Abstract
OBJECTIVE
Small-for-gestational-age (SGA) neonates, infants of diabetic mothers (IDM) and very-low-birth weight premature neonates (VLBW) are reported to have increased risk for developing iron deficiency and possibly associated neurocognitive delays.
STUDY DESIGN
We conducted a pilot study to assess iron status at birth in at-risk neonates by measuring iron parameters in umbilical cord blood from SGA, IDM, VLBW and comparison neonates.
RESULTS
Six of the 50 infants studied had biochemical evidence of iron deficiency at birth. Laboratory findings consistent with iron deficiency were found in one SGA, one IDM, three VLBW, and one comparison infant. None of the infants had evidence of iron deficiency anemia.
CONCLUSIONS
Evidence of biochemical iron deficiency at birth was found in 17% of screened neonates. Studies are needed to determine whether these infants are at risk for developing iron-limited erythropoiesis, iron deficiency anemia or iron-deficient neurocognitive delay.
INTRODUCTION
Iron deficiency at critical times during brain development is associated with long-term neurocognitive problems.1–4 A causal relationship between iron status and brain development is supported by animal models of experimentally induced fetal or neonatal iron deficiency. In the setting of iron deficiency anemia, subsequent iron supplementation corrects the anemia but does not overcome the neurocognitive deficits.1–4 Importantly, evidence from animal model systems suggest that during periods of severe neonatal iron limitation, iron is preferentially routed for erythropoiesis at the expense of brain iron accretion.5
Certain risk factors for the development of iron deficiency in early infancy have been identified. These include (1) small-for-gestational-age neonates (SGA, < 10th percentile weight for gestation), (2) infants of diabetic mothers (IDM) and (3) very-low-birth weight preterm neonates (VLBW, < 1500 g at birth).6,7 These three groups are also at increased risk for neurocognitive deficiencies. It is not clear if iron deficiency in these neonates contributes to the neurocognitive deficiencies.
Neonates born to women with iron deficiency anemia during pregnancy generally have serum iron levels and hematocrits in the same range as neonates born to iron-replete women. However, neonates born to iron-deficient mothers have lower serum ferritin levels indicating decreased iron stores.8,9 On the basis of these and other reports, it has been suggested that compensatory mechanisms regulating transplacental iron trafficking prevents iron deficiency anemia in the infant at birth.10,11 However, protection of the neonate from iron deficiency sufficient to cause neurocognitive delay might be compromised by pathological conditions that could significantly diminish maternal-to-fetal iron transport.9–12 Perhaps the iron deficiency observed in infants born SGA or IDMs is related to such pathology. Infants born preterm do not have the advantage of the large accretion of iron that normally occurs in the third trimester of gestation, possibly contributing to an increased risk of iron deficiency at birth.9–13
Iron deficiency can be grouped into three categories according to severity; (1) biochemical iron deficiency with normal erythropoiesis, (2) biochemical iron deficiency plus iron-limited erythropoiesis but without anemia and (3) biochemical iron deficiency with iron deficiency anemia.14 Biochemical iron deficiency can be identified by a low serum ferritin and a low serum iron. Iron-limited erythropoiesis can be recognized by a fall in both reticulocyte hemoglobin content15,16 and mean corpuscular volume, without a fall in hemoglobin or hematocrit.14,17 Iron deficiency anemia is the next stage of severity, where hemoglobin and hematocrit values decrease. It is unclear what percentage (if any) of SGA, IDM or VLBW neonates at birth, fall into these categories. As a step toward the identification of a target population for early iron intervention, we conducted a pilot study in which we assessed the iron status in the cord blood of a cohort of SGA, IDM, VLBW and healthy comparison neonates.
MATERIALS AND METHODS
This was a prospective pilot study conducted in two Intermountain Healthcare neonatal intensive care units; McKay-Dee Hospital, Ogden, UT and Intermountain Medical Center, Murray, UT. The protocol was approved by the Intermountain Healthcare Institutional Review Board as a de-identified, data-only study with appropriate privacy protection. Inter-mountain Healthcare is a not-for-profit system that owns and operates 22 hospitals in Utah and Idaho.
