Abstract
Adverse early-life experiences, including various forms of early-life stress, have consistently been linked with vulnerability to cognitive and emotional disorders later in life. Understanding the mechanisms underlying the enduring consequences of early-life stress is an active area of research, because this knowledge is critical for developing potential interventions. Animal models of early-life stress typically rely on manipulating maternal/parental presence and care, because these are the major source of early-life experiences in humans. Diverse models have been created, and have resulted in a wealth of behavioral outcomes. Here we focus on recent findings highlighting early-life stress-induced behavioral disturbances, ranging from hippocampus-dependent memory deficits to problems with experiencing pleasure (anhedonia). The use of naturalistic animal models of chronic early-life stress provides insight into the spectrum of cognitive and emotional outcomes and enables probing the underlying mechanisms using molecular-, cellular-, and network-level approaches.
Introduction
Mental illnesses and cognitive disorders commence predominantly early in life [1,2], suggesting the need to explore events early on that predispose and contribute to disease onset. Epidemiological data indicate that various forms of early-life stress in humans can have life-long impacts, ranging from memory deficits and poor executive functioning [3–5] to more explicitly stress-related disorders such as depression, anxiety, and post-traumatic stress disorders [6–11]. Adverse early-life conditions, including poverty, loss of a parent, substance abuse by the mother or maternal depression, are consistently associated with vulnerability to various psychopathologies later in life [12–15]. Understanding the mechanisms for the enduring consequences of early-life stress on brain function has been an active area of neuroscience research, as this knowledge is critical for identifying clinically plausible therapeutic strategies. This review will focus on the behavioral outcomes of early-life stress, with a particular emphasis on new findings emerging within the past few years, and conclude with a unifying theory for how these profound changes may occur.
What is early-life stress?
The type and severity of the perturbations that cause early-life stress seem to govern its consequences. In humans, chronic early-life stress has both physical and emotional components, but the emotional aspects are dominant. Among the most influential studies of the effects of early-life stress are those of institutionally raised children, where chronic impoverished care was associated with cognitive and emotional problems [4,16]. Notably, the associated consequences were partially reversed by fostering, thus highlighting the importance of early-life care per se [4,16–19]. In large part, human early-life stress stems from abnormal patterns of maternal care, ranging from neglect to inconsistency and lack of sensitivity [18,20–22]. In order to study early-life stress, animal models have aimed to recapitulate these conditions by manipulating maternal interactions with offspring.
Modeling early-life stress
In mammals, including humans, monkeys and rodents, maternal input has perhaps the most significant influence on the environment experienced during development [20,22–25]. Thus, most animal models of early-life stress have manipulated maternal interaction, disrupting either the quantity or quality of maternal care early in life (see [26,27] for recent reviews). Non-human primates, whose brains and sociality most closely resemble those of humans, have provided useful insights into the development of complex psychiatric disorders. The seminal work of Harlow and colleagues using maternally-isolated rhesus monkeys as a model was the first to demonstrate that maternal-infant interactions are required for normal cognitive and emotional development [23,28,29]. More recently, using a model of maternal maltreatment in rhesus monkeys, Sanchez and colleagues suggested that this adverse early-life experience affects the development of brain systems involved in stress responses, resulting in emotional reactivity and abusive parenting in adulthood [30,31]. Although primate models of early-life stress continue to provide important insights, the many practical and ethical concerns associated with the use of primates preclude their widespread use. The majority of early-life stress models, including the ones discussed here, employ rodents. Rodents are obviously incapable of reproducing the rich repertoire of human development and cognitive and emotional outcomes. Nevertheless, the major similarities in the role of maternal care, in the stress system, and the ready availability of cognitive and emotional tasks to probe behavior have rendered rodents a tractable model for studying the behavioral outcomes of early-life stress.
As is the case in humans, maternal care plays a critical role in rodent development. The rodent dam is vital for not only providing nutrition and safety in the nest, but also providing important sensory signals and relaying environmental cues to the pups [32–35]. Simply removing the dam for extended periods of time would lead to hypothermia and starvation; thus, many models of early-life stress have used intermittent maternal separation (MS). This paradigm decreases the quantity of maternal care and results in intermittent stress [36,37]. Although MS models have provided a wealth of data on the effects of decreased maternal interaction on pup development, some of the outcomes of this manipulation have been less consistent [38–42]. In addition, the paradigm may differ from relevant human conditions: When infants and children grow up in adverse conditions such as severe poverty, famine, war or with drug-abusing mothers, the stress is typically chronic rather than intermittent, and the mother is typically present and her behavior may be stress-provoking to the child [22,43–45].
