Abstract
We report the solution NMR structure of RHE_CH02687 from Rhizobium etli. Its structure consists of two β-sheets that together with two short and one long α-helix form a hydrophobic cavity. This protein shows a high structural similarity to the prokaryotic protein YndB from Bacillus subtilis, and the eukaryotic protein Aha1. NMR titration experiments confirmed that RHE_CH02687, like its homolog YndB, interacted with flavonoids, giving support for a biological function as a flavonoid sensor in the symbiotic interaction between R. etli and plants. In addition, our study showed no evidence for a direct interaction between RHE_CH02687 and HtpG, the R. etli homolog of Hsp90.
Keywords: AHSA1 family, hydrophobic cavity, NMR titration, flavonoid binding, symbiotic
INTRODUCTION
Rhizobium etli, mainly including the strains Rhizobium etli CFN42, Rhizobium etli bv. mimosae and Rhizobium etli bv. phaseoli, is one of the most predominant bacteria that can form symbiotic relationship with legumes, and plays an important role in providing nutrients for plants by transforming nitrogen into ammonia.1 The complete genome of R. etli CFN42 contains a circular chromosome and six large plasmids.2 The gene RHE_CH02687 is located in the circular chromosome of R. etli CFN42 and has high conservation within rhizobia. RHE_CH02687 is, to date, annotated as a hypothetical conserved protein in the UniProt database (Q2K6S8-RHIEC) with unknown function. Sequence similarity searches using PSI-BLAST revealed that RHE_CH02687 belongs to the activator of Hsp90 ATPase homolog 1-like protein (AHSA1) family (Pfam ID: PF08327), one of the four largest families in the Bet v1-like superfamily (Pfam ID: PF00407). In the AHSA1 family, to date, there are 39 proteins with known structures including human protein Aha1 and YndB from B. subtilis, whose functions have been explored.
Aha1 stimulates the ATPase activity of Hsp90 by interacting with the middle domain of Hsp90, and regulating the interaction kinetics between Hsp90 and its client proteins.3 Another member of AHSA1 family, YndB from Bacillus subtilis, was reported to bind to several flavonoid molecules, and was suggested to be involved in the symbiotic relationship between plants and B. subtilis.4 Flavonoids, a superfamily of plant secondary metabolites, could be divided into several subclasses including chalcones, flavones, flavonols, flavanones, isoflavones, isoflavans, among others.5 A well-known role of flavonoids in the interaction between plants and soil bacteria, is mediating signal exchange during the establishment of the symbiotic relationship between plants and rhizobia.6 Specific flavonoids produced by plant roots could encourage the gathering of compatible rhizobia around the rhizosphere, induce or inhibit the transcription of nodulation (nod) genes, and regulate the phytoalexin resistance of rhizobia to enable successful infection.5,6 So far, the identified symbiosis-related flavonoids include more than thirty kinds of molecules from chalcones, flavones, flavonols, flavanones, and isoflavones classes.5 For instance, methoxychalcone, a type of chalcone-derived flavonoid produced by alfalfa, is a strong nod gene inducer of Sinorhizobium meliloti,7 while luteolin, a type of flavone-derived flavonoid, activates nod gene expression and enhances nodulation of S. meliloti.8 On the other hand, some plant-produced flavonoids can also function as phytoalexins, which may play a role in inhibition of incompatible rhizobia and therefore play an important role in the determination of the rhizobial host-range, potentially leading to diversification in the plant rhizosphere.9 It is known that one type of flavonoid sensing in the rhizobia is mediated by their binding to NodD proteins and the subsequent transcriptional promotion of nod genes.9
In this short note, we present the solution structure of R. etli protein RHE_CH02687. Structural comparison suggested that RHE_CH02687 is similar to the eukaryotic protein Aha1 (PDB ID: 1X53), and the prokaryotic protein YndB (PDB ID: 2KTE) from B. subtilis. Our NMR titration experiments confirmed that RHE_CH02687, similar to YndB, interacts with both flavone and chalcone, which are precursors or intermediates for the production of flavonoid molecules, that are used by plants as signals for infection or as phytoalexins for selection of compatible symbionts in their root nodules. Our findings suggest that RHE_CH02687 is involved in the symbiotic relationship between R. etli and plants. In addition, no direct evidence was obtained in our study for the interaction between RHE_CH02687 and high temperature protein G (HtpG), the homolog of Hsp90 in R. etli, which indicates that any putative interaction between RHE_CH02687 and HtpG may be more complicated, and awaits further investigation.
