Abstract
Clinical heterogeneity in cystic fibrosis (CF) often causes diagnostic uncertainty in infants without symptoms and in older patients with milder phenotypes. We performed a cross-sectional evaluation of a comprehensive set of clinical and laboratory descriptors in a physician-defined cohort (N = 376; Children’s Hospital of Wisconsin and the American Family Children’s Hospital CF centers in Milwaukee and Madison, WI, USA) to determine the robustness of categorizing CF (N = 300), cystic fibrosis transmembrane conductance regulator (CFTR)-related disorder (N = 19), and CFTR-related (CRMS) metabolic syndrome (N = 57) according to current consensus guidelines. Outcome measures included patient demographics, clinical measures, sweat chloride levels, CFTR genotype, age at diagnosis, airway microbiology, pancreatic function, infection, and nutritional status. The CF cohort had a significantly higher median sweat chloride level (105 mmol/l) than CFTR-related disorder patients (43 mmol/l) and CFTR-related metabolic syndrome patients (35 mmol/l; p ≤ 0.001). Patient groups significantly differed in pancreatic sufficiency, immunoreactive trypsinogen levels, sweat chloride values, genotype, and positive Pseudomonas aeruginosa cultures (p ≤ 0.001). An automated classification algorithm using recursive partitioning demonstrated concordance between physician diagnoses and consensus guidelines. Our analysis suggests that integrating clinical information with sweat chloride levels, CFTR genotype, and pancreatic sufficiency provides a context for continued longitudinal monitoring of patients for personalized and effective treatment.
Keywords: CFTR phenotype–genotype correlation, CFTR-opathies, cystic fibrosis, cystic fibrosis metabolic syndrome, cystic fibrosis-related disorder
Although cystic fibrosis (CF) is a monogenic autosomal recessive disorder caused by mutations in the gene encoding cystic fibrosis transmembrane conductance regulator (CFTR), clinical heterogeneity causes diagnostic uncertainty in infants without symptoms and in older patients with milder phenotypes; in CF, as in other disorders, genotype does not necessarily predict phenotype. Before widespread newborn screening (implemented nationwide in the USA in 2010), CF was typically diagnosed in young children based on clinical features, elevated sweat chloride levels ≥60 mmol/l, and/or documentation of two disease-causing mutations in CFTR (1). Despite the advent of newborn screening and improved knowledge about CFTR genetics (2), CF diagnosis remains complex for many reasons (1, 3), such as inconclusive sweat chloride values (4, 5), CFTR mutations of uncertain pathogenicity (2), and differential expression of CFTR or modifier effects (6). Additionally, classes I–III CFTR mutations that typically lead to classic cases of CF may not cause symptoms in infants and young children (7, 8). Thus, the CF phenotype ranges from the absence of disease symptoms to severe, life-shortening lung disease (9).
Concurrent with the rapid implementation of nationwide newborn screening for CF, patients with CFTR-associated abnormalities such as CFTR-related disease (CFTR-RD) and CFTR-related metabolic syndrome (CRMS), but not CF, were recognized. However, the literature contains limited data on these cases, and no prospective longitudinal studies have been reported. Recently established diagnostic categories attempt to close this gap, but with mixed results in terms of accepted validity, implementation, and physician compliance with current consensus guidelines (7, 10, 11). CFTR-RD is a clinical entity that is associated with CFTR dysfunction but does not fulfill the diagnostic criteria for CF. CFTR-RD encompasses symptomatic individuals who have sweat chloride values <60 mmol/l and up to two CFTR mutations, at least one of which is not clearly categorized as a CF-causing mutation (7, 12, 13). The designation CRMS was established (and is generally used in the USA) to address asymptomatic infants with high levels of immunoreactive trypsinogen (IRT; >96th percentile), one CFTR mutation, and sweat chloride levels <60 mmol/l (10, 14, 15). However, this term was unacceptable in Europe, where another designation (CF screen positive, inconclusive diagnosis) was recently proposed (16). This array of genetic diseases will require substantial genetic and clinical data to resolve (3). As summarized in a recent editorial (3), ‘with such a complex family of CFTR-associated disorders and limited data on long term outcomes, it is not surprising that confusion and controversy have surfaced internationally regarding both diagnosis and clinical management.’
Consensus practice guidelines, including genetic counseling, for patients categorized as CFTR-RD or CRMS have been developed based on recommendations from the Cystic Fibrosis Foundation (CFF) and the European Cystic Fibrosis Society (13, 14). Inconsistencies and misclassification of patients indicate that current guidelines are difficult, if not impossible, to apply (3, 4, 17). Furthermore, consensus guidelines do not address the classification and treatment of asymptomatic patients with two CFTR mutations or normal sweat chloride levels, nor do they provide guidance on the use of rapidly developing genomic technologies (3).
To assess the current applicability of CFF and European Cystic Fibrosis Society consensus proposals (7, 10, 12–14); here, we evaluated a comprehensive set of clinical and laboratory descriptors for 376 patients who were either identified and systematically monitored (1994–2012) through the Wisconsin Newborn-Screening Program with elevated IRT levels or who presented with symptoms associated with CF. We examined patient demographics, clinical measures, sweat chloride levels, CFTR genotype, age at diagnosis, airway microbiology, pancreatic function, infection, and nutritional status. In addition to patients whose findings met current diagnostic criteria for CF, our cohort included patients found to have two CFTR mutations and normal sweat chloride levels; these patients pose a diagnostic challenge for clinicians and are not addressed in current guidelines.
Methods
Patient demographics
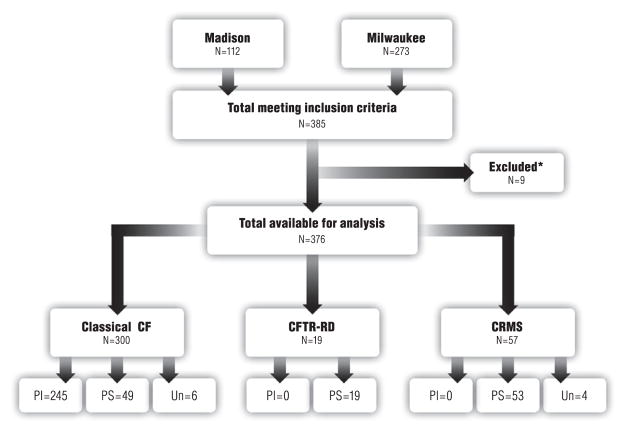
We performed a cross-sectional evaluation of 376 individuals diagnosed and followed from their time of referral to the Children’s Hospital of Wisconsin and the American Family Children’s Hospital CF centers in Milwaukee and Madison, Wisconsin, USA (Fig. 1). Participants were referred to our CF centers through routine newborn screening, which began in Wisconsin in 1994, or because they displayed clinical symptoms consistent with CF. Wisconsin includes IRT, DNA, and sweat chloride evaluations in its CF newborn-screening algorithm, as described elsewhere (1, 18). IRT data on 660,443 newborns born between 1 July 1994 and 30 June 2012 were collected from Wisconsin State Laboratory of Hygiene databases and de-identified for analysis.