A more recently emerging model involves provoking chronic changes in the quality of maternal care by the use of cages with limited nesting and bedding material (LBN) during postnatal days (P)2–9 [26,46,47]. This ‘simulated poverty’ induces stress in the dams [48], and profoundly alters maternal behaviors, such that they are fragmented and unpredictable [49,50]. Notably, the overall duration of maternal nurturing behaviors as well as their general quality (e.g., arched-back nursing) are little changed [24,48–50]. This approach has provoked chronic, unpredictable and uncontrollable “emotional stress” in the pups [26,46–49,51–53]. There is little evidence of physical stress in the pups, with no hypothermia and modest weight changes [26]. Thus, the early-life stress that is generated seems to be a direct effect of the fragmented, unpredictable sensory signals from the mothers [22,26,50,51], resulting in persistent elevation of plasma corticosterone and adrenal hypertrophy [46,49,54]. All of these signs of stress disappear after dams and pups are returned to routine cages on P10. Still, the experience in the LBN cages during this critical window results in long-lasting consequences on cognitive and emotional function. The LBN model has been found to provoke robust and generally reproducible cognitive and emotional outcomes, leading to its adoption by dozens of laboratories around the world [51–53,55–63]. Thus, this review will focus primarily on the LBN model and its behavioral outcomes. Notably, an obvious challenge for both human and animal-model studies of early-life stress and its life-long consequences is the presence of additional factors that might influence these outcomes, including genetics and individual differences in resilience/vulnerability. These are likely some of the factors promoting ambiguous or contradictory results in both human and animal-model studies.
Modeling the behavioral outcomes of early-life stress
The specific later-life consequences of early-life stress in humans are modeled in rodents using standardized cognitive and emotional tests that have been designed to optimize translation to the human condition. For example, rodent tests of depressive-like behavior, such as the forced-swim test (FST), have been validated to show improvement with human antidepressants [64]. Human cognitive function, though much more complex than in rodents, is subserved by areas of the brain that are homologous in the rodent: e.g., the Morris water maze memory task in rodents is analogous to spatial navigation and memory in humans and both are hippocampus-dependent [65]. Much research employing animal models has focused on hippocampus-dependent memory, because of the availability of standardized tests and well-characterized neural substrates, molecules and mechanisms. We describe some of these findings, and regret that space limitations prevent us from discussing executive functions and other prefrontal cortex-dependent behaviors in the context of early-life stress [66–68].
For any model of early-life stress, the detection of a behavioral outcome depends on several variables. The first set of variables pertains to the timing, nature and severity of the stress. Secondly, the age at which animals are tested, whether during adolescence, adulthood, or aging, can determine outcome. Third, the type and difficulty of the test that is used to measure behavioral outcomes is important. For example, a rigorous test such as object location memory (OL) might uncover subtle deficits not apparent in a less challenging test, such as object recognition memory (OR) [69]. These caveats are illustrated below.
A spectrum of cognitive consequences of chronic early-life stress
Diverse cognitive effects of early-life stress have been reported. For example, MS stress on postnatal day 9 has led to improved memory in the active avoidance test [70], whereas the same manipulation on postnatal day 4 has led to impaired memory in the same test [70]. This latter finding is more in line with the majority of the MS literature, which includes reports of impairments in the Morris Water maze test and OR [40,71]. There may be several possible bases for these divergent outcomes, including the potential that mild or predictable stress might be a positive experience [72]. More likely, these diverse results derive from mechanisms depending on the developmental timing of the separation [36].
Memory impairments have been the common outcome in rodents exposed to chronic early-life stress in the LBN paradigm. For example, in a rigorous and hippocampus-dependent test of OL memory, an overt impairment in spatial memory was found as early as adolescence in LBN rats [69]. A less rigorous memory task for OR found comparable performance in LBN vs. Control adolescent rats. However, an acute-stress “challenge” imposed 24 hours prior to the test led to memory problems only in the LBN rats, thus unmasking a latent cognitive vulnerability [69]. The memory deficits after chronic early-life stress also progressed over the lifespan of LBN rats, so that deficits in OR memory emerged by middle-age [69]. At this age, hippocampus-dependent memory deficits were also present using the Morris water maze task [54,73]. Timing of testing is thus an important factor in determining the cognitive outcomes of early-life stress.
