Abstract
Objective
To examine the association of specific adipose tissue depots and risk of incident cancer in the Dallas Heart Study (DHS).
Patients and Methods
Individuals without prevalent cancer in the DHS underwent quantification of adipose depots: visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) and liver fat by MRI, and lower body subcutaneous fat (LBF) by DXA from January 1, 2000, through December 31, 2002, and were followed for the development of cancer for up to 12 years. Multivariable Cox proportional hazards modeling was performed to examine the association between fat depots and cancer.
Results
Among 2,627 participants (median age 43 years; 69% non-white), 167 (6.4%) developed cancer. The most common primary sites of cancer were breast (in women) and prostate (in men). In multivariable models adjusted for age, sex, race, smoking, alcohol use, family history of malignancy and body mass index, 1-standard deviation increase in VAT was not associated with increased risk of cancer: hazard ratio (HR) 0.94 (95% confidence interval (CI) 0.77–1.14). In contrast, each 1-standard deviation increase in LBF was associated with a reduced incidence of cancer; HR 0.69 (95% CI 0.52–0.92) in the fully adjusted model.
Conclusions
In this study, adiposity-associated cancer risk was heterogeneous and varied by fat depot: VAT was not independently associated with incident cancer and LBF appeared to protect against cancer development. Further studies of the adiposity-cancer relationship, including serial assessments, are needed to better elucidate this relationship.
Keywords: visceral adipose tissue, body fat, lower body fat, ectopic fat, obesity, cancer, risk, incidence
Introduction
Obesity, as defined by body mass index (BMI) ≥30 kg/m2, is associated with an increased incidence of, and mortality from, cancer.1,2 This association may be stronger for certain obesity-associated cancers such as those of the breast, endometrium, colon and kidneys.3 However BMI is not a completely representative measure of body fat risk, because distinct fat depots such as visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (SAT) and liver fat (LF) have been associated with differing impacts on metabolic and cardiovascular disease risk.4–8 The relation of these adipose depots with the risk of developing non-cardiovascular chronic conditions, particularly cancer, is not well understood. Studies reporting the risk of cancer in patients who have undergone image-guided measurements of VAT are limited, and have focused on predominantly white or elderly populations, with inconsistent results.4,9 Although SAT has been shown to have a neutral association with cancer, LF and lower body fat (LBF) have not been studied in this regard. We aimed to study the relationship between specific fat depots and the risk of incident cancer among relatively young, multiethnic participants in the Dallas Heart Study (DHS).
Materials and Methods
Study Population
Details on the design of the DHS have been previously described.10 Briefly, the DHS is a single site, multiethnic, population based probability sample of Dallas County residents (aged 18–65 years) with deliberate oversampling of black participants. The current study population was drawn from 3072 participants who completed DHS phase 1 (DHS-1) visits from January 1, 2000, through December 31, 2002, which included a computer-assisted survey, anthropometric and blood pressure measurements, laboratory testing, and imaging assessments. Participants without imaging assessment of VAT were excluded. Since cancer diagnoses were made through linkage to the state cancer registry, participants who had moved out of Texas prior to 2012 were censored at the date they were last known to be a Texas resident. Of the remaining participants, those with history of or present diagnosis of malignancy were also excluded. To account for cancers that may have been undetected at baseline, new cases of cancer diagnosed within 1 year after date of enrollment to DHS were excluded from the analysis (blanking period). After these exclusions, 2,627 participants were eligible for follow-up (Supplemental Figure). All participants provided written informed consent, and the University of Texas Southwestern Medical Center Institutional Review Board approved the protocol.
