Abstract
Context
Immunochemical staining of sentinel lymph nodes (SLNs) and bone marrow identifies breast cancer metastases not seen with routine pathologic or clinical examination.
Objective
To determine the association between survival and metastases detected by immunochemical staining of SLNs and bone marrow from patients with early-stage breast cancer.
Design, Setting, and Patients
From May 1999 to May 2003, 126 sites in the American College of Surgeons Oncology Group Z0010 trial enrolled women with clinical T1–T2, N0, M0 invasive breast carcinoma in a prospective observational study.
Interventions
All patients underwent breast-conserving surgery and SLN dissection; bone marrow aspiration at the time of operation was initially optional and subsequently mandatory (March 2001). SLN specimens (hematoxylin-eosin negative) and bone marrow specimens were sent to a central laboratory for immunochemical staining; treating clinicians were blinded to results.
Main Outcome Measures
Overall survival (primary end point) and disease-free survival (a secondary end point).
Results
Of 5119 (98.3%) SLN specimens, 3904 (76.3%) were tumor-negative by hematoxylin-eosin staining. Of 3326 SLN specimens examined by immunohistochemistry, 349 (10.5%) were tumor-positive. Of 3413 bone marrow specimens examined by immunocytochemistry, 104 (3.0%) were positive. At a median follow-up of 6.3 years (through April 2010), 435 patients had died and 376 had disease recurrence. Immunohistochemical evidence of SLN metastases was not significantly associated with overall survival (5-year rates: 95.7% (95% CI, 95.0%–96.5%) for immunohistochemical positive and 95.1% (95% CI, 92.7%–97.5% for immunohistochemical negative disease, P=0.64), unadjusted hazard ratio [HR], 0.90; 95% confidence interval [CI], 0.59–1.39; P=.64). Bone marrow metastases were associated with decreased overall survival (5-year rates: 95.0% (95% CI, 94.3%–95.8%) and 90.1% (95% CI, 84.5%–96.1%), respectively (P=.01) (unadjusted HR, 1.94; 95% CI, 1.02–3.67; P=.04), but neither immunohistochemical evidence of tumor in SLNs (adjusted HR, 0.88; 95% CI, 0.45–1.71; P=.70) nor immunocytochemical evidence of tumor in bone marrow (adjusted HR, 1.83; 95% CI, 0.79–4.26; P=.15) was statistically significant on multivariable analysis.
Conclusion
Among women receiving breast-conserving therapy and SLN dissection, immunochemical evidence of SLN metastasis was not associated with decreased overall survival over a median of 6.3 years whereas, occult bone marrow metastasis, although rare, was associated with decreased survival.
Trial Registration
ClinicalTrials.gov number, NCT00003854
Introduction
Sentinel lymph node dissection (SLND) has revolutionized the approach to early-stage breast cancer by allowing minimally invasive axillary staging and more intensive examination of the sentinel lymph node (SLN). This has led to the detection of micrometastases1 and isolated tumor cells (ITC) of uncertain significance. Some older retrospective studies linked occult metastases to decreased survival,2–6 but patients were not treated with current standards of adjuvant systemic therapy and their stage of disease generally was higher than for contemporary populations. A long-term prospective study of occult metastases in SLNs from 790 contemporary patients with early breast cancer showed that micrometastases and ITC did not reduce survival.7 By contrast, in the 3884 patients enrolled in the National Surgical Adjuvant Breast and Bowel Project’s (NSAPB’s) B-32 study, occult metastases were associated with a statistically significant 1.2% decrease in 5-year survival.8
Occult metastases in the bone marrow of breast cancer patients have more consistently been associated with decreased survival.9–12 Pooled data from nine clinical studies suggest that patients with bone marrow micrometastases fare worse than those without bone marrow metastases.12 Again, many of these studies focused on larger tumors and more advanced disease than currently seen in the U.S. Data are lacking for early-stage breast cancer managed by SLND and multimodality therapy.
The American College of Surgeons Oncology Group (ACOSOG) initiated the Z0010 trial in 1999 to determine the prevalence and significance of occult metastases in the SLNs and bone marrow of patients who underwent breast-conserving surgery, SLND, and whole breast irradiation for treatment of clinical T1 or T2 node-negative breast cancer (Supplementary Figure 1). The Z0010 study also identified node-positive subjects who became candidates for enrollment in ACOSOG’s Z0011 trial.13 Here we report the major end points of Z0010: prevalence of occult metastases in SLNs and bone marrow, overall survival and disease-free survival (DFS).
