Abstract
OBJECTIVE
Very preterm infants hospitalized in the neonatal intensive care unit (NICU) experience alterations in sensory experiences. Defining types, timing and frequency of sensory-based interventions that optimize outcomes can inform environmental modifications. The objective of this study was to conduct an integrative review on sensory-based interventions used with very preterm infants in the NICU to improve infant and parent outcomes.
STUDY DESIGN
The data sources include MEDLINE, CINAHL, Cochrane Library and Google Scholar. Studies were identified that used sensory-based interventions in the NICU with preterm infants born ≤32 weeks gestation, were published in a peer-reviewed journal between 1995 and 2015, and measured outcomes related to infant and parent outcomes. Studies were extracted from electronic databases and hand-searched from identified reference lists.
RESULTS
Eighty-eight articles were identified (31 tactile, 12 auditory, 3 visual, 2 kinesthetic, 2 gustatory/olfactory and 37 multimodal). There was evidence to support the use of kangaroo care, music and language exposure, and multimodal interventions starting at 25 to 28 weeks postmenstrual age. These interventions were related to better infant development and lower maternal stress, but not all findings were consistent. Limitations included lack of consistent outcome measures, study quality and gaps in the literature.
CONCLUSIONS
Most research identified interventions that were done for short periods of time. It is unclear what the potential is for improving outcomes if positive sensory exposures occur consistently throughout NICU hospitalization. Until more research defines appropriate sensory-based interventions to use with infants born very preterm in the NICU, information from this review can be combined with expert opinion and parent/family values to determine best practice.
INTRODUCTION
Very preterm infants, those born ≤ 32 weeks estimated gestational age (EGA), have a high incidence of long-term morbidity, which is not fully explained by clinical course or the presence of brain injury.1–8 A less-studied influence on long-term outcome is the neonatal intensive care unit (NICU) environment, which may influence neural development of very preterm infants during a sensitive period of brain growth.9 The NICU environment can be fraught with excess light and sound,10–12 as well as other chaotic types of stimulation, when infants lack mature coping mechanisms. As such, minimal stimulation, interaction and/or reduction of environmental exposures have become common NICU practices.10,13,14
Based on current practice models that aim to minimize sensory exposure and facilitate parent–child interaction, hospitals throughout the United States and abroad are renovating NICU spaces to include private rooms. However, it may be important to focus attention on the sensory environment in private NICU rooms where near-complete sensory abatement is possible. We previously investigated differences in neurodevelopmental outcomes in very preterm infants hospitalized in an open ward NICU compared with private rooms,15 and found alterations in brain structure by term equivalent age and significantly poorer language outcomes in preterm infants in low stimulation private rooms.15 These results complement other studies that have demonstrated the importance of development during the NICU period16 and the need for positive sensory exposures to optimize outcomes.17,18 A recent editorial also questioned whether NICU infants are at risk for sensory deprivation in low-stimulation private rooms.19
While noxious sensory stimulation during periods of medical fragility may be detrimental to health,12,20,21 the appropriate amount of optimal sensory stimulation for very preterm infants is poorly defined, understood and implemented. Positive sensory exposures can have lifelong implications on learning, memory, emotions and developmental progression.22 Further, it is well understood that infants receive multidimensional sensory exposures in utero in the final months of pregnancy,23 but the very preterm infant in the NICU misses potentially important, timed exposures that may facilitate neural pathways. An intentional, enhanced sensory environment has the potential to improve infant experiences and promote optimal outcomes for both infants and parents. Careful consideration of an appropriate sensory-based intervention plan should include interventions that have evidence to support their use in this vulnerable population. Important considerations include (1) ensuring that sensory interventions are appropriately timed according to the infant's readiness to accept and benefit from stimuli, based on the sequential order of development and maturation of the sensory system;16 (2) making adaptations available for infants with limiting medical conditions; and (3) ensuring that the amounts and types of interventions across different levels of maturation are defined. However, before an intentional enhanced sensory environment can be defined, a comprehensive review of current evidence is critical. While there are existing reviews of developmental care, neurodevelopmentally supportive care, kangaroo care and single intervention exposures,24–31 to date, reviews on the current state of the science on multisensory exposure for very preterm infants across postmenstrual age (PMA) in the NICU are lacking.
MATERIALS AND METHODS
Purpose
The purpose of this integrative review was to identify evidence for sensory exposures for very preterm infants in the NICU in relation to their impact on neurodevelopmental outcomes of the infant as well as outcomes of the parents. It was of interest to also define the type, amount and timing of sensory-based interventions in the NICU.
Procedures
An integrative review was used to highlight the most relevant evidence related to sensory exposure in the NICU from a range of clinical research methodologies. Various study designs (systematic reviews, randomized controlled trials, quasi-experimental, crossover or single-group repeated measure studies) published in the last 20 years were included. Studies prior to 1995 were excluded to eliminate studies that were done well before current advances in NICU culture, practice and care. The population of interest was very preterm infants born ≤ 32 weeks gestation who were hospitalized in the NICU and had a sensory-based intervention that commenced prior to 36 weeks PMA. Very preterm infants (born > 32 weeks gestation) were the population of interest, as we sought to define optimal sensory exposures among the most fragile and vulnerable infants who were hospitalized in the NICU for significant periods of time. Infants born 432 weeks gestation were excluded, as sensory exposures in the NICU are shorter in duration, many times lasting only a few days or 1 week, consistent with shorter length of stay, in comparison with very preterm infants, who can be hospitalized in the NICU for several weeks or months. Studies that imposed a quantifiable environmental sensory exposure during the NICU stay were included. Studies that identified unimodal interventions were described in their respective categories (tactile, auditory, vestibular, kinesthetic, visual or olfactory/gustatory), while interventions that included more than one type of sensory exposure or those that compared one sensory exposure with a different type of sensory exposure were included in the multimodal category. The comparison group received no identified sensory intervention or standard of care, varying levels of the same or similar intervention, or a different sensory exposure. interventions could be performed by health-care workers, study investigators or parents. Relevant outcomes included infant behavioral outcomes, infant physiology, maternal mental health and parental outcomes. Physiology and behavior were included as outcomes, as it was felt that they could be tied into development in the very preterm infant. Samples of healthy infants were excluded, as this review was intended to define sensory exposures for infants who represent medically complex very preterm infants in the NICU.
Studies with sample size < 30 and no a priori calculation of power that was met were excluded. This was done to focus energies on studies with adequate sample size in order to make inferences from statistical analyses investigating associations. Finally, studies that included outcomes of pain or breastfeeding were excluded, as it was felt that they warranted their own review. See Table 1 for the exclusion criteria for this review. See Table 2 for search criteria and keywords.
Table 1.
Study exclusion criteria
| Population |
| Populations with mean or median gestational age greater than 32 weeks |
| Populations with mean or median postmenstrual age greater than 36 weeks at time of intervention |
| Populations with a purposeful sample of healthy infants (defined as 3 or more of the following factors: never on oxygen, never on medications, no intraventricular hemorrhage or other perinatal brain injury, or if Apgar scores were >7 at 1 or 5 min) |
| Interventions |
| Interventions aimed at reduction of external stimuli (e.g. headphones to reduce noise) |
| Interventions aimed at reducing pain (e.g. during heel stick or endotracheal suctioning) |
| Breastfeeding interventions |
| Therapeutic touch (non-touch, energy-balancing technique) |
| Pacifier-activated sound (includes use of a learning element) |
| Vibrating pacifiers (includes use of a learning element) |
| Breathing bear (no direct intervention to the infant) |
| NIDCAP (interventions individualized for each infant rather than a uniform, quantifiable intervention) |
| Non-relevant outcomes |
| Apnea |
| Incidence of retinopathy of prematurity |
| Breastfeeding measures or feeding outcomes |
| Study design and other factors |
| Studies published before 1995 |
| Studies with a sample size < 30 without an a priori power calculation or sample size not attained |
| Observational studies |
| Pilot or feasibility studies |
| Studies without a comparison group (case reports or case series) |
| Systematic reviews that included studies with different EGA and PMA criteria |
| Primary studies included as part of a relevant systematic review |
| Non-English language studies |
| Studies not published in a peer-reviewed journal (conference abstracts or dissertations) |
| Studies with unclear or incomplete methods, statistical analysis or results |
Abbreviations: EGA, estimated gestational age; NIDCAP, Newborn Individualized Developmental Care and Assessment Program; PMA, postmenstrual age.
