Abstract
Objectives
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves motor symptoms in advanced Parkinson's disease. STN DBS may also affect emotion, possibly by impacting a parallel limbic cortico-striatal circuit. The objective of this study was to investigate changes in prefrontal cortical activity related to DBS during an emotion induction task.
Materials and Methods
We used near infrared spectroscopy to monitor prefrontal cortex hemodynamic changes during an emotion induction task. Seven DBS patients were tested sequentially in the stimulation-on and stimulation-off states while on dopaminergic medication. Patients watched a series of positive, negative, and neutral videos. The general linear model was used to compare prefrontal oxygenated hemoglobin concentration between DBS states.
Results
Deep brain stimulation was correlated with prefrontal oxygenated hemoglobin changes relative to the stimulation off state in response to both positive and negative videos. These changes were specific to emotional stimuli and were not seen during neutral stimuli.
Conclusions
These results suggest that STN stimulation influences the prefrontal cortical representation of positive and negative emotion induction.
Keywords: Parkinson's disease, subthalamic nucleus, emotion, deep brain stimulation, near infrared spectroscopy
INTRODUCTION
Deep brain stimulation (DBS) of the bilateral subthalamic nuclei (STN) is an effective and accepted treatment for advanced Parkinson's disease (PD).1 However, a growing body of evidence demonstrates clinically significant emotional disturbances following DBS surgery.2 In addition to improving motor symptoms of Parkinson's disease, STN DBS may alter emotion processing, impairing emotion recognition,3-5 altering emotion induction,6-8 and affect mood and depression symptoms.9,10 STN DBS therefore presents an opportunity to investigate the role of the STN in emotion induction.
Changes in emotion processing may be explained by the perturbation of limbic circuits caused by STN DBS. The STN has specific sensorimotor, associative, and limbic substructures with indistinct boundaries.11 Changes in STN local field potentials area observed during viewing of positive and negative stimuli and may correlate with valence.12,13 The STN limbic subdivision receives projections from anterior cingulate cortex (ACC), orbitofrontal cortex, nucleus accumbens, ventral pallidum, globus pallidus externus, and the ventral tegmental area and projects to substantia nigra, globus pallidus, and ventral tegmental area,14 and as such is positioned to modulate limbic signaling.
STN DBS alters cortical activity in limbic regions. Decreased activity in ACC, middle frontal gyrus, insula, and superior temporal gyrus and increased activity in parahippocampal gyrus, posterior cingulate gyrus, and medial frontal gyrus were reported with STN DBS.15 Impaired recognition of fearful facial expressions in PD patients correlated with decreased OFC activity during the resting state with DBS on.16 To our knowledge, no study has directly examined DBS related changes in cortical activity specifically during an emotion task. The goal of our study was to determine whether STN DBS affects the prefrontal cortical representation of emotion induction. We hypothesized that STN DBS would alter prefrontal oxyhemoglobin changes during positive and negative emotion induction.
MATERIALS AND METHODS
Participants
Participants were recruited from a randomized controlled study of DBS in early PD (FDA Investigational Device Exemption G050016, ClinicalTrials.gov NCT00282152) and had been implanted with bilateral STN DBS.17 Participants in this study had been treated with medication for between 6 months and 4 years at the time of the study and were classified as Hoehn and Yahr stage II off medication. The Vanderbilt Institutional Review Board approved our study (071210). Participants provided written informed consent. Participants were tested in the DBS on and off conditions (DBSon/DBSoff) and were on dopaminergic medication during testing.
Levodopa equivalent dose was calculated using the conversion L-dopa (mg) + (L-dopa + entacapone/tolcapone (mg)) × 1.33 + rotigotine (mg) × 5 + ropinirole (mg) × 20 + pramiprexole (mg) × 67 + cabergoline (mg) × 67 + pergolide (mg) × 100.18,19 For all patients in the study Unified Parkinson's Disease Rating Scale motor scores (UPDRS III) were obtained during a week long period of observation. Scores were taken at the beginning of the week in a stimulation-on and medication-on state and at the end of the week in the stimulation-off and medication-off state.
All patients underwent neuropsychological testing prior to inclusion in the DBS for early PD study, with evaluations including the Dementia Rating Scale-2 and the Beck Depression Inventory II (BDI-II). At the time of testing for the current study, the Wechsler Test of Adult Reading, the Wechsler Abbreviated Intelligence Scale, and the Profile of Mood States were also completed. The Profile of Mood States was obtained at each testing session to determine whether there was a difference in mood disturbance score prior to the emotion induction task in the different testing conditions.