Patients and Methods
Study Design
Z0010, a prospective observational study of patients undergoing breast-conserving therapy and SLND, was approved by the National Cancer Institute and the institutional review boards of participating institutions. Prior to participating, surgeons were required to perform 20 consecutive SLND procedures with SLN identification and accuracy rates ≥ 85% based on completion axillary lymph node dissection (ALND), or complete a postgraduate program with SLND training.14 Treating clinicians were blinded to the immunochemical status of SLNs and bone marrow specimens.
Patients
Women planning to undergo breast-conserving therapy for clinical T1–T2, N0, M0 invasive breast carcinoma were eligible. Participants were required to have a negative pregnancy test and a functional status (ECOG/ZUBROD) score of ≤ 2. Exclusion criteria were neoadjuvant therapy, pre-pectoral breast implants, concurrent bilateral malignancies, disease not amenable to lumpectomy, and previous axillary surgery. Written informed consent was obtained prior to registration.
Interventions
Bilateral anterior iliac crest bone marrow aspiration biopsy (optional before March 2001) was performed immediately before SLND and lumpectomy. SLND technique was at the discretion of the surgeon. Preoperative lymphoscintigraphy or intraoperative gamma counting was required when tumors were completely medial to the medial edge of the areola. If lumpectomy margins were positive for tumor, re-excision was performed and negative margins were confirmed by the pathologist.
Adjuvant therapies
Whole-breast irradiation specified in the protocol excluded a third supraclavicular field. The total dose for the breast was 45 to 50 Gy administered in tangential fields with coplanar posterior borders. Adjuvant systemic therapy was determined by treating clinicians based on primary tumor factors and results of hematoxylin-eosin staining.
Immunohistochemical Staining of SLN Specimens
Immunohistochemistry was performed at a central laboratory on hematoxylin-eosin–negative SLNs, with results blinded to clinicians. SLNs were formalin-fixed and paraffin-embedded, and blocks were cut into 5-μm sections. Paraffin removal was performed in 10 mmol/L sodium citrate buffer (pH 6) heated at 110°C for 30 minutes in a pressure cooker in a microwave oven. Slides were brought to room temperature, blocked with horse serum for 20 minutes, and incubated with primary antibody in blocking buffer for 1 hour. Two mouse monoclonal antibodies against cytokeratin were used as the primary immunohistochemical detection system: AE-1 (Signet, Dedham, MA) against low and intermediate type 1 acidic keratins, and CAM5.2 (Becton Dickinson, San Jose, CA) against cytokeratins 8 and 18. Subsequently, slides were washed three times with phosphate-buffered saline (PBS) and incubated with biotinylated secondary antibody (antimouse). After washing in PBS three times to remove unbound secondary antibody, slides were stained by 30-minute incubation with avidin-biotin-horseradish peroxidase complexes (Vector Laboratories, Burlingame, CA). The chromogen amino-ethyl-carbazole (AEC) (Sigma-Aldrich, St. Louis, MO) was used as substrate. Slides were counterstained with hematoxylin. Breast cancer tissue known to be cytokeratin-positive was used as a control.
Immunocytochemical Staining of Bone Marrow Specimens
Bone marrow aspirates were sent to the central laboratory, processed, and stained according to our previously published protocol for bone marrow immunocytochemistry.15 Briefly, mononuclear cells were separated using the Ficoll density gradient method and centrifuged onto slides. Slides were stained with a cytokeratin cocktail (AE-1 and CAM 5.2), and chromogen Fast Red (Biocare Medical, Concord, CA) was used to detect the presence of epithelial cells.
Histopathologic and Cytopathologic Evaluation of SLN and Bone Marrow Specimens
Pathologists blinded to clinical information assessed cytokeratin-stained SLN (red-brown) and bone marrow (red) cells for morphologic characteristics of malignancy (size, nuclear pleomorphism, and increased nuclear:cytoplasmic ratio). All SLN and bone marrow specimens with candidate cells and over 10% of randomly selected negative specimens were re-reviewed by a second pathologist.
Over 55% of SLN cases underwent re-review. In cases without consensus, slides were re-reviewed by both initial observers. In rare cases in which consensus was still not reached, a third pathologist served as arbitrator. Only cases with occult metastases identified by multiple observers were scored positive.
All bone marrow slides containing immunocytochemistry-positive and/or suspicious cells were sent to the National Institutes of Health (NIH) for re-review by a cytopathologist. If the central laboratory did not agree with the NIH assessment, an additional review by a third pathologist was performed. Only cases in which multiple reviewers agreed that tumor cells were present were finally scored as positive. Overall, 95% of cases showed complete or near agreement (suspicious vs. positive), and only 5% of cases showed discordance (positive vs. negative).