Demographics, lifestyle and other risk factors were determined from a baseline questionnaire. BMI was calculated as weight (kilograms) divided by the square of height (meters). Waist circumference (WC) and hip circumference (HC) were measured in centimeters and waist hip ratio (WHR) was calculated as ratio of WC/HC. The hypertriglyceridemic waist phenotype was defined by WC ≥90 cm and with serum triglyceride levels ≥2.0 mmol/L.11 Hypertension was defined as BP ≥140/90 mm Hg or taking antihypertensive medication(s). Diabetes mellitus was defined as a fasting serum glucose ≥126 mg/dl, self-reported diabetes, or taking hypoglycemic medication. Smoking was defined as cigarette use within the previous 30 days and/or a lifetime history of having smoked ≥100 cigarettes. Alcohol use was determined in grams/week by self-report. Comorbid conditions were determined from self-report, medication history and clinical assessment. Fasting blood samples were obtained from participants and collected in EDTA-containing tubes and stored at −80°C. Samples were analyzed for high-sensitivity C-reactive protein (hs-CRP), interleukin (IL)-6, adiponectin, leptin, and insulin levels.5,8
Body Fat Distribution Measurements
Participants were scanned by a 1.5-T MRI scanner (Intera, Philips Healthcare, Best, the Netherlands). Retroperitoneal, intraperitoneal, and SAT abdominal fat masses were quantified by a single MRI slice taken at the L2–L3 level using manual contours, as previously validated against cadaveric samples.12 Areas were converted to mass (kilograms, kg) using previously determined regression equations.13 VAT was defined as the combination of both retroperitoneal and intraperitoneal fat masses.8 Subjects also underwent 1H-magnetic resonance spectroscopy for hepatic triglyceride quantification (LF) as previously described.14 Participants were also scanned by DEXA, which was performed with a Delphi W scanner (Hologic, Bedford, Massachusetts) with a fan beam to determine fat and lean mass.15 LBF (kg) was quantified from the total fat mass from the lower extremities.
Cancer Outcomes
DHS was systematically linked to the Texas Cancer Registry (TCR) to determine cancer cases in the cohort.16 The TCR is a population based registry of the State of Texas, and meets quality data standards of both the National Program of Cancer Registries (Center for Disease Control and Prevention) and North American Association of Central Cancer Registries (NAACCR). The Texas Cancer Incidence Reporting Act mandates health care facilities including hospitals, ambulatory surgical centers and cancer treatment centers to report to the TCR. All cancer cases identified by the TCR were classified as ‘prevalent’ or ‘incident’ based on date of cancer diagnosis in relation to date of enrollment to DHS. In cases with more than one known cancer, only the first cancer was included. Carcinoma in situ and skin cancers were not included. Cancers of the gastrointestinal tract in close proximity to visceral fat depots were classified as ‘visceral cancers’ and included colorectal, pancreatic, liver, gall bladder, esophageal, stomach, small intestinal and anal cancers. ‘Obesity-associated cancers’ were defined as per the National Cancer Institute Obesity and Cancer Fact Sheet and included breast and endometrial cancers in women, along with esophageal, pancreatic, gall bladder, colorectal, kidney and thyroid cancer in men and women.17
Participants in the DHS undergo telephone calls from study coordinators along with regular data collection regarding place of residence and vital status. Thus we were able to establish their residence and mortality at multiple points throughout the study period. Patients were followed until non-cancer death, date they were last known to be a Texas resident, incident cancer, or December 2012 (when the TCR was last queried).
Statistical Analysis
Baseline demographic, clinical, laboratory, and imaging variables are expressed as median (25th, 75th percentile) or proportions as appropriate. Deaths due to non-cancer causes were treated as competing events in time- to event analyses according to the methodology of Fine and Gray. Cumulative incidence curves for the relation of sex-specific quartiles of VAT to time to incident cancer were constructed using the method described by Prentice,18 and Gaynor,19 and compared using the likelihood ratio test in a Cox model that accounts for competing risk. Cox proportional hazards models were used to examine the unadjusted and multivariable adjusted associations between measures of adiposity and incident cancer and are reported as hazard ratios (HR) and 95% confidence intervals (CI). Adipose measures were analyzed both continuously per unit standard deviation (SD) increase and as sex-specific quartiles. VAT was the primary exposure. SAT, LF, LBF and WC were secondary exposures. The primary outcome was any incident cancer. Secondary outcomes included development of obesity- and visceral-associated cancers. Cox proportional hazards models were constructed such that the unadjusted model (model 1) was univariable in continuous analysis, and sex-specific in quartile analysis. Models were sequentially adjusted for age and race (and sex in the case of continuous analysis) (model 2), family history of cancer; smoking and alcohol use (model 3) and BMI (model 4). Sensitivity analyses were performed by including cancers diagnosed within the 1-year blanking period, after excluding lung and esophageal cancers (associated with lower BMI), and hematological cancers from the analysis and after excluding breast and prostate cancers (associated with screening procedures) from the analysis. Since the association between ectopic fat depots and cancer may be mediated via obesity, we also analyzed the relation of BMI, WC, WHR and the hypertriglyceridemia-WC index with incident cancer. Two-sided P<.05 were considered significant. All analyses were performed using SAS version 9.2 (SAS Corporation, Cary, North Carolina).
