation that lead to the recruitment of the macrophages [51]. Once in the proximity of the tumor, the macrophages release metalloproteinases, such as MMP-9. MMP-9 cleaves the components in the extracellular matrix, releasing VEGFA from its sequestered state and inducing angiogenesis around the tumor, as seen in hyperplastic islet of Langerhans [51]. By contrast, we speculate that the recruitment process of TAMs may not occur in tumors harboring VEGFA gene amplification, as the additional VEGFA secreted by surrounding cells may no longer provide a growth advantage. The observation that VEGFA amplified/polysomic CRCs had both reduced numbers of CD68-positive and/or CD163+ macrophages and lower PD-L1 stromal expression may further support our hypothesis, since PD-L1 has been reported to be overexpressed in macrophages [51].
Although our cohort in the study was small and the markers used for the identification of macrophages may be imperfect, we found a consistent pattern of macrophages at the tumor front using two markers, in agreement with a previous study [18]. It would be of interest to investigate whether the adverse prognosis associated with the extent of macrophage infiltration in CRC and other cancer types [19–21] overlaps with the aggressive nature of CRCs associated with VEGFA amplification.
Conclusions
In summary, we have identified an association between VEGFA gene amplification in CRC and reduced macrophages, PD-1-positive infiltrating lymphocytes and PD-L1 stromal expression at the tumor front. Further studies are needed to clarify the role of VEGFA on the interaction of CRCs and their tumor microenvironment and to provide mechanistic insight into these observations.
Supporting information
(XLS)
(XLSX)
Acknowledgments
We acknowledge the excellent technical support of D. Tas and P. Hirschmann from the Institute of Pathology (Basel) and would like to thank A. Lacombe and V. Carafa for the technical support.
Data Availability
All data are available in the body of the manuscript or in the supplementary tables.
Funding Statement
Salvatore Piscuoglio is funded by Swiss National Science Foundation (Ambizione grant number PZ00P3_168165); The study was supported by grants from Oncosuisse KLS-3639-02-2015 (Luigi Maria Terracciano) and KLS-3169-02-2013 (Luigi Tornillo). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. LT is currently employed by GILab AG. GILab AG provided support in the form of salary for author LT, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, et al. (2014) Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 23: 2965–2970. doi: 10.1158/1055-9965.EPI-14-0654 [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, et al. (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28: 3167–3175. doi: 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sznol M, Chen L (2013) Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer—response. Clin Cancer Res 19: 5542 doi: 10.1158/1078-0432.CCR-13-2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, et al. (2012) The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One 7: e47045 doi: 10.1371/journal.pone.0047045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom A, Erlandsson A, Delbro D, Wijkander J (2014) Conditioned media from macrophages of M1, but not M2 phenotype, inhibit the proliferation of the colon cancer cell lines HT-29 and CACO-2. Int J Oncol 44: 385–392. doi: 10.3892/ijo.2013.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor-associated macrophages. Immunol Today 13: 265–270. doi: 10.1016/0167-5699(92)90008-U [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229: 176–185. doi: 10.1002/path.4133 [DOI] [PubMed] [Google Scholar]
- 10.Tran TH, Amiji MM (2015) Targeted delivery systems for biological therapies of inflammatory diseases. Expert Opin Drug Deliv 12: 393–414. doi: 10.1517/17425247.2015.972931 [DOI] [PubMed] [Google Scholar]
- 11.Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G (2014) Functional Relationship between Tumor-Associated Macrophages and Macrophage Colony-Stimulating Factor as Contributors to Cancer Progression. Front Immunol 5: 489 doi: 10.3389/fimmu.2014.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanmee T, Ontong P, Konno K, Itano N (2014) Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 6: 1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun T, Yang Y, Luo X, Cheng Y, Zhang M, et al. (2014) Inhibition of tumor angiogenesis by interferon-gamma by suppression of tumor-associated macrophage differentiation. Oncol Res 21: 227–235. doi: 10.3727/096504014X13890370410285 [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A (2006) Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25: 315–322. doi: 10.1007/s10555-006-9001-7 [DOI] [PubMed] [Google Scholar]
- 15.Sica A, Invernizzi P, Mantovani A (2014) Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology 59: 2034–2042. doi: 10.1002/hep.26754 [DOI] [PubMed] [Google Scholar]
- 16.Galvan-Pena S, O'Neill LA (2014) Metabolic reprograming in macrophage polarization. Front Immunol 5: 420 doi: 10.3389/fimmu.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, et al. (2013) Tumor associated macrophages and neutrophils in cancer. Immunobiology 218: 1402–1410. doi: 10.1016/j.imbio.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, et al. (2007) High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 13: 1472–1479. doi: 10.1158/1078-0432.CCR-06-2073 [DOI] [PubMed] [Google Scholar]