MATERIALS AND METHODS
(1) Protein expression, purification and uniformly [13C, 15N] labeling
The RHE_CH02687 gene from R. etli CFN42 encoding the full-length protein (M1-R152) was cloned into the NESG-modified pET21 expression vector including a short C-terminal His6 tag (LEHHHHHH).10 The U-[13C,15N]-labeled (NC) and U-15N, 5% biosynthetically-directed 13C-labeled (NC5) RHE_CH02687 samples were prepared. Briefly, BL21 (DE3) E. coli cells containing above plasmid were grown in MJ minimal medium at 37 °C until the OD600 was 0.6–0.8. At this point, 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) was added, and the incubation temperature was shifted to 17 °C for protein expression. Overnight cell culture was harvested by centrifugation at 6000 rpm, 4°C for 15 minutes, and lysed by sonication. The clarified supernatant containing RHE_CH02687 was purified using an ÄKTAxpress™ (GE Healthcare) with a Ni-affinity column (HisTrap IMAC HP™ column) followed by a gel filtration column (HiLoad 26/60 Superdex 75). The purified protein was concentrated to a final concentration of 0.7 mM in the NMR buffer containing 10% D2O (v/v), 20 mM MES, 100 mM NaCl, 5 mM CaCl2, 10 mM DTT, and 0.02% NaN3 at pH 6.5.
(2) Rotational correlation time (τc) estimate
Average 15N relaxation times were determined from 1D 15N-edited T1 and T2 (CPMG) experiments on the NC5 sample recorded on a Varian Inova 600 MHz at 298 K. Longitudinal T1 relaxation delays were 50, 100, 200, 300, 400, 600, 800, 1000, 1500, and 2000 ms; transverse T2 relaxation CPMG delays were 10, 20, 30, 50, 70, 100, 130, 170, 210, and 250 ms; both experiments had 1.5 s recycle delays. T1 and T2 relaxation times were obtained by integration from 8.5 to 10.5 ppm, and an isotropic rotational correlation time (τc) of 9.1 ns was derived from T1 and T2 measurements for NC5 RHE_CH02687 following the literature equation.11 From the linear fit of τc versus protein molecular weight (MW) for a series of standard proteins,12 the corresponding fit molecular weight (MW) of RHE_CH02687 is 18.7 kDa (Supporting Information Fig. S1). This result indicated that RHE_CH02687 was predominantly monomeric (calculated MW = 17.8 kDa for NC5 RHE_CH02687) under the NMR study conditions.
(3) Chemical shift assignments and structure calculation
A Varian Inova 600 MHz spectrometer and a Bruker Avance III 850 MHz spectrometer were used to record the NMR data at 298 K. NMR data collected for chemical shift assignments and structural calculation were as follows: 2D 1H-15N HSQC and 1H-13C HSQC, 3D HNCO, HNCA, HN(CO)CA, HNCACB, CBCA(CO)NH, H(CC)(CO)NH-TOCSY, (H)CC(CO)NH-TOCSY, H(C)CH-COSY, H(C)CH-TOCSY, two 13C-edited NOESY-HSQC optimized for either aliphatic or aromatic carbons, and 15N-edited NOESY-HSQC on the NC sample, 3D (H)CCH-TOCSY and 4D 13C-13C-HMQC-NOESY-HMQC on the NC sample in D2O, 2D constant time 1H-13C HSQC (CT-HSQC) on the NC5 sample. The mixing time for all NOESY spectra was 70 ms. The backbone and side chain resonances were automatically assigned using the PINE server from NMRFAM,13 followed by manual validation and correction. Stereospecific assignments of isopropyl methyl groups of Leu and Val residues were determined from 2D 1H-13C CT-HSQC spectrum on the NC5 sample.14 Overall, non-proline backbone cross peaks of amide protons and nitrogens were completely assigned and assignments were 98.5% complete for all 1H, 13C and 15N chemical shifts (Supporting Information Fig. S2). Chemical shift assignments have been deposited in the BioMagResDB (BMRB) under the accession number 17530.