Fig. 1.
Flow chart of the study population. PI, pancreatic insufficient; PS, pancreatic sufficient; Un, pancreatic sufficiency status unknown. *Excluded patients were lost to follow-up.
We sought to compare patient classification by treating pediatric pulmonologists with classifications recommended by current consensus guidelines. We therefore evaluated pilocarpine iontophoresis sweat chloride levels tested in a standardized manner in accordance with CFF guidelines (18, 19), pancreatic status based on levels of stool elastase, CFTR mutation class, and information about the phenotypes of CFTR mutations (7, 10, 12, 20). CF patients were included only if they had been evaluated at the CF Center at least once per year. Yearly visits were prospectively scheduled; clinical evaluation was performed and laboratory data were obtained, including sputum culture. To be included in our analyses, patients needed to have at least one sweat chloride value and/or known CFTR genotype and follow-up at least once per year.
Individuals with sweat chloride levels ≥60 mmol/l, pancreatic insufficiency, and/or two disease-causing CFTR mutations (classes I–III) were classified as CF. Patients in whom residual pancreatic function was evident upon evaluation of stool elastase levels (pancreatic insufficiency was defined as stool elastase <200 μg pancreatic elastase per gram of stool) were also categorized as CF. In addition, CF categorization included patients with the following class IV and V mutations previously described as resulting in phenotypes consistent with CF: 3849+10KbC>T, 2789+5G>A, p.Arg117His in cis with 5T, and p.Arg347Pro (13). However, patients with p.Arg117His with 7T/9T mutations were not considered to have classical CF (21).
CFTR-RD was defined as a symptomatic infant/child with either sweat chloride concentrations of 30–59 mmol/l (age <6 months) or 40–59 mmol/l (age ≥6 months) on at least two occasions and/or full CFTR sequencing with two CFTR mutations in trans, of which no more than one is known to cause CF, or a sweat chloride concentration <30 mmol/l (age <6 months) or <40 mmol/l (age ≥6 months). When genetic testing revealed two mutations and intermediate or normal sweat chloride levels, additional family evaluation (parental haplotyping) was performed to confirm that the mutations were in trans. Other patients classified as CFTR-RD were referred to our CF centers from other states that had not implemented newborn screening and/or came to clinical attention because of symptoms in one organ system such as congenital bilateral absence of the vas deferens, bronchiectasis, or pancreatitis.
Asymptomatic hypertrypsinogenemic infants with sweat chloride levels <60 mmol/l and/or at least one CFTR mutation with a non-classical CF genotype (classes IV and V) were classified as exhibiting CRMS. Individuals were also classified as CRMS if they harbored CFTR mutations R117H (p.Arg117His) in cis with 7T and/or 5T in cis with 11 or more TG repeats. This classification was consistent with published guidelines (10, 12) recommending the CRMS diagnosis for asymptomatic infants with intermediate sweat chloride levels on at least two occasions and fewer than two CF-causing mutations, or normal sweat chloride levels and two CFTR mutations, no more than one of which is disease-causing, as described above. Exceptions to this categorization included five patients whose IRT values at birth were unknown due to adoption and unknown parentage, home births in which IRT levels were not measured, and/or referral to our center from states in which CF newborn screening was not implemented. In these five cases, diagnoses were based on the appearance or absence of clinical symptoms, sweat chloride levels, and genotype. When a diagnosis of CRMS was made in infancy and the patient continued to lack symptoms consistent with CF, the patient remained categorized as CRMS. Although Borowitz et al. (10) originally implied that the CRMS diagnosis was restricted to infancy, its usage has extended to older children and indeed has needed to be applied at later ages (4, 17, 22).
This study was approved by the Institutional Review Board of the Children’s Hospital of Wisconsin (CHW 07/72, GC 390, CTSI 847). Informed written consent was obtained from parents/legal guardians. This study was deemed exempt from review by the University of Wisconsin-Madison’s Institutional Review Board.
CFTR genotyping
CFTR genotyping was performed at the time of diagnosis or later when the subject was diagnosed prior to the availability of genetic testing. Patients identified via newborn screening were evaluated by the Wisconsin Newborn Screening Laboratory (1) for the panel of 23 CFTR mutations recommended by the American College of Medical Genetics (23). Additional genetic testing was carried out for patients with one identified mutation, including expanded mutation panel testing (Genzyme Genetics, Cambridge, MA), modified temporal temperature gradient electrophoresis of CFTR (Ambry Genetics, Aliso Viejo, CA), and multiplex ligation-dependent probe amplification for deletions and duplications (Ambry Genetics). Current CFTR mutations were defined according to the CFTR2 database (2, 24) or the Human Genome Variation Society (legacy name) nomenclature as available (http://www.hgvs.org/).
Data collection and clinical assessment
We utilized data from clinical and laboratory databases (Sunrise Clinical Data Manager) and PortCF (the United States CF patient registry) (25) to compare physician diagnoses with consensus-based diagnoses in terms of sweat chloride levels, CFTR genotype, age at diagnosis, airway microbiology, pancreatic status, and other relevant clinical symptoms and CF co-morbidities such as nutritional status (weight, length, height, and body mass index) as defined by CFF practice guidelines (7, 14). Failure to thrive was defined as weight/length ratio <10th percentile in patients younger than 2 years of age and body mass index <10th percentile for age in children older than 2 years or a lack of weight gain over a 3–6 month period. Sputum microbiological data were obtained through Sunrise Clinical Manager medical record system and cross-checked with PortCF. If a paper copy of microbiology results was unavailable or no record existed in Sunrise Clinical Manager, PortCF data were utilized exclusively for evaluation of the year’s culture results.
