Abstract
Objective
We assessed whether requiring >1 medication for blood pressure control is associated with adverse pregnancy outcomes.
Study Design
Retrospective cohort of 974 singletons with chronic hypertension at a tertiary care center. Subjects on >1 antihypertensive agent were compared to those on 1 agent < 20 weeks gestational age with results stratified by average blood pressure (<140/90 and ≥140/90 mmHg) from prenatal visits. The primary maternal outcome was preeclampsia; the primary neonatal outcome was small for gestational age (<10th percentile).
Result
Among women with blood pressure ≥140/90 mmHg, women on multiple agents had the greatest risk of preeclampsia, severe preeclampsia, antenatal admissions to rule out preeclampsia, preterm birth < 35 weeks and composite neonatal adverse outcomes.
Conclusion
Compared to use of a single agent when blood pressure is ≥140/90 mmHg, use of multiple agents increases adverse risks, while no such finding exists when blood pressure is controlled below 140/90 mmHg.
Introduction
Chronic hypertension complicates up to 5% of pregnancies in the United States; this number is expected to increase as average age and body mass index at first pregnancy increases.1 Chronic hypertension in pregnancy is associated with adverse maternal and fetal outcomes.2 Women with mild chronic hypertension, defined as blood pressures 140-150/90-109 mm Hg, in pregnancy have an increased risk of developing superimposed preeclampsia (10-20%), abruptio placentae (0.7-1.5%) and fetal growth restriction (8-16%) compared to the general obstetrics population (3-5%, ≤ 1%, and 10%, respectively).3,4,5,6 Women diagnosed with severe hypertension, defined as ≥160/110 mm Hg, in pregnancy have even greater rates of complications with the risk of superimposed preeclampsia reaching 50%, abruptio placenta 5-10%, preterm birth 62-70%, and fetal growth restriction 31-40%. 7
The current ACOG Task Force on Hypertension in Pregnancy recommends that pharmacologic anti-hypertensive therapy be used in women with severe chronic hypertension due to the increased risk of serious maternal complications.8 Additionally, ACOG and the joint NICHD/ACOG/SMFM task force recommend that timing of delivery in chronic hypertension be based in part on the control of blood pressure. Women with poorly controlled chronic hypertension often require multiple antihypertensive agents to achieve non-severe blood pressures. If blood pressure remains uncontrolled, however, early delivery is necessary.8, 9
While requiring multiple antihypertensive agents to achieve a non-severe blood pressure is a surrogate marker of chronic hypertension severity and thus a marker of adverse outcomes, it is unclear whether the number of agents required to achieve blood pressure control is important in determining outcomes. Prior studies suggest that exposure to antihypertensive medication and multiple antihypertensive medications is associated with adverse outcomes, but these studies are limited by their lack of data on blood pressures during pregnancy.10-14 The objective of this study is to determine the impact of the number of antihypertensive agents used during pregnancy stratified by blood pressure control achieved. We hypothesized that requiring more than one medication for blood pressure control is associated with increased risks of adverse maternal and fetal pregnancy outcomes.
Materials and Methods
This was a retrospective cohort study of all singleton pregnancies with the diagnosis of chronic hypertension delivered at the University of Alabama at Birmingham (UAB) from January 1, 2000 to July 1, 2014. Institutional review board approval was obtained. Subjects were identified by a diagnosis of chronic hypertension in the searchable electronic medical record. The diagnosis of chronic hypertension was confirmed by either current or prior treatment with antihypertensives or elevated blood pressures on two occasions prior to 20 weeks. Standardized chart abstraction forms were used to abstract data from the medical charts by individuals trained in chart abstraction. Data collected included detailed information of maternal demographics, medical and obstetrical history, prenatal blood pressure and anti-hypertensive medication logs, labor and delivery events and neonatal outcomes.
All women were managed by institutional protocol under the supervision of Maternal Fetal Medicine specialists. At UAB, blood pressures during the time period examined were typically treated to achieve systolic blood pressures less than 150 mm Hg and diastolic blood pressures 90 mm Hg. Although first line agents included methyldopa, labetalol, and nifedipine, a patient’s pre-pregnancy antihypertensive regimen may have been continued if not contraindicated during pregnancy. Typically, maximum doses of a single agent were reached prior to initiating a second agent, although exceptions to this may have included dosages limited by side effects.
