Abstract
Shigella flexneri, the etiologic agent of bacillary dysentery, invades epithelial cells as well as macrophages and dendritic cells and escapes into the cytosol soon after invasion. Dissection of the global gene expression profile of the bacterium in its intracellular niche is essential to fully understand the biology of Shigella infection. We have determined the complete gene expression profiles for S. flexneri infecting human epithelial HeLa cells and human macrophage-like U937 cells. Approximately one quarter of the S. flexneri genes showed significant transcriptional adaptation during infection; 929 and 1,060 genes were up- or down-regulated within HeLa cells and U937 cells, respectively. The key S. flexneri virulence genes, ipa-mxi-spa and icsA, were drastically down-regulated during intracellular growth. This theme seems to be common in bacterial infection, because the Ipa-Mxi-Spa-like type III secretion systems were also down-regulated during mammalian cell infection by Salmonella enterica serovar Typhimurium and Escherichia coli O157. The bacteria experienced restricted levels of iron, magnesium, and phosphate in both host cell types, as shown by up-regulation of the sitABCD system, the mgtA gene, and genes of the phoBR regulon. Interestingly, ydeO and other acid-induced genes were up-regulated only in U937 cells and not in HeLa cells, suggesting that the cytosol of U937 cells is acidic. Comparison with the gene expression of intracellular Salmonella serovar Typhimurium, which resides within the Salmonella-containing vacuole, indicated that S. flexneri is exposed to oxidative stress in U937 cells. This work will facilitate functional studies of hundreds of novel intracellularly regulated genes that may be important for the survival and growth strategies of Shigella in the human host.
Shigella flexneri is a facultative intracellular pathogen which is responsible for human bacillary dysentery, a disease characterized by acute rectocolitis (42). The disease process and pathogenesis are associated with an invasive phenotype that is largely characterized by the ability of bacteria to invade nonphagocytic epithelial cells and to cause the apoptosis of macrophages (66). All pathogenic Shigella strains possess a virulence plasmid which encodes the invasion plasmid antigens IpaACDB and the Mxi-Spa-type III secretion system (TTSS), required for the invasion of the colorectal epithelium (1, 68). The initial entry point is thought to be M cells in the lymphoid follicle-associated epithelium at the early stage of epithelial translocation (67). Following translocation across M cells, the bacteria invade epithelial cells from the basolateral side and infect neutrophils, dendritic cells, and resident macrophages.
Infection of both epithelial cells and macrophages has been shown to be dependent on secreted Ipa proteins (34, 73, 82). The bacteria escape from the phagocytic vacuole shortly after entry into all of these cells (25), but the outcome of the invasion process depends on the cell target. In macrophages and dendritic cells, the escape of the bacteria into the cytosol is mediated by the secretion of Ipa protein effectors, particularly IpaB. IpaB also activates caspase 1 (also known as interleukin-1 [IL-1]-converting enzyme), thereby triggering the apoptosis of these cells and the production of IL-1β and IL-18, two cytokines that initiate mucosal inflammation by recruiting polymorphonuclear leukocytes and monocytes to the site of infection (16, 70, 90). Unlike the situation in macrophages, the invasion of epithelial cells does not lead to apoptosis, although the bacteria also require secreted Ipa proteins to escape into the cytosol. Infected epithelial cells remain viable for many hours postinfection, although intracellular growth does cause a degree of metabolic stress to the host cells (39). Additionally, S. flexneri is able to polymerize host actin via the unipolarly expressed IcsA protein, resulting in efficient intra- and intercellular motility (35).
In addition to the plasmid-borne virulence determinants, S. flexneri requires many chromosomally carried genes to survive and to grow in the host cell cytosol, a niche unique among the enteric gram-negative pathogens. The cytosolic milieu is a reducing environment, due to the activity of glutathione reductase, which maintains reduced glutathione at a concentration of about 10 mM and which is necessary to prevent protein folding in the cytosol. To survive and to grow in this reducing environment, S. flexneri requires the periplasmic protein folding catalyst DsbA to balance the redox potential (86). Furthermore, the cytosol is thought to be limiting for the essential metal iron; consistent with this notion, three iron transport systems, Iuc, Sit, and Feo, are required for intracellular growth (63). In contrast, it has been reported that the TonB-dependent transport of heme and enterobactin is not required for the invasion, survival, and multiplication of S. dysenteriae in the cell cytosol. The fact that TonB is essential for intracellular growth suggests a distinct role in intracellular survival which is unrelated to the sequestration of iron (61). Other studies with transposon mutagenesis and in vivo expression technology have identified a number of genes required for plaque formation by S. flexneri on epithelial cell monolayers (3, 83). Furthermore, green fluorescent protein (GFP)-based technology has been used to identify eight S. flexneri genes that are induced during infection of Henle cells; of these, uhpT and pstS are responsible for glucose-6-phosphate acquisition and phosphate acquisition, respectively, and the Salmonella sitA paralog was recently shown to be involved in iron acquisition (63) (Table 1).
TABLE 1.