Infection with respiratory pathogens
Infection was classified as two positive microbiological growths on oropharyngeal, sputum, and/or bronchoalveolar lavage specimens within the year of evaluation. As Children’s Hospital of Wisconsin and the American Family Children’s Hospital are accredited CF Care Centers, patients received standard CF care for antibiotic treatment as outlined by guidelines for detection and recording of infection (26).
Algorithm development and validation
Recursive partitioning (CART®, Salford Systems, San Diego, CA,) a statistical methodology that creates a regression tree according to prognostic significance, was used to perform a second, automated categorization of patients according to sweat chloride levels (categorized as 0–29, 30–59, or ≥60 mmol/l), with number of p.Phe508del mutations, pancreatic sufficiency, and gender as covariates. This unique secondary diagnostic categorization was independent of the clinical diagnosis from current practice guidelines. In developing an algorithm for patient classification, we implemented an automated bootstrap approach that excluded 10 randomly selected samples of the inclusion cohort. Optimization was performed using a Gini splitting rule, which is a function of the proportion and (1-proportion) of cases assigned to each node by a binary split; no node was split if it contained fewer than 15 patients, and the final node was required to contain five or more patients. Utilizing the recursive partitioning decision analysis software, categorization as CF, CFTR-RD, or CRMS was used as the outcome. The results of this analysis were then compared with the physician diagnostic classification.
Statistical analysis
Non-parametric Kruskal–Wallis and Mann–Whitney tests were used to compare continuous variables for CF groups, and chi-squared or Fisher–Halton exact tests were used to compare categorical variables. Pearson’s and Spearman’s correlations were used to examine relationships between continuous variables, as appropriate. We separated the two most common genotypes within our CRMS cohort, p.Phe508del/5T-12TG and p.Phe508del/R117H-7T (p.Arg117His), into subgroups for further analysis of sweat chloride means and ranges. p-values <0.05 were considered significant. Cytel StatX-act (Cytel Studio, version 8, Cytel Inc., Boston, MA), Salford CART (version 6, Salford Systems), and SPSS (version22, IBM Corporation, Armonk, NY) were used for all statistical analyses.
Results
Demographics
Universal newborn screening, standard mutation panels, and/or referral for evaluation of clinical symptoms allowed us to initially identify cases of CF (300/376, 80%), CRMS (57/376, 15%), and CFTR-RD (19/376, 5%) in Wisconsin. The CF cohort had a higher median age at diagnosis than the CFTR-RD and CRMS cohorts (Table 1) because a greater percentage of those patients were referred and diagnosed before universal newborn screening was implemented in surrounding states and/or because the patients presented with symptoms later (Table 1). Several adult patients were diagnosed and followed as part of Wisconsin’s 20-year history of newborn-screening implementation program (27–31).
Table 1.
Demographic information for the three patient cohorts
| CF N = 300
|
CFTR-RD N = 19
|
CRMS N = 57
|
|||||
|---|---|---|---|---|---|---|---|
| N | N (%) or median (range) | N | N (%) or median (range) | N | N (%) or median (range) | p-value | |
| Age of diagnosis, in years | 282 | 0.11 (0.01–54.8) | 18 | 0.06 (0.0–16.0) | 54 | 0.07 (0.02–16.0) | 0.013 |
| 0–8 | 262 (93) | 17 (94) | 52 (96) | 0.529 | |||
| 9–11 | 4 (1) | 1 (6) | – | ||||
| 12–17 | 8 (3) | – | 2 (4)a | ||||
| ≥18 | 8 (3) | – | – | ||||
| Gender | 300 | 19 | 57 | 0.938 | |||
| Male | 148 (49) | 10 (53) | 29 (50) | ||||
| Female | 152 (51) | 9 (47) | 28 (50) | ||||
| State of birth | 298 | 17 | 51 | 0.062 | |||
| Wisconsin | 245 (82) | 16 (94) | 49 (96) | ||||
| Illinois | 26 (9) | – | – | ||||
| Other | 27 (9) | 1 (6) | 2 (4) | ||||
| p.F508del mutation status | 294 | 19 | 57 | ≤0.001 | |||
| 0 copies | 19 (7) | 7 (37) | 9 (16) | ||||
| 1 copy | 133 (45) | 12 (63) | 48 (84) | ||||
| 2 copies | 142 (48) | – | – | ||||
| IRT (ng/ml) | 130 | 171 (32–481) | 12 | 106 (64–161) | 39 | 94 (47–262) | ≤0.001 |
| Sweat chloride level at diagnosis, mmol/l | 266 | 105 (33–149) | 18 | 43 (23–87) | 52 | 35 (14–58) | ≤0.001 |
| <30 | – | 4 (22) | 14 (27) | ≤0.001 | |||
| 31–59 | 11 (4) | 12 (67) | 38 (73) | ||||
| ≥60 | 255 (96) | 2 (11) | – | ||||
| Pseudomonas aeruginosa culture | 300 | 19 | 57 | ≤0.001 | |||
| Negative | 61 (20) | 13 (68) | 35 (61) | ||||
| Positive | 239 (80) | 6 (32) | 22 (39) | ||||
| Pancreatic status | 294 | 19 | 53 | ≤0.001 | |||
| Insufficientb | 245 (83) | – | – | ||||
| Sufficient | 49 (17) | 19 (100) | 53 (100) | ||||
| Failure to thrive | 293 | 19 | 52 | ≤0.001 | |||
| No | 212 (72) | 16 (84) | 51 (98) | ||||
| Yes | 81 (28) | 3 (16) | 1 (2) | ||||
CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; CRMS, CFTR-related metabolic syndrome.
Newborn screening began in Wisconsin in 1984 as a randomized controlled trial; these patients were diagnosed at that time and subsequently followed.
Pancreatic insufficiency was defined as < 200 μg pancreatic elastase per gram of stool.
IRT and sweat chloride levels
As expected, IRT levels were significantly higher in CF, CFTR-RD, and CRMS patients than in a normal birth population cohort (mean 24.6 ng/ml, p ≤ 0.001). IRT levels were significantly higher in CF patients (median 171 ng/ml, range: 32–481 ng/ml) than in CFTR-RD patients (median 106 ng/ml, range: 64–161 ng/ml) or CRMS patients (94 ng/ml, range: 47–262 ng/ml; p ≤ 0.001; Table 1). Individuals with CF had significantly (p ≤ 0.001) higher sweat chloride levels (median 105 mmol/l, range: 33–149 mmol/l) than subjects classified as CFTR-RD (median 43 mmol/l, range: 23–87 mmol/l) or CRMS (median 35 mmol/l, range: 14–58 mmol/l; Table 1). Ninety-six percent of subjects with CF had sweat chloride levels ≥60 mmol/l, compared with 2% of CFTR-RD patients [categorized based on CFTR genotype, p.Phe508del/R117H-7T (p.Arg117His), and symptoms] and 0% of CRMS patients (Table 1). If these patients’ diagnoses had been based solely on sweat chloride levels, these CFTR-RD patients would have been misdiagnosed as CF (7, 32).