Patients with a singleton gestation who met the criteria for the diagnosis of chronic hypertension and were being treated during pregnancy with a single anti-hypertensive agent or multiple anti-hypertensive agents were included in this study. Patients diagnosed with major medical comorbidities such as systemic lupus erythematosus, renal disease, cardiac disease, sickle cell disease and cystic fibrosis, and pregnancies complicated by congenital malformations were excluded. As diabetes is a frequent comorbidity of hypertension, diabetes was included. Only the first eligible pregnancy in the time period was included in the analysis.
The exposure was defined as receiving either one (single agent) or more than one (multiple agents) antihypertensive initiated prior to 20 weeks gestation. We defined the exposure prior to 20 weeks to eliminate the possibility that preeclampsia diagnosis was the reason for initiating a second or third blood pressure agent. Subjects were assigned to testing group based on the greatest concomitant antihypertensive agents used at any point during the pregnancy. Because blood pressure control is closely correlated with maternal and neonatal outcomes, and because the number of blood pressure medications are closely linked to blood pressures measured, we elected a priori to stratify analyses by the average blood pressure <140/90 mm Hg or ≥140/90 mm Hg.11 Blood pressures were recorded at every prenatal visit. The average of all recorded blood pressures was used in analysis. The number and frequency of prenatal visits varied among subjects. The primary maternal outcome examined was superimposed preeclampsia. Secondary maternal outcomes were superimposed preeclampsia with severe features, cesarean delivery (CD) and antepartum hospitalizations to rule out superimposed preeclampsia. Superimposed preeclampsia was defined as chronic hypertension (≥140/90 mm Hg) and proteinuria (either protein/creatinine ratio ≥0.3 or a 24-hour urine protein ≥300 mg). In the absence of proteinuria, laboratory abnormalities consistent with severe features (platelets <100,000/mL, AST≥80 mU/mL (twice the upper limit of normal for our laboratory), creatinine≥1.2 mg/dL) was required for the diagnosis of superimposed preeclampsia with severe features. Blood pressures ≥160/110 mm/Hg in the absence of proteinuria and laboratory abnormalities were not classified as superimposed preeclampsia with or without severe features for the purposes of this study. As neurologic symptoms were not abstracted from the chart review, they were not considered as part of the diagnosis of superimposed preeclampsia. The primary neonatal outcome was small for gestational age, defined as weight <10th percentile for gestational age. Secondary neonatal outcomes measured were preterm birth prior to 35 weeks gestational age and a composite outcome of perinatal death, assisted ventilation, umbilical cord pH <7, 5-minute Apgar ≤3 and neonatal seizures.
Distributions of variables were tested for normality using visual inspection of the histogram and the Kolmogorov-Smirnov test. Exposure groups were then compared using analysis of variance (ANOVA), Kruskall-Wallis, or chi squared test as appropriate. Potentially confounding variables of the exposure-outcome association were identified in the stratified analyses. Multivariable logistic regression models were then developed to better estimate the effect of medications on outcomes of interest while adjusting for potentially confounding variables. Clinically relevant covariates for initial inclusion were selected using results of the stratified analyses, and factors were removed in a backward stepwise fashion, based on significant changes in the exposure adjusted odds ratio or significant differences between hierarchical models using the likelihood ratio test. The statistical analysis was performed using STATA, version 13 Special Edition (College Station, TX).
Results
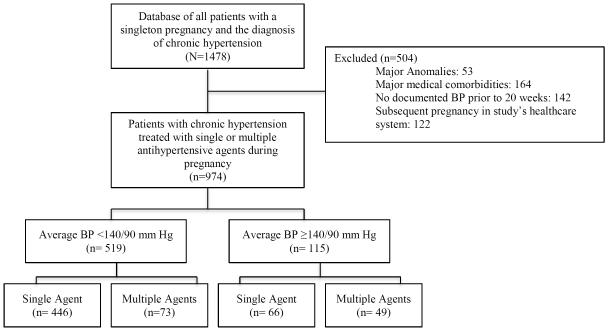
Of 1,478 pregnancies complicated by chronic hypertension identified over the study period, 504 were excluded (53 for major anomalies, 23 for no diagnosis of chronic hypertension, 164 for major medical problems other than diabetes, 142 for no documented blood pressures prior to 20 weeks, and 122 for being a subsequent pregnancy) (Figure 1). Of the remaining 974 subjects, 634 (65%) were prescribed an antihypertensive agent during pregnancy. Of these, 446 (70.3%) were on a single agent with average blood pressures <140/90 mm Hg, 73 (11.5%) were on multiple agents with average blood pressure <140/90 mm Hg, 66 (10.4%) were on a single agent with average blood pressures >140/90 mm Hg, and 49 (7.7%) were on multiple agents with average blood pressures >140/90 mm Hg.