Levels of expression of key S. flexneri genes in HeLa or U937 cells
| Gene name
|
Gene expression levels in the following cells at the indicated time (h), relative to those in LB medium:
|
Function or comments | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common | Systematic | HeLa
|
U937
|
|||||||
| 2 | 4 | 6 | 8 | 1 | 3 | 5 | ||||
| bioA | SF0725 | 4.2 | 3.7 | 4.4 | 4.4 | 2.5 | 3.8 | 3.4 | Biotin synthesis | 62a |
| cydC | SF0845 | 0.9 | 0.6 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | Electron transport respiration | 83 |
| dksA | SF0137 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | IcsA presentation, filamentation | 47 |
| fhuA | SF0142 | 2.9 | 3.1 | 3.5 | 3.8 | 3.7 | 3.6 | 2.9 | Iron transport | 62a |
| htrA (degP) | SF2098 | 0.6 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.4 | DTDP-rhamnosyl transferase | 58 |
| ispAb | SF0358 | 0.6 | 0.6 | 0.7 | 0.7 | 0.6 | 0.6 | 0.6 | Intracellular separation | 38 |
| lysAc | SF2848 | 2.9 | 1.6 | 1.5 | 1.6 | 2 | 2.4 | 2.5 | Lysine biosynthesis | 62a |
| msbB2b | CP0238 | 1.4 | 1.2 | 1.5 | 1.4 | 1.6 | 1.8 | 1.6 | Lipid A biosynthesis? | 13 |
| phoA | SF0230 | 2.9 | 3.1 | 4.4 | 4.3 | 5.3 | 6.1 | 5.6 | Phosphate acquisition | 62a |
| pstS | SF3727 | 2.5 | 3.9 | 4.3 | 4.7 | 3.6 | 4.5 | 5.7 | Phosphate acquisition | 62a |
| rfbG | SF1236 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | O-antigen synthesis | 48 |
| rfcc | SF2097 | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.7 | O-side-chain polymerase | 64 |
| rfec | SF3858 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 | O-antigen synthesis | 65 |
| sitA | SF1365 | 12.2 | 9 | 10.2 | 8.5 | 9.3 | 7.4 | 6.8 | Iron transport | 62a |
| sodA | SF3985 | 0.8 | 0.4 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | Superoxide dismutase | 20 |
| sodBc | SF1684 | 0.4 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | 0.4 | Sensitive to phagocyte killing | 20 |
| tonB | SF1255 | 1.9 | 1.9 | 2.8 | 3 | 2.1 | 2.7 | 2.5 | Energy transducer | 61 |
| uhpT | SF3795 | 15.5 | 7.3 | 4.1 | 4.4 | 5.4 | 4.6 | 4.9 | Sugar phosphate transporter | 62a |
| vacBc | SF4334 | 1.9 | 1.8 | 1.9 | 2 | 2.5 | 2.6 | 1.6 | RNase R activity | 9 |
| vacJ | SF2424 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | Lipoprotein, small plaques | 75 |
| virKb | CP0237 | 0.6 | 0.6 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | Posttranscriptional regulation of VirG | 51 |
| vpsCb | SF3233 | 1.1 | 1.2 | 0.9 | 1 | 1.6 | 1.3 | 1 | ipa secretion | 28 |
| wzzB (rol)b | SF2089 | 0.7 | 0.6 | 0.9 | 0.9 | 0.7 | 1 | 1 | O-antigen synthesis, plaque formation | 49 |
| ydiC (sufA) | SF1714 | 3.7 | 3.1 | 2 | 1.6 | 1.1 | 0.7 | 0.9 | Iron transport | 62a |
Microarray analysis gave levels of induction similar to those reported by Runyen-Janecky et al. for Henle epithelial cells (62): uhpT, 8-fold; bioA, 5-fold; pstS, 17-fold; phoA, 14-fold; ydiC, 6-fold; fhuA, 3-fold; and sitA, 8-fold. The only exception was lysA, which was reported to be induced 3-fold.
The change in gene expression was not significant in comparison to the results for the LB medium sample but are included for reference purposes.
The change from the results for the LB medium sample was significant even though the difference was less than threefold.
The knowledge accumulated over past decades regarding Shigella-host interactions shows the value of taking a cellular microbiological approach to the study of bacterial disease. However, what has been lacking is an appreciation of all of the strategies used by Shigella to survive and to proliferate in the host cytosol. In 2002, Jin et al. published the first genome sequence for S. flexneri (30), and this has now allowed us to construct cDNA microarrays to identify all of the Shigella genes that show transcriptional adaptation during infection of epithelial cells and macrophages. We have used these data to compare the gene expression of S. flexneri with that of Salmonella enterica serovar Typhimurium, an enteric pathogen radically different from S. flexneri in terms of its intracellular survival strategy. These exciting data will strengthen the understanding of the biology of microbe-host interactions for both of these medically important pathogens.
MATERIALS AND METHODS
Bacterial strains.
