Abstract
Tissue inhibitor of metalloproteinase 1 (TIMP-1)-deficient mice are resistant to Pseudomonas aeruginosa corneal infections. Corneas healed completely in TIMP-1-deficient mice, and infections were cleared faster in TIMP-1-deficient mice than in wild-type littermates. Genetic suppression studies using matrix metalloproteinase (MMP)-deficient mice showed that MMP-9, MMP-3, and MMP-7 but not MMP-2 or MMP-12 are needed for resistance. Increased resistance was also seen during pulmonary infections. These results identify a novel pathway regulating infection resistance.
Twenty-five matrix metalloproteinases (MMPs) comprise the major extracellular matrix-degrading activities in mammals. They can remodel matrix during development, tumor metastasis, and cell migration but have nonmatrix substrates as well. MMPs are inhibited posttranslationally by four tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) and by α2-macroglobulin (33).
Many correlative studies have suggested that MMP production was associated with inflammation and that MMP inhibition had an anti-inflammatory effect (3-5, 21, 26, 29). More recently, studies with MMP mutant mice have confirmed that MMPs play an important role in promoting inflammation and immunity: overexpression of MMP-1 in lungs promotes a spontaneous emphysema-like response (6), MMP-12 loss attenuates macrophage migration and lung damage caused by cigarette smoke (27), MMP-7 participates in proteolytic activation of antibacterial defensins by epithelial cells (32) and chemoattraction of inflammatory cells to the lung (16), and MMP-3 loss diminishes T-cell-dependent delayed type hypersensitivity responses, while the loss of MMP-9 delays the resolution of those responses (30). TIMP expression has also been associated with a variety of infectious and noninfectious inflammatory conditions, including those affecting the eye (12, 14, 34), and TIMP-3 regulates tumor necrosis factor alpha (TNF-α) production (18). Collectively, these data highlight varied and complex roles for components of the TIMP-MMP axis during immune and inflammatory responses; however, TIMPs have not been directly shown to regulate responses to infection. To study the role of TIMP-1 in inflammation and immunity and its possible involvement in other physiological processes, mice deficient in TIMP-1 were generated and evaluated for responses during corneal and pulmonary infections with Pseudomonas aeruginosa.
Preparation of Timp1 mutant mice.
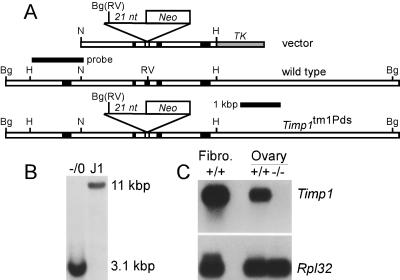
A replacement vector (Fig. 1A) targeted to the Timp1 locus in embryonic stem (ES) cells (Fig. 1B) produced a null allele in mice (Fig. 1C). The replacement vector included a 3.3-kb NdeI to HindIII 5′ arm; a 21-mer oligonucleotide (5′-CTGATCAGCTGACTCGAGG-3′) at an EcoRV site in the third coding exon that abolished the EcoRV site generating BamHI, BglII, and XhoI sites; a G418 drug resistance cassette at the XhoI site; and a TK marker. Electroporated ES clones were screened by Southern blot analysis with a 1,400-nucleotide HindIII-NdeI Timp1 5′ fragment as a probe. Mutant mice were made by injecting blastocysts with targeted ES cells.
FIG. 1.
Targeting Timp1 with a replacement vector. (A) Vector includes 1.6 kb of Timp1 sequences 5′ of an EcoRV (RV) site within coding exon 3, a stop codon-containing oligonucleotide inserted at the EcoRV site replacing it with a BglII (Bg) site, a neo marker 3′ of the mutating oligonucleotide, 1.6 kb of 3′ Timp1 sequences, and a herpes simplex virus TK marker. Before electroporation into J1 ES cells, the vector was linearized 5′ of the Timp1 sequences. Homologous recombination generated the genomic structure shown. (B) A Southern blot of DNA from ES clones digested with BglII revealed an 11-kb wild-type band in the control (J1) and a 3.1-kb mutant band in the homologous recombinants. Because Timp1 is X-linked and J1 cells are from males, a single mutant band is present in targeted clones. (C) Northern blot of RNA from wild-type (+/+) embryonic fibroblasts and ovaries of wild-type and Timp1 mutant (−/−) mice hybridized to human TIMP1 cDNA and mouse Rpl32 cDNA as a loading control.