Figure 1.
Flow diagram of patients with chronic hypertension who had pregnancies within the study’s health system between 2000 and 2014.
The four exposure groups were similar with respect to age, nulliparity, government insurance coverage, marital status, tobacco use, pre-pregnancy body mass index (BMI), aspirin use during current pregnancy, prior preterm delivery, indications for preterm delivery and the diagnoses of pregnancy-induced hypertension (PIH) in a prior pregnancy and diabetes (pregestational and gestational) (Table 1). Women on multiple agents were more likely to be black. Women who were treated with a single antihypertensive agent during pregnancy were diagnosed with chronic hypertension for a significantly shorter time prior to current pregnancy and were less likely to have been on an antihypertensive prior to pregnancy (p <0.01) compared to those on multiple agents. Expected differences in average systolic and diastolic blood pressure prior to 20 weeks based on number of antihypertensive agents used were seen. The average systolic and diastolic blood pressures were significantly less when blood pressure were controlled to <140/90 mm Hg than ≥140/90 mm Hg irrespective of single or multiple antihypertensive agents used.
Table 1.
Maternal Baseline Characteristics by BP Control and Number of Medications
| Single Agent <140/90 |
Multiple Agent <140/90 |
Single Agent ≥140/90 |
Multiple Agent ≥140/90 |
p | |
|---|---|---|---|---|---|
| n=446 | n=73 | n=66 | n=49 | ||
| Age | 30.7 ± 5.8 | 31.9 ± 5.3 | 31.6 ± 6.3 | 32.4 ± 5.6 | 0.09 |
| Race | <0.01 | ||||
| Black | 306 (68.6%) | 63 (86.3%) | 49 (74.2%) | 43 (87.8%) | |
| White | 117 (26.2%) | 8 (11.0%) | 11 (16.7%) | 6 (12.2%) | |
| Other | 23 (5.2%) | 2 (2.7%) | 6 (9.1%) | 0 | |
| Nulliparous | 114 (25.6%) | 15 (20.6%) | 24 (36.4%) | 16 (32.7%) | 0.12 |
|
Government
Insurance |
316 (70.9%) | 44 (60.3%) | 41 (62.1%) | 29 (59.2%) | 0.12 |
|
Number of
Prenatal Visits |
15 (12-18) | 17 (13-20) | 13.5 (10-18) | 15 (10-18) | <0.01 |
| Unmarried | 306 (68.9%) | 48 (65.8%) | 45 (68.2%) | 30 (61.2%) | 0.73 |
| Tobacco | 84 (19.1%) | 8 (11.0%) | 8 (12.3%) | 12 (25.0%) | 0.12 |
| BMI | 39.2 ± 11.3 | 38.9 ± 9.8 | 40.7 ± 11.0 | 43.2 ±11.3 | 0.13 |
|
Years since CHTN
Diagnosis |
3 (1-7) | 4 (2-9) | 3 (1-5) | 8 (3-10) | <0.01 |
|
Medications Prior
to Pregnancy |
275 (61.9%) | 66 (91.7%) | 36 (54.6%) | 33 (68.8%) | <0.01 |
| Aspirin | 42 (9.4%) | 6 (8.2%) | 5 (7.6%) | 5 (10.2%) | 0.95 |
|
Prior Preterm
Delivery |
132 (29.6%) | 27 (37.0%) | 21 (31.8%) | 18 (36.7%) | 0.50 |
|
Indications for
Preterm Delivery |
0.08 | ||||
| Spontaneous | 24 (30%) | 3 (17.7%) | 1 (5.3%) | 1 (5.3%) | |
| Hypertension | 49 (61.3%) | 13 (76.47%) | 17 (89.5%) | 15 (79.0%) | |
|
Non-reassuring
Fetal Status |
7 (8.8%) | 1 (5.9%) | 1 (5.3%) | 3 (15.8%) | |
|
PIH In a Prior
Pregnancy |
149 (33.4%) | 20 (27.4%) | 16 (24.2%) | 17 (34.7%) | 0.72 |
| Diabetes | 0.68 | ||||
