Abstract
The development of the neurokinin-1 receptor-deficient (NK1R−/−) mouse permitted inquiry into the regulation of secretory immunoglobulin A (S-IgA) responses by substance P (SP) after oral immunization with a Salmonella enterica serovar Typhimurium vector expressing colonization factor antigen I (CFA/I) from enterotoxigenic Escherichia coli. In NK1R−/− mice, mucosal and serum IgA anti-CFA/I fimbrial responses were augmented, while secreted IgG anti-CFA/I fimbrial responses remained unaffected compared to those of BALB/c (NK1R+/+) mice. Supportive antibody-forming cells were present in the small intestinal lamina propria and spleen. To gain insight as to why the augmented S-IgA responses occurred, minimally, the responses were not attributed to differences in vaccine colonization of Peyer's patch (PP) and spleen or in their respective tissue weights. However, these S-IgA responses were supported by increased numbers of PP CD4+ T helper (Th) cells secreting interleukin-5 (IL-5) and IL-6 and splenic CD4+ Th cells secreting IL-6 compared to NK1R+/+ mice. Challenge of naive NK1R−/− mice with wild-type Salmonella showed improved median survival compared to naive NK1R+/+ mice. Data from peritoneal macrophage infection studies suggest that this survival is in part contributed by increased IL-10 production. Oral vaccination with Salmonella CFA/I or Salmonella vector showed no significant differences in conferred protection against wild-type challenge for either NK1R−/− or NK1R+/+ mice. Thus, these studies suggest that SP mediation contributes to proinflammatory responses to Salmonella infections.
Salmonella enterica serovar Typhimurium has been manipulated as a live vaccine vector (1, 9, 10, 34, 41) and offers the advantage of efficient presentation of protein or DNA vaccines, and it is particularly as well adept as other bacterial (13, 30, 42, 43) and viral (29) vectors at targeting mucosal inductive sites. Generally, these vectors tend to bias host immunity along T helper type 1 (Th1)-cell-dependent pathways because of their invasive properties or intracellular requirement. Yet we (34, 35) and others (7, 19) have shown that Salmonella vectors can convert Th1-type biases to Th2-type biases by mode of passenger antigen expression (1, 34). It has been established that the extracellular secretion of enterotoxigenic Escherichia coli fimbrial adhesin colonization factor antigen I (CFA/I) produces a biphasic Th cell response supporting enhanced production of secretory immunoglobulin A (S-IgA) antibodies (Abs) (34). This production is evidenced by an early, rapid induction of interleukin-4 (IL-4)- and IL-5-dependent responses followed by an incremental induction of Th1-cell (gamma interferon [IFN-γ])-dependent responses. These Th2-cell cytokines are believed to be responsible for the enhanced S-IgA responses obtained with this vaccine (34) as opposed to Th1-cell-dependent mechanisms induced by conventional Salmonella vaccines (27, 40, 45). How S-IgA responses are supported in the absence of potent Th2-type cytokines remains to be resolved, but one study (31) suggests that IL-6 and IL-10 may contribute to S-IgA responses against tetanus toxoid in IL-4-deficient mice.
Substance P (SP) is an 11-amino-acid neuropeptide with an amidated C terminus (16). While generally recognized for its ability to contract smooth muscle cells or as a pain signal neurotransmitter (16), SP has been shown to augment IgA responses (6, 32, 33, 44). Interestingly, leukocytes express native SP or neurokinin-1 receptor (NK1R) (24, 32, 38). With the availability of NK1R on mononuclear cells, this evidence suggests that SP should influence lymphocytes. Related to IgA production, SP was found to enhance IgA secretion either directly (6, 32) or indirectly via T cells (33, 44).
We pose the question of whether a deficiency in NK1R expression impacts mucosal immunity. To address this question, studies focused on our Salmonella-CFA/I vaccine, showing a biphasic CD4+-Th-cell response with concomitant stimulation of elevated S-IgA Abs as a means to probe mucosal responses. Our results from oral immunization of NK1R−/− mice with Salmonella-CFA/I vaccine show enhanced mucosal and systemic IgA responses to CFA/I fimbriae. These increases in IgA were supported by increased numbers of IL-5- and IL-6-producing CD4+ Th2 cells. Examination of the ability of these vaccines to protect NK1R−/− mice showed no significant differences in levels of protection in NK1R-competent mice. However, innate resistance to wild-type Salmonella was significantly enhanced in naive NK1R−/− mice, suggesting that in the absence of SP, mediation or amplification of proinflammatory responses (21, 22) may be subdued.
MATERIALS AND METHODS
Mice.
Breeder pairs of homozygous NK1R−/− mice on a BALB/c background were kindly provided by Craig Gerard, Children's Hospital, Boston, Mass. (5) and were bred and maintained at the Montana State University Animal Resource Center. BALB/c (NK1R+/+) mice were obtained from Frederick Cancer Research Facility, National Cancer Institute (Frederick, Md.). All mice were maintained in horizontal laminar flow cabinets; sterile food and water were provided ad libitum. All animal care and procedures were in accordance with institutional policies for animal health and well-being.
Oral immunization with Salmonella vaccines.
The S. enterica serovar Typhimurium-CFA/I vector vaccine strain H696 expresses functional CFA/I fimbriae on the vector's cell surface (14). This expression is maintained by a plasmid with a functional asd gene to complement the lethal chromosomal Δasd mutation in the parent Salmonella strain allowing stabilized CFA/I expression in the absence of antibiotic selection (47). NK1R+/+ and NK1R−/− mice (10 mice per group) pretreated with an oral 50% saturated sodium bicarbonate solution received a single oral dose of 5 × 109 CFU of the Salmonella-CFA/I construct or its isogenic control strain H647, which lacks the CFA/I operon. Confirmation of dose administered was accomplished by plating serial dilutions of the vaccines onto Luria-Bertani agar plates.
Ab ELISA.
CFA/I-specific endpoint titers from dilutions of immune sera or fecal extracts (34) were determined by an enzyme-linked immunosorbent assay (ELISA), as previously described, using purified CFA/I fimbriae antigen (15). Endpoint titers were expressed as the reciprocal dilution of the last sample dilution giving an absorbance of ≥0.1 optical density units above the optical density at 415 nm of negative controls after a 1-h incubation.
Lymphoid cell isolation.
Groups of mice (5 to 10 mice per group) were euthanized 4 weeks after oral immunization to collect lymphoid tissues. Splenic and Peyer's patch (PP) lymphocytes were isolated by conventional methods using Dounce homogenization (8, 34), yielding >95% viability using trypan blue exclusion. Enriched CD4+-T-cell fractions were isolated by a negative selection procedure (34).