Results
The study cohort consisted of 2,627 cancer-free individuals at inception. Characteristics of the overall cohort are presented in Table 1. One hundred and sixty- seven individuals (6.4%) developed an incident cancer of which 53% were female and 66% were non-white. There were a total of 129 (4.9%) non-cancer deaths in the cohort over the study period.
Table 1.
Baseline characteristics of participants in the DHS (data are reported as median (interquartile range) or number (%), as appropriate)
| Characteristic | Overall (n=2627) |
|---|---|
| Clinical characteristics | |
| Age (years) | 43 (36, 51) |
| Male | 1209 (46.0%) |
| Race | |
| Black | 1293 (49.2%) |
| White | 822 (31.3%) |
| Hispanic | 456 (17.4%) |
| Other | 56 (2.1%) |
| Smoking | 730 (27.8%) |
| Alcohol use | 1836 (70.0%) |
| Diabetes mellitus | 282 (10.8%) |
| Hypertension | 852 (32.9%) |
| Hyperlipidemia | 355 (13.2%) |
| Physical activity (MET-min/wk) | 145 (0, 599) |
| Family history of cancer | 583 (22.2%) |
| Biochemical characteristics | |
| High-sensitivity C-reactive protein (mg/dL) | 2.7 (1.2, 6.3) |
| Interleukin-6 (pg/mL) | 16.97 (0.0–35.76) |
| Adiponectin (ug/mL | 14.42 (9.60–21.43) |
| Leptin (ng/mL) | 11.90 (5.2, 25.3) |
| Insulin (uIU/mL) | 12.2 (7.3, 20.1) |
| Measures of adiposity | |
| Body weight (kg) | 82.10 (69.9–97.1) |
| Body mass index (kg/m2) | 29.07 (25.21, 33.93) |
| Waist circumference (cm) | 97 (87, 108.5) |
| Waist hip ratio | 0.90 (0.84–0.96) |
| Abdominal subcutaneous adipose tissue (kg) | 4.17 (2.79, 6.26) |
| Liver fat (%) | 3.60 (2.11, 6.64) |
| Lower body fat (kg) | 8.69 (6.15, 11.91) |
| Only women | (n= 1418) |
| Postmenopausal state | 488 (34.7%) |
| Oral contraceptive use | 1079 (76.3%) |
| Hormone replacement therapy use | 180 (29.5%) |
Of the 167 patients who developed cancer, 69 (41%) were ‘obesity- associated’ cancers and 25 (15%) were gastrointestinal or ‘visceral’ cancers. The most common primary cancer sites in women and men were breast (25%) and prostate (20%), respectively. Further details regarding the primary site of cancer are presented in Table 2. These data are similar to the general distribution of cancers seen in the overall TCR registry.20 Those who developed cancer were more likely to be older, have a family history of cancer and higher prevalence of diabetes mellitus, hypertension and hyperlipidemia compared with those that did not develop cancer. They were also more likely to have higher VAT levels and WC. No significant differences in serum biomarker levels were observed.
Table 2.