We analyzed 50 umbilical cord blood samples drawn from 10 SGA, 10 IDM, 10 VLBW and 20 comparison deliveries. The intent of this pilot study was to provide initial data needed for power analysis calculations for future larger studies. As such, sampling was based upon availability over the timeframe of support. Umbilical cord blood samples were obtained only when a study nurse or study neonatologist was available to draw the sample. The collected data included no laboratory tests ordered by medical caretakers. The only information obtained from the infants’ charts was birth weight, gender, gestational age and presence of maternal diabetes. SGA was defined as < 10th percentile for weight. The results of the study tests were not provided to the caretakers or included in the medical records.
Umbilical cord blood was drawn after placental delivery, as described.18 The following laboratory studies were performed at the Intermountain Healthcare Central Clinical Laboratory, Murray, UT, in accordance with Intermountain Healthcare Laboratory Services standard operating procedures and with the manufacturer’s instructions; serum iron, transferrin, iron binding capacity, iron percent saturation, serum ferritin and complete blood count with reticulocyte parameters (Sysmex, Kobe, Japan). The Sysmex quality control procedures were performed daily as recommended by the manufacturer. The following studies were performed at ARUP Laboratories, Salt Lake City, UT, in accordance with ARUP standard operating procedures and manufacturer’s instructions; soluble transferrin receptor and zinc protoporphyrin to heme ratio. Ferritin index was determined for each infant by calculating the ratio of soluble transferrin receptor/log ferritin.
Results of the iron studies were entered into REDCap (Research Electronic Data Capture) for workflow and analysis by an authorized Intermountain Healthcare data analyst (VLB). Medians and interquartile ranges were used to express values. Statistical analysis used the R Foundation package (Statistical Computing, Vienna, Austria). The mixed effects model used the NIME program, version 3.1–105, also from the R package. Statistical significance was set as P < 0.05.
RESULTS
We began the study in December 2015 and completed it in May 2016. Fifty umbilical cord samples were drawn; 20 from healthy comparison neonates of 31 to 40 weeks gestation, 10 from SGA neonates 33 to 40 weeks gestation, 10 from IDM neonates 36 to 39 weeks gestation and 10 from VLBW neonates 25 to 31 weeks gestation.
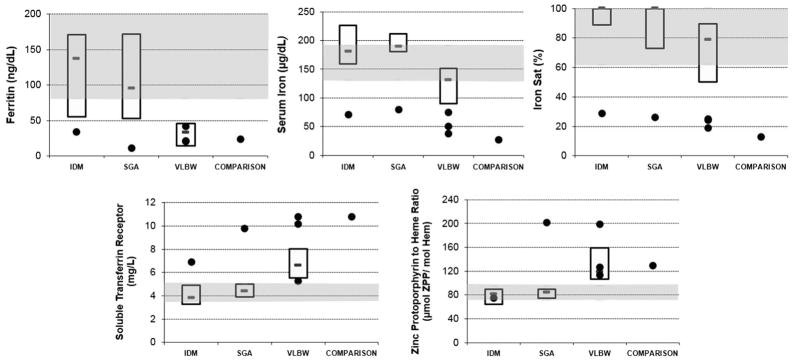
Five of the 30 in the at-risk groups and 1 subject of 20 in the comparison group had a pattern consistent with biochemical iron deficiency; specifically, low values (outside of the interquartile range from the comparison infants) for serum iron, percent iron binding saturation, and serum ferritin, and high values for soluble transferrin receptor and zinc protoporphyrin to heme ratio. Figure 1 displays the five values related to biochemical iron deficiency, Figure 2 shows two values related to iron-limited erythropoiesis and Figure 3 shows two values related to iron deficiency anemia. In all three figures, median and interquartile ranges of the groups of SGA, IDM and VLBW neonates are plotted, the shaded zone represents the interquartile range of the comparison neonates, and the six with the pattern of biochemical iron deficiency are shown as individual dots.
Figure 1.