Lymphocyte isolation from small intestinal lamina propria (iLP) was performed as previously described (8). Briefly, intestines were extracted from the mouse, the PP were carefully removed, and fecal material and mucous were flushed from the intestine by using RPMI 1640 medium. Intestines were minced into ∼1-mm pieces, shaken vigorously in complete medium (CM) (RPMI 1640, 10% fetal bovine serum [HyClone, Logan, Utah], 10 mM HEPES buffer, 10 mM nonessential amino acids, 10 mM sodium pyruvate, 10 U of penicillin-streptomycin/ml) to remove remaining mucous and fecal material, and the waste was filtered through a mesh screen. Intestinal tissues were then placed in RPMI 1640 medium containing 5% fetal bovine serum (HyClone) and 2 mM dithiothreitol (Sigma-Aldrich) in a 50-ml Teflon flask containing a magnetic stir bar and gently agitated on a stir plate at room temperature for 5 to 10 min. The supernatant was discarded, and dithiothreitol was rinsed from the intestinal pieces with RPMI 1640 medium. Intestinal tissues were returned to the Teflon flask, 50 U of collagenase type IV solution/ml containing 0.08 U of DNase/ml, as previously described (8), was added, and the suspension was agitated at 37°C. After 10 min, the supernatant containing iLP cells was removed and washed, and fresh collagenase was added to the remaining intestine. The process was repeated two more times, and isolated cells were washed in CM and resuspended in a 40% Percoll solution (Pharmacia, Uppsala, Sweden) and then layered over a 60% Percoll solution and subjected to gradient centrifugation. Lymphocytes at the interface layer were washed and resuspended in CM.
Ab ELISPOT assays.
Antibody-forming cells (AFC) were enumerated by using IgA and IgG CFA/I-specific ELISPOT assays (8, 34, 35). Mixed cellulose ester membrane-bottomed microtiter plates (Multi-Screen-HA; Millipore Corp., Bedford, Mass.) were coated with 1.0 μg of purified CFA/I fimbriae/ml in sterile phosphate-buffered saline (PBS). For total IgA or IgG AFC determinations, wells were coated with 5 μg of goat anti-mouse IgA or IgG (H chain specific) Abs (Southern Biotechnology Associates, Birmingham, Ala.)/ml in sterile PBS overnight at room temperature. The plates were blocked at 37°C for 2 h with CM. A total of 100 μl of cells from each tissue at varying concentrations (2 × 106 to 1.25 × 105 lymphocytes/ml) was added to the wells, and the plates were incubated at 37°C overnight. Cells were removed, and the plates were washed as previously described (34, 35). For detection of AFC responses, 100 μl of 1.0 μg of goat anti-mouse IgG and IgA-horseradish peroxidase conjugates (Southern Biotechnology Associates)/ml was added to the wells, and the plates were incubated overnight at 4°C. After washing, the wells were developed with 100 μl of AEC (Moss, Inc., Pasadena, Calif.), and the reaction was allowed to continue until spots developed (∼30 min). The reaction was stopped with H2O, the plates were allowed to dry overnight, and AFC were enumerated by counting under a low-power dissecting microscope (Leica, Buffalo, N.Y.).
Cytokine ELISPOT assays.
Cytokine secretion by stimulated lymphocytes was detected by using cytokine-specific ELISPOT assays (34, 35, 45). Splenic and PP CD4+ T cells were cultured at 5 × 106 cells/ml with equal numbers of feeder cells (T-cell-depleted splenic mononuclear cells used as a source of antigen-presenting cells) treated with mitomycin C (50 μg/ml; Sigma-Aldrich Chemical Co., St. Louis, Mo.) (35) either in medium only or with 10 μg of CFA/I fimbriae or alkaline-treated Salmonella nonlipopolysaccharide (non-LPS) extracts/ml (28) for 2 to 3 days at 37°C. The Salmonella non-LPS extracts were prepared as previously described (28, 35). Subsequently, the cells were analyzed by cytokine-specific ELISPOT assays.
Macrophage isolation and infection with Salmonella.
BALB/c mice were given a single 1.0-ml intraperitoneal injection of expired thioglycolate medium (Difco, Detroit, Mich.), and 3 days later, the peritoneum of each mouse was washed with RPMI 1640 (Gibco BRL-Life Technologies, Grand Island, N.Y.) containing 2% fetal calf serum without antibiotics. Peritoneal cells were washed twice in the same medium without antibiotics. Macrophage infection with the attenuated Salmonella H647 strain or with the wild-type H71 strain was performed as previously described (36) with varying bacilli-to-macrophage ratios (0.01 to 1.0) for 1 h at 37°C. Wells were washed twice with complete medium without antibiotics and then incubated with 50 μg of gentamicin (Life Technologies)/ml for 30 min at 37°C. After the wells were washed twice as described above, fresh complete medium without antibiotics (1.0 ml/well) was added, and cells were incubated for an additional 8 h. Supernatants were collected and frozen until analysis by cytokine ELISA.
Cytokine ELISA.
To detect IL-18 production, microtiter wells were coated with 2.0 μg of rat anti-mouse IL-18 (clone 74; MBL Co., Ltd., Woburn, Mass.)/ml in PBS overnight at room temperature. After blocking was done as described above, culture supernatants or dilutions of recombinant IL-18 (MBL) were added and incubated overnight at 4°C. After washing was done as described above, a 1:2,000 dilution of biotinylated rat anti-mouse IL-18 (clone 93-10C; MBL) was incubated for 2 h at 37°C. After washing, a 1:1,000 dilution of horseradish peroxidase-goat anti-biotin (Vector Laboratories, Burlingame, Calif.) was added for 1 h at 37°C. After washing, wells were developed with the substrate 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)diammonium (Moss), and values for the test samples were extrapolated from an IL-18 standard curve. ELISAs specific for tumor necrosis factor alpha (TNF-α), IL-12p70, IL-12p40, and IL-10 were conducted as previously described (36).
Serovar Typhimurium colonization.
NK1R−/− and NK1R+/+ mice were orally immunized with the Salmonella-CFA/I construct or its isogenic Salmonella vector. Two weeks after infection, spleens and PP were removed aseptically and weighed. Tissues were stroke homogenized 10 times in 1.0 ml of sterile deionized water for complete cell lysis. Prior to serial log dilutions, samples were completely mixed by vortexing. Diluted tissue samples were plated onto MacConkey's agar (Difco) and incubated overnight at 37°C, and colonies were subsequently counted.
Serovar Typhimurium challenge studies.