NOE-based inter-proton distance constraints were determined automatically for RHE_CH02687 using CYANA 3.0. Input for CYANA consisted of chemical shift assignments, NOESY peak lists from four NOESY spectra with peak intensities, the constraints for backbone phi (φ) and psi (ψ) torsion angle derived from chemical shifts of backbone atoms using the TALOS+ software program.15 Manual and iterative refinements of NOESY peak picking lists were guided using NMR RPF quality to assess “goodness of fit” between calculated structures and NOESY peak lists.16 Towards the end of the iterative structure calculation process, hydrogen bond constraints for the NH and CO distances were introduced based on identification of proximity of potential donors and receptors in earlier structure calculations. The 20 lowest energy structures calculated by CYANA 3.0 were further refined using restrained molecular dynamics in explicit water CNS 1.2 and the PARAM19 force field, using the final NOE-derived distance constraints, hydrogen bond constraints, and TALOS-derived dihedral angle constraints. The final NMR ensemble of 20 structures and all restraints used in the structure calculations have been deposited in the Protein Data Bank (PDB ID 2LAK). Structural statistics and global structure quality factors were computed using PSVS version 1.4 (Table 1).17
Table 1.
Structural statistics for RHE_CH02687 a
| Conformationally-restricting constraints b | |
|---|---|
| Distance constraints | |
| Total | 1505 |
| Intra-residue (i=j) | 375 |
| Sequential (|i−j|=1) | 383 |
| Medium-range (1<|i−j|<5) | 232 |
| Long-range (|i−j|≥5) | 515 |
| Hydrogen bond constraints | |
| Long-range (|i−j|≥5)/total | 36/80 |
| Dihedral angle constraints | 138 |
| Residue constraint violations b | |
| Average number of distance violations per structure | |
| 0.1–0.2Å | 6 |
| 0.2–0.5 Å | 1.75 |
| >0.5Å | 0 |
| Average RMS distance violation/constraint (Å) | 0.01 |
| Maximum distance violation (Å) | 0.35 |
| Average number of dihedral angle violations per structure | |
| 1–10° | 0.7 |
| >10° | 0 |
| Average RMS dihedral angle violation/constraint (degree) | 0.13 |
| Maximum dihedral angle violation (degree) | 2.6 |
| RMSD from average coordinates b,c | |
| Backbone/Heavy atoms (Å) | 0.7/1.2 |
| Ramachandran plot statistics b,c | |
| Most favored/Allowed regions (%) | 93.3/6.7 |
| Disallowed regions (%) | 0 |
| Global quality scores(raw/Z-score) b | |
| Verify3D | 0.33/−2.09 |
| Prosall | 0.29/−1.49 |
| Procheck(phi-psi) c | 0.11/0.75 |
| Procheck(all) c | 0.17/1.01 |
| Molprobity clash | 22.67/−2.36 |
| RPF Scores d | |
| Recall/Precision | 0.99/0.92 |
| F-measure/DP-score | 0.95/0.82 |
Structural statistics were computed for the ensemble of 20 deposited structures.
Calculated using the PSVS 1.4 program. Residues (1–160) were analyzed.
Ordered residues ranges (with sum of phi and psi > 1.8): 10–18, 20–28, 31–36, 67–77, 93–100, 105–112, 115–139.
