SUMMARY:
The effect of T1 signal on FSL voxel-based morphometry modulated GM density and FreeSurfer cortical thickness is explored. The techniques rely on different analyses, but both are commonly used to detect spatial changes in GM. Standard pipelines show FSL voxel-based morphometry is sensitive to T1 signal alterations within a physiologic range, and results can appear discordant between FSL voxel-based morphometry and FreeSurfer cortical thickness. Care should be taken in extrapolating results to the effect on brain volume.
Cortical segmentation methodologies vary and are used throughout neuroimaging research as well as, increasingly, in clinical care. Two commonly used methods to study cortical GM are FMRIB Software Library voxel-based morphometry (FSL-VBM [http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/fslvbm/index.html])1 and FreeSurfer (http://surfer.nmr.mgh.harvard.edu) cortical thickness (CT).2 FSL-VBM outputs a 3D map of modulated GM density (mGM), and FreeSurfer-CT analysis outputs 1D measurements around the cortical ribbon. Both are commonly interpreted as informing cortical volume, though there are instances where volume was increased based on one technique and decreased based on the other,3,4 suggesting that factors beyond brain volume contribute to results.
GM and WM contrast naturally contributes to successful segmentation; however, the extent to which changes in cortical T1 signal affect mGM and CT is not known. Understanding this relationship is critical for an appropriate interpretation. This study explored the effect of subtle T1 signal alterations within a physiologic range on FSL-VBM1 and FreeSurfer-CT2 by using standard processing pipelines. We also illustrate discordance between the techniques through individual clinical examples.
Materials and Methods
This study is in compliance with our Department of Radiology institutional review board. MPRAGE was performed at 3T (Skyra [Siemens, Erlangen, Germany]; FOV, 256 × 256 mm2; resolution, 1 × 1 × 1 mm3; matrix, 256 × 256; sections, 192; TR, 2100 ms; TE, 3.19 ms; TI, 900 ms; bandwidth, 260 Hz/pixel; flip angle, 8°).
Signal Intensity Simulation
MPRAGE images from a healthy 25-year-old man were used to generate simulated signal changes within the frontal operculum (Fig 1). Forty percent of voxels within the ROI were randomly selected for signal intensity alteration of up to ±20% (5% increments), covering a physiologic T1 range of GM. Pearson correlation coefficient was measured (5% significance level).
Fig 1.
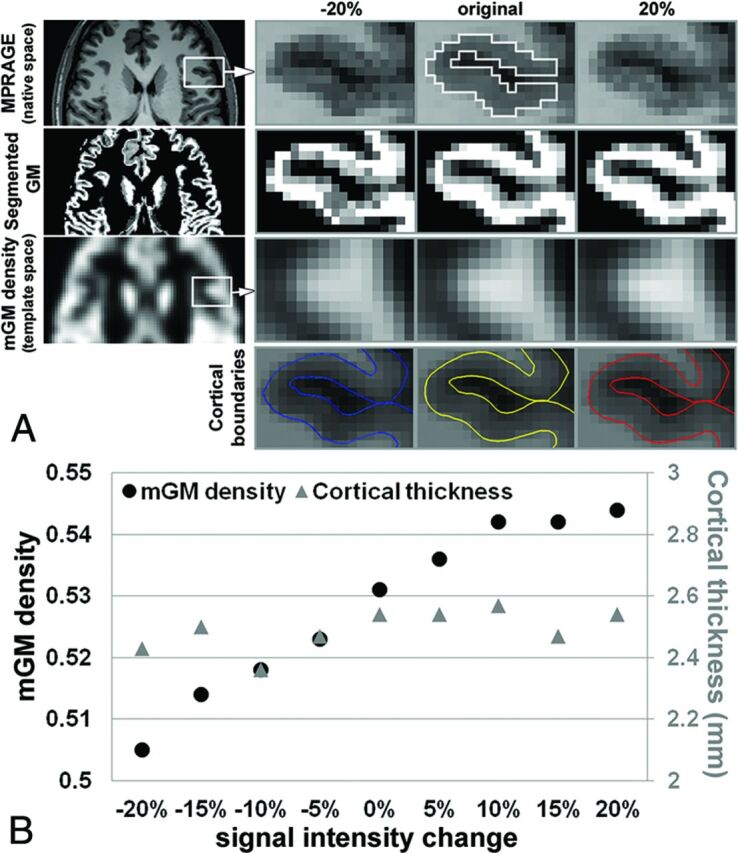
Simulated cortical signal change. A, First row (left to right), Representative MPRAGE in a healthy control patient (25-year-old man). Forty percent of voxels through 7 slices within the ROI were randomly selected and used to simulate changes in T1 signal intensity via decreasing and increasing voxel intensity to 20% in increments of 5%. Selected ROI from the frontal operculum for simulating signal change is shown in the original image (white solid line). Second row, Corresponding segmented GM maps in the native space. Third row, mGM maps in the template space and, Fourth row, corresponding cortical boundaries from FreeSurfer. B, High correlation between signal intensity and mGM is observed (black dots [R = 0.964; P < .001]), but no relationship is seen between signal intensity and CT (gray triangles).