Wild-type serovar Typhimurium strain H71 was used as previously described (11, 35). Groups of NK1R−/− and NK1R+/+ mice were given sterile PBS (vehicle) orally or immunized orally with either Salmonella-CFA/I or Salmonella vector only. Four weeks after immunization, mice were pretreated with 50% saturated sodium bicarbonate solution and then infected with the H71 strain (5 × 107 CFU/0.2 ml) (50% lethal dose, 5 × 104 CFU). The amount of bacteria given was confirmed by plating serial dilutions of bacterial suspensions onto Luria-Bertani agar plates. Mice were observed twice daily, and the extent of survival was recorded for 3 weeks.
Statistical analysis.
The Student's t test was used to evaluate differences between variations in Ab titers, cytokine production levels, tissue weights, and extent of colonization. The Kaplan-Meier method (GraphPad Prism; GraphPad Software, Inc., San Diego, Calif.) was applied to obtain the survival fractions following infection with a lethal dose of wild-type serovar Typhimurium. Using the Mantel-Haenszel log-rank test, the P value for statistical differences between vehicle and vaccines and the performance of these vaccines between NK1R−/− and NK1R+/+ mice were discerned at the 95% confidence interval.
RESULTS
Oral immunization of NK1R−/− mice with Salmonella-CFA/I results in enhanced antigen-specific mucosal and serum IgA responses.
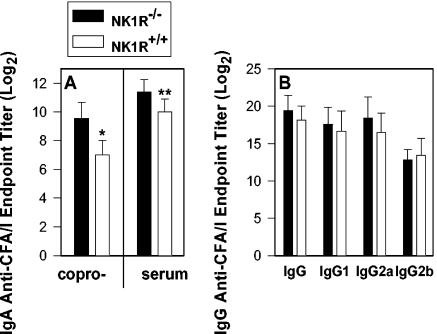
Since SP has been previously shown to augment IgA Ab responses (6, 32, 33, 44), the role of NK1R in intestinal immunity was tested. NK1R−/− and NK1R+/+ mice were orally immunized with either an attenuated Salmonella construct expressing CFA/I (strain H696) or the Salmonella vector only (strain H647). Four weeks after immunization, coproantibody and serum IgA titers were measured by using a CFA/I-specific ELISA. Both the coproantibody and serum IgA responses were significantly increased compared to those obtained for NK1R+/+ mice (5.8-fold [P < 0.001] and 2.6-fold [P = 0.007], respectively) (Fig. 1A). This augmentation in IgA responses was more apparent than the augmentation in the serum IgG, IgG1, IgG2a, and IgG2b responses since these responses did not vary as a consequence of NK1R expression (Fig. 1B).
FIG. 1.
Oral immunization of NK1R−/− mice with the Salmonella-CFA/I construct elicits increased mucosal and serum IgA responses but no change in serum IgG or IgG subclass antibody responses to CFA/I fimbriae. NK1R−/− and NK1R+/+ mice were vaccinated with a single dose of Salmonella vector with the cfaABCE insert strain H696. Fecal and serum IgA (A) and serum IgG anti-CFA/I (B) Ab titers (log2) were measured by standard CFA/I-specific ELISA. Each value represents the means of data from 10 mice ± standard deviations. *P < 0.001, which represents the statistical difference in mucosal IgA anti-CFA/I fimbriae levels; **, P = 0.007, which represents the statistical difference in serum IgA anti-CFA/I fimbriae levels between NK1R−/− and NK1R+/+ mice orally immunized with the Salmonella-CFA/I construct.
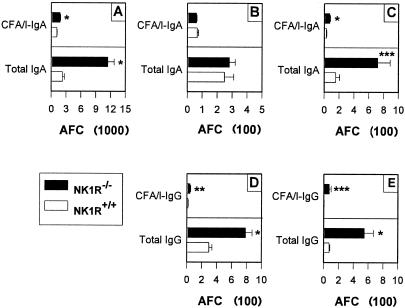
The observed mucosal IgA responses were supported by increases in iLP anti-CFA/I AFC as assessed by Ab ELISPOT. Significant increases in the number of IgA anti-CFA/I and total IgA AFC were detected in the iLP by ∼60% (P < 0.001) and by ∼5-fold (P < 0.001), respectively (Fig. 2A), and in the spleen by ∼2.6-fold (P < 0.001) and ∼4.7-fold (P = 0.034), respectively. CFA/I-specific and total IgG AFC were moderately enhanced in the PP by ∼2.8-fold (P = 0.005) and ∼2.6-fold (P ≤ 0.001), respectively, and in the spleen by ∼7.3-fold (P = 0.001) and ∼14.5-fold (P = 0.019), respectively.
FIG. 2.
Oral immunization of NK1R−/− mice with the Salmonella-CFA/I vaccine shows enhanced numbers of IgA AFC in the iLP and spleen and IgG AFC in PP and spleen. iLP (A), PP (B, D), and splenic (C, E) IgA (A to C) and IgG (D, E) AFC were harvested 4 weeks after oral immunization and evaluated for antigen-specific and total antibody responses by the ELISPOT method. No significant differences in PP IgA AFC were observed between NK1R+/+ and NK1R−/− mice. Values are expressed as the means ± standard errors of the means (SEM) of three experiments (AFC/106 lymphocytes). *P ≤ 0.001; **P = 0.005; ***P ≤ 0.034.
Oral immunization of NK1R−/− mice with the Salmonella CFA/I vaccine induces increases in IL-5 and IL-6 CFA/I fimbriae-specific CD4+-Th-cell responses.