Interestingly, 4/18 (22%) CFTR-RD patients and 14/52 (27%) CRMS patients had normal sweat chloride values (<30 mmol/l) in concert with known CFTR mutations (Table 2). Unlike the low penetrance of R117H (p.Arg117His) reported in a French population (21), 16/18 (89%) patients classified as either CFTR-RD or CRMS in our cohort had at least one R117H (p.Arg117His) mutation. Within the CFTR2 database (http://www.cftr2.org), 793/35,000 (2%) patients had the R117H (p.Arg117His) mutation. With the exception of the five patients lacking newborn-screening data (see Patient demographics in Methods), this group of patients (with sweat chloride levels <30 mmol/l and classified as CFTR-RD and CRMS) came to clinical attention because of elevated IRT levels on newborn screening.
Table 2.
Normal sweat chloride levels with CFTR mutations
| ID | Group | IRT (ng/ml) | Sweat chloride (mmol/l) | Mutation 1 | Mutation 2 |
|---|---|---|---|---|---|
| 1 | CFTR-RD | 102 | 27.9 | F508 | R117H-7T/9T |
| 2 | CFTR-RD | 100 | 22.7 | F508 | R117H-7T/9T |
| 3 | CFTR-RD | ND | 28.7 | F508 | P704L |
| 4 | CFTR-RD | 64 | 29.3 | F508 | R117H-7T/9T |
| 5 | CRMS | ND | 30.9 | F508 | R117H-7T/9T |
| 6 | CRMS | ND | 18.3 | F508 | R117H-7T/9T |
| 7 | CRMS | ND | 25.6 | F508 | R117H-7T/9T |
| 8 | CRMS | 66 | 21.8 | F508 | R117H-7T/9T |
| 9 | CRMS | ND | 14.3 | R553X | A349V |
| 10 | CRMS | 57 | 23 | R117H | R117H-7T |
| 11 | CRMS | 69 | 23 | R117H | R117H-7T |
| 12 | CRMS | 117 | 29 | F508 | R117H-7T/9T |
| 13 | CRMS | 135 | 26 | F508 | R117H-7T/9T |
| 14 | CRMS | 114 | 19 | N1303K | R117H-7T/9T |
| 15 | CRMS | 47 | 21 | F508 | R117H-7T/9T |
| 16 | CRMS | 104 | 22 | F508 | R117H-7T/9T |
| 17 | CRMS | 65 | 27 | F508 | R117H-7T/9T |
| 18 | CRMS | 104 | 30.9 | F508 | R117H-7T/9T |
CFTR-RD, cystic fibrosis transmembrane regulator-related disease; CRMS, cystic fibrosis transmembrane regulator-related metabolic syndrome; IRT, immunoreactive trypsinogen; ND, not determined (and unknown).
Mutation 1 is the more common and primary mutation. CFTR mutations were defined in the text according to the CFTR2 databse or the Human Genome Variation Society (legacy name) nomenclature (http://www.hgvs.org/). IRT and sweat chloride measurements are reported from the time point closest to diagnosis.
CFTR genotype
Complete lists of mutations in both alleles for the CF, CFTR-RD, and CRMS cohorts appear in Tables S1, S2, and S3, respectively (Supporting Information). Descriptions of CF genotypes typically assume that mutations with residual function are phenotypically dominant. For the CF cohort, 275/300 (92%) of subjects carried p.Phe508del (class II) as the more common mutation; 3/300 (3%) carried the G551D (p.Gly551Asp, class III) mutation as the more common mutation, and 2/300 (3%) carried the N1303K (p.Asn1303Lys, class II) mutation as the more common mutation. Similarly, 12/19 (63%) and 48/57 (84%) of the CFTR-RD and CRMS cohorts, respectively, harbored p.Phe508del as the more common mutation. Only 1/57 (2%) CRMS patients carried the R117H-7T (p.Arg117His, class IV) mutation as the more common mutation.
Patients with a single p.Phe508del mutation and a class IV or V mutation with residual CFTR function exhibited milder disease phenotypes, consistent with previous observations (Tables 1 and 3) (33–35). In the CF cohort, 143/300 (48%) of individuals had p.Phe508del as their secondary mutation, followed by 9/300 (3%) with G551D (p.Gly551Asp) and 14/300 (5%) with G542X (p.Gly542X, class I). In the CFTR-RD cohort, 7/19 (37%) harbored R117H-7T (p. Arg117His) as the secondary mutation, followed by 3/19 (16%) with 5T-12TG (class V). CRMS patients were likely to have R117H-7T (p.Arg117His) (36/57; 63%) or 5T-12TG (4/57; 7%) as their secondary mutation, while 2/57 (4%) CRMS patients carried the 5T-11TG mutation (class V).
Table 3.
Integration of clinical and laboratory information for patients with classical CF (N = 300), CFTR-RD (N = 19), and CRMS (N = 57)
| Disease type | Symptoms | Sweat chloridea | IRT | CFTR mutation class | Pancreatic status | FTT | Colonizationb |
|---|---|---|---|---|---|---|---|
| CF | + | Majority >60 mmol/l | ↑ | I–IIIc | >80% insufficient | >25% | >80% PA > 20% MRSA |
| CFTR-RD | + | Majority <60 mmol/l | ↑ | IV–V | Sufficient | ~20% | <35% PA 0% MRSA |
| CRMS | − | All <60 mmol/l | ↑ | IV–V | Sufficient | ~2% | <40% PA < 5% MRSA |
CF, cystic fibrosis; CFTR-RD, cystic fibrosis transmembrane regulator-related disease; CRMS, cystic fibrosis transmembrane regulator-related metabolic syndrome; FTT, failure to thrive; IRT, immunoreactive trypsinogen; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa.
Sweat chloride measurements are report.