- 19.Herrera M, Herrera A, Dominguez G, Silva J, Garcia V, et al. (2013) Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci 104: 437–444. doi: 10.1111/cas.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, et al. (2011) Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica 96: 269–276. doi: 10.3324/haematol.2010.031542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, et al. (2011) Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol 179: 1157–1170. doi: 10.1016/j.ajpath.2011.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, et al. (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 49: 2233–2242. doi: 10.1016/j.ejca.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 23.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, et al. (2013) Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One 8: e82870 doi: 10.1371/journal.pone.0082870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, et al. (2009) Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15: 971–979. doi: 10.1158/1078-0432.CCR-08-1608 [DOI] [PubMed] [Google Scholar]
- 25.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, et al. (2006) The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 8: 190–198. doi: 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang MJ, Kim KM, Bae JS, Park HS, Lee H, et al. (2013) Tumor-infiltrating PD1-Positive Lymphocytes and FoxP3-Positive Regulatory T Cells Predict Distant Metastatic Relapse and Survival of Clear Cell Renal Cell Carcinoma. Transl Oncol 6: 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, et al. (2007) Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 56: 1173–1182. doi: 10.1007/s00262-006-0266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, et al. (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13: 2151–2157. doi: 10.1158/1078-0432.CCR-06-2746 [DOI] [PubMed] [Google Scholar]
- 29.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, et al. (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11: 2947–2953. doi: 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- 30.Richendollar BG, Pohlman B, Elson P, Hsi ED (2011) Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol 42: 552–557. doi: 10.1016/j.humpath.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 31.Sun S, Fei X, Mao Y, Wang X, Garfield DH, et al. (2014) PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother 63: 395–406. doi: 10.1007/s00262-014-1519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, et al. (2005) Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer 104: 2084–2091. doi: 10.1002/cncr.21470 [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, et al. (2006) Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 108: 19–24. doi: 10.1016/j.acthis.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Teng F, Kong L, Yu J (2016) PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9: 5023–5039. doi: 10.2147/OTT.S105862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlajnic T, Andreozzi MC, Schneider S, Tornillo L, Karamitopoulou E, et al. (2011) VEGFA gene locus (6p12) amplification identifies a small but highly aggressive subgroup of colorectal cancer [corrected] patients. Mod Pathol 24: 1404–1412. doi: 10.1038/modpathol.2011.96 [DOI] [PubMed] [Google Scholar]
- 36.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549–580. doi: 10.1124/pr.56.4.3 [DOI] [PubMed] [Google Scholar]
- 37.Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13: 871–882. doi: 10.1038/nrc3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan P, Ji YN, Yu LK (2013) VEGF is associated with the poor survival of patients with prostate cancer: a meta-analysis. Transl Androl Urol 2: 99–105. doi: 10.3978/j.issn.2223-4683.2013.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, He D, Li Z, Zhang X, Pan D, et al. (2015) Overexpression of vascular endothelial growth factor indicates poor outcomes of glioma: a systematic review and meta-analysis. Int J Clin Exp Med 8: 8709–8719. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, et al. (2009) Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol 4: 1094–1103. doi: 10.1097/JTO.0b013e3181a97e31 [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, et al. (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 42.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, et al. (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101: 4878–4886. doi: 10.1182/blood-2002-07-1956 [DOI] [PubMed] [Google Scholar]
- 43.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, et al. (2015) VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212: 139–148. doi: 10.1084/jem.20140559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin EY, Li JF, Bricard G, Wang W, Deng Y, et al. (2007) Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol 1: 288–302. doi: 10.1016/j.molonc.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, et al. (1995) Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res 55: 4140–4145. [PubMed] [Google Scholar]
- 46.Quagliata L, Andreozzi M, Kovac M, Tornillo L, Makowska Z, et al. (2014) SH2D4A is frequently downregulated in hepatocellular carcinoma and cirrhotic nodules. Eur J Cancer 50: 731–738. doi: 10.1016/j.ejca.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 47.Piscuoglio S, Lehmann FS, Zlobec I, Tornillo L, Dietmaier W, et al. (2012) Effect of EpCAM, CD44, CD133 and CD166 expression on patient survival in tumours of the ampulla of Vater. J Clin Pathol 65: 140–145. doi: 10.1136/jclinpath-2011-200043 [DOI] [PubMed] [Google Scholar]
- 48.Yin S, Huang J, Li Z, Zhang J, Luo J, et al. (2017) The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS One 12: e0170042 doi: 10.1371/journal.pone.0170042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei J, Xiao Z, Guo C, Pu Q, Ma L, et al. (2016) Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 7: 34217–34228. doi: 10.18632/oncotarget.9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M, Shao JH, Miao YJ, Cui W, Qi YF, et al. (2014) Tumor cell-activated CARD9 signaling contributes to metastasis-associated macrophage polarization. Cell Death Differ 21: 1290–1302. doi: 10.1038/cdd.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, et al. (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2: 737–744. doi: 10.1038/35036374 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLSX)
Data Availability Statement
All data are available in the body of the manuscript or in the supplementary tables.