Table 2.
Search criteria, keywords and sample search strategy
| Subject | Keywords | MeSH Terms |
|---|---|---|
| Auditory | music therapy, music, Bach, Mozart, lullaby, singing, maternal AND (heartbeat OR voice OR speech OR sound), auditory stimulation, adult talk, parent talk | Music Therapy; Singing; Acoustic Stimulation; Voice; Speech |
| Gustatory/Olfactory | colostrum, oral immune therapy, sucrose, flavor, gustatory, taste, oral hygiene, oral care, buccal care, smell, scent, olfactory, odor | Colostrum; Sucrose; Taste Perception; Taste; Flavoring Agents; Oral Hygiene; Smell; Olfactory Perception; Odors |
| Kinesthetic | kinesthetic, range of motion, physical therapy, exercise, physical activity, physiotherapy, passive limb movement, extension AND flexion | Kinesthesis; Range of Motion; Articular; Musculoskeletal Manipulations; Motor Activity; Physical Therapy Modalities; Movement; Exercise |
| Tactile | tactile stimulation, touch, tactile, massage, skin contact, skin-to-skin, kangaroo care, kangaroo mother care, acupressure | Touch; Tactile stimulation; M technique; TAC TIC; Massage |
| Vestibular | rock, bounce, swing, hammock, vestibular | Vestibule; Labyrinth; Motion; Proprioception |
| Vision | Eye contact, eye engagement, visual contact, visual engagement, eye-to-eye, mobile, light AND (cycled OR exposure OR dim OR reduction), visual AND (stimulus OR toy OR intervention OR novelty OR pattern) | Photic Stimulation; Pattern Recognition; Visual; Color Perception; Lighting; Light |
| Multimodala | multimodal, multiple sensory, ATVV | |
| combined with | ||
| Infant | infant, newborn, neonate, preterm, premature, low birth weight, LBW, VLBW, ELBW | Infant; Infant, Premature; Infant, Low Birth Weight |
| Sample search strategy | ||
| Population | 1 Infant[mh] OR infanta[tiab] OR newborna[tiab] OR neonata[tiab] | |
| 2 preterma[tiab] OR pre-terma[tiab] OR prematura[tiab] OR ‘low birthweight’[tiab] OR ‘low birth weight’[tiab] OR lbw[tiab] OR vlbw[tiab] | ||
| 3 1 AND 2 | ||
| 4 Infant, Premature[mh] | ||
| 5 Infant, Low Birth Weight[mh] | ||
| 6 #3 OR #4 OR #5 | ||
| Interventions | 7 Music therapy[mh] OR musica[tiab] OR Bach[tiab] OR Mozart[tiab] OR lullaba[tiab] OR Singing[mh] OR singing[tiab] | |
| 8 Mothers[mh] OR mothera[tiab] OR maternal[tiab] | ||
| 9 Voice[mh] OR voice[tiab] | ||
| 10 Speech[mh] OR speech[tiab] | ||
| 11 ‘sound simulation’[tiab] | ||
| 12 #9 OR #10 OR #11 | ||
| 13 #8 AND #12 | ||
| 14 Acoustic Stimulation[mh] | ||
| 15 ‘auditory stimulation’[tiab] | ||
| 16 heartbeata[tiab] OR ‘heart beat’[tiab] | ||
| 17 ‘adult talk’[tiab] OR ‘parent talk’[tiab] | ||
| 18 #7 OR #13 OR #14 OR #15 OR #16 OR #17 | ||
| Combined | 19 #6 AND #18 | |
Searches of individual sensory categories also generated multimodal studies for inclusion.
Search strategy
A systematic search for studies published from January 1995 to October 2015 was performed using databases including MEDLINE (via PubMed), CINAHL (Cumulative Index to Nursing and Allied Health Literature), the Cochrane Library and Google Scholar. Reference lists of included studies were also searched for relevant literature. Searches were performed separately for each type of sensory exposure.
Study screening
One reviewer (authors, AH or RG) screened studies for inclusion. Studies were screened first by title. In situations where the title was unclear, the abstract was retrieved for review. The full text articles of potentially relevant studies were reviewed for final inclusion. If relevance of an intervention or inclusion of a study was unclear, it was resolved through discussion with the review team (authors: RP, AH, RG and JS).
Data extraction
One reviewer performed data extraction (authors, AH or RG) that was checked for accuracy by a second reviewer (authors, AH or RG). Extracted information included study design, sample size, country of origin, intervention (including frequency, duration, timing), EGA at birth, PMA at intervention, study inclusion/exclusion criteria and study outcomes and results. When results from the same sample were reported in multiple publications, they were reported together in this review as a single study. When it was unclear if samples came from the same cohort, authors were contacted for confirmation.
Study quality
Assessment of study quality was independently performed by two reviewers (AH and RG), and disagreements regarding study quality were resolved by discussion among the two reviewers until consensus was achieved. Systematic reviews were assessed for methodological quality using the Documentation and Appraisal Review Tool (DART).32 The remaining studies were assessed for quality using a modified version of a tool developed by the United Kingdom's National Institute for Health and Care Excellence (NICE).33 The tool evaluates studies for selection bias (randomization, allocation concealment, group comparability at baseline), performance bias (groups received the same care, blinding of participants and health-care workers), attrition bias (equal follow-up time, completion of treatment, complete outcome data), detection bias (appropriate length of follow-up, precise definition of outcomes, valid and reliable outcomes, blinding of investigators or outcome assessor) and other bias (statistical methods, issues related to specific study designs). Each factor was rated as yes/adequate, no/inadequate or unclear. Several of these factors were not relevant for single-group repeated measures studies.
Synthesis of findings
Given the significant heterogeneity of studies and their outcomes, study findings could not be combined quantitatively but were summarized qualitatively. Evidence related to each type of sensory intervention was defined across each PMA to determine at what age of maturity evidence existed to support specific interventions.
RESULTS
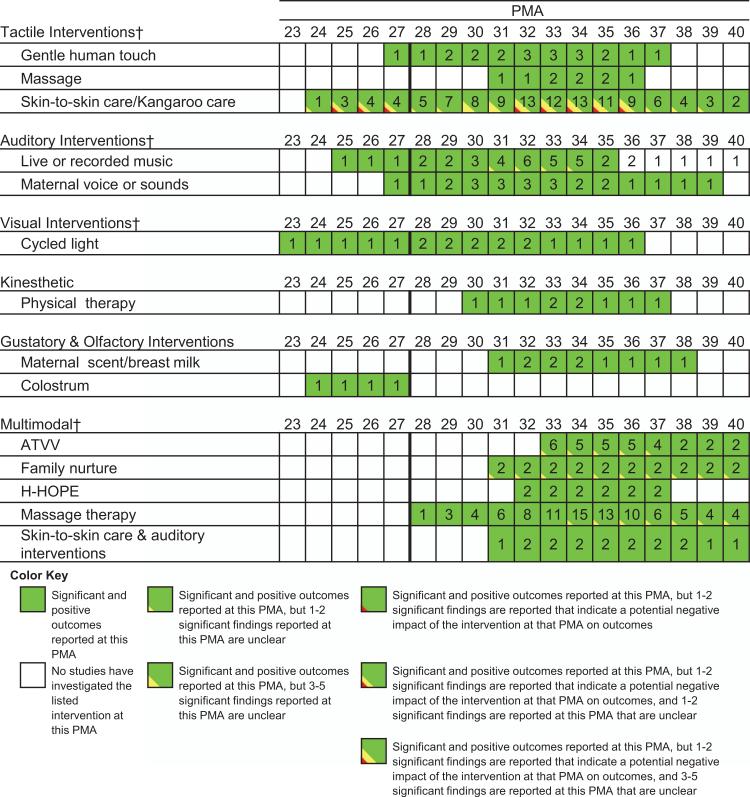
See Figure 1 for a breakdown of the articles reviewed during the integrative review process. See Figure 2 for evidence of the different types of interventions that have been studied across different PMA. See Supplementary Appendix SA for the 88 studies included in this review. See Supplementary Appendix SB for the quality assessment tables.