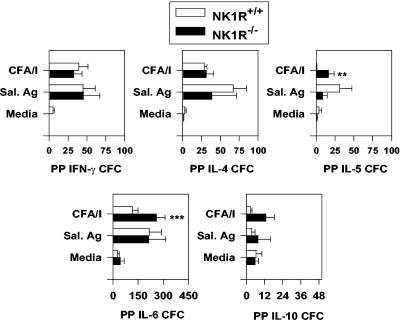
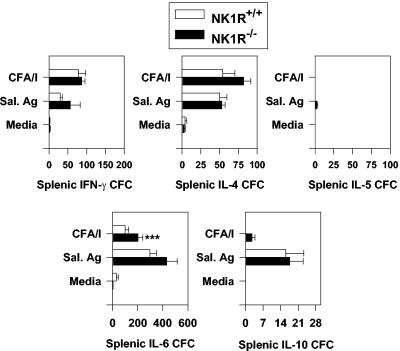
To determine why the mucosal IgA responses were enhanced, CD4+-Th-cell responses from NK1R−/− and NK1R+/+ mice orally immunized with the Salmonella-CFA/I vaccine were evaluated. To determine the source of the CFA/I-specific CD4+ Th cells, both PP and splenic CD4+ T cells were enriched and assayed for in vitro antigen restimulation for the production of Th1 and Th2 cell cytokines. These cells were cultured by conventional means (34) and restimulated in vitro with purified CFA/I fimbriae. After 2 to 3 days of in vitro antigen restimulation, CD4+ Th cells were analyzed by cytokine ELISPOT assays to quantify CFA/I-specific cytokine responses (Fig. 3 and 4). Immune NK1R−/− PP CD4+ T cells showed elevated numbers of IL-5 and IL-6 cytokine-forming cells (CFC), representing increases of >20-fold (P = 0.014) and 2.3-fold (P = 0.034), respectively, compared to those induced by CFA/I-restimulated NK1R+/+ PP CD4+ T cells (Fig. 3). NK1R−/− IL-4 and IL-10 CFC were evaluated and showed no significant differences from NK1R+/+ mice as did the IFN-γ CFC. No differences in PP CFC responses to intracellular Salmonella antigens were observed (Fig. 3). Likewise, splenic CD4+-T-cell responses were evaluated, and the IL-6 CFC responses to CFA/I fimbriae were augmented in NK1R−/− mice by twofold (P < 0.05) (Fig. 4). No differences between NK1R−/− and NK1R+/+ splenic IL-4, IL-5, IL-10, or IFN-γ CFC were observed when CD4+ T cells were restimulated with CFA/I fimbriae by using non-LPS Salmonella antigens (Fig. 4). Thus, the observed augmentation of CFA/I-specific IgA responses was supported by increases in Th2-type cytokine production.
FIG. 3.
Mucosal CD4+-Th2-cell anti-CFA/I responses are enhanced in NK1R−/− mice orally immunized with the Salmonella-CFA/I vaccine. Four weeks after oral immunization, NK1R−/− and NK1R+/+ mice were evaluated for their CD4+-Th-cell responses by the cytokine ELISPOT method. Immune PP CD4+ Th cells were isolated and cocultured with mitomycin C-treated feeder cells for 2 days with medium only, with 10 μg of purified CFA/I fimbriae/ml, or with 10 μg of NaOH Salmonella (non-LPS) extracts/ml. CD4+ T cells were then evaluated for IFN-γ, IL-4, IL-5, IL-6, and IL-10 responses and compared to similarly restimulated NK1R+/+ murine responses. The number of IL-5 and IL-6 CFC were significantly increased in NK1R−/− mice. Depicted are the means ± SEM of CFC/106 CD4+ T cells from a total of three experiments. **P = 0.014; ***P = 0.037. Sal. Ag, Salmonella antigen.
FIG. 4.
Splenic IL-6 anti-CFA/I responses are enhanced in NK1R−/− mice orally immunized with the Salmonella-CFA/I vaccine. As described in the legend of Fig. 3, NK1R−/− and NK1R+/+ mice were evaluated for their CD4+-Th-cell responses by the cytokine ELISPOT method. Immune splenic CD4+ Th cells were isolated and cocultured with mitomycin C-treated feeder cells for 2 days with medium only, with 10 μg of purified CFA/I fimbriae/ml, or with 10 μg of NaOH Salmonella (non-LPS) extracts/ml. CD4+ T cells were then evaluated for IFN-γ, IL-4, IL-5, IL-6, and IL-10 responses and compared to similarly restimulated NK1R+/+ murine responses. The number of IL-6 CFC in response to CFA/I fimbrial stimulation was significantly increased in NK1R−/− mice. Depicted are the means ± SEM of CFC/106 CD4+ T cells from a total of three experiments. ***P < 0.05.
Increased S-IgA responses are not attributed to enhanced colonization of NK1R−/− mice by Salmonella-CFA/I.
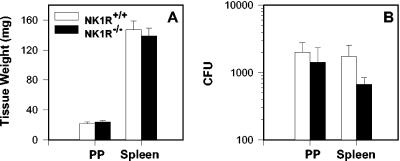
To assess whether the observed increases in S-IgA responses were attributed to enhanced colonization of NK1R−/− mice by Salmonella-CFA/I, tissue weights of PP and spleens were measured from Salmonella vector-infected and Salmonella-CFA/I-infected NK1R+/+ and NK1R−/− mice. These evaluations were conducted to assess whether NK1R−/− mice showed increased susceptibility to infection with our Salmonella vaccine. Evaluation of weights at 2 weeks after immunization with Salmonella-CFA/I showed no significant increases in PP or spleens in either mouse (Fig. 5A). Likewise, no significant differences in the extent of vaccine colonization were observed 2 weeks postimmunization (Fig. 5B). PP and spleens were procured, and serial dilutions of homogenates were examined on MacConkey's agar to determine CFU levels. NK1R−/− mice orally immunized with the Salmonella-CFA/I vaccine failed to show a significant difference in the extent of colonization compared to similarly immunized NK1R+/+ mice. This evidence suggests that NK1R−/− mice show no preferential bias or increased susceptibility to infection by Salmonella-CFA/I, indicating that the observed increased S-IgA responses were not attributed to increased vaccine colonization.
FIG. 5.
Differences in S-IgA responses are not attributed to the extent of H696 vaccine colonization in the PP or spleen. Determination of tissue weights (A) and tissue colonization studies (B) were performed to determine whether the NK1R−/− mice (n = 8) showed increased susceptibility to vaccine colonization. No differences in tissue weights and extent of colonization were observed 2 weeks after oral immunization compared to those of orally immunized NK1R+/+ mice (n = 8).
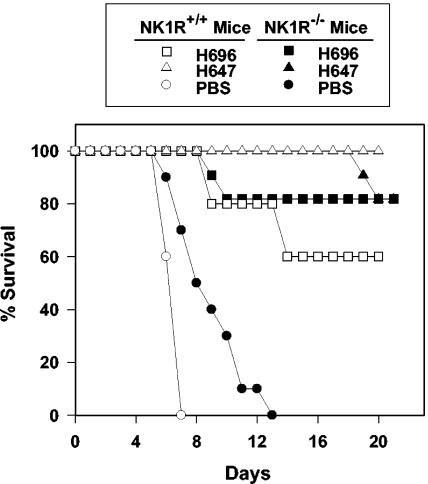
Orally immunized NK1R−/− mice show increased resistance to wild-type Salmonella challenge.