Statistical Analysis
The primary end point of Z0010 was overall survival from initial diagnosis. Patients not known to have died were censored at date of last follow-up. A secondary end point was DFS from diagnosis until first recurrence (any site) or death; patients without known recurrence were censored at date of last follow-up or death. The study was powered to evaluate the prognostic significance of immunohistochemistry-detected SLN metastases among women with hematoxylin-eosin–negative SLNs, with the assumption that 75% of women would have hematoxylin-eosin–negative nodes and that 10% of these women would have immunohistochemistry-positive SLN(s). A target sample size of 5300 women, including those with nodal metastases detected by hematoxylin-eosin, provided 90% power to detect a hazard ratio (HR) of 1.7 (immunohistochemistry-positive SLNs versus immunohistochemistry-negative SLNs) with a 0.05 two-sided significance level.
Comparisons between groups used chi-square tests for categorical variables and appropriate two-sample tests (t-test or Wilcoxon rank sum) for continuous variables. Kaplan-Meier estimates and curves were used to summarize overall survival and DFS. The primary analysis was a log-rank comparison of overall survival between groups. Curves displayed cumulative incidence rather than event-free survival.16 Univariable and multivariable models were constructed using Cox proportional hazards regression; the prespecified multivariable analyses were adjusted for known prognostic variables (age, tumor type, lymphovascular invasion, estrogen receptor status) and for variables expected to affect survival (adjuvant systemic therapy). Analyses were performed by ACOSOG Statistical Unit with SAS statistical analysis software, version 9.2 (SAS Institute, Cary, NC); all tests were two-sided and P values <.05 were considered significant.
Results
Sentinel Lymph Node Dissection
Between May 10, 1999, and May 30, 2003, 5538 patients at 126 institutions enrolled in Z0010. Of these, 185 were ineligible (multicentric disease, incorrect pathology, absence of pre-treatment pregnancy test, and regulatory violations) and 143 did not have the prescribed operation. Of 5210 eligible patients, 5119 (98.3%) had SLNs identified; specified mapping agents were blue dye alone (N=751), radioisotope alone (N=296), and blue dye plus radioisotope (N=4064). There was no statistically significant difference in SLN identification rates among different SLND techniques.
Study Population
Most patients were over age 50 (68.9%) with clinical stage I (83.3%) invasive ductal carcinoma (80.1%) (Table 1). Median tumor size was 1.4 cm (range 0 to 19 cm), and 81.2% of patients had estrogen-receptor–positive tumors. ALND was performed in 107 (2.1%) women with hematoxylin-eosin–negative SLNs.
Table 1.
Age and tumor characteristics of patients whose sentinel lymph nodes stained negative by hematoxylin and eosin (H&E) and were subsequently examined by immunohistochemistry (IHC).
| Variable | Tumor Status of Sentinel Lymph Node | P value | |
|---|---|---|---|
|
| |||
| Negative by H&E and IHC (N=2977) | Positive by IHC (N=349) | ||
|
| |||
| Age, in years | |||
| median (min, max) | 57 (23, 95) | 54 (27, 87) | |
| ≤ 50, n (%) | 835(28.1) | 125(35.8) | .003 |
| > 50, n (%) | 2141(71.9) | 224(64.2) | |
| missing, n | 1 | 0 | |
|
| |||
| Tumor type, n (%) | |||
| ductal | 2387 (80.3) | 262 (75.1) | |
| lobular | 226 (7.6) | 45 (12.9) | |
| both | 77 (2.6) | 14 (4.0) | .002 |
| other | 284 (9.6) | 28 (8.0) | |
| missing | 3 | 0 | |
|
| |||
| Lymphovascular invasion, n (%) | |||
| absent | 1921 (90.4) | 217(83.1) | .0003 |
| present | 205(9.6) | 44(16.9) | |
| missing | 851 | 88 | |
|
| |||
| Tumor size, in cm | |||
| median (minimum, maximum) | 1.2 (0,19) | 1.5 (0.1,5.0) | |
| ≤ 1.0, n (%) | 1260 (45.1) | 101 (30.7) | |
| 1.1 to 2.0, n (%) | 1202 (43.1) | 161 (48.9) | <.0001 |
| > 2.0, n (%) | 330 (11.8) | 67 (20.4) | |
| missing, n | 185 | 20 | |
|
| |||
| Estrogen receptor status, n (%) | |||
| positive | 2225 (81.1) | 268 (83.5) | .30 |
| negative | 518 (18.9) | 53 (16.5) | |
| missing | 234 | 28 | |
|
| |||
| Progesterone receptor status, n (%) | |||
| positive | 1828 (67.6) | 219 (70.0) | .40 |
| negative | 875(32.4) | 94 (30.0) | |
| missing | 274 | 36 | |
Use of adjuvant therapy in patients with hematoxylin-eosin–negative SLNs was as follows: 2956 of 3247 (91.0%) women received whole breast radiation, 2743 of 3289 (83.4%) women received systemic chemotherapy (2061 of 2479 [83.1%] with immunohistochemistry-negative and 269 of 299 [90.0%] with immunohistochemistry-positive SLNs), 2230 of 3289 (67.8%) women received hormonal therapy (1678 of 2479 [67.7%] with immunohistochemistry-negative and 216 of 299 [72.2%%] with immunohistochemistry-positive SLNs), and 2498 of 3247 (76.9%) women received whole breast radiation plus adjuvant systemic therapy (1902 of 2462 [77.3%] with immunohistochemistry-negative and 235 of 299 [78.6%] with immunohistochemistry-positive SLNs).