Microbiological growth on oropharyngeal, sputum, and/or bronchoalveolar lavage specimens from the time poinst closest to diagnosis.
Exceptions noted in manuscript text.
Within our CFTR-RD cohort, we identified CFTR mutations that had been previously characterized as classes III and IV and consistent with CFTR-RD (36). As noted above, 7/19 (37%) individuals had at least one R117H-7T allele (p.Arg117His). Of these, 3/19 (16%) individuals had at least one 5T-12TG allele (Table S2). As shown in Table 2, the seven subjects carrying R117H-7T (p.Arg117His) had a median sweat chloride level of 31 mmol/l (range: 22.7–45.5 mmol/l), and the three subjects harboring 5T-12TG had a median sweat chloride level of 44 mmol/l (range: 38.1–54.9 mmol/l). These observations confirm previous reports that the R117H (p. Arg117His) and 5T-12TG mutations often correspond to mild forms of CF with few or no pulmonary symptoms (2, 37, 38).
CFTR deletions and duplications were identified in five patients with CF (Table S1). No patients with CFTR-RD or CRMS carried deletions or duplications (Tables S2 and S3, respectively); these individuals with CFTR deletions or duplications expressed clinical symptoms similar to those of patients homozygous for p.Phe508del.
Utility of automated patient re-categorization
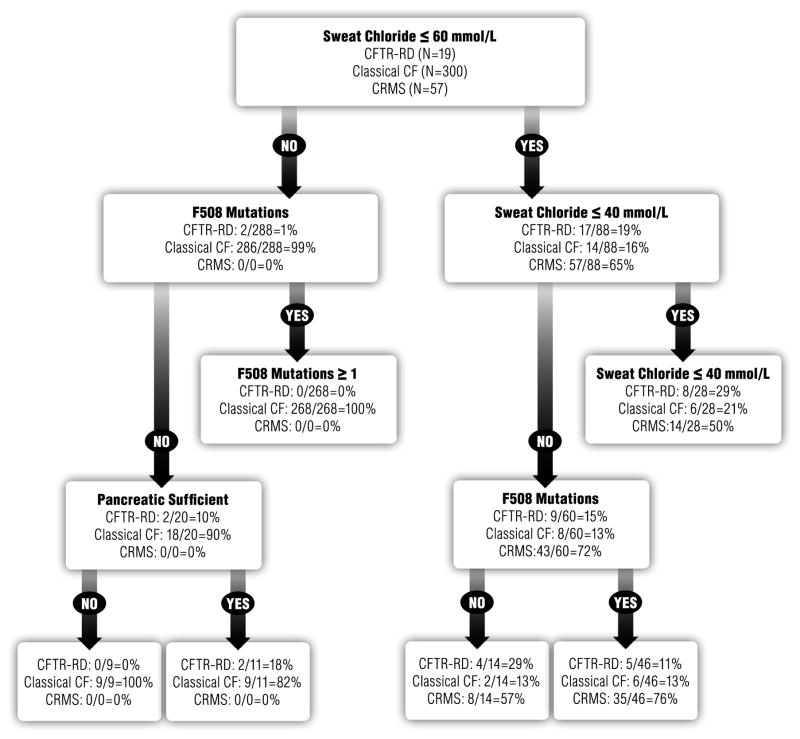
To test the utility of applying our comprehensive dataset for extending CF-related diagnoses beyond current consensus-based guidelines, we pooled all patients and their data and used recursive partitioning to create a regression tree, allowing us to perform an automated secondary re-categorization that was blinded to the initial patient classification by the physician but based on all available measurements. Classification tree analyses randomly selected the cut-off values to substratify and define the patient groups. This automated analysis successfully differentiated CRMS from CFTR-RD and CF on the basis of sweat chloride levels, pancreatic insufficiency or sufficiency, and the number of p.F508 alleles (Fig. 2). However, the algorithm assigned no patient to a different diagnosis group, confirming that our physicians used consensus guidelines when diagnosing, nor did any patient exhibit subsequent signs or symptoms that would indicate a need for different classification. In the CART model, sweat chloride levels ≤60 mmol/l were the best initial delineator of the three clinical categories (CF, CFTR-RD, and CRMS). Continued analyses of recursive partitioning showed that the number of p.Phe508del mutations, sweat chloride levels ≤40 mmol/l, and pancreatic sufficiency further clarified clinical categorizations (Fig. 2).
Fig. 2.
Patient classification algorithm. This algorithm considers sweat chloride values and pancreatic sufficiency measurements obtained closest to the time of diagnosis.
Other distinguishing clinical features in the three cohorts
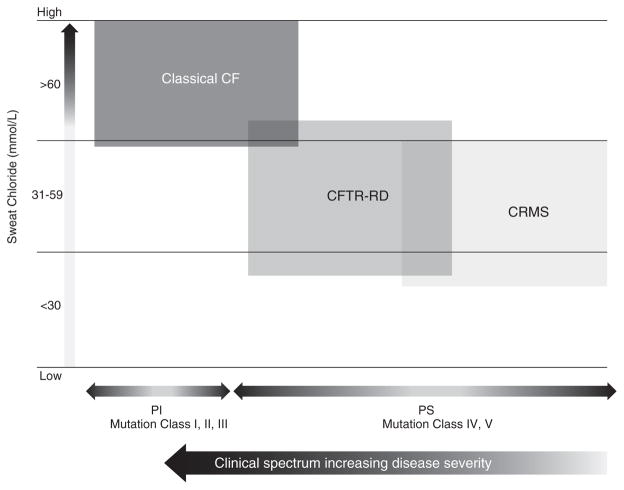
As expected, 83% (245/294) of the CF cohort was pancreatic insufficient, while none of the CFTR-RD and CRMS patients were insufficient (p ≤ 0.001; Table 1). Failure to thrive was most evident in the CF group (81/294; 28%; Table 1). In contrast, 16% (3/19) of CFTR-RD patients and 2% (1/52) of CRMS patients were diagnosed with failure to thrive, rates that were significantly lower than in the CF group (p ≤ 0.001; Table 1). Eighty percent (239/300) of CF patients had bacterial cultures positive for Pseudomonas aeruginosa. P. aeruginosa was also found in CFTR-RD (6/19; 32%) and CRMS patients (22/57; 39%; Table 1). Within the Milwaukee cohort, 22% of CF patients yielded cultures of methicillin-resistant Staphylococcus aureus, compared with none of the CFTR-RD patients and only 2% of the CRMS patients (p ≤ 0.001; data not shown). Figure 3 graphically summarizes the clinical characteristics of our CF, CFTR-RD, and CRMS cohorts according to sweat chloride level, pancreatic status, mutation classification, and clinical spectrum (3).