To test the importance of NK1R in protection against wild-type Salmonella challenge, Salmonella-CFA/I-immunized, Salmonella vector-immunized, and unimmunized NK1R−/− and NK1R+/+ mice were orally challenged with a lethal dose of wild-type Salmonella strain H71 (11, 35). Mice that were vaccinated were given the challenge dose 4 weeks after the oral immunization. None of the unimmunized NK1R+/+ (n = 10) and NK1R−/− mice (n = 10) survived, as expected; however, the attrition rate between the two mouse groups was significantly different (P = 0.002). All of the nonvaccinated NK1R+/+ mice succumbed to the challenge by day 7. In contrast, nonvaccinated NK1R−/− mice survived longer, succumbing to challenge with a median survival time of 9 days as opposed to 7 days for the NK1R+/+ mice; however, none of the NK1R−/− mice survived beyond day 13 (Fig. 6). Salmonella vector-vaccinated or Salmonella-CFA/I-vaccinated NK1R−/− mice (n = 11) showed no significant differences in survival compared to similarly vaccinated NK1R+/+ mice (n = 10). Likewise, both the Salmonella vector and Salmonella-CFA/I vaccine were equally efficacious in NK1R−/− mice. In contrast, the Salmonella vector was slightly more efficacious than the Salmonella-CFA/I vaccine (P = 0.029) in NK1R+/+ mice (Fig. 6). For both vaccines in NK1R+/+ and NK1R−/− mice, vaccination significantly improved survival compared to unvaccinated control mice (P ≤ 0.001). Thus, these studies suggest that SP contributes to proinflammatory responses and that survival is delayed in the absence of the NK1R.
FIG. 6.
NK1R−/− mice show increased resistance to wild-type serovar Typhimurium challenge. NK1R−/− (n = 11) and NK1R+/+ (n = 10) mice were orally immunized with PBS, Salmonella vaccine vector, strain H647, or Salmonella-CFA/I vector vaccine strain H696, and 4 weeks later, they were lethally challenged with 1,000 50% lethal doses of wild-type serovar Typhimurium strain H71. NK1R−/− mice (PBS vaccinated) showed increased resistance to wild-type Salmonella challenge compared to similarly challenged NK1R+/+ mice (P = 0.002). NK1R−/− mice orally immunized with the Salmonella-CFA/I vaccine or the Salmonella vector had equally protective capacities compared to that of similarly immunized NK1R+/+ mice; however, NK1R+/+ mice orally vaccinated with the Salmonella vector had slightly more protective capacity against wild-type Salmonella challenge than NK1R+/+ mice orally immunized with Salmonella-CFA/I (P = 0.029). Overall, both NK1R−/− and NK1R+/+ mice orally vaccinated with either Salmonella vaccine showed improved median survival compared to challenged, naive mice (P ≤ 0.001).
Increased IL-10 production by Salmonella-infected peritoneal macrophages.
To address the increased resistance to Salmonella infection by NK1R−/− mice, infection of peritoneal macrophages with Salmonella was performed to assess whether TNF-α, IL-12, and IL-18 were differentially expressed in NK1R−/− and NK1R+/+ mice. Various infection ratios of less than one bacillus per macrophage were used as previously described for 8 h (36), since ratios of >1.0 bacillus per macrophage cause cell death. No or minimal differences in TNF-α, IL-12p70, and IL-18 were observed when macrophages were infected with the attenuated Salmonella vector strain H647 or with wild-type Salmonella strain H71 (Table 1). However, significant increases (P ≤ 0.05) in IL-10 production by peritoneal macrophages derived from NK1R−/− mice, but not NK1R+/+ mice, were observed (Table 1). In addition, increased levels of IL-12p40 were also observed. Thus, the increased anti-inflammatory cytokine IL-10 may account for the increased protection against wild-type Salmonella challenge.
TABLE 1.
Infection of NK-1R−/− peritoneal-macrophages with an attenuated or wild-type Salmonella strain induces IL-10 and IL-12p40
| Salmonella straina | Infection ratio (Salmonella:macrophage)b | Cytokine | Cytokine production (ng/ml) (mean ± SD) in
|
P valuec | |
|---|---|---|---|---|---|
| NK1R+/+ mice | NK1R−/− mice | ||||
| H647 | 0.85:1 | TNF-α | 2.42 ± 1.02 | 2.31 ± 0.7 | - |
| 0.17:1 | 0.47 ± 0.052 | 0.494 ± 0.281 | - | ||
| 0.085:1 | 0 | 0 | - | ||
| - | 0 | 0 | - | ||
| 0.85:1 | IL-12p70 | 0 | 0 | - | |
| 0.17:1 | 0 | 0 | - | ||
| 0.085:1 | 0 | 0 | - | ||
| - | 0 | 0 | - | ||
| 0.85:1 | IL-12p40 | 0.44 ± 0.228 | 1.2 ± 0.21 | - | |
| 0.17:1 | 0.45 ± 0.174 | 1.2 ± 0.151 | 0.013 | ||
| 0.085:1 | 0.196 ± 0.017 | 0.424 ± 0.331 | 0.005 | ||
| - | 0 | 0 | - | ||
| 0.85:1 | IL-18 | 0.169 ± 0.136 | 0.184 ± 0.01 | - | |
| 0.17:1 | 0 | 0 | - | ||
| 0.085:1 | 0 | 0 | - | ||
| - | 0 | 0 | - | ||
| 0.85:1 | IL-10 | 0.118 ± 0.01 | 0.332 ± 0.132 | <0.05 | |
| 0.17:1 | 0 | 0.263 ± 0.071 | 0.003 | ||
| 0.085:1 | 0 | 0.336 ± 0.146 | 0.016 | ||
| - | 0 | 0 | - | ||
| H71 | 0.83:1 | TNF-α | 15.4 ± 1.28 | 13.3 ± 2.75 | - |
| 0.083:1 | 14.1 ± 1.16 | 10.1 ± 0.527 | 0.006 | ||
| 0.010:1 | 1.02 ± 0.663 | 0 | - | ||
| - | 0 | 0 | - | ||
| 0.83:1 | IL-12p70 | 0 | 0 | - | |
| 0.083:1 | 0 | 0 | - | ||
| 0.010:1 | 0 | 0 | - | ||
| - | 0 | 0 | - | ||
| 0.83:1 | IL-12p40 | 0.583 ± 0.145 | 0.980 ± 0.058 | 0.012 | |
| 0.083:1 | 0.544 ± 0.031 | 0.424 ± 0.050 | 0.024 | ||
| 0.010:1 | 0.088 ± 0.027 | 0.073 ± 0.031 | - | ||
| - | 0 | 0 | - | ||
| 0.83:1 | IL-18 | 0.340 ± 0.102 | 0.254 ± 0.094 | - | |
| 0.083:1 | 0.184 ± 0.039 | 0.205 ± 0.042 | - | ||
| 0.010:1 | 0.200 ± 0.035 | 0.210 ± 0.096 | - | ||
| - | 0 | 0 | - | ||
| 0.83:1 | IL-10 | 0 | 0.180 ± 0.167 | <0.05 | |
| 0.083:1 | 0.030 ± 0.040 | 0.119 ± 0.023 | 0.029 | ||
| 0.010:1 | 0 | 0 | - | ||
| - | 0 | 0 | - | ||
Strain H647 is ΔaroD S. enterica serovar Typhimurium; strain H71 is wild-type S. enterica serovar Typhimurium.