DBS Surgery and Contact Location
Deep brain stimulation electrodes model 3389 (Medtronic Inc.) were bilaterally implanted using standard stereotactic methods and incorporating intraoperative microelectrode recording as previously described.20
Following surgery, CT scans were obtained on the same day to confirm electrode location. Postoperative CT scans were coregistered with preoperative MRI scans in order to localize electrodes. We subsequently identified the contact of stimulation being used after a period of clinical optimization. The location of active contacts in anterior commissure – posterior commissure space was tabulated.
Emotion Induction Task
A series of video clips was chosen from a database of video segments characterized for emotion induction properties.21 We used video stimuli because film has been shown to be one of the most effective methods of emotion induction.22 Participants viewed a series of 8 emotional video clips at each session with 4 positive and 4 negative valence clips shown in random order. Emotional video clips were 1.6-4.6 minutes in length. There were 2 different blocks of videos so that subjects did not view the same set of videos in the 2 testing sessions. A 30 second neutral valence video between consecutive emotional video clips served as a control. Because of constraints of the schedule of the early PD study that subjects were recruited from, we were unable to randomize the order of testing states. Patients were tested with DBS on and then again with DBS off 24 hours later, 30 minutes to 4 hours after DBS had been turned off.
NIRS
We used near infrared spectroscopy (NIRS) (22 channel Hitachi ETG-4000) to monitor changes in oxyhemoglobin (oxyHb) concentration during the emotion induction task. NIRS uses light in the near infrared spectrum to monitor changes in oxyHb levels and can detect cortical activity similar to the functional magnetic resonance imaging (fMRI) blood oxygenation level dependent response.23 We chose to use NIRS because it is noninvasive, compatible with implanted electrodes, and has good motion tolerance for the tremor that is inevitable in testing patients with DBS turned off. NIRS has previously been used to demonstrate increased oxyHb in bilateral ventrolateral prefrontal cortex regions during negative and decreased oxyHb in left dorsolateral prefrontal cortex area during positive emotion induction.24 Regions such as OFC involved in STN limbic circuitry are accessible to NIRS.25
Data Analysis
Raw data were processed using Matlab (MathWorks, Natick MA). Temporal downsampling from 10 Hz to 1 Hz and data conversion to oxyHb, deoxyHb, and total hemoglobin levels using the modified Beer-Lambert Law, and normalization were first performed. Data were then converted into a format compatible with Brain Voyager QX (Brain Innovation, Maastricht). Linear drift correction and further statistical analyses were performed with Brain Voyager QX. The general linear moved was used to compare NIRS signals between DBS states. To determine the effect of DBS on baseline oxyHb, the signal during neutral stimuli for DBSoff was subtracted from that for DBSon. To determine the emotion induction specific effect, the signal during emotional relative to neutral stimuli during DBSoff was subtracted from this signal during DBSon, generating statistical parametric maps for the effect of DBS on oxyHb concentration during emotional stimuli. A false discovery rate set at 0.05 was utilized to correct for multiple comparisons.
RESULTS
Participants
There were 7 participants, 1 woman and 6 men. Demographic and clinical variables are presented in table 1.
Table 1.
Demographic and clinical information
| Mean (SD) | |
|---|---|
| Age | 64 (7.5) |
| Education (years) | 15 (2.6) |
| Disease duration (years) | 3.7 (1.8) |
| Time since surgery (months) | 14.3 (7.7) |
| Levodopa equivalent dose | 331 (192.7) |
| UPDRS III on DBS and dopaminergic medication | 23.3 (10.9) |
| UPDRS III off DBS and dopaminergic medication | 42.3 (13.7) |
| Baseline DRS-2 | 14.3 (1.0) |
| WTAR | 111.4 (9.6) |
| WAIS | 112.1 (10.8) |
| Baseline BDI-II | 6.1 (4.1) |
| POMS | |
| DBSon | 60.7 (10.6) |
| DBSoff | 66.7 (5.5) |
BDI-II: Beck Depression Inventory II, DRS-II: Dementia Rating Scale II, POMS: profile of mood states, UPDRS III: Unified Parkinson Disease Rating Scale motor section, WASI: Wechsler Abbreviated Scale of Intelligence, WTAR: Wechsler Test of Adult Reading
Neuropsychological tests were within normal ranges (table 1). In particular, BDI-II scores fell within the minimal depression range of 0-13. The Profile of Mood States total mood disturbance score was 60.7 in the DBSon condition and 66.7 in the DBSoff condition (p = 0.43, student's t test), suggesting that there was no difference in mood state prior to emotion induction task in the different testing conditions (table 1).