RPF scores reflected the goodness-of-fit of the final ensemble of structures including disordered residues to the NMR data.
(4) NMR titrations with flavonoids
NMR titrations of RHE_CH02687 with flavonoids including flavone and chalcone were performed on the Bruker Avance III 600 MHz instrument at 298 K. Flavonids are insoluble in the NMR buffer used for the protein structural study but are soluble in DMSO. However, the solvent effect on chemical shift perturbations caused by DMSO, at a concentration of 10% (v/v), for example, was too large to be acceptable for NMR titration experiments (Supporting Information Fig. S3a). Further testing determined that 2 μL of DMSO in the final 300 μL (0.7%) NMR buffer led to negligible solvent effects on the protein 1H and 15N chemical shifts (Supporting Information Fig. S3b). To solubilize flavonoids in the NMR buffer, either flavone or chalcone was pre-dissolved in DMSO-d6 at the concentration of 90.0 mM for the titration experiments. Mixtures containing 0.6 mM 15N-labeled RHE_CH02687 sample and a final flavone or chalcone concentration of 0.6 mM were prepared separately by addition of 2 μL of flavonoid stock solution to 298 μL of protein solution in NMR buffer.
RESULTS AND DISCUSSION
RHE_CH02687 is a 16.5 kDa protein that belongs to protein family AHSA1 (Pfam PF08327), one of the four largest subfamilies in the Bet v1-like clan. The PSI-BLAST search of RHE_CH02687 against the UniProt Knowledgebase identified 872 sequences with an E-value cut-off 1.0 × 10−3. Among them, there are 102 proteins with an E-value of 8.0 × 10−41 or lower, which belong to the organisms of order Rhizobiales, with the sequence identity greater than 60%. The sequence identities of RHE_CH02687 with sequences of homologs from other organisms are relatively low. For example, the homolog TN53_36565 from Streptomyces sp. WM6386, OK006_6424 from Actinobacteria bacterium OK006, ASG23_00810 from Cellulomonas sp. Leaf395, and AN963_24995 from Brevibacillus choshinensis, share only up to 55%, 47%, 45% and 42% sequence identity with RHE_CH02687 (with E-values of 1.0 × 10−33, 1.0 × 10−26, 1.0 × 10−24 and 2.0 × 10−30), respectively [Fig. 1(A)].
Figure 1.

Sequence alignment and NMR structures of RHE_CH02687 from Rhizobium etli CFN42. (A) Alignment of RHE_CH02687 with four homologues from other organisms: TN53_36565 from Streptomyces sp. WM6386, OK006_6424 from Actinobacteria bacterium OK006, ASG23_00810 from Cellulomonas sp. Leaf395, and AN963_24995 from Brevibacillus choshinensis. Alignment was rendered using ESPript with default settings for similarity calculations. Secondary structural elements from RHE_CH02687 are indicated above the amino acid residue number in the sequence. Identical (red box with white letters) and similar (red letters) amino acids are denoted. (B) Superposition of the Cα traces of the final ensemble of 20 conformers from the solution NMR structure of RHE_CH02687, α-helices and β-strands are colored in blue, and loop regions in gray. The disordered C-terminal His6 tag is not shown for clarity. (C) Stereo-view of cartoon representation of RHE_CH02687 structure with the lowest overall energy. All α-helices are colored red, and β-strands are colored green.