Increased infection resistance in Timp1 mutants.
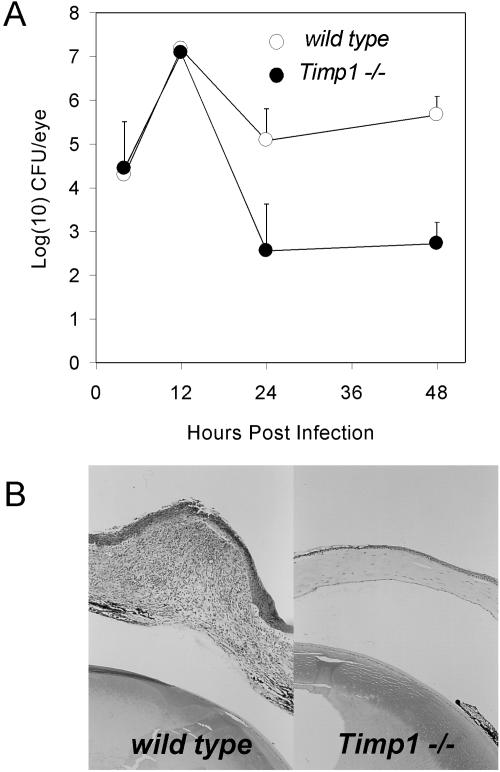
TIMP-1 has been implicated in erythropoiesis (9), wound healing (25), steroidogenesis (2), and tumor metastasis. However, the loss of TIMP-1 in mice caused no detectable changes in these processes (22, 28) or in kidney fibrosis (8), but modest changes were observed in corneal neovascularization (35) and left ventricular geometry (24). However, TIMP-1-deficient mice had dramatically improved bacterial clearance relative to wild-type mice after induction of corneal infections with a human corneal isolate of P. aeruginosa (Fig. 2A). For corneal infections, we grew nonmucoid P. aeruginosa strain 6389 overnight on tryptic soy agar plates prepared with Noble agar, collected bacteria in sterile proteose peptone (2%), anesthetized mice with tribromoethanol, made three 1-mm scratches with a 26-gauge needle, and placed 5 μl of bacteria (107 CFU) on the corneas. We measured bacterial burdens several times within 5 days after infection by plate count of whole eyes homogenized in 2% proteose peptone. At 4 h after infection of strain 129S4/Jae mice, eyes from wild-type and mutant animals had identical numbers of bacteria, indicating that the initiation of the infection was not altered by the Timp1 mutation. By 12 h after infection, when a burst of P. aeruginosa replication had occurred, comparable numbers of bacteria were recovered from mutant and wild-type eyes, indicating that there was no intrinsic barrier to bacterial replication in mutant mice. By 24 and 48 h after infection, when histological sections revealed that neutrophil infiltration into infected corneas had begun and then peaked, respectively (data not shown), there were 2 to 3 orders of magnitude fewer viable bacteria in eyes of mutant mice than in eyes of wild-type mice. Statistical analysis of bacterial burden was done by analysis of variance (ANOVA). Strain 129 mice recover completely from these infections and are considered resistant. C57BL/6 mice can clear infections too, but they undergo corneal perforation because of unresolved inflammation and are considered infection sensitive (23). By 16 days after infection, wild-type C57BL/6 mice maintained a persistent inflammatory response and underwent perforation; however, TIMP-1-deficient littermates resolved the inflammation completely, and corneal integrity was restored (Fig. 2B). These results, from two different measures, demonstrated dramatically increased resistance to P. aeruginosa corneal infection in TIMP-1-deficient mice.
FIG. 2.
Increased resistance to P. aeruginosa in eyes of Timp1−/− mice. Mice on the 129S4/SvJae background were infected with 106 CFU of P. aeruginosa (strain 6389)/ml. (A) At several times after infection, the infected eyes were removed, and bacterial burdens were assayed by plate count of whole-eye homogenates. Each point represents the mean of results from at least four animals. The P values are <0.003 and <0.0001 at 24 and 48 h postinfection, respectively. (B) Histological sections of corneas from representative mutant and wild-type mice on the C57BL/6 background taken 16 days after infection.