Figure 1.
Flow diagram of articles identified in the integrative review.
Figure 2.
Different interventions studied across PMA. This figure demonstrates where evidence currently exists related to sensory exposures at different PMA and defines when interventions were deemed beneficial (green), unclear (yellow) or potentially negative (red). There were many outcomes studied that did not reach statistical significance, and these are not represented here in this figure. Studies across this review exhibited a large variety of outcomes, making them challenging to compare quantitatively. Also, many studies found statistically significant results that did not appear to be clinically significant (e.g., SpO2 higher in one condition (97.13%) than another (96.38%, P = 0.01));72 therefore, it is unclear what these outcomes mean in a global, developmental context. Numbers in squares represent the number of studies that have investigated the listed intervention for each PMA. Some studies did not list sufficient information to estimate PMA at intervention and, thus, were not included in this table (see Supplementary Appendix SA).
All articles that were included in the review and their details are available in Supplementary Appendix SA. Many of the outcomes were related to physiology or sleep, which did not have a direct and clear tie to development or parent outcomes. In addition, some of the significant differences that were reported had questionable clinical significance. Therefore, only outcomes that included clinically relevant developmental outcomes or parent mental health are synthesized below in the text.
Tactile
Thirty-one articles, representing 26 different cohorts, were identified on tactile sensory interventions.34–64 Three studies were on gentle human touch,34–37 two on massage38–40 and 21 on kangaroo care.41–64 Gentle human touch treatment length and duration ranged from 10 to 15 min over a course of 5 to 15 days. Tactile massage, which included tactile only portions with no kinesthetic component, consisted of 15-min treatments three times per day for 9 to 10 days.38–40 The duration of kangaroo care interventions ranged from 30 min51 to continuous kangaroo care after the infant stabilized.60 In three studies the treatment length and duration were unspecified.45,58,60
One study investigated the impact of gentle human touch on developmental outcomes, and no difference in behavioral organization was found.35 One study investigated the effects of massage on the mother and found better mother–infant interaction.38 There were 11 studies that investigated the impact of kangaroo care on infant development or parent mental health. Infants receiving kangaroo care looked more intently at a stimulus with less gaze aversion46,48 and demonstrated better mental development at 6, 12 and 24 months as well as better cognitive development at 5 and 10 years.47 Parents who participated in kangaroo care demonstrated fewer depressive symptoms and a better mood,45,47,48 some decreases in measures of stress,63 better mother–infant interaction46,50 and better maternal self-esteem.50 Mothers who provided kangaroo care also were more adept at providing a developmentally appropriate environment for the infant.46 However, not all studies found significant differences in outcomes among those receiving kangaroo care. No differences in infant social interaction48 as well memory, social emotional health and developmental outcome at age 1 year54 have been reported. No differences in IQ at 5 and 10 years47 have also been reported. Other studies reported no differences in maternal depression,47,54 stress,54,56,63 anxiety,54 parent attitude toward baby or parent emotion,53 and parent interaction with infant.54,63 Negative responses to kangaroo care included some alterations in temperature stability when kangaroo was done between 25 and 27 weeks PMA, some bradycardic and hypoxic events starting at 32 weeks PMA44 and poorer sleep states.42,44,49,59,61,64 However, there are inconsistent findings with others reporting better temperature stability, improved physiology and sleep following kangaroo care.42,44,49,50,61
Auditory
Twelve articles, representing 11 different cohorts, pertained to auditory interventions,65–76 including 2 on live music/singing,72,73 5 on recorded music/singing/maternal voice66,68,70,71,74 and 2 on recorded maternal biological sounds.75,76 Treatment lengths ranged from 45 s to 45 min and were done one to four times per day over a course of 1 to 21 days.65–76 There were differences across studies regarding whether the auditory exposure was live or recorded. Music was related to improved feeding behaviors and less parent stress.72 Maternal voice was related to fewer stress responses, better neurobehavior at term and better developmental outcome at 3 and 6 months.74
Visual
Three articles pertained to visual interventions,77–79 one of which was a systematic review consisting of eight articles.78 No studies investigated the effects of visual stimulation using objects or people to focus visual attention and pursuit. All studies reported on the effects of cycled light. Cycled light was started at birth for several studies, and the intervention continued throughout hospitalization. No differences in neurobehavior at 32 and 38 weeks PMA were observed in relation to cycled light compared with other light environments.78
Kinesthetic
Two articles pertained to kinesthetic interventions, both of which specifically investigated physical therapy.80,81 One of the articles was a systematic review consisting of 11 studies.80 Treatment duration varied, but most included specific exercises performed five times, repeated five times per week over 4 weeks. There were no studies that assessed the impact of physical activity/kinesthetic interventions on neurodevelopmental outcomes.80
Gustatory and olfactory
Three articles pertained to olfactory/gustatory interventions, all of which specifically investigated the effects of oropharyngeal colostrum, breast milk odor or mother's scent.82–84 Treatment duration ranged from every 3 h for 3 days to continuously until discharge. There were no studies that specifically investigated the effect of gustatory/olfactory stimulation on infant development or maternal mental health outcomes.
Multimodal
Thirty-seven articles on multimodal interventions, representing 32 different cohorts, were identified as part of this review.18,85–120 Eight articles were on the Auditory, Tactile, Vestibular, Visual (ATVV) intervention originally described by Rosemary White-Traut, two on the Family Nurture intervention,99,113 three on the Hospital to Home: Optimizing the Premature Infant's Environment (H-HOPE) intervention,114,115,120 one on massage with aromatic oil,112 three on use of kangaroo care coupled with auditory stimuli87,108,111 and 20 described a massage intervention that was coupled with a kinesthetic component.85,86,89–98,100,102–107,109
ATVV interventions were related to better developmental outcomes,18 improved tolerance of handling31 and better feeding.117,119 ATVV has also been related to mothers having a more rapid decline in depressive symptoms and less parenting stress.101 However, several studies also reported no significant differences in outcomes of infants receiving ATVV, including no differences in neurobehavior and neurodevelopment118 and no differences in infant responsiveness.101
The Family Nurture Intervention reported no difference in relationship to maternal caregiving behaviors among those receiving the intervention.99,113
Only one study reports parent and infant outcomes in relation to the H-HOPE; trends are reported, but no statistically significant differences in parent child interaction were found.114,115,120
Massage interventions, which include a kinesthetic component, were related to significant decreases in maternal stress behaviors,100,104 better neurobehavior103 and better mental development at age 2 years.105,107 However, some studies also reported no differences in psychomotor development105,107 or father's stress104 among those receiving multimodal massage.