Results of iron studies assessing biochemical iron deficiency (ferritin, serum iron, iron saturation, soluble transferrin receptor and zinc protoporphyrin to heme ratio). The shaded areas show the upper and lower interquartile range of the comparison neonates. The boxes show the upper and lower interquartile range (and median value) of the groups (n =10) of SGA, IDM and VLBW neonates. Single dots are the six neonates with the pattern of test results suggesting iron deficiency at birth. IDM, infant of diabetic mother; SGA, small-for-gestational-age; VLBW, very-low-birth weight.
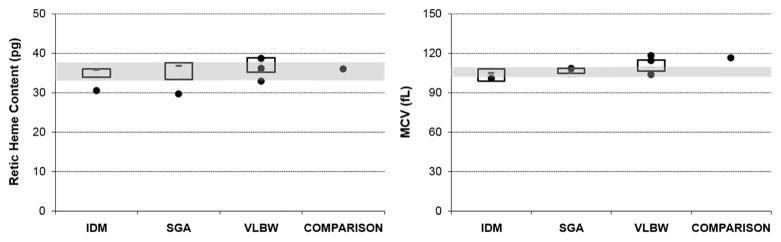
Figure 2.
Results of iron studies assessing iron-limited erythropoiesis (reticulocyte hemoglobin content and mean corpuscular volume). The shaded areas show the upper and lower interquartile range of the comparison neonates. The boxes show the upper and lower interquartile range (and median value) of the groups (n =10) of SGA, IDM and VLBW neonates. Single dots are the six neonates with the pattern of test results suggesting iron deficiency at birth. IDM, infant of diabetic mother; SGA, small-for-gestational-age; VLBW, very-low-birth weight.
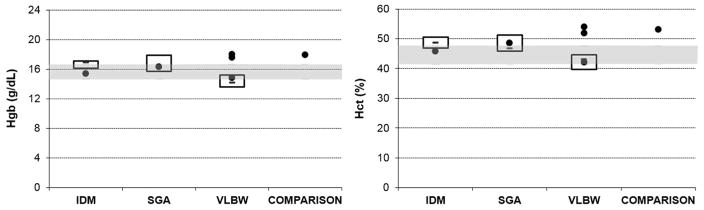
Figure 3.
Results of iron studies assessing iron deficiency anemia (hemoglobin and hematocrit). The shaded areas show the upper and lower interquartile range of the comparison neonates. The boxes show the upper and lower interquartile range (and median value) of the groups (n =10) of SGA, IDM and VLBW neonates. Single dots are the six neonates with the pattern of test results suggesting iron deficiency at birth. IDM, infant of diabetic mother; SGA, small-for-gestational-age; VLBW, very-low-birth weight.
The values of each iron test, from the six infants with an iron-deficient pattern, are shown in Table 1. The interquartile range for ferritin index from comparison neonates was 1.7 to 2.41. The ferritin index of the six infants with an iron-deficient pattern ranged from 3.27 to 9.41. Three of the six infants with an iron-deficient pattern had reticulocyte hemoglobin levels below the interquartile range of the comparison infants. None of the six had iron deficiency anemia, as their hemoglobin and hematocrit values were within the reference intervals for gestational age.15,19,20
Table 1.