| Gestational | 61 (13.8%) | 9 (12.7%) | 10 (15.2%) | 3 (6.5%) | |
| Pregestational | 89 (20.1%) | 16 (22.5%) | 9 (13.6%) | 10 (21.7%) | |
|
Average Systolic
BP <20 weeks |
125 ± 8 | 126 ± 8 | 144 ± 6 | 148 ± 10 | <0.01 |
|
Average Diastolic
BP <20 weeks |
75 ± 7 | 76 ± 7 | 87 ± 8 | 89 ± 7 | <0.01 |
Data presented as mean ± standard deviation, median (interquartile range), or n (%) as appropriate
The incidence of superimposed preeclampsia was significantly different across the four exposure groups, increasing as both number of medications and blood pressure increased but with a dramatic increase seen as blood pressures increased above 140/90 regardless of the number of medications (p<0.01, Table 2). Similarly, the incidence of superimposed preeclampsia with severe features increased as both the number of medications and blood pressure increased (p=0.02) as did the incidence of admissions to assess for superimposed (p<0.01). The incidence of SGA, preterm birth <35 weeks, and the composite neonatal outcome all increased as both the number of medications and blood pressure increased (p<0.01).
Table 2.
Maternal and Neonatal Outcomes by BP Control and Number of Medications
| Single Agent <140/90 |
Multiple Agent <140/90 |
Single Agent ≥140/90 |
Multiple Agent ≥140/90 |
p | |
|---|---|---|---|---|---|
| Superimposed Preeclampsia |
88 (19.7%) | 17 (23.3%) | 22 (33.3%) | 18 (36.7%) | <0.01 |
| Superimposed Severe PE |
53 (11.9%) | 10 (13.7%) | 10 (15.2%) | 14 (28.6%) | 0.02 |
| Cesarean Delivery |
212 (47.5%) | 30 (41.1%) | 32 (48.5%) | 26 (53.1%) | 0.61 |
| Admissions to r/o PE |
30 (6.7%) | 13 (17.8%) | 10 (15.2%) | 8 (16.3%) | <0.01 |
| SGA | 67 (15.0%) | 16 (21.9%) | 15 (22.7%) | 14 (29.2%) | 0.04 |
| PTB <35 weeks |
81 (18.5%) | 17 (23.9%) | 20 (32.3%) | 19 (42.2%) | <0.01 |
| Composite Neonatal |
49 (11.0%) | 12 (16.4%) | 12 (18.2%) | 16 (32.7%) | <0.01 |
We then compared each group to the reference group of single agent, blood pressures <140/90 while adjusting for confounding factors (Table 3). After adjusting for confounding variables, women on multiple blood pressure agents with blood pressures <140/90 were not at significantly increased odds of superimposed preeclampsia, superimposed preeclampsia with severe features, SGA, preterm birth <35 weeks, or the primary neonatal outcome compared to women with blood pressures <140/90 on a single antihypertensive agent. Women on a single agent and blood pressures ≥140/90 were at increased odds of admissions to rule out superimposed preeclampsia and preterm birth <35 weeks, but not at increased odds of superimposed preeclampsia, superimposed preeclampsia with severe features, SGA or the primary neonatal outcome. Women on multiple agents and blood pressure ≥140/90 were at increased odds of superimposed preeclampsia, superimposed preeclampsia with severe features, preterm birth <35 weeks, and composite neonatal outcome compared to women on a single agent with blood pressures <140/90.
Table 3.