Infection ratio confirmed by plate count. -, no infection.
-, not significant.
DISCUSSION
SP is primarily thought of in its neurological context (reviewed in reference 16) rather than for its ability to augment host immune responses (reviewed in reference 37). As such, SP has been found to be associated with a number of inflammatory diseases (20, 26, 39, 46). In support of these findings, SP induces production of proinflammatory cytokines TNF-α, IL-1, IL-6, and IL-12 by macrophages (22, 25). Such findings lead us to suspect that SP may play an important role in bacterial infections. At least for Salmonella infections, it was shown that increases in NK1R expression do occur (21), suggesting that SP may be important for protection against Salmonella (21). Mice treated with the SP receptor antagonist spantide II showed increased susceptibility to infection by Salmonella (21). While it may initially appear that our results obtained with the NK1R−/− mice may contradict these findings, these mice showed an increased time of median survival to Salmonella infection. Aside from the possible differences in the Salmonella serovars used in these two studies, the innate cells in the NK1R−/− mice are not capable of expressing NK1R and thus may have enhanced compensatory mechanisms in place to address this deficiency. As found with our macrophage studies of Salmonella infection, no or minimal differences in TNF-α and IL-18 were observed, suggesting that NK1R−/− macrophages are capable of eliciting proinflammatory cytokines. Neither source of macrophages was able to produce IL-12p70 in response to low infection ratios, consistent with previous reports (4, 36). However, increased IL-10 and IL-12p40 production by NK1R−/− macrophages was observed, which may account for the improved median survival time by dampening the inflammatory response. Increases in IL-10 production were also observed in gammaherpesvirus 68-infected NK1R−/− mice (12). In contrast, treatment with the SP antagonist may impact a number of physiological systems simultaneously and may not be able to induce the compensatory mechanisms in the presence of this antagonist. Nonetheless, in both studies, it was shown that SP and its receptor play an important role in protection against this enteric bacterium. This concept of lessened inflammation was also suggested in previous studies examining the role of L-selectin upon gut innate immunity to oral Salmonella challenge (36).
Regarding the adaptive immune response to Salmonella challenge, oral immunization of NK1R−/− or NK1R+/+ mice with the attenuated Salmonella vector did not show significant differences in protection against wild-type Salmonella challenge. Likewise, oral immunization with the Salmonella-CFA/I vaccine showed similar level of protection in both NK1R−/− and NK1R+/+ mice when subjected to wild-type challenge. Significant differences in the extent of protection conferred by oral immunization of NK1R+/+ mice were noted only when the mice were immunized with the Salmonella vector rather than Salmonella-CFA/I vaccine. Perhaps the anti-inflammatory attributes of this vaccine were insufficient for protection against wild-type challenge.
To understand the heightened S-IgA responses in NK1R−/− mice, cytokine analysis was performed to determine whether Th2-type cytokines may be induced after oral immunization with Salmonella-CFA/I. Cytokine profiling studies revealed that in the PP, IL-5 CFC were particularly enhanced in the NK1R−/− mice compared to NK1R+/+ PP showing no IL-5 production 4 weeks after oral immunization. Moreover, IL-6 production by NK1R−/− PP and splenic CD4+ T cells was increased compared to that of NK1R+/+ mice. While IL-4 CFC levels were not different in response to vaccination in these mice, the production of these other anti-inflammatory cytokines suggests that the proinflammatory responses would be lessened. The IFN-γ CFC levels in response to purified CFA/I fimbriae were not significantly different between the Salmonella-CFA/I-vaccinated mouse groups, but as a whole, the IFN-γ CFC were not particularly elevated compared to the results obtained from mice orally vaccinated with the Salmonella vector as evidenced in previous studies (34, 35). These increases in IL-5 and IL-6 CFC also suggest that these cytokines accounted for the improved S-IgA anti-CFA/I fimbriae responses, since it is well established that IL-5 and IL-6 are known to enhance IgA production (2, 3).
A particularly interesting finding from this work was that in the absence of the NK1R, IgA responses were increased. As stated above, the observed increases in IL-5 and IL-6 production must have enhanced the CFA/I-specific IgA responses; however, it was previously shown that B-cell expression of NK1R supported increases in IgA production (32). While the latter studies were conducted in vitro in the absence of T cells, at least in vivo, SP alteration of B cells may be minimal, as those studies suggested. In fact, in the PP, SP-containing nerve fibers avoided B-cell areas and infiltrated T-cell zones (17, 23). Moreover, IgG responses to CFA/I fimbriae were not affected by the absence of NK1R. If the majority of IgG is derived from the systemic compartment, this finding is not out of the ordinary. Given that SP is believed to influence Ab responses at a more regional or compartmentalized level, the lack of a systemic influence is in agreement with the notion that SP influences the mucosal compartments more. The levels of SP in the gut are found to be second in concentration only to those of the brain (16) and are also elevated in the lungs (18). Thus, our findings are consistent with the notion that SP can influence mucosal IgA responses, and the observed changes in this study suggest that SP contributes to the proinflammatory responses which generally downregulate anti-inflammatory cytokines in the absence of a functional NK1R.
In agreement with previous studies, oral vaccination with Salmonella-CFA/I elicits elevated S-IgA Abs supported by specific increases in Th2-type cytokines (34, 35, 47). This result may be attributed to the repetitive nature of enterotoxigenic E. coli fimbrial antigens in provoking Th2 cell development (1, 34) in that it resembles soluble immunization with adjuvant. Why this Salmonella vaccine does not behave as traditional Salmonella vaccines do remains unclear, but it is obvious that the presence of the CFA/I fimbriae does alter the course of host recognition. Perhaps the fimbria can stimulate anti-inflammatory responses or simply fails to stimulate inflammatory cytokines as previously observed (36), whereby as little as 1 bacillus of Salmonella vector per 80 macrophages is sufficient to stimulate elevated levels of IL-1 and TNF-α. In contrast, infection ratios in excess of 10 to 1 are necessary to substantially induce such cytokine responses by Salmonella-CFA/I. Studies are continuing to address why Salmonella-CFA/I vaccine behaves as it does.