DBS Placement and Settings
All patients were stimulated at a rate of 130Hz and with a pulse width of 60μsec. Table 2 demonstrates the location of the active contacts in AC-PC coordinate space and the amplitude of stimulation used. Average amplitude of stimulation was 1.8V. The mean location of the active contact was X=11.18 (standard deviation 2.09), Y=−3.02 (2.24), Z=−3.15 (3.35), consistent with the region we generally target for STN DBS. All active contacts were located within the STN.
Table 2.
Stimulation parameters and AC-PC location of active contacts
| Subject | Left Side | Right Side | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active Contacts | Amp. (V) | Location X | Location Y | Location Z | Active Contacts | Amp. (V) | Location X | Location Y | Location Z | |
| 1 | 2−, C+ | 1.8 | 8.01 | −6.20 | 5.83 | 2−, C+ | 1.8 | 11.59 | −4.09 | −5.38 |
| 2 | 3−, C+ | 2.2 | 13.74 | −0.09 | 1.68 | 0−, C+ | 1.7 | 7.10 | −4.82 | −2.81 |
| 3 | 2−, C+ | 2.0 | 11.98 | −1.39 | 0.95 | 3−, C+ | 2.0 | 11.90 | −1.03 | 4.73 |
| 4 | 3−, C+ | 1.7 | 10.15 | −4.08 | −4.65 | 2−, C+ | 1.7 | 14.13 | 0.15 | −6.76 |
| 5 | 1−, C+ | 1.8 | 13.62 | −2.08 | −4.05 | 2−, C+ | 1.8 | 12.00 | −3.6 | −4.14 |
| 6 | 1−, C+ | 1.4 | 10.69 | −4.70 | −5.26 | 0−, C+ | 1.4 | 11.5 | −7.1 | −6.27 |
| 7 | 2−, C+ | 2.0 | 8.82 | −1.67 | −2.66 | 2−, C+ | 1.7 | 11.31 | −1.58 | −3.64 |
NIRS
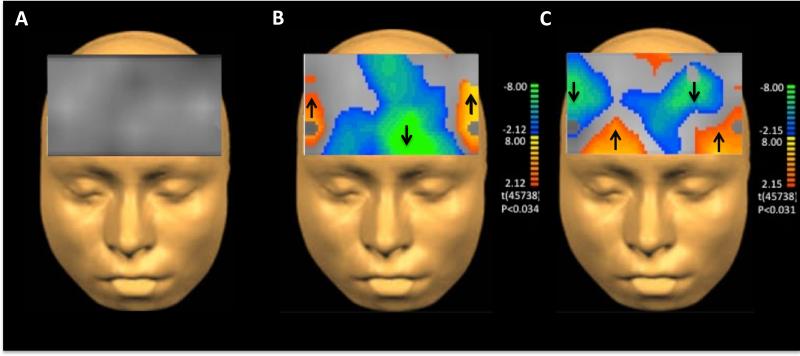
In the resting state, DBSon did not cause significant changes in prefrontal oxyHb compared to DBSoff (figure 1A). During emotion induction, STN stimulation was correlated with decreased frontopolar oxyHb concentration while viewing positive valence videos compared to the off stimulation state (p=0.034, figure 1B). STN stimulation also altered oxyHb concentration during negative video viewing with decreased frontopolar and right lateral prefrontal cortex oxyHb concentration and increased bilateral inferior ventrolateral prefrontal cortex oxyHb concentration compared to DBSoff (p=0.031, figure 1C). These findings suggest that STN DBS alters prefrontal activity specifically during emotional stimuli.
Figure 1.