Adjusted Odds of Adverse Outcomes Compared to Single Agent, BP<140/90 mm Hg
| Multiple Agent <140/90 |
Single Agent ≥140/90 | Multiple Agent ≥140/90 |
|
|---|---|---|---|
| Superimposed Preeclampsia* |
1.35 (0.75-2.46) | 2.04 (1.15-3.63) | 2.27 (1.19-4.28) |
| Superimposed Severe PE* |
1.34 (0.64-2.81) | 1.33 (0.63-2.83) | 2.86 (1.41-5.77) |
| Cesarean Delivery† | 0.89 (0.51-1.53) | 1.04 (0.60-1.81) | 1.25 (0.64-2.45) |
| Admissions to r/o PE‡ | 3.18 (1.56-6.51) | 2.44 (1.12-5.31) | 3.21 (1.36-7.58) |
| SGA§ | 1.36 (0.65-2.86) | 1.40 (0.68-2.88) | 1.82 (0.81-4.07) |
| PTB <35 weeks∥ | 1.35 (0.73-2.46) | 1.97 (1.09-3.56) | 3.05 (1.59-5.83) |
| Composite Neonatal ** | 1.63 (0.82-3.25) | 1.72 (0.86-3.45) | 3.84 (1.96-7.50) |
Adjusted for history of preeclampsia, nulliparity
Adjusted for prior vaginal delivery, diabetes, tobacco use
Adjusted for diabetes
Adjusted for race, body mass index, diabetes, and tobacco use
Adjusted for prior preterm delivery, nulliparity
Adjusted for nulliparity
Discussion
In this large cohort, increasing number of medications and blood pressures ≥140/90 were associated with increased risks of maternal complications and adverse neonatal outcomes, including superimposed preeclampsia, superimposed preeclampsia with severe features, preterm birth <35 weeks, and a composite adverse neonatal outcome. Our results imply that blood pressures ≥140/90 mm Hg are associated with a greater risk of maternal complications and adverse fetal outcomes, regardless of the number of medications used, although those patients on multiple agents whose blood pressures are >140/90 are at the highest risk.
Prior studies have shown significant differences in pregnancy outcomes between women with mild chronic hypertension (140-159 mm Hg systolic, 90-109 mm Hg diastolic) and those with severe hypertension (systolic ≥160 and/or diastolic ≥110) during pregnancy. The CHIPS Trial, a randomized control trial of less tight versus tight control of hypertension, showed no significant difference in serious adverse outcomes between “tight” blood pressure control (target diastolic blood pressure, 100 mm Hg) “less-tight” blood pressure control (target diastolic blood pressure, 85 mm Hg) .10,11 However, this study did not report on differences in outcomes based on how many antihypertensive agents were required to achieve blood pressure control. Requiring multiple blood pressure medications for blood pressure control in pregnancy is considered a marker of more severe disease, as evidenced by the recommendations to deliver women requiring medication up to one week earlier than women not requiring medication.12 However, this is largely based on clinical opinion. Our findings suggest that patients requiring multiple antihypertensive agents to achieve the same blood pressure are not at increased risk of adverse events compared to those only requiring a single antihypertensive agent to achieve the same blood pressure; however, poorly controlled blood pressures in spite of multiple agents is associated with an increased risk of adverse outcomes.
In a sub-group analysis of a large prospective cohort study by Ray et al, adverse fetal outcomes were measured in singleton gestations with hypertension. Women were exposed to no antihypertensive treatment, single beta-blocker therapy, single non-beta blocker therapy and combined beta-blocker plus non-beta-blocker therapy. The results indicated that combined antihypertensive therapy was strong indicator for preterm birth and a moderate marker of small for gestational age fetuses. This study, however, has several differences from our study. Ray et al. focused primarily on the administration of antihypertensive agents and did not evaluate blood pressure control. In their case, the administration of antihypertensive medications was considered “therapy” even with a one-time dose of an intravenous medication. Additionally, they did not distinguish preeclampsia treatment from the treatment of chronic hypertension.13
Su et al used a population-based cohort to investigate the impact of antihypertensive therapy on the fetus among women with chronic hypertension. Combined therapy (alpha blocker plus beta blocker) increased the risk of low birth weight (AOR 2.22, 95% CI 1.29-3.84), preterm birth (AOR 2.13, 95% CI 1.25 to 3.63) and small for gestational age (AOR 2.11, 95% CI 1.39 to 3.19) compared to untreated women with chronic hypertension. Although antihypertensive medication use was associated with increased risk of low birth weight, preterm birth and small for gestational age compared to untreated women with chronic hypertension, the absolute incidence of these adverse outcomes appeared similar between women on combination therapy and single therapy.14
The advantage of our large retrospective study is its ability to offer detailed patient-level data, enabling the stratification of patient outcomes by blood pressure control actually achieved. In addition, the study uses a strict definition of superimposed preeclampsia, allowing for precise measurement of maternal outcomes.