Iron status measurements at birth from comparison neonates categorized as iron replete (n =19) vs iron-deficient infants (n =6) at birth
| Patient group | Biochemical measures of iron deficiency
|
Measures of iron-deficient erythropoiesis
|
Measures of iron deficiency anemia
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ferritin (ng ml −1) | Iron (μg dl −1) | Sat (%) | Soluble transferrin receptor (mg l −1) | ZPP to heme ratio | Retic hemoglobin content (pg) | MCV (fl) | Hgb (g dl −1) | Hct (%) | |
| SGA, n = 1 | 11 | 79 | 26 | 9.8 | 202 | 29.6 | 108.2 | 16.3 | 48.6 |
| IDM, n = 1 | 35 | 71 | 29 | 6.9 | 75 | 30.5 | 100.9 | 15.4 | 45.7 |
| VLBW, n = 1 | 21 | 75 | 25 | 10.8 | 199 | 36.1 | 114.3 | 17.6 | 51.8 |
| VLBW, n = 1 | 22 | 51 | 24 | 10.2 | 127 | 32.8 | 118.2 | 18.0 | 53.9 |
| VLBW, n = 1 | 42 | 38 | 19 | 5.3 | 114 | 38.6 | 103.7 | 14.8 | 42.1 |
| Preterm (31 w), n = 1 | 24 | 28 | 13 | 10.8 | 130 | 36.0 | 116.2 | 17.9 | 53.0 |
| Interquartile range comparison group n = 19 | 81–200 | 130–190 | 61–100 | 3.6–5.0 | 70–95 | 33.2–37.5 | 101.9–109.7 | 14.7–16.7 | 41.4–47.7 |
Abbreviations: Hct, hematocrit; Hgb, blood hemoglobin concentration; IDM; infant of diabetic (type I or type II) mother; MCV, mean corpuscular volume; Sat, saturation of iron binding with iron; SGA, small-for-gestational-age ( < 10th percentile weight for gestational age at birth); VLBW, very-low-birth weight ( < 1500 g); ZPP, zinc protoporphyrin. Individual values are shown for the six judged as having biochemical iron deficiency. Median and interquartile ranges are shown for the comparison neonates.
Correlation analyses and linear regression were performed across all infants to assess any effect of gestational age and/or birth weight on the iron studies. Serum iron, % iron binding saturation, serum ferritin, mean corpuscular volume and zinc protoporphyrin to heme ratio were influenced by gestational age, whereas soluble transferrin receptor level was not. The mean corpuscular volume and the zinc protoporphyrin to heme ratio both tended to fall as the gestational age increased (R2 = 0.45 and 0.31, respectively). The serum iron and the serum ferritin slightly increased as the gestational age increased (R2 = 0.26 and 0.25). Birth weight did not generally have an effect on iron measurements independent of the effect of gestational age. The only exception was serum ferritin; increasing gestational age was associated with higher serum ferritin levels (R2 = 0.25); however, at any given gestational age, increasing birth weight was associated with lower serum ferritin levels (R2 = 0.25).
DISCUSSION
Several observations have led to a previous assumption that iron deficiency at birth is extremely rare or almost non-existent.14 The observations are: (1) maternal iron deficiency does not result in low serum iron levels of their neonates,8,9 (2) the fetus is an excellent scavenger of maternal iron, even at the expense of very-low maternal iron stores21 and (3) maternal iron supplementation during pregnancy does not affect fetal ferritin levels22 or soluble transferrin receptor levels.23 Thus, a familiar assertion related to iron status in neonates was that iron deficiency will not be present at birth but it can develop over the first few months after birth in infants with low-iron stores. However, subsequent studies have shown that iron deficiency can exist at birth in some neonates, including those infants whose mothers have iron deficiency anemia.8,9 VLBW premature neonates do not have the advantage of the large accretion of iron during the last trimester of pregnancy, which may lead to iron deficiency at birth.14 SGA infants and IDM are also considered to be at risk for iron deficiency at birth,24 but this assumption had not previously been well documented.
Our present observations from this pilot suggest that some neonates (12%, 6 of 50) have evidence of biochemical iron deficiency at birth. Specifically, they demonstrate a pattern of low values for serum iron, iron saturation, and ferritin, and high values for soluble transferrin receptor, zinc protoporphyrin to heme ratio and ferritin index. Each of these laboratory changes supports the diagnosis of biochemical iron deficiency.