Results of SLN and Bone Marrow Immunochemistry
Of 5119 patients with an SLN specimen, 1215 (23.7%) had SLN metastases by hematoxylin-eosin examination. Of the remaining 3904 (76.3%) patients, 3326 (85.2%) had SLNs assessed by immunohistochemistry; 349 (10.5%) specimens contained occult metastases. Specimens were not assessed by immunohistochemistry if they contained inadequate tissue (121 [3.1%] patients) or were not sent for processing (457 [11.7%] patients).
Of 3413 (66.7%) patients who underwent bone marrow biopsy, 104 (3.0%) had occult metastases by immunocytochemistry. Autologous SLN and bone marrow specimens from 2205 patients showed no concordance with respect to occult metastases (kappa statistic, −0.01 [95% CI, −0.07–0.05]) (Table 2).
Table 2.
Immunochemical concordance of autologous bone marrow and sentinel lymph node specimens.
| Immunohistochemical staining of sentinel lymph node | Total | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Immunocytochemical staining of bone marrow | Positive | 6(0.3) | 62(2.8) | 68(3.1) |
| Negative | 238(10.8) | 1899(86.1) | 2137(96.9) | |
| Total | 244(11.1) | 1961(88.9) | ||
Kappa statistic, −0.01 (95% confidence interval, −0.07–0.05)
Increasing tumor size was associated with SLN metastases identified by hematoxylin-eosin staining or immunohistochemistry. In hematoxylin-eosin–negative SLNs, median tumor size was 1.5 cm (interquartile range [IQR], 1.0–2.0) versus 1.2 cm (IQR, 0.9–1.7) for specimens with versus without immunohistochemical metastases (P<.0001). There was no significant relationship between tumor size and occult metastases in the bone marrow; median tumor size was 1.4 cm (IQR, 0.83–1.98) versus 1.4 cm (IQR, 1.0–2.0) for specimens with versus without metastases (P=.87).
SLN and Bone Marrow Status and Survival
All women were followed up until April 21, 2010, when study data were frozen for analysis. At a median follow-up of 6.3 years, there were 435 deaths and 376 women with disease recurrence. Less than 10% of women had overdue follow-up.
Among patients with hematoxylin-eosin–negative SLNs, there was no significant difference in overall survival associated with immunohistochemistry-negative versus immunohistochemistry-positive SLNs (P=.64); 5-year rates were 95.7% (95% CI, 95.0%–96.5%) and 95.1% (95% CI, 92.7%–97.5%), respectively (Figure 1a). Likewise, there was no statistically significant difference in DFS associated with immunohistochemistry-negative versus immunohistochemistry-positive SLNs (P=.82); 5-year rates were 92.2% (95% CI, 91.1%–93.2%) and 90.4% (87.2%–93.8%), respectively (Figure 1b).
Figure 1.
Figure 1a. Cumulative incidence of death for patients whose sentinel lymph node specimens were hematoxylin and eosin (H&E) negative and immunohistochemistry (IHC) negative versus H&E negative and IHC positive.
Figure 1b. Cumulative incidence of recurrence or death for patients whose sentinel lymph node specimens were hematoxylin and eosin (H&E) negative and immunohistochemistry (IHC) negative versus H&E negative and IHC positive.
Immunohistochemical evidence of SLN metastases was not associated with reduced overall survival on univariable analysis (unadjusted HR, 0.90 [95% CI, 0.59–1.39]; P=.64) or multivariable analysis (adjusted HR, 0.88 [95% CI, 0.45–1.71]; P=.70). Age >50 years and tumor size >1 cm were independently associated with reduced overall survival.