The good signal-to-noise ratio and broad chemical shift dispersions in the 2D 1H-15N HSQC spectrum for protein RHE_CH02687 (NC sample) suggests that this protein was well-folded and suitable for structure determination using NMR spectroscopy (Supporting Information Fig. S2). Figure 1(B) shows the superposition of Cα traces of the final ensemble of 20 conformers from the solution NMR structure of RHE_CH02687. The RMSD for backbone atoms and heavy atoms in the ordered regions was 0.7 and 1.2 Å, respectively. All secondary structural elements were well defined as shown in Figure 1(C), including seven β-strands (β1, 10-17; β2, 42-44; β3, 52-54; β4, 66-70; β5, 74-77; β6, 93-100; β7, 105-112) and three α-helices (α1, 21-29; α2, 31-36; α3, 117-139) with the secondary structural order of N-β1-α1-α2-β3-β4-β5-β6-β7-α3-C. The strands, β1, β7, β6, β5 and β4 formed a five-stranded antiparallel β-sheet, while β2 and β3 formed another antiparallel β-sheet. Together with three α-helices, these strands form the helix-grip fold, like other proteins in Bet v1-like superfamily4, creating one large hydrophobic cavity with a volume of 1303 Å3 as calculated using the CASTp server.18 RHE_CH02687 exhibits high structural similarity to both Aha1 and YndB with Z-scores of 10.3 and 8.2 when aligned, even though the sequence identity levels are only 19.6% and 14.7%, respectively (Supporting Information Fig. S4). To explore the biochemical function of RHE_CH02687, NMR titration experiments were carried out with flavonoids and HtpG, the R. etli homolog of Hsp90.
Given that the identified flavonoid molecules in the crosstalk between rhizobia and plants are numerous, flavone and chalcone that serve as the main skeletons for production of the flavone and chalcone classes of flavonoid molecules through modifications such as glycosylation, malonylation, methylation, hydroxylation, acylation, and prenylation, were used to determine the flavonoid-binding activity of RHE_CH02687. Chemical shift perturbations (CSPs)19 greater than the cut-off value of 0.1 ppm were observed for backbone 1H-15N cross peaks from 14 residues (R13, W37, G39, T40, E41, V54, N55, V86, H111, E118, C120, H123, E124 and Y130) when RHE_CH02687 was titrated with flavone [Fig. 2 (A)], whereas these and five more cross peaks (T29, M38, G122, E125 and L131) had significant CSPs when it was titrated with chalcone [Fig. 2 (B)]. Upon mapping to the solution structure of RHE_CH02687, those residues with significant CSPs were predominantly located either in the secondary structure elements or in the loops that formed the hydrophobic cavity [Fig. 2 (C)]. Among these binding sites, five residues (T29, W37, G39, H111 and H123) are highly conserved as analyzed using the ConSurf server [Fig. 2(D)].20 Our titration experiments confirmed that RHE_CH02687 interacts with both of the flavonoids, flavone and chalcone. In addition, the hydrophobic cavity volume in RHE_CH02687 (1303 Å3) is similar in size to that of YndB (894Å3), which might be expected considering their similar flavonoid binding functions. Considering the essential roles of flavonoids in the crosstalk between rhizobia and plants, we speculate that RHE_CH02687 represents a novel flavonoid-binding protein involved in the symbiotic relationship between R. etli and plants, in a way that is different that the NodD-mediated flavonoid binding and regulation of the nod genes.9 The detailed roles of RHE_CH02687 in the crosstalk between rhizobia and plants, like flavonoid sensing involved in the infection of plant roots to form the root nodules, or functions as stress sensors responding to flavonoid phytoalexins to facilitate rhizobia survival, remains to be further explored. On the other hand, RHE_CH02687 showed no direct interaction with HtpG from our in vitro experiments including NMR titration (Supporting Information Fig. S5) and native gel (data not shown), which suggests that any putative interaction between RHE_CH02687 and HtpG may be more complicated, and awaits further exploration.
Figure 2.