Recent findings for emotional consequences of chronic early-life stress
A variety of emotional problems, based on rodent tasks considered indicative of depression or anxiety, have been reported after early-life stress [26,74–76]. More recently, anhedonia, a reduced capacity to experience pleasure which commonly heralds depression or schizophrenia in humans [77], has been identified following early-life stress. Already during adolescence, anhedonia, apparent both as a significant reduction in sucrose preference and a reduction of peer-play, was found in late-adolescent LBN rats [50]. This anhedonia was not accompanied in adolescent rats by overt anxiety-like behavior or depressive-like behavior. Increased anxiety-like behaviors in the elevated-plus maze test were found later in adulthood [55], but these were no longer found during middle-age (i.e., 12 months of age in rats) [78]. These effects of age on anxiety and other emotional outcomes are not surprising, because in humans, the emergence and waning of anxiety and depression are highly age-dependent [79,80].
Adolescent anhedonia after early-life stress has since been confirmed in a separate LBN cohort in a different laboratory, as indicated by decreased M&M consumption as well as reduced lever pressing for cocaine (S. Mahler, personal communication). Adolescent anhedonia after early-life MS has also been found by some authors [38,40], including one report of decreased cocaine self-administration [81], in accord with the attenuated drug-seeking in the LBN model. However, others reported increased or unchanged sucrose preference following MS [38–42].
The anxiety- and depression-like behaviors resulting from MS have been variable, ranging from increased anxiety in the elevated-plus maze test [55] and increased immobility in the FST [40] to no changes in either test [82,83]. For further details, the reader is referred to a recent review summarizing the emotional consequences of MS imposed at different developmental ages [26].
These differing results may be due to variation in the timing of the MS during development [84], as well as differences in the age of testing. The timing of the stress is important for emotional outcomes: Indeed for the LBN model, when it was imposed later during development, on P8–P12, increased immobility in the FST was reported during adolescence [60]. Furthermore, for all experimental models, the procedures employed for emotional testing (e.g., lighting during the elevated-plus maze test [85]) can affect the outcome. Accordingly, an effort should be made in the field to standardize behavioral testing procedures as much as possible, and recognize the importance of timing of developmental stress and of testing age when interpreting results.
Although the majority of emotional consequences of chronic early-life stress have been negative, there is some evidence for positive outcomes following stressful experiences that are challenging but not overwhelming, so-called “stress inoculation” [72]. For example, Lyons and colleagues have demonstrated that exposure of newly weaned squirrel monkeys to brief intermittent maternal separations decreased subsequent anxiety and stress-responsivity. This resilience to later stress did not seem to be maternally mediated or related to changes in maternal care, unlike the rodent models discussed above [86].
Conclusions
Stress has profound effects on the brain, manifesting as altered behavioral outcomes. This is especially true when the stress occurs during vulnerable developmental periods. Brain maturation involves multiple dynamic processes that are regulated both by genetic factors and environmental input [87–90]. Many of these processes continue during postnatal life. Although it is impossible to directly compare rodent and human brain development and their trajectories, there is excellent information about comparative development of specific brain regions across species. For example, hippocampal development in the full-term human neonate is similar to that of a P5–P7 rat [91], providing common context to studies targeting early-life stress and other manipulations.
The transducing mechanisms that convert the experience of early-life stress to overt behavioral changes remain unclear. Abnormal maturation [54,92–94] or rewiring of neuronal connectivity in the underlying brain networks [95] have been proposed. For example, abnormal maternal care and chronic early-life stress have been shown to result in increased number and function of excitatory synapses to stress-sensitive neurons in the hypothalamus [57], promoting vulnerability to future stress signals. In contrast, reduced excitatory synapse number and function has been reported after ‘optimal’ early-life experiences, such as augmented maternal care [96]. These changes in synaptic activity are sufficient to program long-term changes in neuronal gene expression, maintained via epigenetic alterations of the chromatin [97,98]. Thus, increased excitatory input early in life may sensitize the central components of the neuroendocrine stress system to subsequent stress, predisposing to stress-related emotional disorders. Other structural changes, including stunting, atrophy or hypertrophy of dendritic structure, and altered connectivity, might take place in the amygdala and hippocampus [73], as well as pleasure centers of the brain, contributing to widespread circuit-level dysfunction (Figure 1).