Fig. 3.
Clinical descriptors of our cohort capture the overlaps and distinctions among CF, CFTR-RD, and CRMS. Sweat chloride measurements are from the time point closest to diagnosis. CF, cystic fibrosis; CFTR-RD, cystic fibrosis transmembrane conductance regulator-related disorder; CRMS, CFTR-related metabolic syndrome; PI, pancreatic insufficient; PS, pancreatic sufficient.
Discussion
Utilizing the largest cohort of CF patients described to date, we characterized patient clinical status and investigated novel methods to distinguish CF patients from those with CFTR-RD and CRMS. Our initial definitions of CFTR-RD and CRMS were in accordance with those endorsed by the CFF consensus report (12, 14), as were the diagnoses of our physicians, but the spectrum of clinical phenotypes and the pleiotropy of clinical presentations complicate diagnosis and prognosis (3, 39), prompting us to expand our analysis to address the complex relationship between genotype and phenotype in CF. Our data from Wisconsin suggest that in a typical US population, the distribution of CFTR-associated disorders is approximately 80% CF, 15% CRMS, and 5% CFTR-RD, similar to a previous analysis of CF Foundation registry data (17). It has become increasingly well known from recent studies in Wisconsin and elsewhere, as well as from data in the CF Foundation Patient Registry data, that ~1/3 of CF patients diagnosed early through newborn-screening experience growth retardation (40). The 28% of patients in the CF group with failure to thrive despite comprehensive nutritional care is in accordance with international experience (41) and suggests that care providers should better understand the factors (42) that limit growth and devote additional efforts to improve follow-up management to prevent failure to thrive.
In contrast to other recent investigations, our large study population was non-migrating, lived in a defined geographic region, and underwent complete genetic and clinical characterization with the clinical categorizations confirmed by blinded algorithmic assessment. Classification tree analyses randomly selected the cut-off values to substratify and define the patient groups. None of the patients migrated to a different group in our automated analysis, nor did any patient exhibit any subsequent signs or symptoms that would indicate a need for different classification. The n and % were adequate for each division, as defined in Figure 2.
Due to the spectrum of clinical heterogeneity, current newborn CF mutation panels remain a screening tool that cannot supply definitive diagnostic parameters based solely on allelic mutations. While based on rigorous assessment of the impact of each mutation on CFTR function in conjunction with phenotype standards, a recent categorization scheme identifying CFTR mutations as ‘CF causing’ or of ‘variable clinical significance’ (2) has limited use in actual clinical decision processes. Therefore, currently, clinical evaluation of sweat chloride levels together with pancreatic function and CFTR mutations remains key for differentiating CF, CFTR-RD, and CRMS (Fig. 3). Our automated recursive partitioning analysis did not alter the diagnosis of any patient (Fig. 2), suggesting that integrating clinical information with sweat chloride levels, CFTR genotype, and pancreatic sufficiency provides the clinician with a context for patient classification and prognosis. Centers that do not have extensive experience with newborn screening and the nuances of diagnostic classification in the newborn-screening era may misclassify patients; there is evidence in the CFF registry of poor adherence to published guidelines (personal communication, Dr Bruce Marshall, Vice President of Clinical Affairs of CFF). Specifically, ~40% of patients within the CFF patient registry who met criteria for CRMS were diagnosed with CF (11), which may indicate a need to increase the monitoring of patients with CRMS, as the progression of this disorder is unknown. Longitudinal data, which are beyond the scope of the present investigation, may extend the definitions of CF-related disorders beyond current consensus-based guidelines and may empower clinicians to craft monitoring and treatment regimens that are more appropriate to nuanced-disease categorizations.
Phenotypic differences exist between individuals with CF and those with CFTR-RD and CRMS (Table 3; Fig. 3); however, disease progression remains unknown for CFTR-RD and CRMS. In one report, 11% of patients who had elevated IRT levels but were classified as CF screen positive, inconclusive diagnosis later developed CF (4). We are currently following our CRMS patients longitudinally to establish such a reference.
In the absence of a widely used and universally accepted set of diagnostic parameters for CFTR-RD or CRMS, we encourage the use of a working algorithm. CF is likely in cases of abnormal IRT levels, intermediate sweat chloride levels suggestive of abnormal ion transport, and two CFTR mutations. In such cases, an intensive search for a CFTR mutation in the proband and consideration of parental haplotyping are warranted, with follow-up at a CF center. Conversely, although demonstration of only one CFTR mutation and intermediate or normal sweat chloride levels does not rule out CFTR-RD or CRMS, it does appear to exclude a diagnosis of CF, as long as an appropriate mutation panel has been used based on the patient’s race and ethnicity, and should prompt more thorough investigation of alternative diagnoses. Sweat chloride levels <30 mmol/l in children younger than 6 months and <40 mmol/l in children 6 months or older are considered normal. In contrast, our data suggest that in rare instances (Table 2), individuals with CFRD or CRMS exhibit sweat chloride levels in this ‘normal’ range due to the presence of CFTR mutations that have a subtle functional impact and an indeterminate clinical impact. Importantly, individuals with elevated IRT levels, one CFTR mutation, and a sweat chloride level in the normal range are carriers and do not have CRMS or CFTR-RD.
Note that this algorithm does not incorporate pulmonary function testing because most children diagnosed through newborn screening lack respiratory symptoms and have normal lung function (43). Our results concur with previous consensus statements and a case report that suggested reserving the CRMS term for newborns with elevated IRT levels that lack clinical symptoms diagnostic of CF (10). We have retained this terminology for patients who remain asymptomatic.
A limitation of the present cross-sectional investigation is that we were unable to predict whether patients with CRMS and CFTR-RD will continue to exhibit an absence of symptoms or mild clinical courses with regard to pulmonary disease, pancreatic function, airway inflammation, and bacterial infection into adulthood, or whether they will eventually develop symptoms more consistent with CF. Although 94% of individuals with CFTR-RD and 96% of patients with CRMS in our cohorts are younger than 8 years of age at present, and only two patients are older than 18 years, none have shown evidence of bronchiectasis. However, the majority of the examined cohorts were drawn from pediatric centers with young populations of patients that lacked development of the CFTR-RD co-morbidities described by Bombieri et al. (12), such as congenital absence of the vas deferens, pancreatitis, and/or bronchiectasis, hindering our ability to investigate the long-term clinical courses associated with CFTR-RD and CRMS. Longitudinal integration of clinical, genetic, and non-genetic information from recently adopted universal CF newborn-screening programs may assist the further development of algorithms that predict and accurately classify the phenotypic variability of CF, CFTR-RD, and CRMS.