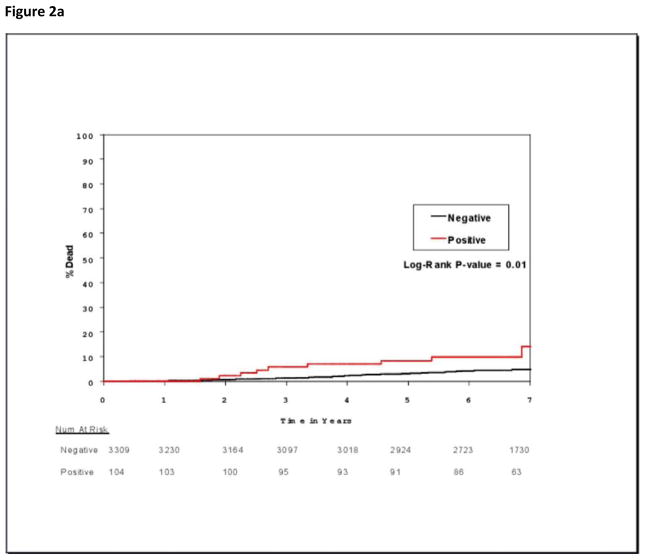
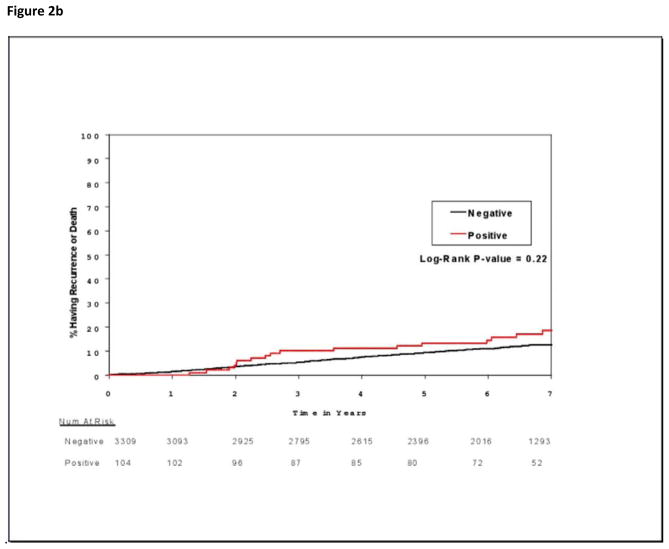
Occult bone marrow metastases were significantly associated with increased mortality (Figure 2a) but not with increased recurrence (Figure 2b). At 5 years, mortality rates for patients with immunocytochemistry-negative and immunocytochemistry-positive bone marrow specimens were 5.0% (95% CI, 4.2%–5.7% ) and 9.9% (95% CI, 3.9%–15.5%), respectively (P=.01); there were 247 deaths in 3309 patients with negative specimens and 15 deaths in 104 patients with positive specimens. Corresponding overall survival rates were 95.0% (95% CI, 94.3%–95.8%) and 90.1% (95% CI, 84.5%–96.1%), respectively (P=.01). There were 377 DFS events in 3309 patients with negative specimens and 17 DFS events in 104 patients with positive specimens. Five-year DFS rates for these patients with immunocytochemistry-negative and immunocytochemistry-positive specimens were 90.8% (95% CI, 89.7%–91.8%) and 86.7% (95% CI, 80.3%–93.7%), respectively (P=.22). Univariable analysis linked bone marrow metastases to reduced overall survival, but multivariable analysis assigned significance only to age >50 years and tumor size >1.0 cm (Table 3). However, because HR was not significantly reduced by the additional clinicopathologic and treatment variables (unadjusted HR, 1.94 [95% CI, 1.02–3.67], P=.04 on univariable analysis; adjusted HR,1.83 [95% CI, 0.79–4.26], P=.15 on multivariable analysis), absence of multivariable significance is consistent with the limited number of immunocytochemistry-positive specimens.
Figure 2.
Figure 2a. Cumulative incidence of death for patients whose bone marrow specimens were negative or positive for occult metastases by immunocytochemistry.
Figure 2b. Cumulative incidence of recurrence or death for patients whose bone marrow specimens were negative or positive for occult metastases by immunocytochemistry.
Table 3.
Univariable and multivariable models for overall survival of women whose sentinel lymph nodes stained negative by hematoxylin and eosin.