Stimulation state affected prefrontal oxygenated hemoglobin concentration during emotional stimuli. Areas of color indicate regions of significant change; warm colors indicate significant increase while cool colors indicate significant decrease in prefrontal oxyHb. Arrows indicate the direction of change. A During neutral stimuli there were no significant changes in prefrontal activity in the DBSon compared to the DBSoff state. B, C Images represent the difference in prefrontal activation between DBSon compared to DBSoff during emotional relative to neutral videos. B During positive valence stimuli DBS patients had significantly less frontal polar oxygenated hemoglobin concentration in the DBSon state than the DBSoff state. C During negative stimuli the DBSon state was correlated with decreased frontopolar and right lateral prefrontal cortex oxyHb concentration and increased bilateral inferior ventrolateral prefrontal cortex oxyHb concentration compared to DBSoff
DISCUSSION
We show that STN stimulation alters prefrontal hemodynamics during an emotion induction task but not during emotionally neutral stimuli. These findings suggest that STN DBS alters limbic circuits and prefrontal activity specifically during emotion induction.
These findings support a role for the STN in limbic circuitry. The STN has sensorimotor, associative, and limbic subdivisions whose borders are not sharply demarcated.11 The STN is a subcentimeter structure, and spread of current from contacts in the sensorimotor subdivision to the limbic subdivision may occur, resulting in changes in prefrontal cortical activity via modulation of limbic cortico-basal ganglia-thalamocortico circuits. The STN also has reciprocal direct projections with prefrontal cortex 26 which may be modulated by STN DBS and contribute to the changes in prefrontal activity seen during emotion induction.
Prefrontal cortex plays an important role in emotion processing.27,28 Prefrontal activity has a strong contribution to representation of induction of discrete positive and negative valence emotions.29 NIRS has previously been used to demonstrate changes in prefrontal activity correlated with emotional responses to music.30 The prefrontal cortex additionally contributes to emotional control and regulation.31 The DBS dependent changes we observed in prefrontal activity during emotion induction may denote differences in primary representation of emotion related to differences in strength of emotion induction with DBS or alternatively may represent DBS related changes in emotional regulation.
In keeping with the changes in prefrontal activity we observed, clinical changes in mood states may occur after STN DBS. The observed changes are complex and heterogeneous. Both decreased intensity of negative emotion induction and greater mood and emotion induction have been reported with STN DBS.6-8 Increased, decreased, and unchanged rates of depression have all been reported.7,9,10,32,33 The heterogeneous effects of STN DBS on emotion may be related to different locations of contacts stimulated.11,34,35 Interaction between medication and stimulation status may also contribute.36 Due to limitations in time and study design we were unable to investigate the interaction between medication and stimulation state, for example by also testing patients in the medications off and stimulation on and off states. While our within subject comparison analysis helps to control for the effect of medication, further studies with patients in these additional states may help to elucidate the interaction between dopaminergic medication and DBS. Finally, underlying mood state may impact findings; STN activity in response to emotionally charged pictures correlates with Beck Depression Inventory scores.37 An additional limitation of our study is the small sample size, and due to this we were unable to examine for the effect of time after surgery on the described findings. Overall, the effects of STN DBS on emotion are complex and heterogeneous, reflecting the complexity and poorly understood nature of emotions and emotion induction, but clearly exist. Our findings of altered prefrontal activity during an emotion induction task illustrate a physiological change during DBS specific to emotion induction that may contribute to some of these findings.
CONCLUSION
In this study we show that STN stimulation is correlated with unique patterns of changes in prefrontal metabolism in PD patients during an emotion induction task. This finding has important implications for the changes in emotion recognition and experience reported in PD patients treated with STN DBS. Additional study may help to further describe the mechanism of these findings.
Acknowledgments
Sources of Financial Support: The study was funded by a National Institutes of Health R21 grant (grant number R21NS070136-02). Funding from NIH grants RO1 EB006136 and RO1 NS095291 09 also contributed to the project.
Footnotes
Authorship Statement: Dr. Bick designed and conducted the study. She was responsible for data collection, analysis, and interpretation, and drafted the paper. Dr. Folley participated in study design and data analysis. Dr. Mayer participated in data collection and analysis. Dr. Park assisted with study design and provided expertise in the collection and analysis of NIRS data. Dr. Charles assisted with patient recruitment, study oversight, and manuscript preparation. Dr. Camalier contributed to data analysis and manuscript preparation. Dr. Pallavaram assisted with data analysis and localization of active DBS contacts. Dr. Konrad participated in study design and manuscript preparation. Dr. Neimat contributed to study design, data analysis, and manuscript preparation. All authors critically reviewed the manuscript and approved the final version.