During a state of low iron availability, serum levels of iron and ferritin typically fall. The consequent decreased iron delivery to the erythron is reflected in an increase in soluble transferrin receptor and the ratio of zinc protoporphyrin to heme. Soluble transferrin receptor is the cleaved extracellular portion of transferrin receptor-1 released into the serum. An elevated level is a stable marker of iron deficiency, even during an inflammatory state.17 Likewise an elevated zinc protoporphyrin to heme ratio indicates iron limitation for heme production. In a state of iron deficiency, protoporphyrin IX, the immediate precursor of heme, will incorporate zinc instead of iron, increasing the ratio of zinc protoporphyrin to heme.25 However, the zinc protoporphyrin to heme ratio at birth must be interpreted with caution and in the context of the clinical setting and other laboratory markers, as they are often elevated compared with levels in children and adults, thought to be due to physiologic increased erythropoiesis in the newborn.26
Evidence from animal model systems suggest that biochemical iron deficiency during fetal development might have adverse consequences even in the absence of fetal anemia.1–5 We found biochemical iron deficiency in 5 of the 30 screened neonates in at-risk groups and in 1 of 20 in the comparison group. Three of the six infants with evidence of biochemical iron deficiency had reticulocyte hemoglobin levels below the interquartile range of the comparison infants, which may be suggestive of early iron-limited erythropoiesis. We did not find evidence of iron deficiency anemia at birth. Other markers of iron metabolism might have utility in assessing iron status, particularly in the setting of inflammation. The changes in iron status with inflammation are largely mediated by an increase in the serum iron regulatory hormone hepcidin and are characterized by a high or normal ferritin but low serum iron.27–30 As such, future studies may benefit from measurement of hepcidin along with markers of inflammation.
Various risk factors, including SGA, IDM, may have varying effects on the iron status in preterm vs term infants.31 We found that serum ferritin increased with increasing gestational age, but that birth weight was inversely related to serum ferritin at any given gestational age. It is likely that with increased body mass, additional red blood cells are needed to increase oxygen carrying capacity to the larger tissue area. IDM may be large-for-gestational age and can have polycythemia. The additional red blood cells produced in this state require iron, thus storage forms of iron might be lower in these neonates.
There are a number of limitations to this study. First, because study subjects were de-identified, we have no information on the iron status of the mothers, on expression of placental iron transport, or on the subsequent clinical courses of the 50 neonates. However, the information obtained from this pilot study will be useful in designing larger studies, which will include evaluation of maternal iron status and assessment of the effect of iron supplementation. Akkermans et al.32 studied the iron status of late preterm infants at 6 weeks of age and showed that over 30% had developed iron deficiency anemia. We speculate that many infants who are identified to be iron deficient at birth will go on to develop iron-limited erythropoiesis or iron deficiency anemia prior to the initiation of iron supplementation.
Second, all but two infants from our comparison group were greater than 36 weeks gestation at birth, thus our interquartile comparison ranges for each iron study may not apply to infants of younger gestational ages. Gestational age-specific reference ranges may be necessary to appropriately interpret biomarkers of iron status in preterm infants, and many of these reference ranges are not well established.7
Third, the small numbers and variable gestational ages in our comparison group (n = 20) reduces confidence in our reference intervals. Our control values for serum ferritin, however, are similar to those published by Siddappa et al.33 and Lorenz et al.,29,34 our control zinc protoporphyrin to heme ratios are similar to those published by Miller et al.35 and Cheng et al.,25 our control soluble transferrin receptor values are similar to those reported by Sweet et al.23,36 and our RBC indices, reticulocyte hemoglobin content, and hemoglobin and hematocrit values are similar to the larger reference intervals studies published by our group.15,19,20 Moreover, the trend we observed toward an increase in ferritin with increasing gestational age is similar to that observed by Siddappa et al.,33 and the fall in zinc protoporphyrin to heme ratio we observed with increasing gestational age is similar to that reported by Cheng et al.25 Also the lack of effect of gestational age on soluble transferrin receptor level, which we found, was previously reported by Sweet et al.23 Among our six samples with biochemical iron deficiency, the ferritins were all well below the 5th percentile confidence limits for ferritin reported by Siddappa et al.,33 and our four highest zinc protoporphyrin to heme ratios were all greater than the 95th percentile confidence limits reported by Chen et al.25 Thus, by comparison to published studies of others, as well as by comparison to our own controls, we are reasonably certain that our six neonates were indeed iron deficient at birth.