Kangaroo care plus singing as well as kangaroo care plus live harp were also shown to be related to decreased maternal anxiety.87,108
Vestibular
No studies pertaining to isolated vestibular stimulation met inclusion criteria. However, see section on multimodal stimulation for articles that used vestibular stimulation in conjunction with other sensory exposures.18,88,101,110,116–119
DISCUSSION
Key findings of this review include that there is a growing body of evidence supporting the use of early tactile, auditory, kinesthetic, visual, olfactory/gustatory and multimodal sensory-based interventions in the NICU with very preterm infants. However, there are significant differences in sensory exposures, outcomes, dosages and timing of sensory interventions across the literature that make it challenging to combine studies for a cohesive understanding of appropriate sensory exposures across PMA. Consistent relationships of sensory exposures to outcomes were not observed across studies. In addition, there are gaps in our understanding of appropriate timing of interventions, and several studies fail to elucidate the PMA that sensory interventions commenced. In addition, there is little evidence to suggest there are improved long-term outcomes related to sensory interventions. Finally, studies identifying sensory-based interventions contain many methodological issues that make it difficult to appropriately interpret results across studies. However, current evidence identified in this review can be combined with expert clinical opinion and patient/family values to identify the ideal landscape for sensory-based interventions for very preterm infants in the NICU. Such work can lay the foundation for establishing sensory exposure guidelines that outline the type, dose, timing and frequency of appropriate sensory-based interventions for future investigation.
There is little evidence to suggest there are improved long-term outcomes from sensory interventions, which was the main focus of this review. An absence of evidence does not mean these interventions do not improve long-term outcomes. There is some evidence in this review to support the use of kangaroo care, music and language exposure, and multimodal interventions starting at 25 to 28 weeks PMA. Such interventions have been demonstrated to have positive relationships with infant development, sleep and physiology, as well as lower maternal stress. However, most of the research identified interventions that were done for short periods of time over only a few days. For example, some interventions were limited to 1 day for 1 to 1.5 h, others to 45 s twice per day over a course of 2 to 6 weeks, and still others ranged from 15 to 45 min over a course of 1 to 6 days.30,41,49,51,65–67,69,70,87,90–96,100,103,104,108 It remains unclear what the potential is for improving outcomes if such sensory exposures occurred consistently throughout NICU hospitalization. Moreover, evidence related to vestibular, kinesthetic and olfactory/gustatory interventions is not as well defined. There are also significant gaps in the literature related to most interventions. Despite the gaps in the literature, outlining where evidence exists, as done in our PMA tables can enable a better understanding of where more research is needed.
While some evidence to support the benefits of sensory-based interventions, as well as existing gaps, were identified in this review, very few risks of conducting sensory interventions were uncovered. As most NICUs in the United States are converting their spaces to ones with private rooms, sensory abatement is possible if families are not present and not at the center of care. Therefore, it is important that we build appropriate models of care within the new environments to optimize outcomes for very preterm infants and their families. This review identified some inconsistent benefits of sensory-based interventions. While more research is needed, it should not keep us from providing age-appropriate positive sensory exposures to very preterm infants. The development and implementation of a clinical practice guideline on sensory-based interventions can aid in guiding parent participation and health-care professionals in fostering important early interactions in order to optimize both infant and parental outcomes. Our next step will be to take the evidence identified in this review and couple it with expert clinical opinion and patient/family values in order to develop a clinical practice guideline. Appropriately timed sensory interventions that are supported by current evidence and places the parents at the center of the infant's care, which can be done within the context of developmental care across PMA.
This is the first review, to our knowledge, identifying the literature on multiple modes of sensory-based interventions in the NICU for very preterm infants. Other reviews related to neonates focus on developmental care, neurodevelopmentally supportive care or individual interventions.24,25,28,29 Individualized developmental care is a system of care in which there is continuous assessment of infant behavior mixed with modifications to caregiving of the preterm infant; a clinical practice guideline for individualized developmental care was established in 2007.121 This work is important, as it aids in the understanding of modifying the environment early in development to support the unique needs of the preterm infant, provides guidance on how to assess and when/how to intervene, gives specific criteria for light levels (cycling, avoiding direct light) and defines maximum intensity (not to exceed 50 decibels) of sound. A review of developmental care was conducted by Symington in 2006 and includes components of positioning, clustering care, modifications of sensory stimuli and individualized developmental care interventions.25 In a systematic review of neurodevelopmentally supportive care, 42 elements of care were identified, which included interventions such as family centered care, flexion positioning, modifying caregiving and reducing environmental stimuli.24 There were also elements of sensory interventions identified with this review, which include components of olfactory stimulation, reducing sensory monotony, kangaroo care, positive tactile stimulation, teaching parents to interact with the preterm infant, uterine environment, and day and night cycle. However, previous reviews do not address the appropriate dosage and timing of sensory exposures, which this review attempts to better define.
Limitations of included studies
There is a possibility of publication bias, where only studies reporting positive outcomes were published and included in this review. In addition, most studies included multiple outcome measures, many of which did not reach statistical significance. Many outcomes that did have statistical significance were challenging to interpret and many may not have been clinically significant. We included multiple research designs in an effort to capture all appropriate literature related to improving the sensory environment, so lower quality non-randomized designs could have biased the review findings. Of the studies that were randomized, many did not specify their methods clearly or report allocation concealment. This, in addition to incomplete or weak assessments of participants at baseline, placed many of these studies at high risk for selection bias. While participants could not be blinded, and it may be difficult to blind parents and health-care workers to the intervention, few studies attempted to blind the outcomes assessor. Completeness of treatment and follow-up was also difficult to ascertain, as studies infrequently reported the number of infants by group with complete outcomes data and reasons for loss to follow-up. Most interventions were very short and were not conducted across the majority of hospitalization. In addition, many studies did not give clear descriptions of inclusion criteria including EGA at birth, and the PMA at the start and end of intervention. Finally, generalizability of many of the studies is limited.
Limitations of this review
Owing to resource limitations, this review did not include non-English language studies or non- published literature, and only one reviewer screened studies and performed data extraction. Exclusion of studies with a sample size less than 30 may have excluded relevant literature, though this was an attempt to exclude lower quality studies with convenience sampling and limited external validity. Articles published more than 20 years ago were excluded, so it is possible that important research from a time when there were rapid transformations in providing or minimizing sensory stimuli in the NICU has been excluded. The size and scope of this review also did not allow us to follow-up with individual study authors in situations where methods or data were missing or unclear. When possible, we attempted to rate the methodological quality as unclear and explained our reason for judgment in these cases. In addition, this review is limited by lack of common interventions and outcomes, making it difficult to combine results into a cohesive whole. Finally, this review did not include literature on sensory exposures among infants born between 33 and 36 weeks gestation, which eliminated many research articles that define sensory exposures on healthier preterm infants. A review on this other population of preterm infants is warranted to define appropriate exposures, but the intent of the current review was to identify literature that defines the impact of sensory exposures in very preterm infants, who have different vulnerabilities and spend significant amounts of time in the NICU at the start of their lives.
In conclusion, early positive sensory exposures have been identified as being safe and potentially important for optimizing infant and parent outcomes in the NICU. However, a cohesive plan of sensory exposure is difficult to establish solely from the literature. This review is an important start of identifying the evidence that can support early sensory exposures in the NICU. Coupling these findings with expert clinical opinion, as well as parent input, could lead to the development of a clinical practice guideline that can inform appropriate sensory exposures across PMA in the NICU that aims to optimize outcomes. Defining a clinical practice guideline for sensory-based interventions is the next step.