MMP-9, MMP-7, and MMP-3 are needed for infection resistance in Timp1 mutants.
To determine whether the increased resistance phenotype was due to the loss of proteinase inhibitory activity in Timp1−/− mice, we repeated the corneal infections in 129S4/Jae mice that were treated with BB-94, a synthetic metalloproteinase inhibitor (7). We first injected animals intraperitoneally with 40 mg of BB-94 (British Biotech, Oxford, England)/kg of body weight daily for 4 days prior to infection and on the day of infection using suspension of BB-94 (3.0 mg/ml) in phosphate-buffered saline (PBS) containing 0.01% Tween 40. Over 99% of the enhanced bacterial clearance by mutant mice was suppressed by BB-94 treatment (Table 1).
TABLE 1.
Suppression of the infection resistance phenotype in Timp1 mutants by a synthetic proteinase inhibitora
| Timp1 genotype | Treatment | n | CFU/eye | CFU/eye relative to Timp1−/− mice | P value |
|---|---|---|---|---|---|
| −/0 | PBS | 6 | 1.9 × 102 | 1 | 7 × 10−6 |
| +/0 | PBS | 7 | 1.5 × 105 | 790 | |
| −/0 | BB-94 | 5 | 6.5 × 104 | 1 | 0.02 |
| +/0 | BB-94 | 7 | 2.7 × 105 | 4 |
Wild-type (+/0) or mutant (−/0) male mice treated with a proteinase inhibitor (BB-94) or saline (PBS) were infected in the cornea with P. aeruginosa strain 6389. Twenty-four hours later, eyes were removed and bacterial burdens were measured. The number of mice (n), the mean values for CFU per eye, and the ratio of CFU/eye relative to PBS-treated Timp1 mutants are shown. P values were calculated by ANOVA. Percent suppression was calculated using the formula 100 × [1 − (BB-94 ratio +/0:−/0)/(PBS ratio +/0:−/0)] and was 99% after BB-94 treatment. The P values are for the comparison of wild-type and mutant mice. The P value for the difference between the PBS-treated +/0 and BB-94-treated −/0 mice is 0.2.
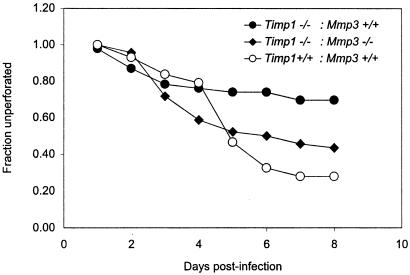
Because BB-94 inhibits MMPs as well as the non-MMP proteinase tumor necrosis factor alpha-converting enzyme (TACE) that releases membrane-bound TNF-α from the surfaces of cells (1, 19), this pharmacological approach did not identify the specific proteinases influencing the infection phenotypes in Timp1−/− mice. However, because TIMP-1 is inactive against TACE (15), it was likely that that the Timp1 mutant phenotype was mediated most directly by an MMP. Nonetheless, to identify the proteinases responsible for the Timp1 mutant phenotype, we took a genetic approach, reasoning that if one or more MMPs are required for the mutant phenotype, then the increased resistance to infection should be suppressed in mice harboring mutations in both Timp1 and the critical proteinases. To perform this analysis, we bred Timp1 mutants with animals carrying lesions in Mmp2 (11), Mmp3 (20), Mmp7 (31), Mmp9 (17), and Mmp12 (10). These animals were available on either the C57BL/6 background or on different 129 backgrounds. Guided by the results shown in Fig. 2, the controls and suppression tests used the assay appropriate for the specific strain background: bacterial burden assays for strain 129 mice and kinetics of corneal perforation for C57BL/6 mice. For each cross, control infections were performed with wild-type progeny and mice with only the Timp1 mutation to verify that the Timp1 mutant phenotype could be observed on these backgrounds. On each strain background, Timp1 mutants exhibited significantly increased resistance to infection relative to the wild-type controls, consistent with our earlier data with Timp1−/− mice that had not been crossed into Mmp mutant backgrounds (Table 2, Fig. 3, and data not shown). In Timp1 Mmp9 double mutants, resistance to infection was indistinguishable from the corresponding wild-type control animals but significantly lower than resistance in the Timp1 single mutants on the same background. This result indicated that MMP-9 was essential for the Timp1 phenotype (Table 2). In Timp1 Mmp7 double mutants and Timp1 Mmp3 double mutants, resistance to infection was significantly higher than in the strain-matched wild-type controls but significantly lower than in the Timp1 single mutant controls. This result indicated that while these two MMPs also contributed to the Timp1 mutant phenotype, they were not by themselves responsible for all of the resistance (Table 2 and Fig. 3). Suppression analysis using Mmp2 and Mmp12 mutants revealed that they do not contribute to the Timp1−/− phenotype (data not shown). Statistical analysis of corneal perforation data was by log rank testing of all data points in each experiment.