If subsequent studies show that biochemical iron deficiency at birth is an authentic pathological entity and is present in a significant proportion of certain at-risk populations, there may be an opportunity for a more personalized and precision medicine approach to iron supplementation in infancy. For instance, neonates identified as iron deficient at birth could participate in randomized trials to assess the value of additional or earlier iron supplementation as compared with current practice guidelines37 on important hematologic, neurocognitive and behavioral outcomes.
Acknowledgments
We thank the Metabolics Core from the University of Utah for assistance with mass spectrometry (DK110858). Funding was provided by the Division of Neonatology, University of Utah, ARUP Laboratories, Salt Lake City, UT, and Intermountain Healthcare, Salt Lake City, UT. This work was supported in part by the US National Institutes of Health (DK030534 to Diane Ward).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Callahan LS, Thibert KA, Wobken JD, Georgieff MK. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci. 2013;35:427–436. doi: 10.1159/000354178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran PV, Dakoji S, Reise KH, Storey KK, Georgieff MK. Fetal iron deficiency alters the proteome of adult rat hippocampal synaptosomes. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1297–R1306. doi: 10.1152/ajpregu.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr. 2011;2:112–121. doi: 10.3945/an.110.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:1–10. doi: 10.3389/fnhum.2013.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamora TG, Guiang SF, 3rd, Widness JA, Georgieff MK. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res. 2016;79:922–928. doi: 10.1038/pr.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay K, Yadav RK, Kishore SS, Garewal G, Jain V, Narang A. Iron status at birth and at 4 weeks in term small-for-gestation infants in comparison with appropriate-for-gestation infants. J Matern Fetal Neonatal Med. 2011;7:886–890. doi: 10.3109/14767058.2010.536866. [DOI] [PubMed] [Google Scholar]
- 7.Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36:27–42. doi: 10.1016/j.clp.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hokama T, Takenaka S, Hirayama K, Yara A, Yoshida K, Itokazu K, et al. Iron status of newborns born to iron deficient anaemic mothers. J Trop Pediatr. 1996;42:75–77. doi: 10.1093/tropej/42.2.75. [DOI] [PubMed] [Google Scholar]
- 9.Emamghorashi F, Heidari T. Iron status of babies born to iron-deficient anaemic mothers in an Iranian hospital. East Mediterr Health J. 2004;10:808–814. [PubMed] [Google Scholar]
- 10.Gambling L, Lang C, McArdle HJ. Fetal regulation of iron transport during pregnancy. Am J Clin Nutr. 2011;94:1903 S–1907 S. doi: 10.3945/ajcn.110.000885. [DOI] [PubMed] [Google Scholar]
- 11.McArdle HJ, Lang C, Hayes H, Gambling L. Role of the placenta in regulation of fetal iron status. Nutr Rev. 2011;69:S17–S22. doi: 10.1111/j.1753-4887.2011.00428.x. [DOI] [PubMed] [Google Scholar]
- 12.Balesaria S, Hanif R, Salama MF, Raja K, Bayele HK, McArdle H, et al. Fetal iron levels are regulated by maternal and fetal Hfe genotype and dietary iron. Haematologica. 2012;97:661–669. doi: 10.3324/haematol.2011.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev. 2016;74:421–431. doi: 10.1093/nutrit/nuw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodnough LT, Nemeth E. Clinical features of iron deficiency. In: Greer JP, Arber DA, Glader B, List AF, Means RT Jr, Paraskevas F, et al., editors. Wintrobe’s Clinical Hematology. 13. Lippincott/Williams & Wilkins; Philadelphia, PA, USA: 2014. pp. 626–627. [Google Scholar]
- 15.Christensen RD, Henry E, Bennett ST, Yaish HM. Reference intervals for reticulocyte parameters of infants during their first 90 days after birth. J Perinatol. 2016;36:61–66. doi: 10.1038/jp.2015.140. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz L, Arand J, Büchner K, Wacker-Gussmann A, Peter A, Poets CF, et al. Reti-culocyte haemoglobin content as a marker of iron deficiency. Arch Dis Child Fetal Neonatal Ed. 2015;100:F198–F202. doi: 10.1136/archdischild-2014-306076. [DOI] [PubMed] [Google Scholar]