| Variable | Number of: | Univariable | Multivariable* | |||
|---|---|---|---|---|---|---|
| Patients | Deaths | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | ||||||
| ≤ 50 years | 1131 | 46 | 1.00 (ref) | 1.00 (ref) | ||
| > 50 years | 2771 | 251 | 2.24 (1.64–3.07) | <.0001 | 2.26 (1.30–3.94) | .004 |
| Tumor type | ||||||
| ductal | 3094 | 251 | 1.00 (ref) | 1.00 (ref) | ||
| lobular | 319 | 18 | 0.67 (0.41–1.08) | .10 | 0.84 (0.36–1.97) | .69 |
| both | 97 | 9 | 1.16 (0.60–2.26) | .66 | 1.97 (0.70–5.49) | .20 |
| other | 389 | 19 | 0.58 (0.36–0.93) | .023 | 1.19 (0.51–2.76) | .68 |
| Lymphovascular invasion | ||||||
| absent | 2380 | 187 | 1.00 (ref) | 1.00 (ref) | ||
| present | 301 | 35 | 1.52 (1.06–2.19) | 0.022 | 1.03 (0.54–1.96) | .93 |
| Tumor size | ||||||
| ≤ 1.0 cm | 1602 | 99 | 1.00 (ref) | 1.00 (ref) | ||
| 1.1 to 2.0 cm | 1609 | 122 | 1.20 (0.93–1.55) | .15 | 2.22 (1.34–3.68) | .002 |
| > 2.0 cm | 455 | 58 | 2.14 (1.56–2.94) | <.0001 | 3.22 (1.70–6.12) | .0003 |
| Estrogen receptor status | ||||||
| negative | 661 | 77 | 1.00 (ref) | 1.00 (ref) | ||
| positive | 2943 | 207 | 0.55 (0.42–0.72) | <.0001 | 0.64 (0.40–1.04) | .07 |
| Adjuvant systemic therapy | ||||||
| no | 546 | 58 | 1.00 (ref) | 1.00 (ref) | ||
| yes | 2743 | 173 | 0.56 (0.42–0.75) | <.0001 | 0.60 (0.34–1.02) | .06 |
| Sentinel lymph node immunohistochemistry | ||||||
| negative | 2977 | 226 | 1.00 (ref) | 1.00 (ref) | ||
| positive | 349 | 23 | 0.90 (0.59–1.39) | .64 | 0.88 (0.45–1.71) | .70 |
| Bone marrow immunocytochemistry | ||||||
| negative | 2471 | 168 | 1.00 (ref) | 1.00 (ref) | ||
| positive | 73 | 10 | 1.94 (1.02–3.67) | .04 | 1.83 (0.79–4.26) | .15 |
adjusted for all variables in the table
Adjuvant systemic therapy did not have a statistically significant association with the outcomes of patients with SLN occult metastases: 5-year overall survival rate was 96.3% (95% CI, 89.4%–100.0%) without adjuvant systemic therapy versus 95.7% (95% CI, 93.2%–98.2%) with adjuvant systemic therapy (P=.74); 5-year DFS rate was 91.4% (95% CI, 80.7%–100.0%) without adjuvant systemic therapy versus 91.0% (95% CI, 87.5%–94.7%) with adjuvant systemic therapy (P=.87).
Discussion
Z0010 is the largest prospective trial to assess immunochemically detected metastases in the SLNs and bone marrow of women with early-stage breast cancer. Occult SLN metastases were detected in 10.5% of patients with hematoxylin-eosin–negative SLNs but were not associated with survival. Occult bone marrow metastases were associated with decreased overall survival only when clinicopathologic factors were not considered.
Z0010 was undertaken in part to resolve conflicting data from large retrospective studies of patients with occult metastases (immunohistochemistry-positive/hematoxylin-eosin–negative) in the ALND specimen. The Ludwig Breast Cancer Study Group identified occult metastases in 20% of patients, about one third of whom received adjuvant systemic therapy as part of the randomized Ludwig Trial V.6 Occult metastases were associated with decreased survival for postmenopausal but not premenopausal women, and the overall decrease was not significant. In a study of over 200,000 patients in the Surveillance, Epidemiology and End Results database, survival rate progressively decreased for patients whose nodes were pN0, pN1mic, and pN1, 17 but the authors acknowledged problems with a large retrospective database. Hansen et al7 reported findings similar to those of Z0010 but unlike the Z0010 study, immunohistochemical results often affected decisions regarding adjuvant systemic therapy. In fact, the variable use of adjuvant systemic therapy in these retrospective studies may account for some differences in results.
A retrospective database review by De Boer et al18 reported outcomes for breast cancer patients treated at eight cancer centers in the Netherlands. The study included 856 node-negative and 856 node-positive (ITC or micrometastases) patients who did not receive adjuvant systemic therapy, and 995 node-positive (ITC or micrometastases) patients who received adjuvant systemic therapy. With a median follow-up of 5.1 years, they noted a significant increase in events among patients with ITC and micrometastases who did not receive adjuvant systemic therapy but not among those who received adjuvant systemic therapy. This analysis is difficult to compare with other studies because DFS included contralateral breast cancer and non-breast malignancies, which are not likely to be biologically related to occult metastases from breast cancer. In fact, their study showed no difference in overall survival with the detection of micrometastases or ITC.