Distribution of incident cancers by primary site, with visceral adipose tissue (VAT) levels
| Type of cancer | Anatomical sites included | Number (% of total) of incident cancers | VAT levels, kg median (IQR) |
|---|---|---|---|
| Breast | Breast | 41 (25) | 2.06 (1.55–2.51) |
| Prostate | Prostate | 33 (20) | 2.73 (1.99–3.27) |
| Lung | Lung | 14 (8) | 2.20 (1.11–2.75) |
| Genitourinary | Gynecological, kidney, urinary bladder | 24 (14) | 2.43 (1.99–2.75) |
| Gastrointestinal | Esophagus, stomach, small intestine, colon, rectum, anus, liver, pancreas, gallbladder | 25 (15) | 2.77 (1.75–3.12) |
| Hematological | Leukemia, Lymphoma (Hodgkin and non-Hodgkin) | 12 (7) | 2.35 (1.76–2.88) |
| Others | Brain, thyroid, head and neck, not otherwise specified | 18 (11) | 2.01 (1.37–2.54) |
| Overall | 167 (100) | 2.34 (1.65, 2.94) | |
Relation with Adiposity Depots
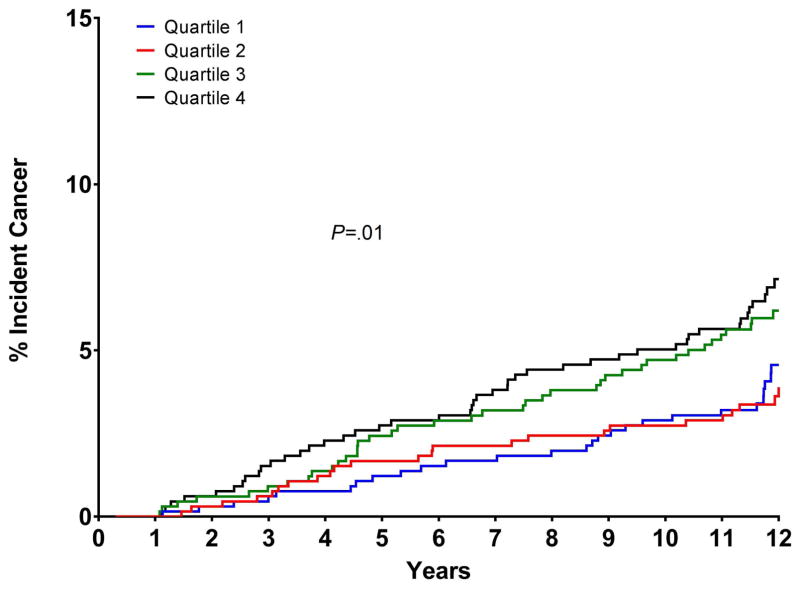
VAT levels among participants with cancers of different sites are presented in Table 2. Characteristics of the cohort across sex-specific VAT quartiles are presented in Supplemental Table 1. The prevalence of hypertension, diabetes and hyperlipidemia increased across the VAT quartiles; as did the age and BMI, WC and WHR. Figure 1 shows the cumulative incidence curves for incident cancer by sex-specific quartiles of VAT; the cumulative rates of incident cancer at 12 years were 4.6% (95% CI 2.8–6.3) for Q1, 3.6% (95% CI 2.2–5.1) for Q2, 6.2% (95% CI 4.3–8.1) for Q3, and 7.1% (95% CI 5.1–9.2) for Q4 (P=.01).
Figure 1.
Cumulative incidence curves for the relation of sex-specific quartiles of visceral adipose tissue to time to incident cancer. Vertical axes show the cumulative percentage of subjects developing cancer in each quartile, horizontal axes represent time since study entry (years).