Supplementary Material
ACKNOWLEDGEMENTS
The project was supported by the University Research Strategic Alliance, the Comprehensive Opportunities for Rehabilitation Researchers (K12 HD055931) and the Gordon and Betty Moore Foundation. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number U54 HD087011 and the Intellectual and Developmental Disabilities Research Center at Washington University. We would like to thank Jessica Roussin, Michael Wallendorf, Mary Bocox, Liana Merz, Geneva Wilson and Jake Gilliland. We also wish to thank Brad Schlaggar, Carolyn Baum, Graham Colditz, Mary Politi, Elizabeth Kruvand and F Sessions Cole. We would also like to thank Katie Ross, Kelsey Dewey, Felicia Foci, Polly Durant, Justin Ryckman, Rachel Harris, Elizabeth Heiny, Gabby Blenden, Lisa Shabosky, Bailey Hall, Anna Annecca and Sarah Wolf.
Footnotes
AUTHOR CONTRIBUTIONS
RGP conceived of the original idea to do an integrative review to inform a clinical practice guideline on sensory-based interventions in the NICU. She was involved with data synthesis, and wrote the first draft of the manuscript. She oversaw all parts of the project and approved the final version of the manuscript submitted. RG and AH conducted the literature review and identified articles appropriate for the integrative review. They were involved in identifying the articles, assessing each for quality and wrote the first draft of the evidence table. They critically reviewed the manuscript's content and approved the final version of the manuscript submitted. LCR assisted with the analysis processes, reviewed and revised the manuscript, and approved the final manuscript as submitted, and was also responsible for reporting the studies in the evidence table and ensured the accuracy of the evidence table. SO assisted with the analysis processes, reviewed and revised the manuscript, and approved the final manuscript as submitted, and made the PMA tables that demonstrate the PMA at which interventions have been investigated. JS was involved in idea conception, study design, data synthesis and ensured accuracy of the studies reported. She oversaw all parts of the project. She provided intellectual content to the manuscript and approved the final version that was submitted.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
REFERENCES
- 1.Anderson P, Doyle LW. Victorian Infant Collaborative Study G. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990 s. JAMA. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51–58. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158(5):766–774. e761. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Goyen TA, Lui K, Woods R. Visual-motor, visual-perceptual, and fine motor outcomes in very-low birthweight children at 5 years. Dev Med Child Neurol. 1998;40(2):76–81. doi: 10.1111/j.1469-8749.1998.tb15365.x. [DOI] [PubMed] [Google Scholar]
- 5.Holsti L, Grunau RVE, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23(1):9–15. doi: 10.1097/00004703-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Maguire CM, Walther FJ, van Zwieten PH, Le Cessie S, Wit JM, Veen S. Follow-up outcomes at 1 and 2 years of infants born less than 32 weeks after Newborn Individualized Developmental Care and Assessment Program. Pediatrics. 2009;123(4):1081–1087. doi: 10.1542/peds.2008-1950. [DOI] [PubMed] [Google Scholar]
- 7.Center for Disease Control and Prevention Reproductive Health, Preterm Birth. 2012 Mar 23; [cited 2012]. Available at: http://www.cdc.gov/reproductive health/maternalinfanthealth/PretermBirth.htm.
- 8.Williams J, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52(3):232–237. doi: 10.1111/j.1469-8749.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- 9.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 10.Lasky RE, Williams AL. Noise and light exposures for extremely low birth weight newborns during their stay in the neonatal intensive care unit. Pediatrics. 2009;123(2):540–546. doi: 10.1542/peds.2007-3418. [DOI] [PubMed] [Google Scholar]
- 11.Kent WD, Tan AK, Clarke MC, Bardell T. Excessive noise levels in the neonatal ICU: potential effects on auditory system development. J Otolaryngol. 2002;31(6):355–360. doi: 10.2310/7070.2002.34358. [DOI] [PubMed] [Google Scholar]
- 12.Noise: a hazard for the fetus and newborn. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics. 1997;100(4):724–727. [PubMed] [Google Scholar]
- 13.McGrath JM. Human factors: the importance of communication to outcomes in the NICU. J Perinat Neonat Nurs. 2013;27(2):108–109. doi: 10.1097/JPN.0b013e3182907e89. [DOI] [PubMed] [Google Scholar]
- 14.Byers JF. Components of developmental care and the evidence for their use in the NICU. MCN Am J Matern Child Nurs. 2003;28(3):174–180. doi: 10.1097/00005721-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J Pediatr. 2014;164(1):52–60. e52. doi: 10.1016/j.jpeds.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graven SN, Browne JV. Sensory development in the fetus, neonate, and infant: introduction and overview. Newborn Infant Nurs Rev. 2008;8(4):169–172. [Google Scholar]
- 17.Leib SA, Benfield DG, Guidubaldi J. Effects of early intervention and stimulation on the preterm infant. Pediatrics. 1980;66(1):83–90. [PubMed] [Google Scholar]
- 18.Kanagasabai PS, Mohan D, Lewis LE, Kamath A, Rao BK. Effect of multisensory stimulation on neuromotor development in preterm infants. Indian J Pediatr. 2013;80(6):460–464. doi: 10.1007/s12098-012-0945-z. [DOI] [PubMed] [Google Scholar]
- 19.Jobe AH. A risk of sensory deprivation in the neonatal intensive care unit. J Pediatr. 2014;164(6):1265–1267. doi: 10.1016/j.jpeds.2014.01.072. [DOI] [PubMed] [Google Scholar]
- 20.Graven SN, Bowen FW, Jr, Brooten D, Eaton A, Graven MN, Hack M, et al. The high-risk infant environment. Part 1. The role of the neonatal intensive care unit in the outcome of high-risk infants. J Perinatol. 1992;12(2):164–172. [PubMed] [Google Scholar]
- 21.Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks' gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr. 2002;140(2):192–199. doi: 10.1067/mpd.2002.121932. [DOI] [PubMed] [Google Scholar]
- 22.Lickliter R. The integrated development of sensory organization. Clin Perinatol. 2011;38(4):591–603. doi: 10.1016/j.clp.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hepper PG, Shahidullah BS. Development of fetal hearing. Arch Dis Child. 1994;71(2):F81–F87. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubbe W, Van der Walt CS, Klopper HC. Integrative literature review defining evidence-based neurodevelopmental supportive care of the preterm infant. J Perinat Neonat Nurs. 2012;26(3):251–259. doi: 10.1097/JPN.0b013e3182650b7e. [DOI] [PubMed] [Google Scholar]
- 25.Symington AJ, Pinelli J. Cochrane Database Syst Rev. Wiley-Blackwell: John Wiley & Sons, Ltd.; 2006. Developmental care for promoting development and preventing morbidity in preterm infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics. 2016;137(1):e20152238–e20152238. doi: 10.1542/peds.2015-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Chang MY, Chang LY, Mu PF. Experiences of parents providing kangaroo care to a premature infant: a systematic review of the qualitative evidence protocol. JBI Database System Rev Implement Rep. 2015;13(9):112–119. doi: 10.11124/jbisrir-2015-2238. [DOI] [PubMed] [Google Scholar]
- 28.Juneau AL, Aita M, Heon M. Review and critical analysis of massage studies for term and preterm infants. Neonat Netw. 2015;34(3):165–177. doi: 10.1891/0730-0832.34.3.165. [DOI] [PubMed] [Google Scholar]
- 29.Smith JR. Comforting touch in the very preterm hospitalized infant: an integrative review. Adv Neonat Care. 2012;12(6):349–365. doi: 10.1097/ANC.0b013e31826093ee. [DOI] [PubMed] [Google Scholar]
- 30.Krueger C. Exposure to maternal voice in preterm infants: a review. Adv Neonat Care. 2010;10(1):13–18. doi: 10.1097/ANC.0b013e3181cc3c69. quiz 19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Standley J. Music therapy research in the NICU: an updated meta-analysis. Neonat Netw. 2012;31(5):311–316. doi: 10.1891/0730-0832.31.5.311. [DOI] [PubMed] [Google Scholar]
- 32.Diekemper RL, Ireland BK, Merz LR. Development of the Documentation and Appraisal Review Tool for systematic reviews. World J Meta-Anal. 2015;3(3):142–150. [Google Scholar]
- 33.(NICE) NIfHaCE The Guidelines Manual 2012. Appendix C: Methodology checklist: randomized controlled trials [cited] Available from http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-c-methodology-checklist-randomised-controlled-trials.