Clinical Examples
Several clinical cases were selected to illustrate concordant and discordant results: 1) acute and chronic infarcts; 2) healthy 19-year-old and 50-year-old patients; and, 3) a 28-year-old man with mild traumatic brain injury (mTBI) and an age- and sex-matched control patient.
Image Analysis
All images underwent denoising.5 Standard FSL-VBM (v1.1) processing steps included brain extraction, manual editing, automated tissue-type segmentation, nonlinear registration to GM template, modulation, and smoothing with isotropic Gaussian kernel (σ, 3 mm). FreeSurfer (v5.3.0) analysis was performed to estimate regional CT.
ROIs were drawn around areas of acute and chronic infarct in the native space and compared with contralateral normal-appearing analogous brain. For the control group and patients with TBI, a precuneal ROI from the Harvard-Oxford atlas was interrogated because previous studies report age-6 and TBI-associated7 morphometry changes in this region. ROIs drawn in the native space were warped to the target space by using transform matrices created by FSL-VBM and FreeSurfer-CT.
Results
Simulation results show a strong correlation between signal intensity and mGM (R = 0.964; P < .001). No correlation is present between signal intensity and CT (Fig 1B).
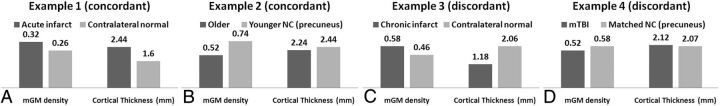
Concordant and discordant results are illustrated in Figs 2 and 3. The acute infarct showed 23% higher mGM and 52% higher CT compared with the contralateral analogous brain. The older healthy patient showed 30% lower mGM and 8% lower CT in the precuneus compared with the younger control patient. Discordant results included area of chronic infarct, demonstrating 26% higher mGM and 43% lower CT compared with the contralateral normal-appearing analogous brain. In the patient with mTBI, mGM was 12% lower and CT was 2% higher in a precuneal ROI compared with a matched control patient.
Fig 2.
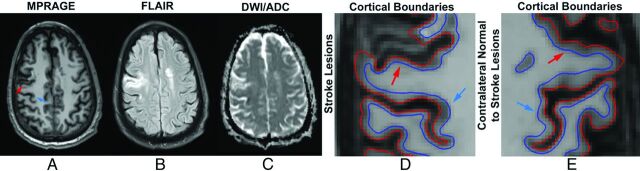
A 71-year-old man with acute onset weakness of the left upper extremity presented with an area of acute infarction (blue arrow) along the deep posterior cortex of the right precentral gyrus involving the hand motor region, with A, relative hypointensity on MPRAGE, B, hyperintensity on FLAIR, and C, restricted diffusion on the ADC map. The patient also has an area of chronic infarction more anteriorly (red arrow), showing A, relative hypointensity on MPRAGE, B, hyperintensity on FLAIR, and C, T2 shine through on the ADC map. ROIs in the affected areas (D) show higher and lower CT, respectively, compared with contralateral analogous brain (E); however, mGM as a marker of cortical volume was higher in the acute infarct, as expected, but also higher in the chronic infarct.