In the NSABP’s B-32 cohort analysis,8 5-year overall survival was 96.4% without occult SLN metastases versus 95.8% with occult metastases. This significant difference was concluded to be insufficient to impact systemic treatment or justify routine immunohistochemistry. This is congruent with conclusions based on Z0010 data. Indeed, data from the two trials also are congruent given the differences between these trials. First, the NSABP B-32 protocol required evaluation of two widely spaced (0.5 mm) sections intended to detect all metastases larger than 1.0 mm plus some metastases smaller than 1.0 mm,8 whereas the Z0010 protocol required standard processing similar to that used in routine pathology laboratory practice. Second, the smaller number of patients with immunohistochemistry-detected micrometastases in Z0010 may have been insufficient to detect a small difference in survival. Third, 78.3% of subjects in B-32 received adjuvant systemic therapy, as compared with 86.2% of women in ACOSOG Z0010; this difference could have attenuated the association between occult metastases and survival in Z0010.
Most patients in Z0010 received adjuvant systemic therapy, reflecting practice patterns in the United States independent of immunohistochemical findings. Thus, although the effect of untreated micrometastases in Z0010 patients is unknown, it is not relevant to current practice. This conclusion is supported by a population-based study of 24,051 patients in Denmark,19 which reported that micrometastasis was the sole indication for administration of chemotherapy in only 2.1% of patients. Decisions regarding adjuvant systemic therapy most often reflect consideration of biologic or molecular factors associated with the primary tumor.20
Occult metastases of breast cancer in bone marrow reportedly occur in 4%–48% of patients and consistently have been associated with decreased overall survival.21, 22,12 These earlier reports included all patients with operable breast cancer and were conducted in an era when patients generally presented with a higher stage of disease. By contrast, Z0010 included only patients with the lowest clinical stage of invasive breast cancer. Because occult bone marrow metastasis is related to stage of disease,9 it is not surprising that the incidence of bone marrow metastases is far lower in Z0010 than in prior studies. Technical differences in the assays also may have contributed to differences among studies; immunochemical staining of bone marrow is challenging. In any case, the excellent overall outcome for all patients enrolled in Z0010 supports the low incidence of bone marrow metastases. Balic et al15 reported a putative stem cell-like phenotype (CD44+CD24−/low) in immunocytochemistry-positive cells from the bone marrow of 65% of Z0010 patients. This suggests that biologic factors in addition to the size of metastasis may determine the tumorigenic potential of metastatic cells.
Recently there has been considerable interest in the detection of circulating tumor cells (CTC) in the peripheral blood of patients with cancer, including breast cancer.23 Studies have used enrichment technologies to isolate and quantify CTC, usually in patients with known systemic metastases. Several studies have shown that monitoring CTC can identify responders and nonresponders to systemic treatment. While these technologies have shown considerable promise in patients with metastatic disease, they do not have the sensitivity required to detect CTC in patients with early-stage disease, such as those in Z0010. Newer and more efficient detection methods may address this issue.24
The findings of Z0010 have important implications for clinical practice. Many laboratories routinely perform multiple sections and immunohistochemistry on hematoxylin-eosin–negative SLNs, even though the College of American Pathologists (CAP) guidelines for SLN processing do not include their use. Data from Z0010 shows that occult metastases detected by immunohistochemistry are not associated with survival differences in patients with the earliest stages of breast cancer. Although longer follow-up might reveal small differences in outcome, these are likely to be of no clinical significance, as demonstrated by findings of NSAPB-B32.
Bone marrow examination with immunocytochemistry may identify high-risk women; however, the incidence in Z0010 was too low to recommend incorporating bone marrow aspiration biopsy into routine practice for patients with the earliest stages of breast cancer. Improved techniques for isolating and detecting occult tumor cells may make their assessment in the bone marrow more efficient and feasible.24
Routine immunohistochemical examination of hematoxylin-eosin–negative SLNs and routine immunocytochemical examination of bone marrow are not clinically warranted for early-stage (clinical T1–2, N0) breast cancer.
Supplementary Material
Acknowledgments
Funding/support: This study was supported by funds from the National Institutes of Health, under the Grants entitled “Bone Marrow and Sentinel Node Micrometastases in Breast Cancer” (NCI R01 CA 85840; R. Cote, Principal Investigator) and “American College of Surgeons Oncology Group” (NCI U10 CA076001).
Footnotes
Prior presentation: Presented in part at the annual meeting of the American Society of Clinical Oncology, June 4–8, 2010, Chicago, IL.
Author contributions: The first author of this manuscript, Dr. Armando Giuliano, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Karla V. Ballman performed and is responsible for the statistical analysis. All authors attest to the fidelity of the article with respect to the full protocol and statistical plan.