As most CF patients are now diagnosed through newborn screening, application of genomic sequencing to this population may provide an unprecedented opportunity for novel prognostic and therapeutic interventions and for the generation of guidelines and platforms for other newborn-screening programs (44), with the caveat that knowledge of the patient’s genotype does not necessarily reveal the patient’s phenotype, prompting the need for additional functional analyses. With discovery of the gene responsible for CF (45), it was hoped that knowledge of a patient’s genotype would predict disease severity. However, there is no consistent phenotype–genotype correlation, perhaps due to protein-activity thresholds, modifier genes, and/or system dynamics (46–49).
A recent description of disease causality in patients with CFTR variants (2) suggests that increased use of sequencing in the clinical setting may address the functional and clinical significance of rare variants, underscoring the importance of collating patient genotype and associated phenotype data for detailed analysis, as we have done here. Close monitoring of these patients over time is warranted to determine whether they eventually develop CF or maintain milder clinical phenotypes. In the newborn-screening era, continued monitoring would strike a balance between the risk of delayed diagnosis of CF, leading to inadequate treatment early in life, and the risk of ‘over-medicalization’ of patients and families with CFTR-RD or CRMS. With improved methods for distinguishing such patients soon after newborn screening, both prognosis and therapeutic planning can be enhanced.
Acknowledgments
We acknowledge the assistance of the Cystic Fibrosis Center staff at Children’s Hospital of Wisconsin in Milwaukee, Wisconsin and American Family Children’s Hospital in Madison, Wisconsin. We thank all of our CF patients and their families; we appreciate their involvement and support. We also thank Rachel Bersie, Katelyn Parker-McGill, and Iqbal Rashid, MD, for their assistance with data collection. We appreciate Dr Bruce Marshall’s thoughtful review of our manuscript. H. L. is supported by 5R21HL102523 R21 and 1DP2OD007031. A. L., M. R., and P. M. F. have been supported by 2R01 DK34108. Support was also provided to H. L. by the Children’s Hospital of Wisconsin Research Institute and by the Ann Hardy Fund, Milwaukee, Wisconsin, USA.
Author contributions: H. L. delineated the hypothesis, conceived and designed the study, performed and oversaw the data analyses, and wrote the manuscript. K. S. helped collect samples and assisted with data collection and analysis. M. N. analyzed the data, and D. H. and A. L. assisted with data collection. S. A. M. critically reviewed the manuscript. O. L., M. R., M. K. D., S. Z. N., P. S., and P. M. F. assisted with data analysis and edited the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s website.
References
- 1.Baker MW, Groose M, Hoffman G, Rock M, Levy H, Farrell PM. Optimal DNA tier for the IRT/DNA algorithm determined by CFTR mutation results over 14 years of newborn screening. J Cyst Fibros. 2011;10:278–281. doi: 10.1016/j.jcf.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosnay PR, Siklosi KR, Van Goor F, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy H, Farrell PM. New challenges in the diagnosis and management of cystic fibrosis. J Pediatr. 2015;166:1337–1341. doi: 10.1016/j.jpeds.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ooi CY, Castellani C, Keenan K, et al. Inconclusive diagnosis of cystic fibrosis after newborn screening. Pediatrics. 2015;135:e1377–e1385. doi: 10.1542/peds.2014-2081. [DOI] [PubMed] [Google Scholar]
- 5.Groman JD, Meyer ME, Wilmott RW, Zeitlin PL, Cutting GR. Variant cystic fibrosis phenotypes in the absence of CFTR mutations. New Engl J Med. 2002;347:401–407. doi: 10.1056/NEJMoa011899. [DOI] [PubMed] [Google Scholar]
- 6.Drumm ML, Konstan MW, Schluchter MD, et al. Genetic modifiers of lung disease in cystic fibrosis. New Engl J Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 7.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock ML, Levy H, Zaleski C, Farrell PM. Factors accounting for a missed diagnosis of cystic fibrosis after newborn screening. Pediatr Pulmonol. 2011;46:1166–1174. doi: 10.1002/ppul.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 10.Borowitz D, Parad RB, Sharp JK, et al. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis trans-membrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J Pediatr. 2009;155:S106–S116. doi: 10.1016/j.jpeds.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren CL, Parad R, Borowitz D. Indeterminate cystic fibrosis newborn screening results. Pediatric Pulmonol. 2015;50:209–210. doi: 10.1002/ppul.23055. [DOI] [PubMed] [Google Scholar]
- 12.Bombieri C, Claustres M, De Boeck K, et al. Recommendations for the classification of diseases as CFTR-related disorders. J Cyst Fibros. 2011;10(Suppl 2):S86–S102. doi: 10.1016/S1569-1993(11)60014-3. [DOI] [PubMed] [Google Scholar]
- 13.Castellani C, Cuppens H, Macek M, Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179–196. doi: 10.1016/j.jcf.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren CL, Desai H, Platt M, Dixon M. Clinical outcomes in infants with cystic fibrosis transmembrane conductance regulator (CFTR) related metabolic syndrome. Pediatr Pulmonol. 2011;46:1079–1084. doi: 10.1002/ppul.21475. [DOI] [PubMed] [Google Scholar]
- 16.Munck A, Mayell SJ, Winters V, et al. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J Cyst Fibros. 2015;14(6):706–713. doi: 10.1016/j.jcf.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Ren CL, Fink AK, Petren K, et al. Outcomes of infants with indeterminate diagnosis detected by cystic fibrosis newborn screening. Pediatrics. 2015;135:e1386–e1392. doi: 10.1542/peds.2014-3698. [DOI] [PubMed] [Google Scholar]
- 18.Kloosterboer M, Hoffman G, Rock M, et al. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics. 2009;123:e338–e346. doi: 10.1542/peds.2008-1681. [DOI] [PubMed] [Google Scholar]