- 34.Bahman Bijari B, Iranmanesh S, Eshghi F, Baneshi MR. Gentle Human Touch and Yakson: the effect on preterm's behavioral reactions. ISRN Nurs. 2012;2012:750363. doi: 10.5402/2012/750363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison LL, Williams AK, Berbaum ML, Stem JT, Leeper J. Physiologic and behavioral effects of gentle human touch on preterm infants. Res Nurs Health. 2000;23(6):435–446. doi: 10.1002/1098-240X(200012)23:6<435::AID-NUR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Im H, Kim E. Effect of Yakson and Gentle Human Touch versus usual care on urine stress hormones and behaviors in preterm infants: a quasi-experimental study. Int J Nurs Stud. 2009;46(4):450–458. doi: 10.1016/j.ijnurstu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Im H, Kim E, Cain KC. Acute effects of Yakson and Gentle Human Touch on the behavioral state of preterm infants. J Child Health Care. 2009;13(3):212–226. doi: 10.1177/1367493509337441. [DOI] [PubMed] [Google Scholar]
- 38.Ferber SG, Feldman R, Kohelet D, Kuint J, Dollberg S, Arbel E, et al. Massage therapy facilitates mother-infant interaction in premature infants. Infant Behav Dev. 2005;28(1):74–81. [Google Scholar]
- 39.Ferber SG, Kuint J, Weller A, Feldman R, Dollberg S, Arbel E, et al. Massage therapy by mothers and trained professionals enhances weight gain in preterm infants. Early Hum Dev. 2002;67(1-2):37–45. doi: 10.1016/s0378-3782(01)00249-3. [DOI] [PubMed] [Google Scholar]
- 40.Chen LL, Su YC, Su CH, Lin HC, Kuo HW. Acupressure and meridian massage: combined effects on increasing body weight in premature infants. J Clin Nurs. 2008;17(9):1174–1181. doi: 10.1111/j.1365-2702.2007.02147.x. [DOI] [PubMed] [Google Scholar]
- 41.Azevedo VM, Xavier CC, Gontijo Fde O. Safety of kangaroo mother care in intubated neonates under 1500 g. J Trop Pediatr. 2012;58(1):38–42. doi: 10.1093/tropej/fmr033. [DOI] [PubMed] [Google Scholar]
- 42.Bauer K, Pyper A, Sperling P, Uhrig C, Versmold H. Effects of gestational and postnatal age on body temperature, oxygen consumption, and activity during early skin-to-skin contact between preterm infants of 25-30-week gestation and their mothers. Pediatr Res. 1998;44(2):247–251. doi: 10.1203/00006450-199808000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Bier JA, Ferguson AE, Morales Y, Liebling JA, Archer D, Oh W, et al. Comparison of skin-to-skin contact with standard contact in low-birth-weight infants who are breast-fed. Arch Pediatr Adolesc Med. 1996;150(12):1265–1269. doi: 10.1001/archpedi.1996.02170370043006. [DOI] [PubMed] [Google Scholar]
- 44.Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin-to-skin (kangaroo) care, respiratory control, and thermoregulation. J Pediatr. 2001;138(2):193–197. doi: 10.1067/mpd.2001.110978. [DOI] [PubMed] [Google Scholar]
- 45.de Macedo EC, Cruvinel F, Lukasova K, D'Antino ME. The mood variation in mothers of preterm infants in Kangaroo mother care and conventional incubator care. J Trop Pediatr. 2007;53(5):344–346. doi: 10.1093/tropej/fmm076. [DOI] [PubMed] [Google Scholar]
- 46.Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics. 2002;110(1 Pt 1):16–26. doi: 10.1542/peds.110.1.16. [DOI] [PubMed] [Google Scholar]
- 47.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75(1):56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Feldman R, Weller A, Sirota L, Eidelman AI. Skin-to-skin contact (Kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Dev Psychol. 2002;38(2):194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- 49.Fohe K, Kropf S, Avenarius S. Skin-to-skin contact improves gas exchange in premature infants. J Perinatol. 2000;20(5):311–315. doi: 10.1038/sj.jp.7200378. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Bang K-S. The effects of kangaroo care on maternal self-esteem and premature infants' physiological stability. Korean J Women Health Nurs. 2011;17(5):454. doi: 10.4069/kjwhn.2011.17.5.454. [DOI] [PubMed] [Google Scholar]
- 51.Legault M, Goulet C. Comparison of kangaroo and traditional methods of removing preterm infants from incubators. J Obstet Gynecol Neonatal Nurs. 1995;24(6):501–506. doi: 10.1111/j.1552-6909.1995.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 52.Maastrup R, Greisen G. Extremely preterm infants tolerate skin-to-skin contact during the first weeks of life. Acta Paediatr. 2010;99(8):1145–1149. doi: 10.1111/j.1651-2227.2010.01806.x. [DOI] [PubMed] [Google Scholar]
- 53.Messmer PR, Rodriguez S, Adams J, Wells-Gentry J, Washburn K, Zabaleta I, et al. Effect of kangaroo care on sleep time for neonates. Pediatr Nurs. 1997;23(4):408–414. [PubMed] [Google Scholar]
- 54.Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin-to-skin contact in extremely preterm infants. Early Hum Dev. 2006;82(7):447–455. doi: 10.1016/j.earlhumdev.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Ramanathan K, Paul VK, Deorari AK, Taneja U, George G. Kangaroo mother care in very low birth weight infants. Indian J Pediatr. 2001;68(11):1019–1023. doi: 10.1007/BF02722345. [DOI] [PubMed] [Google Scholar]
- 56.Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonat Netw. 2000;19(4):31–35. doi: 10.1891/0730-0832.19.4.31. [DOI] [PubMed] [Google Scholar]
- 57.Rojas MA, Kaplan M, Quevedo M, Sherwonit E, Foster L, Ehrenkranz RA, et al. Somatic growth of preterm infants during skin-to-skin care versus traditional holding: a randomized, controlled trial. J Dev Behav Pediatr. 2003;24(3):163–168. doi: 10.1097/00004703-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Samra NM, Taweel AE, Cadwell K. Effect of intermittent kangaroo mother care on weight gain of low birth weight neonates with delayed weight gain. J Perinat Educ. 2013;22(4):194–200. doi: 10.1891/1058-1243.22.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scher MS, Ludington-Hoe S, Kaffashi F, Johnson MW, Holditch-Davis D, Loparo KA. Neurophysiologic assessment of brain maturation after an 8-week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 2009;120(10):1812–1818. doi: 10.1016/j.clinph.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider C, Charpak N, Ruiz-Pelaez JG, Tessier R. Cerebral motor function in very premature-at-birth adolescents: a brain stimulation exploration of kangaroo mother care effects. Acta Paediatr. 2012;101(10):1045–1053. doi: 10.1111/j.1651-2227.2012.02770.x. [DOI] [PubMed] [Google Scholar]
- 61.Smith SL. Physiologic stability of intubated VLBW infants during skin-to-skin care and incubator care. Adv Neonat Care. 2001;1(1):28–40. [Google Scholar]
- 62.Smith SL. Heart period variability of intubated very-low-birth-weight infants during incubator care and maternal holding. Am J Crit Care. 2003;12(1):54–64. [PubMed] [Google Scholar]
- 63.Tallandini MA, Scalembra C. Kangaroo mother care and mother-premature infant dyadic interaction. Infant Mental Health J. 2006;27(3):251–275. doi: 10.1002/imhj.20091. [DOI] [PubMed] [Google Scholar]
- 64.Tornhage CJ, Serenius F, Uvnas-Moberg K, Lindberg T. Plasma somatostatin and cholecystokinin levels in preterm infants during kangaroo care with and without nasogastric tube-feeding. J Pediatr Endocr Met. 1998;11(5):645–651. doi: 10.1515/jpem.1998.11.5.645. [DOI] [PubMed] [Google Scholar]