- 17.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 18.Baer VL, Lambert DK, Carroll PD, Gerday E, Christensen RD. Using umbilical cord blood for the initial blood tests of VLBW neonates results in higher hemoglobin and fewer RBC transfusions. J Perinatol. 2013;33:363–365. doi: 10.1038/jp.2012.127. [DOI] [PubMed] [Google Scholar]
- 19.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123:e333–e337. doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- 20.Christensen RD, Jopling J, Henry E, Wiedmeier SE. The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J Perinatol. 2008;28:24–28. doi: 10.1038/sj.jp.7211852. [DOI] [PubMed] [Google Scholar]
- 21.McArdle HJ, Gambling L, Kennedy C. Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc. 2014;73:9-1.5. doi: 10.1017/S0029665113003637. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77:924–930. doi: 10.1093/ajcn/77.4.924. [DOI] [PubMed] [Google Scholar]
- 23.Sweet DG, Savage GA, Tubman R, Lappin TR, Halliday HL. Cord blood transferrin receptors to assess fetal iron status. Arch Dis Child Fetal Neonatal Ed. 2001;85:F46–F68. doi: 10.1136/fn.85.1.F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Lost-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S91. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng CF, Zerzan JC, Johnson DB, Juul SE. Zinc protoporphyrin-to-heme ratios in high-risk and preterm infants. J Pediatr. 2012;161:81–87. doi: 10.1016/j.jpeds.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 26.Lott DG, Zimmerman M, Labbe RF, Kling PJ, Widness JA. Erythrocyte zinc proto-porphyrin is elevated with prematurity and fetal hypoxemia. Pediatrics. 2005;116:414–422. doi: 10.1542/peds.2004-1601. [DOI] [PubMed] [Google Scholar]
- 27.Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 2012;1823:1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem. 2011;57:1650–1669. doi: 10.1373/clinchem.2009.140053. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz L, Peter A, Poets CF, Franz AR. A review of cord blood concentrations of iron status parameters to define reference ranges for preterm infants. Neonatology. 2013;104:194–202. doi: 10.1159/000353161. [DOI] [PubMed] [Google Scholar]
- 30.Rehu M, Punnonon K, Ostland V, Heinonen S, Westerman M, Pulkki K, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85:345–352. doi: 10.1111/j.1600-0609.2010.01479.x. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy PJ, Zundel HR, Johnson KR, Blohowiak SE, Kling PJ. Impact of growth restriction and other prenatal risk factors on cord blood iron status in prematurity. J Pediatr Hematol Oncol. 2016;38:210–215. doi: 10.1097/MPH.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 32.Akkermans MD, Uijterschout L, Abbink M, Vos P, Rovekamp-Abels L, Boersma B, et al. Predictive factors of iron depletion in late preterm infants at the postnatal age of 6 weeks. Eur J Clin Nutr. 2016;70:941–946. doi: 10.1038/ejcn.2016.34. [DOI] [PubMed] [Google Scholar]
- 33.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz L, Herbst J, Engel C, Peter A, Abele H, Poets CF, et al. Gestational age-specific reference ranges of hepcidin in cord blood. Neonatology. 2014;106:133–139. doi: 10.1159/000360072. [DOI] [PubMed] [Google Scholar]
- 35.Miller SM, McPherson RJ, Juul SE. Iron sulfate supplementation decreases zinc protoporphyrin to heme ratio in premature infants. J Pediatr. 2006;148:44–48. doi: 10.1016/j.jpeds.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 36.Sweet DG, Savage G, Tubman TR, Lappin TR, Halliday HL. Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch Dis Child Fetal Neonatal Ed. 2001;84:F40–F43. doi: 10.1136/fn.84.1.F40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of the preterm infant. In: Kleinman RD, editor. Pediatric Nutrition Handbook. Vol. 5. American Academy of Pediatrics; Chapel Hill, NC, USA: 2004. pp. 23–54. [Google Scholar]