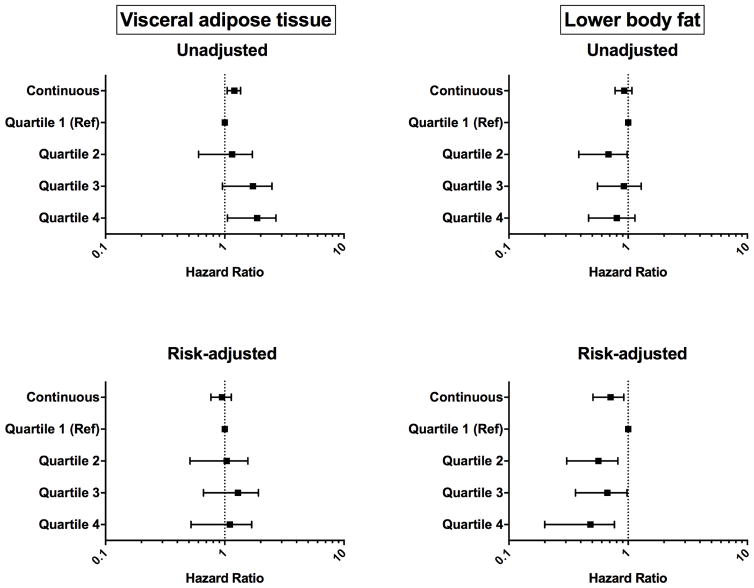
Figure 2 shows the graded associations between continuous and categorical (sex-specific quartiles) measures of VAT, LBF and incident cancer in unadjusted and fully adjusted models. In unadjusted models, each 1-SD increase in VAT was associated with higher risk of cancer (HR 1.20, 95% CI 1.06–1.36) (Supplemental Table 2). However, this association attenuated with adjustment for age, race and sex. The number of incident cancers in each quartile of VAT are presented in Supplemental Table 3. In unadjusted models, there was a non-significant trend toward a lower risk for cancer related to LBF. In fully-adjusted models, each 1-SD increase in LBF was associated with a significantly reduced incidence of cancer; HR (95% CI): 0.69 (0.52–0.92), P=.01 for LBF as a continuous measure, with a similar, graded decrease in risk across LBF quartiles (Figure 2). This association between LBF and cancer was only revealed on addition of BMI to model 4 (Supplemental Table 4). No such association was observed for SAT and liver fat (Supplemental Table 5).
Figure 2.
Forrest plot of the graded risk for incident cancer per 1-unit standard deviation increase in visceral adipose tissue and lower body fat, before and after adjustment for clinical and demographic variables. Model is adjusted for sex, race, age, smoking status, alcohol use, family history of cancer and body mass index. Data is given as hazard ratio (95% confidence intervals).
In analysis stratified by sex and race, no association between VAT and cancer was observed in fully adjusted models (Supplemental Tables 6–9) although the number of events in each subgroup were limited. Mean VAT levels were higher in whites than non- whites (2.30 kg versus 2.09 kg respectively) and in men than women (2.54 kg versus 1.83 kg). Similar to VAT, no significant association between WC, WHR, BMI categories or hypertriglyceridemia- WC phenotype and incident cancer was seen in fully adjusted models (Supplemental Tables 10A, 10B and 10C). Sensitivity analyses performed including incident cancers diagnosed within 1 year of the baseline exam, and after sequentially excluding lung cancers, esophageal cancers and hematological malignancies from the analysis, and after excluding breast and prostate cancer cases from the analysis did not affect the results (Supplemental Tables 11–14). VAT was not associated with increased risk of developing ‘visceral cancers’ or ‘obesity- associated cancers’ in multivariable models (Table 3 and Supplemental Tables 15–16). On subgroup analysis for LBF by sex, the association was significant in women but not men, but formal interaction testing did not show a statistically significant heterogeneity of effect (Supplemental Tables 17–18). Levels of LF were noted to be higher in Hispanics but subgroup analysis of LF in these racial groups did not show a relationship with incident cancer (Supplemental tables 19–20).
Table 3.
Cox proportional hazard models of adiposity depots and incident cancer
| Adiposity measure | Unadjusted model HR (95% CI) | Adjusted model a HR (95% CI) |
|---|---|---|
| All incident cancer | ||
| Visceral adipose tissue | 1.20 (1.06–1.36) | 0.94 (0.77–1.14) |
| Subcutaneous adipose tissue | 1.13 (0.97–1.31) | 1.08 (0.78–1.51) |
| Liver fat | 0.91 (0.79–1.06) | 0.96 (0.93–1.01) |
| Lower body fat | 0.93 (0.79–1.09) | 0.69 (0.52–0.92) |
| Obesity- associated cancer | ||
| Visceral adipose tissue | 1.08 (0.87–1.33) | 1.13 (0.80–1.59) |
| Visceral/gastrointestinal cancer | ||
| Visceral adipose tissue | 1.47 (1.09–1.99) | 1.21 (0.76–1.93) |
Data are per 1-standard deviation increase in adiposity measure and reported as hazard ratio (HR) and 95% confidence interval (95% CI)
Multivariable model adjusted for age, sex, race, smoking, alcohol, family history of cancer and body mass index
Discussion
In this multiethnic cohort study, visceral adiposity as assessed by MRI was not associated with development of incident cancer through follow-up as long as 12 years. We also did not observe an association between VAT and future development of gastrointestinal or obesity-associated cancers, although individual numbers of cancers were relatively small. A protective effect of LBF was observed in our study. This finding is novel and has not been demonstrated previously, and is consistent with observed associations with cardiovascular disease 6 and type 2 diabetes 8 in which LBF is consistently protective. The lack of association between LBF and cancer in univariable analysis was likely due to reverse confounding, such that adjustment for BMI (positively associated with cancer risk) revealed the inverse association of LBF. Further studies elucidating this relationship and its potential implications should be a priority. The other fat depots, SAT and LF, were not associated with cancer in our study.