TABLE 2.
MMP-9 and MMP-7 are needed for the Timp1 mutant corneal phenotypea
| Genotype | n | CFU/eye | CFU/eye relative to Timp1−/− mice | P value | |
|---|---|---|---|---|---|
| Timp1+/+Mmp9+/+ | 32 | 1.5 × 104 | 63a | 0.2 | |
| Timp1−/−Mmp9−/− | 17 | 4.6 × 104 | 19 | 0.01 | |
| Timp1−/−Mmp9+/+ | 32 | 2.4 × 102 | 1a | ||
| Timp1+/+Mmp7+/+ | 30 | 1.6 × 105 | 2,100b | 0.0004 | |
| Timp1−/−Mmp7−/− | 34 | 5.7 × 103 | 75 | 0.0005 | |
| Timp1−/−Mmp7+/+ | 31 | 76 | 1b |
Mice with the indicated genotypes were infected in the cornea with P. aeruginosa strain 6389. Five days after infection, eyes were removed and bacterial burdens were measured. P values were determined by ANOVA. a, P < 1 × 10−5; b, P = 1 × 10−10. The P values listed are for the comparison of data from one row with that from the row below it.
FIG. 3.
Loss of MMP-3 suppresses resistance to infection in Timp1−/− mice. A total of 43 Timp1+/+ Mmp3+/+ animals, 46 Timp1−/− Mmp3+/+ animals, and 46 Timp1−/− Mmp3−/− animals were infected as described in the legend to Fig. 2 and observed daily for signs of corneal perforation. Perforation in Timp1+/+ Mmp3+/+ and Timp1−/− Mmp3−/− animals were indistinguishable (P = 0.3). However, the rates of perforation of Timp1+/+ Mmp3+/+ and Timp1−/− Mmp3+/+ animals were distinct (P = 0.002).
Enhanced resistance to pulmonary infections in Timp1 mutants.
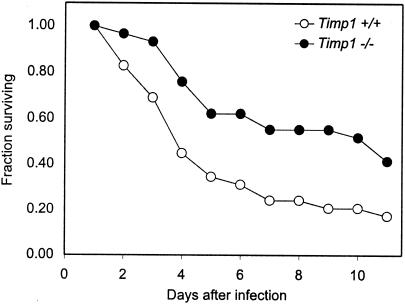
To determine whether TIMP-1-deficient mice also showed enhanced resistance to pulmonary infection, we grew P. aeruginosa strain M57-15 overnight in liquid tryptic soy broth and instilled 107 CFU in 25 μl of solution intratracheally into the right lungs of anesthetized mice. This strain is a mucoid isolate from the lungs of a cystic fibrosis patient. Our assays for bacterial burden and gross appearance of histological sections revealed no differences between Timp1+/+ and Timp1−/− mice at several times after infection. However, survival of mutant animals was significantly improved in TIMP-1-deficient mice (P = 0.008). This result demonstrated that increased resistance to infection in Timp1 mutants is not limited to the cornea (Fig. 4).
FIG. 4.