Figure 1.
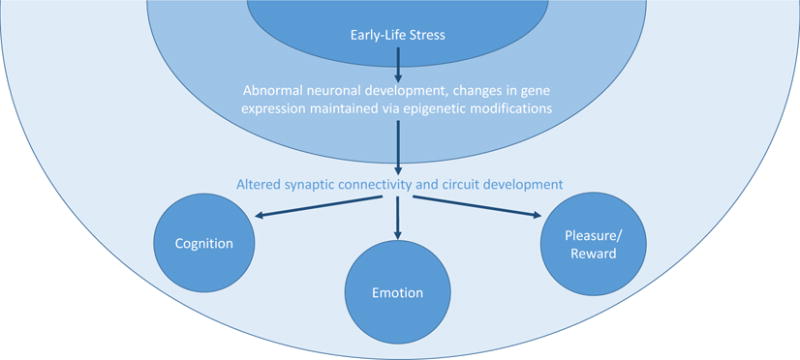
A unifying theoretical framework for how early-life stress can induce long-term changes in behavior. The inciting event is the experience of early-life stress, represented in the first concentric circle. Early-life stress causes a cascade of changes acutely during the perinatal period that results in abnormal neuronal development and changes in gene expression, which are maintained long-term via epigenetic modifications of the chromatin (represented in the second concentric circle)[95,98,102]. These molecular- and cellular-level changes build upon each other to create altered synaptic connectivity and circuit development at the level of the network, ultimately resulting in the observed alterations in cognition, emotion, and pleasure/reward (represented by the 3 nodes within the third concentric circle)[78,93,103,104].
Recognizing the complexity of early-life stress and its long-term consequences allows for the generation of meaningful, novel approaches aiming to improve the human condition. Future work in the field must move beyond the traditional focus on the HPA axis to fully appreciate the vast array of behavioral outcomes and their network and mechanistic underpinnings [13,50,59,99–101]. Comprehensive approaches with multiple levels of analysis and integration of human and animal-model studies are required to probe the consequences of early-life adversity: understanding the underlying processes is a prerequisite for precise, individualized interventions to improve the outcomes of the world’s current and future children.
Highlights.
Early-life stress is linked with vulnerability to cognitive and emotional disorders.
Naturalistic animal models of early-life stress are critical to identify mechanisms.
Recent studies report outcomes ranging from hippocampus-dependent memory deficits to emotional consequences such as anhedonia and depression.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers MH73136, NS29012, P50MH096889] and the Hewitt Foundation for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Transformative neurodevelopmental research in mental illness, Results of the NIMH Workgroup, 2009 NIH. 2009
- 3.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–63. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- 4.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science (80) 2007;318 doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–92. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 6.Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 7.Browne A, Finkelhor D. Impact of child sexual abuse: A review of the research. Psychol Bull. 1986;99:66–77. [PubMed] [Google Scholar]
- 8.Heim C, Newport DJ. Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Lynch M, Cicchetti D. An ecological-transactional analysis of children and contexts: The longitudinal interplay among child maltreatment, community violence, and children’s symptomatology. Dev Psychopathol. 1998;10:235–257. doi: 10.1017/s095457949800159x. [DOI] [PubMed] [Google Scholar]
- 10.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, Walsh CA, Wong MY-Y, Beardslee WR. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 11.Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. J Psychol. 2001;135:17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- 12.Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 14.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 15.Schore AN. Attachment and the regulation of the right brain. Attach Hum Dev. 2000;2:23–47. doi: 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- 16.Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 18.Nelson CA, Bos K, Gunnar MR, Sonuga-Barke EJSV. The neurobiological toll of early human deprivation. Monogr Soc Res Child Dev. 2011;76:127–146. doi: 10.1111/j.1540-5834.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hostinar CE, Gunnar MR. The developmental effects of early life stress: an overview of current theoretical frameworks. Curr Dir Psychol Sci. 2013;22:400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowlby J. Research into the origins of delinquent behaviour. Br Med J. 1950;1:570–3. doi: 10.1136/bmj.1.4653.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18:580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seay B, Hansen E, Harlow HF. Mother-infant separation in monkeys. J Child Psychol Psychiatry. 1962;3:123–132. doi: 10.1111/j.1469-7610.1962.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 24.Rincón-Cortés M, Sullivan RM. Early life trauma and attachment: Immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40:141–153. doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014;56:1675–1688. doi: 10.1002/dev.21230. A recent review describing in detail animal models that target maternal interaction and their later-life consequences, including neuroendocrine, cognitive, and emotional outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Nishi M, Horii-Hayashi N, Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci. 2014;8:166. doi: 10.3389/fnins.2014.00166. A recent review focusing on animal models utilizing maternal separation and their consequences for brain and behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason WA, Harlow HF. Performance of infant rhesus monkeys on a spatial discrimination problem. J Comp Physiol Psychol. 1958;51:71–74. doi: 10.1037/h0040609. [DOI] [PubMed] [Google Scholar]