There is clear data linking incident cancer with higher BMI, 2 but its association with regional fat depots remains less well studied. Britton and colleagues have previously reported the positive association of VAT and incident cancer from the predominantly white Framingham cohort; with a stronger association in men. Murphy and colleagues reported a weak association between VAT and cancer in women and no association in men from an elderly population (mean age 74 years). Our study population was much younger (43 years) and only 6.4% individuals in our cohort developed cancer compared to 24.5% in the study by Murphy which is likely a function of our younger cohort. The lack of an age-independent association of VAT and cancer in our study could potentially be explained by a lack of power brought about by this lower event rate. However, our study was similarly sized and had more events compared to the study by Britton. Age and VAT are highly correlated,21,22 and the unadjusted association between VAT with incident cancer in our study became null after adjusting for age. Our study also had a similar proportion of obesity-associated cancers to the study by Murphy (approximately 40%), making it unlikely that a differential cancer phenotype existed between the studies. In our study, we did observe that higher levels of LBF were associated with a lower risk of the future development of cancer, and this effect was independent of age. Since our study is the first to report a protective relationship between LBF and incident cancer, we are unable to compare our findings with previous studies.
Since the early 1980s, regional body fat distribution, as measured by WC or WHR, has been known to be more strongly correlated with cardiovascular outcomes than BMI.23,24 The emergence of imaging techniques such as computed tomography in the 1980s allowed for distinguishing VAT from subcutaneous adiposity,25,26 which was associated with several metabolic abnormalities and increased risk of thrombosis.27 From a mechanistic standpoint, paracrine release of cytokines from VAT may alter local nuclear transcription and gene regulation inducing cell cycle and transcriptional changes leading to malignancy,28 and systemic effects of adipose may induce various cancers via increased chronic inflammation and adipokine and sex hormone release.29 Differential relations of VAT and cancer in men and women, seen in ours and prior studies, may partially be explained by levels of circulating sex hormones, although we were unable to test this hypothesis in the current study. Furthermore, in our study, we did not see a difference between levels of leptin, insulin and inflammatory markers in individuals who did and did not develop cancer. Studies from the DHS also reported no association between adiponectin and leptin levels and cancer incidence.16,30 This may also partially explain why we did not see an association between VAT and cancer in adjusted models. LBF has been shown to be a ‘protective fat depot’ by virtue of being negatively correlated with metabolic and cardiovascular risk factors15,31 and being inversely associated with incident type 2 diabetes8 and cardiovascular disease.6 It is hypothesized that LBF may act as reservoir for ectopic fat and reduces its physiological impact vis-à-vis its role as buffer for excess triglyceride stores. However, it is unclear if the protective association of LBF on cancer is mediated through its role as a metabolic sink, or if deposition of fat in the lower body compartment per se may decrease risk for cancer. LBF may also represent a protective reservoir for toxic lipophilic carcinogenic pollutants.