Conflict of Interest Statement: Dr. Charles has received income from Medtronic, Ipsen, Teva, Merz, and Allergan for education and consulting services. Dr. Konrad has served as a consultant for Medtronic and FHC, Inc. Dr. Neimat has received income from Medtronic for consulting services. All other authors declare no conflict of interest.
REFERENCES
- 1.Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349(20):1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 2.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord. 2006;12(5):265–272. doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Drapier D, Peron J, Leray E, Sauleau P, Biseul I, Drapier S, et al. Emotion recognition impairment and apathy after subthalamic nucleus stimulation in Parkinson's disease have separate neural substrates. Neuropsychologia. 2008;46(11):2796–2801. doi: 10.1016/j.neuropsychologia.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Biseul I, Sauleau P, Haegelen C, Trebon P, Drapier D, Raoul S, et al. Fear recognition is impaired by subthalamic nucleus stimulation in Parkinson's disease. Neuropsychologia. 2005;43(7):1054–1059. doi: 10.1016/j.neuropsychologia.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Dujardin K, Blairy S, Defebvre L, Krystkowiak P, Hess U, Blond S, et al. Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- 6.Ory S, Le Jeune F, Haegelen C, Vicente S, Philippot P, Dondaine T, et al. Pre-frontal-insular-cerebellar modifications correlate with disgust feeling blunting after subthalamic stimulation: A positron emission tomography study in Parkinson's disease. J Neuropsychol. 2015 doi: 10.1111/jnp.12094. [DOI] [PubMed] [Google Scholar]
- 7.Schneider F, Habel U, Volkmann J, Regel S, Kornischka J, Sturm V, et al. Deep brain stimulation of the subthalamic nucleus enhances emotional processing in Parkinson disease. Arch Gen Psychiatry. 2003;60(3):296–302. doi: 10.1001/archpsyc.60.3.296. [DOI] [PubMed] [Google Scholar]
- 8.Vicente S, Biseul I, Peron J, Philippot P, Drapier S, Drapier D, et al. Subthalamic nucleus stimulation affects subjective emotional experience in Parkinson's disease patients. Neuropsychologia. 2009;47(8-9):1928–1937. doi: 10.1016/j.neuropsychologia.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Berney A, Vingerhoets F, Perrin A, Guex P, Villemure JG, Burkhard PR, et al. Effect on mood of subthalamic DBS for Parkinson's disease: a consecutive series of 24 patients. Neurology. 2002;59(9):1427–1429. doi: 10.1212/01.wnl.0000032756.14298.18. [DOI] [PubMed] [Google Scholar]
- 10.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 11.Mallet L, Schupbach M, N'Diaye K, Remy P, Bardinet E, Czernecki V, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci U S A. 2007;104(25):10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brucke C, Kupsch A, Schneider GH, Hariz MI, Nuttin B, Kopp U, et al. The subthalamic region is activated during valence-related emotional processing in patients with Parkinson's disease. Eur J Neurosci. 2007;26(3):767–774. doi: 10.1111/j.1460-9568.2007.05683.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn AA, Hariz MI, Silberstein P, Tisch S, Kupsch A, Schneider GH, et al. Activation of the subthalamic region during emotional processing in Parkinson disease. Neurology. 2005;65(5):707–713. doi: 10.1212/01.wnl.0000174438.78399.bc. [DOI] [PubMed] [Google Scholar]
- 14.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20(1):128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 15.Le Jeune F, Peron J, Grandjean D, Drapier S, Haegelen C, Garin E, et al. Subthalamic nucleus stimulation affects limbic and associative circuits: a PET study. Eur J Nucl Med Mol Imaging. 2010;37(8):1512–1520. doi: 10.1007/s00259-010-1436-y. [DOI] [PubMed] [Google Scholar]