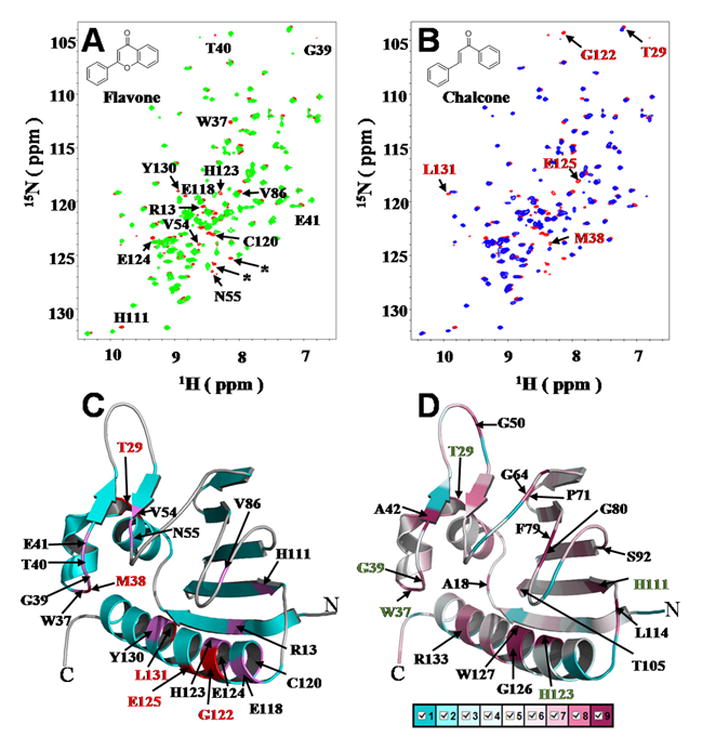
NMR titrations with flavonoids, binding site mapping and residue conservation analysis of RHE_CH02687. (A) Overlay of the 2D 1H-15N HSQC spectra for 0.6 mM 15N-labeled RHE_CH02687 in the absence (red) and presence of 0.6 mM flavone (green), all 14 assigned residues with observed chemical shift perturbations (CSPs) are indicated with single-letter amino acid, two from the unassigned C-terminal His6 tag indicated by asterisk symbols (*); (B) Overlay of the 1H-15N HSQC spectra for 0.6 mM 15N-labeled RHE_CH02687 in the absence (red) and presence of 0.6 mM chalcone (blue), the five additional residues with CSPs for titration with chalcone are indicated here, excluding the 14 residues with CSPs in (A); (C) Binding site mapping onto RHE_CH02687. (D) ConSurf image showing the conserved residues. Residue coloring, representing the degree of residue conservation, ranges from cyan (variable) to magenta (highly reserved). The 19 highly conserved residues are labeled. Among them, five highly conserved residues (T29, W37, G39, H111 and H123) with observed CSPs when titrated with flavonoids (for both flavone and chalcone) were colored with green labels. The molecular structures of flavone and chalcone are shown as insets in (A) and (B).
In summary, the solution structure of Rhizobium etli protein RHE_CH02687 has been solved using NMR spectroscopy. RHE_CH02687 interacts with the flavonoids flavone and chalcone with the binding site located in its highly conserved hydrophobic cavity. Our findings revealed that RHE_CH02687 may play a role related to the symbiotic interaction between R. etli and plants based on its flavonoid-binding activity.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institute of General Medical Sciences of USA (Grant Numbers: U54-GM074958 and U54-GM094597), the Hundred Talent Program by Chinese Academy of Sciences, the Ministry of Science and Technology (Grant number 2016YFA051201) and the National Natural Sciences Foundation of China (Grant Number: 21575155).