Strengths of the study include a multiethnic population cohort with precise imaging assessments of adipose depots and evaluation of novel fat depots not previously studied. Limitations include the observational design which precludes ability to ascribe causation. The cross-sectional nature of fat quantification also does not allow assessment of adiposity change on cancer outcomes. Since DHS participants who were diagnosed with cancer outside the State of Texas may not have been captured through the TCR, we censored participants at the date they were last known to be a Texas resident to avoid ascertainment bias. The relatively small number of incident cancer cases also limits our ability to perform individual analyses for each cancer site and also in sex/race subgroups. Finally, since our study did not include South or East Asians, we are unable to determine the impact of VAT on cancer in these racial groups.
Conclusion
We did not see an association between VAT and incident cancer in our study. In contrast, LBF was significantly and independently associated with lower cancer risk. Further studies including serial adipose depot assessments in a large diverse population with close follow-up for cancer development are needed to better define the relationship between adiposity and cancer.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by grant K23DK106520 from the National Institute of Diabetes and Digestive and Kidney Diseases to Dr. Neeland, by grants UL1DE019584 and PL1DK081182 from the National Institutes of Health, and by grant number UL1TR001105 from the National Center for Advancing Translational Sciences. Dr. Neeland is supported as a Dedman Family Scholar in Clinical Care at UT Southwestern Medical Center.
Cancer data have been provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, 1100 West 49th Street, Austin, TX 78756, http://www.dshs.state.tx.us/tcr/default.shtm, or (512) 776-3080. We thank Mereeja Varghese and Kathleen Wilkinson for their help in working with the DHS.
AG and IJN had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- BMI
body mass index
- DHS
Dallas Heart Study
- LBF
lower body fat
- LF
liver fat
- SAT
subcutaneous adipose tissue
- TCR
Texas Cancer Registry
- VAT
visceral adipose tissue
Footnotes
Conflicts of Interest/Disclosures: No disclosures for any authors
The paper was presented in abstract form at the American Society of Clinical Oncology (ASCO) 2016 meeting in Chicago, IL, USA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 4.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra A, Neeland IJ, Berry JD, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64(10):997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 6.Neeland IJ, Turer AT, Ayers CR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65(19):2150–2151. doi: 10.1016/j.jacc.2015.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenquist KJ, Massaro JM, Pedley A, et al. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100(1):227–234. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy RA, Bureyko TF, Miljkovic I, et al. Association of total adiposity and computed tomographic measures of regional adiposity with incident cancer risk: a prospective population-based study of older adults. Appl Physiol Nutr Metab. 2014;39(6):687–692. doi: 10.1139/apnm-2013-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 11.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 12.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35(8):1490–1496. [PubMed] [Google Scholar]
- 13.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65(2):403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276(5 Pt 1):E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 15.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91(11):4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 16.Beg MS, Saleem S, Turer A, et al. A Prospective Analysis of Plasma Adiponectin and Risk of Incident Cancer: The Dallas Heart Study. J Natl Compr Canc Netw. 2015;13(7):873–878. doi: 10.6004/jnccn.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. [Accessed November 15 OaCRJ, 2012]; http://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet.
- 18.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 19.Gaynor JJ, Erick J, et al. On the Use of Cause-Specific Failure and Conditional Failure Probabilities: Examples From Clinical Oncology Data. J Am Stat Assoc. 1993;88(422):400–09. doi: 10.2307/2290318.. [DOI] [Google Scholar]
- 20. [Accessed January 5, 2015]; https://www.dshs.state.tx.us/tcr/statisticalData/2014FactSheets/Texas-Fact-Sheets-2014.aspx. https://www.dshs.state.tx.us/tcr/statisticalData/2014FactSheets/Texas-Fact-Sheets-2014.aspx.
- 21.Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol (1985) 1992;72(2):787–795. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 22.Pascot A, Lemieux S, Lemieux I, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22(9):1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 23.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72(3):1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289(6454):1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36(1):54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 26.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250(6 Pt 1):E736–745. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 27.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 28.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher EJ, LeRoith D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care. 2013;36(Suppl 2):S233–239. doi: 10.2337/dcS13-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Herman Y, Ayers C, et al. Plasma Leptin Levels and Risk of Incident Cancer: Results from the Dallas Heart Study. PLoS One. 2016;11(9):e0162845. doi: 10.1371/journal.pone.0162845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol. 2004;24(5):923–929. doi: 10.1161/01.ATV.0000125702.26272.f6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.