Timp1−/− mice are resistant to pulmonary challenge with P. aeruginosa. Pulmonary infections were established by instilling into the right lungs of mice 25 μl of solution containing 107 CFU of P. aeruginosa (M57-15). Mice were observed daily during the 11-day period following infection, and numbers of survivors were noted. Infected animals included 17 Timp1+/+ and 29 Timp1−/− animals. All mice were strain matched. Timp1−/− mice had significantly better survival than Timp1+/+ mice (P = 0.008).
Loss of TIMP-1 in mice resulted in increased resistance to corneal and pulmonary infection with P. aeruginosa. Resistance to corneal infection could be suppressed by BB-94, indicating that the phenotype was reversible and due to the loss of the proteinase-inhibiting activity of TIMP-1. The proteinases MMP-9, MMP-7, and MMP-3 are each important for resistance; however, the hierarchy of their importance is unknown. Because BB-94 also inhibits TACE (1, 19), BB-94 suppression of the Timp1−/− phenotype may involve effects mediated by TNF-α release. However, suppression of the Timp1 phenotype by loss of MMPs that do not process TNF-α indicates that TNF-α-mediated effects cannot explain the phenotype of Timp1 mutants. Furthermore, in animals with corneal infections, no circulating TNF-α was detected by enzyme-linked immunosorbent assay (data not shown); however, local production in the cornea was not tested.
The targets of MMP-9, MMP-7, and MMP-3 that lead to improved resistance to infection in TIMP-1-deficient mice are unknown. It is also unknown whether a single common mechanism is affected in the corneal and pulmonary models. It is possible that dysregulated MMP proteolysis directly modifies innate immunity in Timp1 mutants. Consistent with this are data showing that MMP-7 cleaves mature defensins from precursors (32). Alternatively, innate immunity could be affected indirectly. For example, release of syndecan-1 from epithelial cells by MMP-7 has been shown to influence chemokine mobilization and epithelial transmigration of neutrophils (16). It is not likely that the loss of TIMP-1 affects the ability of infections to become established. During the first 12 h of corneal infections, bacteria adhered to and replicated in the infected corneas equally well in both mutant and wild-type mice (Fig. 2A). Furthermore, the resistance phenotype was not apparent until large numbers of inflammatory cells were recruited to infected eyes (data not shown).
In contrast to our results showing that the loss of TIMP-1 is protective against infections are studies showing that mice treated with anti-TIMP-1 rabbit serum apparently have increased susceptibility to infection relative to animals treated with normal rabbit serum (13). In those studies, it is possible that the formation of TIMP-1-containing immune complexes activated serum complement leading to its depletion. Increased susceptibility to infections in those animals could then be more influenced by complement depletion and have nothing to do with TIMP-1 depletion. Assays for serum complement or experiments using antibody against an irrelevant serum protein to control for this were not reported. Furthermore, it is not known whether TIMP-1 was actually depleted from mice or if a feedback mechanism increased local synthesis in critical anatomical sites under conditions of systemic TIMP-1 depletion. These caveats are not concerns with the null genetic alteration in Timp1 we report here.
Our results raise the possibility that TIMP-1 antagonists or MMP agonists may be of therapeutic benefit for augmenting resistance to P. aeruginosa. Such agents may be of greatest utility for individuals with unresolved, antibiotic-resistant pulmonary infections, such as cystic fibrosis patients.
Acknowledgments
This work was supported by Public Health Service grants EY11279 and AI053194 from the National Institutes of Health.
We thank D. Oleszek and K. Somogyi for excellent technical help; Gerald B. Pier for helpful advice; and Steven Shapiro, John Mudgett, Shigeyoshi Itohara, and Lynn Matrisian for mice deficient in MMP-12, MMP-3, MMP-2, and MMP-7, respectively.