- 16.Le Jeune F, Peron J, Biseul I, Fournier S, Sauleau P, Drapier S, et al. Subthalamic nucleus stimulation affects orbitofrontal cortex in facial emotion recognition: a PET study. Brain. 2008;131(Pt 6):1599–1608. doi: 10.1093/brain/awn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charles PD, Dolhun RM, Gill CE, Davis TL, Bliton MJ, Tramontana MG, et al. Deep brain stimulation in early Parkinson's disease: Enrollment experience from a pilot trial. Parkinsonism Relat Disord. 2012;18(3):268–273. doi: 10.1016/j.parkreldis.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosset K, Needleman F, Macphee G, Grosset D. Switching from ergot to nonergot dopamine agonists in Parkinson's disease: a clinical series and five-drug dose conversion table. Mov Disord. 2004;19(11):1370–1374. doi: 10.1002/mds.20210. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Oroz MC, Lopez-Azcarate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson's disease. Brain. 2011;134(Pt 1):36–49. doi: 10.1093/brain/awq301. [DOI] [PubMed] [Google Scholar]
- 20.Kahn E, D'Haese PF, Dawant B, Allen L, Kao C, Charles PD, et al. Deep brain stimulation in early stage Parkinson's disease: operative experience from a prospective randomised clinical trial. J Neurol Neurosurg Psychiatry. 2012;83(2):164–170. doi: 10.1136/jnnp-2011-300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer A, Nils Fdr, Sanchez X, Philippot P. Assessing the effectiveness of a large database of emotion-eliciting films: A new tool for emotion researchers. Cognition & Emotion. 2010;24(7):1153–1172. [Google Scholar]
- 22.Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood inductino procedures: A meta-analysis. European Journal of Social Psychology. 1996;26:557–580. [Google Scholar]
- 23.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17(2):719–731. [PubMed] [Google Scholar]
- 24.Hoshi Y, Huang J, Kohri S, Iguchi Y, Naya M, Okamoto T, et al. Recognition of human emotions from cerebral blood flow changes in the frontal region: a study with event-related near-infrared spectroscopy. J Neuroimaging. 2011;21(2):e94–101. doi: 10.1111/j.1552-6569.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 25.Tobias JD. Cerebral oximetry monitoring with near infrared spectroscopy detects alterations in oxygenation before pulse oximetry. J Intensive Care Med. 2008;23(6):384–388. doi: 10.1177/0885066608324380. [DOI] [PubMed] [Google Scholar]
- 26.Degos B, Deniau JM, Le Cam J, Mailly P, Maurice N. Evidence for a direct subthalamo-cortical loop circuit in the rat. Eur J Neurosci. 2008;27(10):2599–2610. doi: 10.1111/j.1460-9568.2008.06229.x. [DOI] [PubMed] [Google Scholar]
- 27.Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biol Psychol. 2004;67(1-2):219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 29.Saarimaki H, Gotsopoulos A, Jaaskelainen IP, Lampinen J, Vuilleumier P, Hari R, et al. Discrete Neural Signatures of Basic Emotions. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv086. [DOI] [PubMed] [Google Scholar]
- 30.Moghimi S, Kushki A, Guerguerian AM, Chau T. Characterizing emotional response to music in the prefrontal cortex using near infrared spectroscopy. Neurosci Lett. 2012;525(1):7–11. doi: 10.1016/j.neulet.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Raio CM, Orederu TA, Palazzolo L, Shurick AA, Phelps EA. Cognitive emotion regulation fails the stress test. Proc Natl Acad Sci U S A. 2013;110(37):15139–15144. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser I, Kryspin-Exner I, Brucke T, Volc D, Alesch F. Long-term effects of STN DBS on mood: psychosocial profiles remain stable in a 3-year follow-up. BMC Neurol. 2008;8:43. doi: 10.1186/1471-2377-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61(8):1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- 34.Greenhouse I, Gould S, Houser M, Hicks G, Gross J, Aron AR. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson's disease. Neuropsychologia. 2011;49(3):528–534. doi: 10.1016/j.neuropsychologia.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson's disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65(5):586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mondillon L, Mermillod M, Musca SC, Rieu I, Vidal T, Chambres P, et al. The combined effect of subthalamic nuclei deep brain stimulation and L-dopa increases emotion recognition in Parkinson's disease. Neuropsychologia. 2012;50(12):2869–2879. doi: 10.1016/j.neuropsychologia.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Huebl J, Schoenecker T, Siegert S, Brucke C, Schneider GH, Kupsch A, et al. Modulation of subthalamic alpha activity to emotional stimuli correlates with depressive symptoms in Parkinson's disease. Mov Disord. 2011;26(3):477–483. doi: 10.1002/mds.23515. [DOI] [PubMed] [Google Scholar]