A major limitation of the study is the relatively small sample size of subjects who failed to achieve blood pressure control <140/90 mmHg. Due to this small sample size, subjects with blood pressures in the mild hypertension of 140-159/90-109 as well as severe hypertension range were categorized into the same group, making it impossible to distinguish the effects of mild and severe hypertension in our study.
In conclusion, blood pressure control, rather than the number of agents used to achieve that blood pressure control, seems most associated with pregnancy outcomes. Future studies should further stratify blood pressures into normotensive, mild hypertension and severe hypertension to better define outcomes. A larger sample size is likely necessary for this endeavor, due to the few subjects with blood pressure outcomes in the severe range. There continues to be an urgent need for prospective clinical trials investigating both fetal and maternal outcomes in women with chronic hypertension.
Acknowledgements
Dr. Harper is supported by K12HD001258 (PI WW Andrews, NICHD), which partially supports this work.
Footnotes
Conflict of Interest
The authors declare that there does not exist any competing financial interests in relation to the work described.
References
- 1.Vanek M, Sheiner E, Levy A, Mazor M. Chronic hypertension and the risk for adverse pregnancy outcome after superimposed preeclampsia. Int J Gynaecol Obstet. 2004;86:7–11. doi: 10.1016/j.ijgo.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM. Chronic Hypertension in Pregnancy. Obsetrics and Gynecology. 2002;100:369–77. doi: 10.1016/s0029-7844(02)02128-2. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM, Abdella TN, Anderson GD. Pregancy outcomes in 211 patients with mild chronic hypertension. Obstet Gynecol. 1983;61:571–6. [PubMed] [Google Scholar]
- 4.Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal Fetal Medicine Units. N Eng J Med. 1998;339:667–71. doi: 10.1056/NEJM199809033391004. [DOI] [PubMed] [Google Scholar]
- 5.Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. AM J Obstet Gynecol. 1994;171:410–6. doi: 10.1016/0002-9378(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 6.McCowan LM, Buist RG, North RA, Gamble G. Perinatal morbidity in chronic hypertension. Br J Obstet Gynecol. 1996;103:123–9. doi: 10.1111/j.1471-0528.1996.tb09662.x. [DOI] [PubMed] [Google Scholar]
- 7.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynceol. 2000;183:S1–22. [PubMed] [Google Scholar]
- 8.American Congress of Obstetricians and Gynecologists . Hypertension in Pregnancy. American Congress of Obstetricians and Gynecologists; Washington DC: 2013. Task force on hypertension in pregnancy. [Google Scholar]
- 9.The American College of Obstetricians and Gynecologists Committee on Obstetrics Practice. The Society for Maternal-Fetal Medicine Medically Indicated Late-Preterm and Early-Term Deliveries. Apr, 2013. Committee Opinion Number 560.
- 10.Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015 Jan;372(5):407–17. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 11.Ankumah NA, Cantu J, Jauk V, Biggio J, Hauth J, Andrews W, Tita AT. Risk of adverse pregnancy outcomes in women with mild chronic hypertension before 20 weeks of gestation. Obstet Gynecol. 2014;123(5):966–72. doi: 10.1097/AOG.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 12.Spong C. Defining “Term” Pregnancy: Recommendations From the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309(23):2445–2446. doi: 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- 13.Ray JG, Vermeulen MJ, Burrows EA, Burrows RF. Use of antihypertensive medications in pregnancy and the risk of adverse perinatal outcomes: McMaster Outcome Study of Hypertension In Pregnancy 2 (MOS HIP 2) BMC Pregnancy and Childbirth. 2001;1:6. doi: 10.1186/1471-2393-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su C-Y, Lin H-C, Cheng H-C, Yen AM-F, Chen Y-H, et al. Pregnancy Outcomes of Anti-Hypertensives for Women with Chronic Hypertension: A Population-Based Study. PLoS ONE. 2013;8(2):e53844. doi: 10.1371/journal.pone.0053844. [DOI] [PMC free article] [PubMed] [Google Scholar]