Editor: F. C. Fang
REFERENCES
- 1.Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitzner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385:729-733. [DOI] [PubMed] [Google Scholar]
- 2.Boujrad, N., S. O. Ogwuegbu, M. Garnier, C. H. Lee, B. M. Martin, and V. Papadopoulos. 1995. Identification of a stimulator of steroid hormone synthesis isolated from testis. Science 268:1609-1612. [DOI] [PubMed] [Google Scholar]
- 3.Cambray, G. J., G. Murphy, and J. J. Reynolds. 1981. The effects of dexamethasone in vitro on the production of collagenase and inhibitor by synovial and cartilage explants from the joints of rabbits with a proliferative arthritis. Rheumatol. Int. 1:69-72. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael, D. F., G. P. Stricklin, and J. M. Stuart. 1989. Systemic administration of TIMP in the treatment of collagen-induced arthritis in mice. Agents Actions 27:378-379. [DOI] [PubMed] [Google Scholar]
- 5.Cawston, T., P. McLaughlan, R. Coughlan, V. Kyle, and B. Hazleman. 1990. Synovial fluids from infected joints contain metalloproteinase-tissue inhibitor of metalloproteinase (TIMP) complexes. Biochim. Biophys. Acta 1033:96-102. [DOI] [PubMed] [Google Scholar]
- 6.D'Armiento, J., S. S. Dalal, Y. Okada, R. A. Berg, and K. Chada. 1992. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell 71:955-961. [DOI] [PubMed] [Google Scholar]
- 7.Davies, B., P. D. Brown, N. East, M. J. Crimmin, and F. R. Balkwill. 1993. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 53:2087-2091. [PubMed] [Google Scholar]
- 8.Eddy, A. A., H. Kim, J. Lopez-Guisa, T. Oda, and P. D. Soloway. 2000. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int. 58:618-628. [DOI] [PubMed] [Google Scholar]
- 9.Gasson, J. C., D. W. Golde, S. E. Kaufman, C. A. Westbrook, R. M. Hewick, R. J. Kaufman, G. G. Wong, P. A. Temple, A. C. Leary, E. L. Brown, et al. 1985. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature 315:768-771. [DOI] [PubMed] [Google Scholar]
- 10.Hautamaki, R. D., D. K. Kobayashi, R. M. Senior, and S. D. Shapiro. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277:2002-2004. [DOI] [PubMed] [Google Scholar]
- 11.Itoh, T., M. Tanioka, H. Yoshida, T. Yoshioka, H. Nishimoto, and S. Itohara. 1998. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 58:1048-1051. [PubMed] [Google Scholar]
- 12.Kenney, M. C., M. Chwa, A. Alba, M. Saghizadeh, Z. S. Huang, and D. J. Brown. 1998. Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr. Eye Res. 17:238-246. [DOI] [PubMed] [Google Scholar]
- 13.Kernacki, K. A., R. Barrett, and L. D. Hazlett. 1999. Evidence for TIMP-1 protection against P. aeruginosa-induced corneal ulceration and perforation. Investig. Ophthalmol. Vis. Sci. 40:3168-3176. [PubMed] [Google Scholar]
- 14.Kernacki, K. A., D. J. Goebel, M. S. Poosch, and L. D. Hazlett. 1998. Early TIMP gene expression after corneal infection with Pseudomonas aeruginosa. Investig. Ophthalmol. Vis. Sci. 39:331-335. [PubMed] [Google Scholar]
- 15.Lee, M. H., M. Rapti, V. Knauper, and G. Murphy. 2004. Threonine 98, the pivotal residue of tissue inhibitor of metalloproteinases (TIMP)-1 in metalloproteinase recognition. J. Biol. Chem. 279:17562-17569. [DOI] [PubMed] [Google Scholar]
- 16.Li, Q., P. W. Park, C. L. Wilson, and W. C. Parks. 2002. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111:635-646. [DOI] [PubMed] [Google Scholar]
- 17.Liu, Z., J. M. Shipley, T. H. Vu, X. Zhou, L. A. Diaz, Z. Werb, and R. M. Senior. 1998. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J. Exp. Med. 188:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed, F. F., D. S. Smookler, S. E. M. Taylor, B. Fingleton, Z. Kassiri, O. H. Sanchez, J. L. English, L. M. Matrisian, B. Au, W.-C. Yeh, and R. Khokha. 2004. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat. Genet. 36:969-977. [DOI] [PubMed] [Google Scholar]