In summary, the results from this study support the notion that SP contributes to intestinal immunity. The observed increases in S-IgA are linked to increases in CFA/I-specific CD4+ Th2 cells producing IL-5 and IL-6. While Salmonella-CFA/I generally induces elevated Th2-type cytokines, the absence of NK1R showed increased production of the anti-inflammatory cytokine IL-10. Thus, augmentation of anti-inflammatory input would be expected to enhance Th2-type responses as observed. While it is interesting that IL-4 was not specifically altered, other S-IgA-promoting cytokines, IL-5 and IL-6, were observed. This increase in IL-10 also appears to influence the resistance to infection with wild-type Salmonella, as shown by the increased median survival, but had no significant impact upon challenged mice vaccinated with either Salmonella-CFA/I or its isogenic Salmonella vector strain.
Acknowledgments
We thank Nancy Kommers for her assistance in preparing the manuscript and Craig Gerard for providing breeder pairs of the NK1R−/− mice.
This work was supported by U.S. Public Service grants AI-41123 and DE-13812 and in part by Montana Agricultural Station and U.S. Department of Agriculture formula funds.
Editor: J. T. Barbieri
REFERENCES
- 1.Ascón, M. A., D. M. Hone, N. Walters, and D. W. Pascual. 1998. Oral immunization with a Salmonella vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect. Immun. 66:5470-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beagley, K. W., J. H. Eldridge, H. Kiyono, M. P. Everson, W. J. Koopman, T. Honjo, and J. R. McGhee. 1988. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J. Immunol. 141:2035-2042. [PubMed] [Google Scholar]
- 3.Beagley, K. W., J. H. Eldridge, F. Lee, H. Kiyono, M. P. Everson, W. J. Koopman, T. Hirano, T. Kishimoto, and J. R. McGhee. 1989. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J. Exp. Med. 169:2133-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bost, K. L., and J. D. Clements. 1997. Intracellular Salmonella dublin induces substantial secretion of the 40-kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect. Immun. 65:3186-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozic, C. R., B. Lu, U. E. Hopken, C. Gerard, and N. P. Gerard. 1996. Neurogenic amplification of immune complex inflammation. Science 273:1722-1725. [DOI] [PubMed] [Google Scholar]
- 6.Braun, A., P. Wiebe, A. Pfeufer, R. Gessner, and H. Renz. 1999. Differential modulation of human immunoglobulin isotype production by the neuropeptides substance P, NKA, and NKB. J. Neuroimmunol. 97:43-50. [DOI] [PubMed] [Google Scholar]
- 7.Bullifent, H. L., K. F. Griffin, S. M. Jones, A. Yates, L. Harrington, and R. W. Titball. 2000. Antibody responses to Yersinia pestis F1-antigen expressed in Salmonella typhimurium aroA from in vivo-inducible promoters. Vaccine 18:2668-2676. [DOI] [PubMed] [Google Scholar]
- 8.Csencsitis, K. L., and D. W. Pascual. 2002. Absence of L-selectin delays mucosal B cell responses in nonintestinal effector tissues. J. Immunol. 169:5649-5659. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss, R., S. M. Kelly, P. A. Gulig, and K. Nakayama. 1989. Selective delivery of antigens by recombinant bacteria. Curr. Top. Microbiol. Immunol. 146:34-49. [DOI] [PubMed] [Google Scholar]
- 10.Curtiss, R., S. M. Kelly, and J. O. Hassan. 1993. Live oral avirulent Salmonella vaccines. Vet. Microbiol. 37:397-405. [DOI] [PubMed] [Google Scholar]
- 11.Dybing, J. K., N. Walters, and D. W. Pascual. 1999. The role of endogenous IL-18 in resolving wild-type and attenuated Salmonella typhimurium infections. Infect. Immun. 67:6242-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsawa, S. F., W. Taylor, C. C. Petty, I. Marriott, J. V. Weinstock, and K. L. Bost. 2003. Reduced CTL response and increased viral burden in substance P receptor-deficient mice infected with murine γ-herpesvirus 68. J. Immunol. 170:2605-2612. [DOI] [PubMed] [Google Scholar]
- 13.Fennelly, G. J., S. A. Khan, M. A. Abadi, T. F. Wild, and B. R. Bloom. 1999. Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector. J. Immunol. 162:1603-1610. [PubMed] [Google Scholar]
- 14.Girón, J. A., J.-G. Xu, C. R. González, D. Hone, J. B. Kaper, and M. M. Levine. 1995. Simultaneous expression of CFA/I and CS3 colonization factor antigens of enterotoxigenic Escherichia coli by ΔaroC, ΔaroD Salmonella typhi vaccine strain CVD 908. Vaccine 13:939-946. [DOI] [PubMed] [Google Scholar]
- 15.Hall, R. H., D. R. Maneval, Jr., J. H. Collins, J. L. Theibert, and M. M. Levine. 1989. Purification and analysis of colonization factor antigen 1, coli surface antigen 1, and coli surface antigen 3 fimbriae from enterotoxigenic Escherichia coli. J. Bacteriol. 171:6372-6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hokfelt, T., B. Pernow, and J. Wahren. 2001. Substance P: a pioneer amongst neuropeptides. J. Intern. Med. 249:27-40. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa, S., S. P. Sreedharan, E. J. Goetzl, and R. L. Owen. 1994. Immunohistochemical localization of peptidergic nerve fibers and neuropeptide receptors in Peyer's patches of the cat ileum. Regul. Pept. 54:385-395. [DOI] [PubMed] [Google Scholar]
- 18.Joos, G. F., K. O. De Swert, and R. A. Pauwels. 2001. Airway inflammation and tachykinins: prospects for the development of tachykinin receptor antagonists. Eur. J. Pharmacol. 429:239-250. [DOI] [PubMed] [Google Scholar]
- 19.Kang, H. Y., J. Srinivasan, and R. Curtiss III. 2002. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect. Immun. 70:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidd, B. L., J. J. Inglis, K. Vetsika, V. C. Hood, C. De Felipe, H. Bester, S. P. Hunt, and S. C. Cruwys. 2003. Inhibition of inflammation and hyperalgesia in NK-1 receptor knock-out mice. Neuroreport 14:2189-2192. [DOI] [PubMed] [Google Scholar]
- 21.Kincy-Cain, T., and K. L. Bost. 1996. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J. Immunol. 157:255-264. [PubMed] [Google Scholar]