References
- 1.Aguilar OM, Riva O, Peltzer E. Analysis of Rhizobium etli and of its symbiosis with wild Phaseolus vulgaris supports coevolution in centers of host diversification. Proc Natl Acad Sci USA. 2004;101(37):13548–13553. doi: 10.1073/pnas.0405321101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez V, Santamaria RI, Bustos P, Hernandez-Gonzalez I, Medrano-Soto A, Moreno-Hagelsieb G, Janga SC, Ramirez MA, Jimenez-Jacinto V, Collado-Vides J, Davila G. The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc Natl Acad Sci USA. 2006;103(10):3834–3839. doi: 10.1073/pnas.0508502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koulov AV, LaPointe P, Lu B, Razvi A, Coppinger J, Dong MQ, Matteson J, Laister R, Arrowsmith C, Yates JR, 3rd, Balch WE. Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol Biol Cell. 2010;21(6):871–884. doi: 10.1091/mbc.E09-12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark JL, Mercier KA, Mueller GA, Acton TB, Xiao R, Montelione GT, Powers R. Solution structure and function of YndB, an AHSA1 protein from Bacillus subtilis. Proteins. 2010;78(16):3328–3340. doi: 10.1002/prot.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janczarek M, Rachwal K, Marzec A, Grzadziel J, Palusinska-Szysz M. Signal molecules and cell-surface components involved in early stages of the legume-rhizobium interactions. Applied Soil Ecology. 2015;85:94–113. [Google Scholar]
- 6.Hassan S, Mathesius U. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot. 2012;63(9):3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell CA, Hartwig UA, Joseph CM, Phillips DA. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters NK, Frost JW, Long SR. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- 9.Liu CW, Murray JD. The Role of Flavonoids in Nodulation Host-Range Specificity: An Update. Plants (Basel) 2016;5(3) doi: 10.3390/plants5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acton TB, Xiao R, Anderson S, Aramini J, Buchwald WA, Ciccosanti C, Conover K, Everett J, Hamilton K, Huang YJ, Janjua H, Kornhaber G, Lau J, Lee DY, Liu G, Maglaqui M, Ma L, Mao L, Patel D, Rossi P, Sahdev S, Shastry R, Swapna GV, Tang Y, Tong S, Wang D, Wang H, Zhao L, Montelione GT. Preparation of protein samples for NMR structure, function, and small-molecule screening studies. Methods Enzymol. 2011;493:21–60. doi: 10.1016/B978-0-12-381274-2.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay LE, Torchia DA, Bax A. Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry. 1989;28(23):8972–8979. doi: 10.1021/bi00449a003. [DOI] [PubMed] [Google Scholar]
- 12.Rossi P, Swapna GV, Huang YJ, Aramini JM, Anklin C, Conover K, Hamilton K, Xiao R, Acton TB, Ertekin A, Everett JK, Montelione GT. A microscale protein NMR sample screening pipeline. J Biomol NMR. 2010;46(1):11–22. doi: 10.1007/s10858-009-9386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrami A, Assadi AH, Markley JL, Eghbalnia HR. Probabilistic interaction network of evidence algorithm and its application to complete labeling of peak lists from protein NMR spectroscopy. PLoS Comput Biol. 2009;5(3):e1000307. doi: 10.1371/journal.pcbi.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neri D, Szyperski T, Otting G, Senn H, Wuthrich K. Stereospecific nuclear magnetic resonance assignments of the methyl groups of valine and leucine in the DNA-binding domain of the 434 repressor by biosynthetically directed fractional 13C labeling. Biochemistry. 1989;28(19):7510–7516. doi: 10.1021/bi00445a003. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YJ, Powers R, Montelione GT. Protein NMR recall, precision, and F-measure scores (RPF scores): structure quality assessment measures based on information retrieval statistics. J Am Chem Soc. 2005;127(6):1665–1674. doi: 10.1021/ja047109h. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya A, Tejero R, Montelione GT. Evaluating protein structures determined by structural genomics consortia. Proteins. 2007;66(4):778–795. doi: 10.1002/prot.21165. [DOI] [PubMed] [Google Scholar]
- 18.Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006;34(Web Server issue):W116–118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulder FA, Schipper D, Bott R, Boelens R. Altered flexibility in the substrate-binding site of related native and engineered high-alkaline Bacillus subtilisins. J Mol Biol. 1999;292(1):111–123. doi: 10.1006/jmbi.1999.3034. [DOI] [PubMed] [Google Scholar]
- 20.Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, Pupko T, Ben-Tal N. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33(Web Server issue):W299–302. doi: 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