- 29.Seay B, Harlow HF. Maternal separation in the rhesus monkey. J Nerv Ment Dis. 1965;140:434–41. doi: 10.1097/00005053-196506000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta) Behav Neurosci. 2006;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez MM, Mccormack K, Grand AP, Fulks R, Graff A, Maestripieri D, Born J, Ditschuneit I, Schreiber M, Dodt C, et al. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev Psychopathol. 2010;22:45. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine S. Infantile experience and resistance to physiological stress. Science (80) 1957;126:405–405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 33.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci. 1999;19:3982–91. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champagne F, Meaney MJ. Chapter 21: Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 35.Lucassen PJ, Naninck EFG, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–631. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 37.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaels CC, Holtzman SG. Neonatal stress and litter composition alter sucrose intake in both rat dam and offspring. Physiol Behav. 2006;89:735–741. doi: 10.1016/j.physbeh.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 41*.Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–1211. doi: 10.1002/hipo.22302. The first study to show that a “two hit” model of maternal separation combined with corticosterone treatment in adolescence/young adulthood induces sex-specific behavioral outcomes. Males have disrupted spatial memory, whereas females exhibit anhedonia. [DOI] [PubMed] [Google Scholar]
- 42.Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 2002;73:115–122. doi: 10.1016/s0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- 43.Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol. 2003;15:297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- 44.Whipple EE, Webster-Stratton C. The role of parental stress in physically abusive families. Child Abuse Negl. 1991;15:279–291. doi: 10.1016/0145-2134(91)90072-l. [DOI] [PubMed] [Google Scholar]
- 45.Kendall-Tackett KA. Violence against women and the perinatal period: the impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence Abuse. 2007;8:344–53. doi: 10.1177/1524838007304406. [DOI] [PubMed] [Google Scholar]
- 46.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–9. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naninck EFG, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- 48.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, Stern H. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702. doi: 10.1038/tp.2015.200. The first study to fully describe quantitatively the fragmented, unpredictable sequence of maternal behavior in the LBN model of early-life stress, as well as identify the severe anhedonia in late-adolescent LBN rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29:15745–55. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X-D, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, et al. Forebrain CRF₁ modulates early-life stress-programmed cognitive deficits. J Neurosci. 2011;31:13625–34. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segala S, Salum GA, Manfro GG, Silveira PP. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152:2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, Lambert JJ, Belelli D. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci. 2013;33:19534–54. doi: 10.1523/JNEUROSCI.1337-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang ICR. Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress. 2013;16:549–556. doi: 10.3109/10253890.2013.816841. [DOI] [PubMed] [Google Scholar]
- 59.Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A. 2013;110:18274–8. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–65. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 63.Wang X-D, Labermaier C, Holsboer F, Wurst W, Deussing JM, Müller MB, Schmidt MV. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- 64.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 65.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X-D, Liao X-M, Uribe-Mariño A, Liu R, Xie X-M, Jia J, Su Y-A, Li J-T, Schmidt MV, Wang X-D, et al. Stress during a critical postnatal period induces region-specific structural abnormalities and dysfunction of the prefrontal cortex via CRF1. Neuropsychopharmacology. 2014;40:1203–1215. doi: 10.1038/npp.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Cheng Z, Tang H, Jiao H, Sun X, Cui Q, Luo F, Pan H, Ma C, Li B. Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of Wnt signaling. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw121. [DOI] [PubMed] [Google Scholar]