- 22.Kincy-Cain, T., and K. L. Bost. 1997. Substance P-induced IL-12 production by murine macrophages. J. Immunol. 158:2334-2339. [PubMed] [Google Scholar]
- 23.Kulkarni-Narla, A., A. J. Beitz, and D. R. Brown. 1999. Catecholaminergic, cholinergic and peptidergic innervation of gut-associated lymphoid tissue in porcine jejunum and ileum. Cell Tissue Res. 298:275-286. [DOI] [PubMed] [Google Scholar]
- 24.Lai, J. P., S. D. Douglas, and W. Z. Ho. 1998. Human lymphocytes express substance P and its receptor. J. Neuroimmunol. 86:80-86. [DOI] [PubMed] [Google Scholar]
- 25.Lotz, M., J. H. Vaughan, and D. A. Carson. 1988. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 241:1218-1221. [DOI] [PubMed] [Google Scholar]
- 26.Mantyh, C. R., T. S. Gates, R. P. Zimmerman, M. L. Welton, E. P. Passaro, Jr., S. R. Vigna, J. E. Maggio, L. Kruger, and P. W. Mantyh. 1988. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc. Natl. Acad. Sci. USA 85:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroeni, P., B. Villareal-Ramos, and C. E. Hormaeche. 1992. Role of T cells, TNF-α and IFN-γ in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro− Salmonella vaccines. Microb. Pathog. 13:477-491. [DOI] [PubMed] [Google Scholar]
- 28.Mastroeni, P., B. Villarreal-Ramos, J. A. Harrison, R. Demarco de Hormaeche, and C. E. Hormaeche. 1994. Toxicity of lipopolysaccharide and of soluble extracts of Salmonella typhimurium in mice immunized with a live attenuated aroA salmonella vaccine. Infect. Immun. 62:2285-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow, C. D., M. J. Novak, D. C. Ansardi, D. C. Porter, and Z. Moldoveanu. 1999. Recombinant viruses as vectors for mucosal immunity. Curr. Top. Microbiol. Immunol. 236:255-273. [DOI] [PubMed] [Google Scholar]
- 30.Noriega, F. R., G. Losonsky, J. Y. Wang, S. B. Formal, and M. M. Levine. 1996. Further characterization of ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203 as a mucosal Shigella vaccine and as a live-vector vaccine for delivering antigens of enterotoxigenic Escherichia coli. Infect. Immun. 64:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okahashi, N., M. Yamamoto, J. L. Vancott, S. N. Chatfield, M. Roberts, H. Bluethmann, T. Hiroi, H. Kiyono, and J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascual, D. W., J. Xu-Amano, H. Kiyono, J. R. McGhee, and K. L. Bost. 1991. Substance P acts directly upon cloned B lymphoma cells to enhance IgA and IgM production. J. Immunol. 146:2130-2136. [PubMed] [Google Scholar]
- 33.Pascual, D. W., K. W. Beagley, H. Kiyono, and J. R. McGhee. 1995. Substance P promotes Peyer's patch and splenic B cell differentiation, p. 52-59. In J. Mestecky et al. (ed.), Advances in mucosal immunology. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 34.Pascual, D. W., D. M. Hone, S. Hall, F. W. van Ginkel, M. Yamamoto, N. Walters, K. Fujihashi, R. Powell, S. Wu, J. L. Vancott, H. Kiyono, and J. R. McGhee. 1999. Expression of recombinant enterotoxigenic colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect. Immun. 67:6249-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascual, D. W., M. D. White, T. Larson, and N. Walters. 2001. Impaired mucosal immunity in L-selectin-deficient mice orally immunized with a Salmonella vaccine vector. J. Immunol. 167:407-415. [DOI] [PubMed] [Google Scholar]
- 36.Pascual, D. W., T. Trunkle, and J. Sura. 2002. Fimbriated Salmonella enterica serovar Typhimurium abates the initial inflammatory responses by macrophages. Infect. Immun. 70:4273-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascual, D. W. 2004. The role of tachykinins on bacterial infections. Front. Biosci. 9:3209-3217. [DOI] [PubMed] [Google Scholar]
- 38.Payan, D. G., D. R. Brewster, and E. J. Goetzl. 1984. Stereo-specific receptors for substance P on cultured IM-9 lymphoblasts. J. Immunol. 133:3260-3265. [PubMed] [Google Scholar]
- 39.Piedimonte, G., M. M. Rodriguez, K. A. King, S. McLean, and X. Jiang. 1999. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am. J. Physiol. 277:L831-L840. [DOI] [PubMed] [Google Scholar]
- 40.Ramarathinam, L., D. W. Niesel, and G. R. Klimpel. 1993. Salmonella typhimurium induces IFN-γ production in murine splenocytes: role of natural killer cells and macrophages. J. Immunol. 150:3973-3981. [PubMed] [Google Scholar]
- 41.Roberts, M., S. N. Chatfield, and G. Dougan. 1994. Salmonella as carriers of heterologous antigens, p. 27-58. In D. T. O'Hagen (ed.), Novel delivery systems for oral vaccines. CRC Press, Boca Raton, Fla.
- 42.Schafer, R., D. A. Portnoy, S. A. Brassell, and Y. Paterson. 1992. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J. Immunol. 149:53-59. [PubMed] [Google Scholar]
- 43.Soussi, N., G. Milon, J. H. Colle, E. Mougneau, N. Glaichenhaus, and P. L. Goossens. 2000. Listeria monocytogenes as a short-lived delivery system for the induction of type 1 cell-mediated immunity against the p36/LACK antigen of Leishmania major. Infect. Immun. 68:1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanisz, A. M., D. Befus, and J. Bienenstock. 1986. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferation by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J. Immunol. 136:152-156. [PubMed] [Google Scholar]
- 45.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 46.Weinstock, J. V., A. Blum, A. Metwali, D. Elliott, N. Bunnett, and R. Arsenescu. 2003. Substance P regulates Th1-type colitis in IL-10 knockout mice. J. Immunol. 171:3762-3767. [DOI] [PubMed] [Google Scholar]
- 47.Wu, S., D. W. Pascual, J. L. VanCott, J. R. McGhee, D. R. Maneval, Jr., M. M. Levine, and D. M. Hone. 1995. Immune response to Escherichia coli and Salmonella typhimurium vectors that express colonization factor antigen I (CFA/I) of enterotoxigenic E. coli in the absence of the CFA/I positive regulator cfaR. Infect. Immun. 63:4933-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]