- 19.Legrys VA, McColley SA, Li Z, Farrel PM. The need for quality improvement in sweat testing infants after newborn screening for cystic fibrosis. J Pediatr. 2010;157:1035–1037. doi: 10.1016/j.jpeds.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellani C, Southern KW, Brownlee K, et al. European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros. 2009;8:153–173. doi: 10.1016/j.jcf.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Thauvin-Robinet CMA, Huet F, Genin E, et al. The very low penetrance of cystic fibrosis for the R117H mutation: a reappraisal for genetic counseling and newborn screening. J Med Genet. 2009;46:752–758. doi: 10.1136/jmg.2009.067215. [DOI] [PubMed] [Google Scholar]
- 22.Groves T, Robinson P, Wiley V, Fitzgerald DA. Long-term outcomes of children with intermediate sweat chloride values in infancy. J Pediatr. 2015;166(1469–74):e1461–63. doi: 10.1016/j.jpeds.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 23.Watson MS, Cutting GR, Desnick RJ, et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet Med. 2004;6:387–391. doi: 10.1097/01.GIM.0000139506.11694.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosnay PR, Cutting GR. Interpretation of genetic variants. Thorax. 2014;69:295–297. doi: 10.1136/thoraxjnl-2013-204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National cystic fibrosis patient registry annual data report, 2013. Bethesda, Maryland: 2014. [Google Scholar]
- 26.Proesmans M, Balinska-Miskiewicz W, Dupont L, et al. Evaluating the "Leeds criteria" for pseudomonas aeruginosa infection in a cystic fibrosis centre. Eur Resp J. 2006;27:937–943. doi: 10.1183/09031936.06.00100805. [DOI] [PubMed] [Google Scholar]
- 27.Farrell MH, Farrell PM. Newborn screening for cystic fibrosis: ensuring more good than harm. J Pediatr. 2003;143:707–712. doi: 10.1016/j.jpeds.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Farrell PM. Improving the health of patients with cystic fibrosis through newborn screening. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Adv Pediatr. 2000;47:79–115. [PubMed] [Google Scholar]
- 29.Farrell PM. Cystic fibrosis newborn screening: shifting the key question from "should we screen?" to "how should we screen?". Pediatrics. 2004;113:1811–1812. doi: 10.1542/peds.113.6.1811. [DOI] [PubMed] [Google Scholar]
- 30.Farrell PM, Kosorok MR, Laxova A, et al. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. New Engl J Med. 1997;337:963–969. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]
- 31.Farrell PM, Lai HJ, Li Z, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005;147:S30–S36. doi: 10.1016/j.jpeds.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 2005;132:589–595. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 33.Braun AT, Farrell PM, Ferec C, et al. Cystic fibrosis mutations and genotype-pulmonary phenotype analysis. J Cyst Fibros. 2006;5:33–41. doi: 10.1016/j.jcf.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Hamosh A, Corey M. Correlation between genotype and phenotype in patients with cystic fibrosis. The Cystic Fibrosis Genotype-Phenotype Consortium. New Engl J Med. 1993;329:1308–1313. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- 35.Sermet-Gaudelus I, Girodon E, Sands D, et al. Clinical phenotype and genotype of children with borderline sweat test and abnormal nasal epithelial chloride transport. Am J Respir Crit Care Med. 2010;182:929–936. doi: 10.1164/rccm.201003-0382OC. [DOI] [PubMed] [Google Scholar]
- 36.Wilschanski M, Zielenski J, Markiewicz D, et al. Correlation of sweat chloride concentration with classes of the cystic fibrosis transmembrane conductance regulator gene mutations. J Pediatr. 1995;127:705–710. doi: 10.1016/s0022-3476(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 37.Groman JD, Karczeski B, Sheridan M, et al. Phenotypic and genetic characterization of patients with features of "nonclassic" forms of cystic fibrosis. J Pediatr. 2005;146:675–680. doi: 10.1016/j.jpeds.2004.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groman JD, Hefferon TW, Casals T, et al. Variation in a repeat sequence determines whether a common variant of the cystic fibrosis transmembrane conductance regulator gene is pathogenic or benign. Am J Hum Genet. 2004;74:176–179. doi: 10.1086/381001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Boeck K, Wilschanski M, Castellani C, et al. Cystic fibrosis: terminology and diagnostic algorithms. Thorax. 2006;61:627–635. doi: 10.1136/thx.2005.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai HJ, Shoff SM. Classification of malnutrition in cystic fibrosis: implications for evaluating and benchmarking clinical practice performance. Am J Clin Nutr. 2008;88(1):161–166. doi: 10.1093/ajcn/88.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanDevanter DR, Kahle JS, O’Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros. 2015 doi: 10.1016/j.jcf.2015.09.008. pii: S1569-1993(15)00216-7 epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Shoff SM, Ahn HY, Davis L, Lai H, Wisconsin CF Neonatal Screening Group. Temporal associations among energy intake, plasma linoleic acid, and growth improvement in response to treatment initiation after diagnosis of cystic fibrosis. Pediatrics. 2006;177(2):391–400. doi: 10.1542/peds.2004-2832. [DOI] [PubMed] [Google Scholar]
- 43.Sly PD, Gangell CL, Chen L, et al. Risk factors for bronchiectasis in children with cystic fibrosis. New Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 44.Baker MW, Atkins AE, Cordovado SK, Hendrix M, Earley MC, Farrell PM. Improving newborn screening for cystic fibrosis using next-generation sequencing technology: a technical feasibility study. Genet Med. 2015 Feb 12; doi: 10.1038/gim.2014/209. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 46.Accurso FJ, Sontag MK. Gene modifiers in cystic fibrosis. J Clin Invest. 2008;118(3):839–841. doi: 10.1172/JCI35138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dipple KM, Phelan JK, McCabe ER. Consequences of complexity within biological networks: robustness and health, or vulnerability and disease. Mol Genet Metab. 2001;74(1–2):45–50. doi: 10.1006/mgme.2001.3227. [DOI] [PubMed] [Google Scholar]
- 48.Miller MR, Soave D, Li W, et al. Variants in solute carrier SLC26A9 modify prenatal exocrine pancreatic damage in cystic fibrosis. J Pediatr. 2015;166(5):1152–1157. doi: 10.1016/j.jpeds.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dipple KM, McCabe ER. Phenotypes of patients with “simple” Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am J Hum Genet. 2000;66(6):1729–1735. doi: 10.1086/302938. [DOI] [PMC free article] [PubMed] [Google Scholar]