- 65.Arnon S, Shapsa A, Forman L, Regev R, Bauer S, Litmanovitz I, et al. Live music is beneficial to preterm infants in the neonatal intensive care unit environment. Birth (Berkeley, Calif) 2006;33(2):131–136. doi: 10.1111/j.0730-7659.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 66.Cassidy JW. The effect of decibel level of music stimuli and gender on head circumference and physiological responses of premature infants in the NICU. J Music Ther. 2009;46(3):180–190. doi: 10.1093/jmt/46.3.180. [DOI] [PubMed] [Google Scholar]
- 67.Coleman JM, Pratt RR, Stoddard RA, Gerstmann DR, Abel H-H. The effects of the male and female singing and speaking voices on selected physiological and behavioral measures of premature infants in the intensive care unit. Int J Arts Med. 1997;5(2):4–11. [Google Scholar]
- 68.Farhat A, Amiri R, Karbandi S, Esmaily H, Mohammadzadeh A. The effect of listening to lullaby music on physiologic response and weight gain of premature infants. J Neonatal-Perinat Med. 2010;3(2):103–107. [Google Scholar]
- 69.Garunkstiene R, Buinauskiene J, Uloziene I, Markuniene E. Controlled trial of live versus recorded lullabies in preterm infants. Nordic J Music Ther. 2013;23(1):71–88. [Google Scholar]
- 70.Keidar HR, Mandel D, Mimouni FB, Lubetzky R. Bach music in preterm infants: no ‘Mozart effect’ on resting energy expenditure. J Perinatol. 2014;34(2):153–155. doi: 10.1038/jp.2013.138. [DOI] [PubMed] [Google Scholar]
- 71.Krueger C, Parker L, Chiu SH, Theriaque D. Maternal voice and short-term outcomes in preterm infants. Dev Psychobiol. 2010;52(2):205–212. doi: 10.1002/dev.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loewy J. NICU music therapy: song of kin as critical lullaby in research and practice. Ann NY Acad Sci. 2015;1337(1):178–185. doi: 10.1111/nyas.12648. [DOI] [PubMed] [Google Scholar]
- 73.Loewy J, Stewart K, Dassler AM, Telsey A, Homel P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics. 2013;131(5):902–918. doi: 10.1542/peds.2012-1367. [DOI] [PubMed] [Google Scholar]
- 74.Picciolini O, Porro M, Meazza A, Gianni ML, Rivoli C, Lucco G, et al. Early exposure to maternal voice: effects on preterm infants development. Early Hum Dev. 2014;90(6):287–292. doi: 10.1016/j.earlhumdev.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Webb AR, Heller HT, Benson CB, Lahav A. Mother's voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA. 2015;112(10):3152–3157. doi: 10.1073/pnas.1414924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmerman E, Keunen K, Norton M, Lahav A. Weight gain velocity in very low-birth-weight infants: effects of exposure to biological maternal sounds. Am J Perinatol. 2013;30(10):863–870. doi: 10.1055/s-0033-1333669. [DOI] [PubMed] [Google Scholar]
- 77.Guyer C, Huber R, Fontijn J, Bucher HU, Nicolai H, Werner H, et al. Very preterm infants show earlier emergence of 24-hour sleep-wake rhythms compared to term infants. Early Hum Dev. 2015;91(1):37–42. doi: 10.1016/j.earlhumdev.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Morag I, Ohlsson A. Cochrane Database of Systematic Reviews. Wiley-Blackwell: John Wiley & Sons, Ltd.; 2013. Cycled light in the intensive care unit for preterm and low birth weight infants. [Google Scholar]
- 79.Vasquez-Ruiz S, Maya-Barrios JA, Torres-Narvaez P, Vega-Martinez BR, Rojas-Granados A, Escobar C, et al. A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum Dev. 2014;90(9):535–540. doi: 10.1016/j.earlhumdev.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Schulzke SM, Kaempfen S, Trachsel D, Patole SK. Cochrane Database of Systematic Reviews. Wiley-Blackwell: John Wiley & Sons, Ltd.; 2014. Physical activity programs for promoting bone mineralization and growth in preterm infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vignochi CM, Silveira RC, Miura E, Canani LH, Procianoy RS. Physical therapy reduces bone resorption and increases bone formation in preterm infants. Am J Perinatol. 2012;29(8):573–578. doi: 10.1055/s-0032-1310520. [DOI] [PubMed] [Google Scholar]
- 82.Kardas Ozdemir F, Guducu Tufekci F. The effect of individualised developmental care practices on the growth and hospitalisation duration of premature infants: the effect of mother's scent and flexion position. J Clin Nurs. 2014;23(21-22):3036–3044. doi: 10.1111/jocn.12407. [DOI] [PubMed] [Google Scholar]
- 83.Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135(2):e357–e366. doi: 10.1542/peds.2014-2004. [DOI] [PubMed] [Google Scholar]
- 84.Yildiz A, Arikan D, Gozum S, Tastekin A, Budancamanak I. The effect of the odor of breast milk on the time needed for transition from gavage to total oral feeding in preterm infants. J Nurs Scholarsh. 2011;43(3):265–273. doi: 10.1111/j.1547-5069.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 85.Aly H, Moustafa MF, Hassanein SM, Massaro AN, Amer HA, Patel K. Physical activity combined with massage improves bone mineralization in premature infants: a randomized trial. J Perinatol. 2004;24(5):305–309. doi: 10.1038/sj.jp.7211083. [DOI] [PubMed] [Google Scholar]
- 86.Ang JY, Lua JL, Mathur A, Thomas R, Asmar BI, Savasan S, et al. A randomized placebo-controlled trial of massage therapy on the immune system of preterm infants. Pediatrics. 2012;130(6):e1549–e1558. doi: 10.1542/peds.2012-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arnon S, Diamant C, Bauer S, Regev R, Sirota G, Litmanovitz I. Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 2014;103(10):1039–1044. doi: 10.1111/apa.12744. [DOI] [PubMed] [Google Scholar]
- 88.Cameron EC, Maehle V, Reid J. The effects of an early physical therapy intervention for very preterm, very low birth weight infants: a randomized controlled clinical trial. Pediatr Phys Ther. 2005;17(2):107–119. doi: 10.1097/01.pep.0000163073.50852.58. [DOI] [PubMed] [Google Scholar]
- 89.Choi H, Kim SJ, Oh J, Lee MN, Kim S, Kang KA. The effects of massage therapy on physical growth and gastrointestinal function in premature infants: a pilot study. J Child Health Care. 2016;20(3):394–404. doi: 10.1177/1367493515598647. [DOI] [PubMed] [Google Scholar]
- 90.Diego MA, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J Pediatr. 2005;147(1):50–55. doi: 10.1016/j.jpeds.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 91.Diego MA, Field T, Hernandez-Reif M. Temperature increases in preterm infants during massage therapy. Infant Behav Dev. 2008;31(1):149–152. doi: 10.1016/j.infbeh.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diego MA, Field T, Hernandez-Reif M. Preterm infant weight gain is increased by massage therapy and exercise via different underlying mechanisms. Early Hum Dev. 2014;90(3):137–140. doi: 10.1016/j.earlhumdev.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatr. 2007;96(11):1588–1591. doi: 10.1111/j.1651-2227.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 94.Dieter JN, Field T, Hernandez-Reif M, Emory EK, Redzepi M. Stable preterm infants gain more weight and sleep less after five days of massage therapy. J Pediatr Psychol. 2003;28(6):403–411. doi: 10.1093/jpepsy/jsg030. [DOI] [PubMed] [Google Scholar]