Financial disclosure: None reported.
Role of the sponsor: The National Cancer Institute had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Additional contributions: We thank the ACOSOG staff, in particular the leadership of Heidi Nelson, MD (ACOSOG Group Co-Chair, Mayo Clinic, Rochester, MN), David Ota, MD (ACOSOG Group Co-Chair, Duke University, Durham, NC), and Samuel A. Wells, Jr, MD (Senior Clinician, National Cancer Institute, Bethesda, MD). All three of these physicians contributed to study design and/or manuscript review; none received compensation. We also thank the investigators and their site research teams. Finally, we wish to thank the brave patients with breast cancer who participated in this study and their caregivers.
References
- 1.Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995 Sep;222(3):394–399. doi: 10.1097/00000658-199509000-00016. discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Mascarel I, Bonichon F, Coindre JM, Trojani M. Prognostic significance of breast cancer axillary lymph node micrometastases assessed by two special techniques: reevaluation with longer follow-up. Br J Cancer. 1992 Sep;66(3):523–527. doi: 10.1038/bjc.1992.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher ER, Swamidoss S, Lee CH, Rockette H, Redmond C, Fisher B. Detection and significance of occult axillary node metastases in patients with invasive breast cancer. Cancer. 1978 Oct;42(4):2025–2031. doi: 10.1002/1097-0142(197810)42:4<2025::aid-cncr2820420452>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Nasser IA, Lee AK, Bosari S, Saganich R, Heatley G, Silverman ML. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol. 1993 Sep;24(9):950–957. doi: 10.1016/0046-8177(93)90108-s. [DOI] [PubMed] [Google Scholar]
- 5.Trojani M, de Mascarel I, Bonichon F, Coindre JM, Delsol G. Micrometastases to axillary lymph nodes from carcinoma of breast: detection by immunohistochemistry and prognostic significance. Br J Cancer. 1987 Mar;55(3):303–306. doi: 10.1038/bjc.1987.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet. 1999 Sep 11;354(9182):896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 7.Hansen NM, Grube B, Ye X, et al. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009 Oct 1;27(28):4679–4684. doi: 10.1200/JCO.2008.19.0686. [DOI] [PubMed] [Google Scholar]
- 8.Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011 Feb 3;364(5):412–421. doi: 10.1056/NEJMoa1008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991 Oct;9(10):1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 10.Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000 Feb 24;342(8):525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 11.Diel IJ, Kaufmann M, Costa SD, et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst. 1996 Nov 20;88(22):1652–1658. doi: 10.1093/jnci/88.22.1652. [DOI] [PubMed] [Google Scholar]
- 12.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005 Aug 25;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011 Feb 9;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005 Oct;242(4):593–599. doi: 10.1097/01.sla.0000184210.68646.77. discussion 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006 Oct 1;12(19):5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002 May 11;359(9318):1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- 17.Chen SL, Hoehne FM, Giuliano AE. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007 Dec;14(12):3378–3384. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 18.de Boer M, van Deurzen CH, van Dijck JA, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009 Aug 13;361(7):653–663. doi: 10.1056/NEJMoa0904832. [DOI] [PubMed] [Google Scholar]
- 19.Tvedskov TF, Jensen MB, Balslev E, Ejlertsen B, Kroman N. Stage migration after introduction of sentinel lymph node dissection in breast cancer treatment in Denmark: A nationwide study. Eur J Cancer. 2010 Dec 29; doi: 10.1016/j.ejca.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Oakman C, Santarpia L, Di Leo A. Breast cancer assessment tools and optimizing adjuvant therapy. Nat Rev Clin Oncol. 2010 Dec;7(12):725–732. doi: 10.1038/nrclinonc.2010.170. [DOI] [PubMed] [Google Scholar]
- 21.Molino A, Colombatti M, Bonetti F, et al. A comparative analysis of three different techniques for the detection of breast cancer cells in bone marrow. Cancer. 1991 Feb 15;67(4):1033–1036. doi: 10.1002/1097-0142(19910215)67:4<1033::aid-cncr2820670428>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Osborne MP, Wong GY, Asina S, Old LJ, Cote RJ, Rosen PP. Sensitivity of immunocytochemical detection of breast cancer cells in human bone marrow. Cancer Res. 1991 May 15;51(10):2706–2709. [PubMed] [Google Scholar]
- 23.Cristofanilli M, Braun S. Circulating tumor cells revisited. JAMA. 2010 Mar 17;303(11):1092–1093. doi: 10.1001/jama.2010.292. [DOI] [PubMed] [Google Scholar]
- 24.Lin HK, Zheng S, Williams AJ, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010 Oct 15;16(20):5011–5018. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.