Fig 3.
In vivo examples illustrate concordant results between FSL-VBM and FreeSurfer-CT in the ROIs of acute infarct (A) as well as when comparing younger and older healthy control patients (B), and discordant cortical morphometry results in ROIs of chronic infarct (C) and in the precuneus (D) in a patient with mTBI compared with a matched control patient. Of note, prior work reports morphometric changes to the precuneus in aging and traumatic brain injury. The mean values within the ROIs were reported.
Discussion
FSL-VBM is sensitive to T1 signal variations within a clinically relevant range, which was not found to be true for FreeSurfer-CT. Although mGM and CT are completely different measures, both are commonly used to assess GM volume. Discordant results between FSL-VBM and FreeSurfer-CT analyses may result from T1 effects on mGM. This observation is of clinical and research importance because there are myriad conditions that affect T1 signal. Careful interpretation of FSL-VBM is warranted, particularly in the setting of discordant findings.
Several FSL-VBM methodologic steps are worth comment: 1) bias correction may alter T1 signal, and 2) tissue-type segmentation can affect output mGM. All images underwent identical bias correction before both analyses, and we demonstrated the effect of T1 signal change on mGM and CT by applying standard pipelines to recreate commonly used approaches that have widespread availability. Future optimization is warranted to achieve accurate detection of pathology.
Other methods of cortical segmentation are not specifically addressed here. Whether a similar dependence on T1 signal is present in VBM approaches such as SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12) is not known. The cases provided as part of this report are not meant as generalizable results regarding the specific pathologies described, but instead serve as in vivo examples of the phenomenon of discordance in terms of interpreting FSL-VBM and FreeSurfer-CT. Future studies with larger cohorts would be useful to study specific conditions; these results from single patients demonstrate that discordance may be present not only at a group-wise statistical level, but on an individual basis.
Conclusions
In summary, we demonstrated the dependence of mGM on T1 signal. Care should be taken in interpreting mGM results as volume change alone. Used in concert, FSL-VBM and FreeSurfer-CT analyses may be complementary.
ABBREVIATIONS:
- CT
cortical thickness
- mGM
modulated GM density
- mTBI
mild traumatic brain injury
- VBM
voxel-based morphometry
Footnotes
Disclosures: Yvonne Lui—RELATED: Grant: NIH R01 NS039135-11, NIH R21 NS090349, NIH P41 EB017183.* *Money paid to the institution.
This work was supported by grant R01 NS039135-11 from the National Institute for Neurological Disorders and Stroke, a component of the National Institutes of Health. This work was also performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), a National Institute of Biomedical Imaging and Bioengineering Biomedical Technology Resource Center (NIH P41 EB017183).
REFERENCES
- 1. Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 2. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–94 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 3. Hutton C, Draganski B, Ashburner J, et al. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 2009;48:371–80 10.1016/j.neuroimage.2009.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010;53:1135–46 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith SM, Brady JM. SUSAN—A new approach to low level image processing. Int J Comput Vis 1997;23:45–78 10.1023/A:1007963824710 [DOI] [Google Scholar]
- 6. Gaetz W, Roberts TP, Singh KD, et al. Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp 2012;33:2035–46 10.1002/hbm.21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Kierans A, Kenul D, et al. Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology 2013;267:880–90 10.1148/radiol.13122542 [DOI] [PMC free article] [PubMed] [Google Scholar]