- 19.Moss, M. L., S. L. Jin, M. E. Milla, D. M. Bickett, W. Burkhart, H. L. Carter, W. J. Chen, W. C. Clay, J. R. Didsbury, D. Hassler, C. R. Hoffman, T. A. Kost, M. H. Lambert, M. A. Leesnitzer, P. McCauley, G. McGeehan, J. Mitchell, M. Moyer, G. Pahel, W. Rocque, L. K. Overton, F. Schoenen, T. Seaton, J. L. Su, J. D. Becherer, et al. 1997. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385:733-736. [DOI] [PubMed] [Google Scholar]
- 20.Mudgett, J. S., N. I. Hutchinson, N. A. Chartrain, A. J. Forsyth, J. McDonnell, I. I. Singer, E. K. Bayne, J. Flanagan, D. Kawka, C. F. Shen, K. Stevens, H. Chen, M. Trumbauer, and D. M. Visco. 1998. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 41:110-121. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan, M. S., P. E. Desrochers, A. M. Chinnaiyan, D. F. Gibbs, J. Varani, K. J. Johnson, and S. J. Weiss. 1993. In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases 2. Proc. Natl. Acad. Sci. USA 90:11523-11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nothnick, W. B., P. Soloway, and T. E. Curry, Jr. 1997. Assessment of the role of tissue inhibitor of metalloproteinase-1 (TIMP-1) during the periovulatory period in female mice lacking a functional TIMP-1 gene. Biol. Reprod. 56:1181-1188. [DOI] [PubMed] [Google Scholar]
- 23.Preston, M. J., K. A. Kernacki, J. M. Berk, L. D. Hazlett, and R. S. Berk. 1992. Kinetics of serum, tear, and corneal antibody responses in resistant and susceptible mice intracorneally infected with Pseudomonas aeruginosa. Infect. Immun. 60:885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roten, L., S. Nemoto, J. Simsic, M. L. Coker, V. Rao, S. Baicu, G. Defreyte, P. J. Soloway, M. R. Zile, and F. G. Spinale. 2000. Effects of gene deletion of the tissue inhibitor of the matrix metalloproteinase-type 1 (TIMP-1) on left ventricular geometry and function in mice. J. Mol. Cell. Cardiol. 32:109-120. [DOI] [PubMed] [Google Scholar]
- 25.Saarialho-Kere, U. K., E. S. Chang, H. G. Welgus, and W. C. Parks. 1992. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J. Clin. Investig. 90:1952-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shingu, M., Y. Nagai, T. Isayama, T. Naono, and M. Nobunaga. 1993. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin. Exp. Immunol. 94:145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shipley, J. M., R. L. Wesselschmidt, D. K. Kobayashi, T. J. Ley, and S. D. Shapiro. 1996. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA 93:3942-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soloway, P. D., C. M. Alexander, Z. Werb, and R. Jaenisch. 1996. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene 13:2307-2314. [PubMed] [Google Scholar]
- 29.Vernillo, A. T., N. S. Ramamurthy, L. M. Golub, and B. R. Rifkin. 1994. The nonantimicrobial properties of tetracycline for the treatment of periodontal disease. Curr. Opin. Periodontol. 1994:111-118. [PubMed] [Google Scholar]
- 30.Wang, M., X. Qin, J. S. Mudgett, T. A. Ferguson, R. M. Senior, and H. G. Welgus. 1999. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc. Natl. Acad. Sci. USA 96:6885-6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson, C. L., K. J. Heppner, P. A. Labosky, B. L. Hogan, and L. M. Matrisian. 1997. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl. Acad. Sci. USA 94:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 33.Woessner, J. F., Jr. 2001. MMPs and TIMPs. An historical perspective. Methods Mol. Biol. 151:1-23. [PubMed] [Google Scholar]
- 34.Xue, M. L., D. Wakefield, M. D. Willcox, A. R. Lloyd, N. Di Girolamo, N. Cole, and A. Thakur. 2003. Regulation of MMPs and TIMPs by IL-1beta during corneal ulceration and infection. Investig. Ophthalmol. Vis. Sci. 44:2020-2025. [DOI] [PubMed] [Google Scholar]
- 35.Yamada, E., T. Tobe, H. Yamada, N. Okamoto, D. J. Zack, Z. Werb, P. D. Soloway, and P. A. Campochiaro. 2001. TIMP-1 promotes VEGF-induced neovascularization in the retina. Histol. Histopathol. 16:87-97. [DOI] [PubMed] [Google Scholar]