- 95.Field T, Diego M, Hernandez-Reif M, Dieter JN, Kumar AM, Schanberg S, et al. Insulin and insulin-like growth factor-1 increased in preterm neonates following massage therapy. J Dev Behav Pediatr. 2008;29(6):463–466. doi: 10.1097/DBP.0b013e3181856d3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Field T, Diego MA, Hernandez-Reif M, Deeds O, Figuereido B. Moderate versus light pressure massage therapy leads to greater weight gain in preterm infants. Infant Behav Dev. 2006;29(4):574–578. doi: 10.1016/j.infbeh.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez AP, Vasquez-Mendoza G, Garcia-Vela A, Guzman-Ramirez A, Salazar-Torres M, Romero-Gutierrez G. Weight gain in preterm infants following parent-administered Vimala massage: a randomized controlled trial. Am J Perinatol. 2009;26(4):247–252. doi: 10.1055/s-0028-1103151. [DOI] [PubMed] [Google Scholar]
- 98.Haley S, Beachy J, Ivaska KK, Slater H, Smith S, Moyer-Mileur LJ. Tactile/kinesthetic stimulation (TKS) increases tibial speed of sound and urinary osteocalcin (U-MidOC and unOC) in premature infants (29-32weeks PMA). Bone. 2012;51(4):661–666. doi: 10.1016/j.bone.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hane AA, Myers MM, Hofer MA, Ludwig RJ, Halperin MS, Austin J, et al. Family nurture intervention improves the quality of maternal caregiving in the neonatal intensive care unit: evidence from a randomized controlled trial. J Dev Behav Pediatr. 2015;36(3):188–196. doi: 10.1097/DBP.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 100.Hernandez-Reif M, Diego M, Field T. Preterm infants show reduced stress behaviors and activity after 5 days of massage therapy. Infant Behav Dev. 2007;30(4):557–561. doi: 10.1016/j.infbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holditch-Davis D, White-Traut RC, Levy JA, O'Shea TM, Geraldo V, David RJ. Maternally administered interventions for preterm infants in the NICU: effects on maternal psychological distress and mother-infant relationship. Infant Behav Dev. 2014;37(4):695–710. doi: 10.1016/j.infbeh.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Massaro AN, Hammad TA, Jazzo B, Aly H. Massage with kinesthetic stimulation improves weight gain in preterm infants. J Perinatol. 2009;29(5):352–357. doi: 10.1038/jp.2008.230. [DOI] [PubMed] [Google Scholar]
- 103.Mathai S, Fernandez A, Mondkar J, Kanbur W. Effects of tactile-kinesthetic stimulation in preterms—a contolled trial. Indian Pediatr. 2001;38(10):1091–1098. [PubMed] [Google Scholar]
- 104.Matricardi S, Agostino R, Fedeli C, Montirosso R. Mothers are not fathers: differences between parents in the reduction of stress levels after a parental intervention in a NICU. Acta Paediatr. 2013;102(1):8–14. doi: 10.1111/apa.12058. [DOI] [PubMed] [Google Scholar]
- 105.Mendes EW, Procianoy RS. Massage therapy reduces hospital stay and occur-rence of late-onset sepsis in very preterm neonates. J Perinatol. 2008;28(12):815–820. doi: 10.1038/jp.2008.108. [DOI] [PubMed] [Google Scholar]
- 106.Moyer-Mileur LJ, Haley S, Slater H, Beachy J, Smith SL. Massage improves growth quality by decreasing body fat deposition in male preterm infants. J Pediatr. 2013;162(3):490–495. doi: 10.1016/j.jpeds.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Procianoy RS, Mendes EW, Silveira RC. Massage therapy improves neurodevelopment outcome at two years corrected age for very low birth weight infants. Early Hum Dev. 2010;86(1):7–11. doi: 10.1016/j.earlhumdev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 108.Schlez A, Litmanovitz I, Bauer S, Dolfin T, Regev R, Arnon S. Combining kangaroo care and live harp music therapy in the neonatal intensive care unit setting. Isr Med Assoc J. 2011;13(6):354–358. [PubMed] [Google Scholar]
- 109.Smith SL, Lux R, Haley S, Slater H, Beachy J, Moyer-Mileur LJ. The effect of massage on heart rate variability in preterm infants. J Perinatol. 2013;33(1):59–64. doi: 10.1038/jp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Standley JM. The effect of music and multimodal stimulation on responses of premature infants in neonatal intensive care. Pediatr Nurs. 1998;24(6):532–538. [PubMed] [Google Scholar]
- 111.Teckenberg-Jansson P, Huotilainen M, Polkki T, Lipsanen J, Jarvenpaa AL. Rapid effects of neonatal music therapy combined with kangaroo care on prematurely-born infants. Nordic J Music Ther. 2011;20(1):22–42. [Google Scholar]
- 112.Valizadeh S, Hosseini MB, Asghari Jafarabadi M, Ajoodanian N. The effects of massage with coconut and sunflower oils on oxygen saturation of premature infants with respiratory distress syndrome treated with nasal continuous positive airway pressure. J Caring Sci. 2012;1(4):191–199. doi: 10.5681/jcs.2012.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Welch MG, Hofer MA, Stark RI, Andrews HF, Austin J, Glickstein SB, et al. Randomized controlled trial of Family Nurture Intervention in the NICU: assessments of length of stay, feasibility and safety. BMC Pediatr. 2013;13(1):148. doi: 10.1186/1471-2431-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.White-Traut R, Norr KF, Fabiyi C, Rankin KM, Li Z, Liu L. Mother-infant interaction improves with a developmental intervention for mother-preterm infant dyads. Infant Behav Dev. 2013;36(4):694–706. doi: 10.1016/j.infbeh.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.White-Traut R, Rankin KM, Pham T, Li Z, Liu L. Preterm infants' orally directed behaviors and behavioral state responses to the integrated H-HOPE intervention. Infant Behav Dev. 2014;37(4):583–596. doi: 10.1016/j.infbeh.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.White-Traut RC, Nelson MN, Silvestri JM, Cunningham N, Patel M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatr Nurs. 1997;23(2):169–175. 193. [PubMed] [Google Scholar]
- 117.White-Traut RC, Nelson MN, Silvestri JM, Patel M, Berbaum M, Gu GG, et al. Developmental patterns of physiological response to a multisensory intervention in extremely premature and high-risk infants. J Obstet Gynecol Neonatal Nurs. 2004;33(2):266–275. doi: 10.1177/0884217504263289. [DOI] [PubMed] [Google Scholar]
- 118.White-Traut RC, Nelson MN, Silvestri JM, Patel M, Vasan U, Han BK, et al. Developmental intervention for preterm infants diagnosed with periventricular leukomalacia. Res Nurs Health. 1999;22(2):131–143. doi: 10.1002/(sici)1098-240x(199904)22:2<131::aid-nur5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 119.White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy-Rey P, et al. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Dev Med Child Neurol. 2002;44(2):91–97. doi: 10.1017/s0012162201001736. [DOI] [PubMed] [Google Scholar]
- 120.White-Traut RC, Rankin KM, Yoder JC, Liu L, Vasa R, Geraldo V, et al. Influence of H-HOPE intervention for premature infants on growth, feeding progression and length of stay during initial hospitalization. J Perinatol. 2015;35(8):636–641. doi: 10.1038/jp.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandenberg KA. Individualized developmental care for high risk newborns in the NICU: a practice guideline. Early Hum Dev. 2007;83(7):433–442. doi: 10.1016/j.earlhumdev.2007.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.