- 68.Tada H, Miyazaki T, Takemoto K, Takase K, Jitsuki S, Nakajima W, Koide M, Yamamoto N, Komiya K, Suyama K, et al. Neonatal isolation augments social dominance by altering actin dynamics in the medial prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113:E7097–E7105. doi: 10.1073/pnas.1606351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69**.Molet J, Maras PM, Kinney-Lang E, Harris NG, Rashid F, Ivy AS, Solodkin A, Obenaus A, Baram TZ. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016 doi: 10.1002/hipo.22661. The first study to find that a more rigorous (but non-stressful) and hippocampus-dependent object location memory task can unveil memory deficits as early as late adolescence in LBN rats, whereas a less rigorous, less selective objection recogntion memory task does not find differences in cognitive performance unless the animals are challenged with a “second hit” of acute stress, or tested in middle age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmann J, Pryce C, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 71.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 72.Lyons D, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front Behav Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–15. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Baram TZ. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. 2015 doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 76.Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–38. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2010;9:947. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jorm AF. Does old age reduce the risk of anxiety and depression? A review of epidemiological studies across the adult life span. Psychol Med. 2000;30:11–22. doi: 10.1017/s0033291799001452. [DOI] [PubMed] [Google Scholar]
- 81.O’Connor RM, Moloney RD, Glennon J, Vlachou S, Cryan JF. Enhancing glutamatergic transmission during adolescence reverses early-life stress-induced deficits in the rewarding effects of cocaine in rats. Neuropharmacology. 2015;99:168–176. doi: 10.1016/j.neuropharm.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 82.Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res. 2008;61:106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 83.Benetti F, Mello PB, Bonini JS, Monteiro S, Cammarota M, Izquierdo I. Early postnatal maternal deprivation in rats induces memory deficits in adult life that can be reversed by donepezil and galantamine. Int J Dev Neurosci. 2009;27:59–64. doi: 10.1016/j.ijdevneu.2008.09.200. [DOI] [PubMed] [Google Scholar]
- 84.Enthoven L, de Kloet ER, Oitzl MS. Differential development of stress system (re)activity at weaning dependent on time of disruption of maternal care. Brain Res. 2008;1217:62–69. doi: 10.1016/j.brainres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Hogg S. A review of the validity and variability of the Elevated Plus-Maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 86.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci U S A. 2006;103:3000–5. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 88.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (80) 2003;301 doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 89.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bogdan R, Hariri AR. Neural embedding of stress reactivity. 2012;15:1605–1607. doi: 10.1038/nn.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karsten CA, Baram TZ. How does a neuron “know” to modulate its epigenetic machinery in response to early-life environment/experience? Front Psychiatry. 2013;4:89. doi: 10.3389/fpsyt.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korosi A, Shanabrough M, McClelland S, Liu Z-W, Borok E, Gao X-B, Horvath TL, Baram TZ. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–13. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol Med. 2015;21:134–144. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 98**.Singh-Taylor A, Molet J, Jiang S, Korosi A, Bolton JL, Noam Y, Simeone K, Cope J, Chen Y, Mortazavi A, et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.240. [in press]. The first study to demonstrate in a model of augmented maternal care that decreased excitatory synaptic activity in the paraventricular nucleus of the hypothalamus is sufficient to induce epigenetic reprogramming of the chromatin and enable long-term maintenance of gene expression changes in CRH, along with many other genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 100.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101*.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. A recent review highlighting the role of stress throughout the lifespan, including early-life adversity, in antisocial behaviors, as well as the underlying structural, functional, and molecular changes in the brain. [DOI] [PubMed] [Google Scholar]
- 102.Singh-Taylor A, Korosi A, Molet J, Gunn BG, Baram TZ. Synaptic rewiring of stress-sensitive neurons by early-life experience: A mechanism for resilience? Neurobiol Stress. 2015;1:109–115. doi: 10.1016/j.ynstr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bogdan R, Hariri AR. Neural embedding of stress reactivity. Nat Neurosci. 2012;15:1605–7. doi: 10.1038/nn.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]