S. flexneri 2a strain 301 (Sf301) (30) was routinely grown at 37°C on tryptic soy agar supplemented with 0.01% Congo red. Prior to cell infection, liquid cultures were obtained by inoculation of red colonies into Luria broth (LB; Difco) and growth at 37°C with shaking (200 rpm) to early exponential phase (approximate optical density at 600 nm, 0.3). These growth conditions were chosen to ensure that the Shigella Mxi-Spa TTSS was expressed to facilitate cellular invasion.
Cell cultures and bacterial infection.
Human HeLa cervical epithelial and human monoblastic macrophage-like U937 cell lines were grown in Dulbecco minimal essential medium and RPMI 1640 medium, respectively (Gibco). Both media contain 10% fetal bovine serum, 2 mM l-glutamine, and penicillin-streptomycin (Invitrogen). Cells were incubated at 37°C in a humidified 5% CO2 atmosphere as described previously (87). For bacterial infection, both cell lines were grown to 90% confluence on 120-mm-diameter plates. U937 cell cultures were supplemented with phorbol myristate acetate (200 ng ml−1; Sigma) to induce macrophage differentiation for 7 days prior to infection (22). Bacteria were added to cell monolayers at a multiplicity of infection of 100 per cell, and the samples were centrifuged for 10 min at 800 × g. After 30 min of incubation at 37°C, extracellular bacteria were removed by extensive washing with phosphate-buffered saline. A total of 10 ml of fresh medium (Dulbecco minimal essential medium or RPMI 1640 medium) containing gentamicin (50 μg ml−1) was added to each plate, and infected cells were incubated at 37°C for up to 8 and 5 h for HeLa and U937 cells, respectively.
Construction of ShEcoli cDNA microarrays.
The microarrays used in this study are an enhanced version of the Escherichia coli K-12 cDNA microarrays used previously (2, 81). The ShEcoli microarrays have 4,262 open reading frames (ORFs) from E. coli K-12 strain MG1655 (6), 1,125 ORFs from enterohemorrhagic E. coli strain EDL933 (56), and 328 ORFs specific to S. flexneri Sf301 (30). The criterion used to choose E. coli ORFs suitable for analysis of S. flexneri was that only those with more than 80% identity over at least 100 bp to the corresponding S. flexneri ORFs were selected. For ORFs shorter than 100 bp, more than 90% identity was required. We noted that for the S. flexneri Sf301 genome, 613 ORFs, mostly insertion elements, have very closely related paralogs. These ORFs were all excluded from the microarrays. In summary, the extended ShEcoli microarrays allowed us to analyze the expression of 3,564 (93.3%) of the remaining 3,821 ORFs in the S. flexneri Sf301 genome.
Shigella ORFs were amplified with specific primer pairs (Sigma-Genosys) designed by using Array Designer 2 (Premier Biosoft). All primers had a 16-nucleotide common 5′ overhang, TCCTAGGAGCTCTTCT for forward primers and TGCCTAGGGCTCTTCG for reverse primers, designated Adapt-F and Adapt-R, respectively. Our strategy was to amplify all of the coding sequence (CDS) by a two-step PCR procedure involving an initial small-scale PCR (25 μl) with gene-specific primers followed by a larger-scale PCR (100 μl) with the Adapt-F and Adapt-R primers as described by Clements et al. (10). DNA from the PCRs was resuspended in 4 μl of spotting solution containing 50% dimethyl sulfoxide and 0.3× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). PCR products were printed onto GAPS II slides (Corning) by using a Stanford MarkII arrayer (77). The DNA was UV cross-linked to the slides by using a Stratalinker (Stratagene) at 300 mJ. Arrayed slides were blocked prior to hybridization as described previously (14).
RNA extraction.
Bacterial RNA was extracted from infected mammalian cells as described previously (18). At each time point, infected epithelial cells or macrophages were lysed to recover intracellular bacteria by exposure to 0.1% (wt/vol) sodium dodecyl sulfate-0.1% (vol/vol) acidic phenol-19% (vol/vol) ethanol in water for 30 min on ice. The S. flexneri bacteria were not lysed by this procedure, but the mRNA was stabilized and protected from degradation (27, 76). Bacteria were pooled from at least four cell culture plates for each time point, and RNA was prepared by using a Promega SV total RNA purification kit according to the manufacturer's instructions. Bacterial RNA was further purified by DNase I treatment and phenol-chloroform extraction. Control RNAs from bacteria grown to exponential phase in LB and from eukaryotic cells were isolated by using the same RNA purification kit. RNA size chromatography was carried out with an Agilent 2100 Bioanalyzer.
Template labeling and hybridization.
Labeling of RNA and chromosomal DNA was performed as described previously (18). Briefly, RNA (5 μg) was first reverse transcribed into cDNA; cDNA was subsequently fluorescently labeled by random priming with the Klenow fragment. Chromosomal DNA (400 ng) was labeled in a manner similar to that used for cDNA by random priming with the Klenow fragment. After being labeled, samples were hybridized overnight at 65°C. Each labeled RNA sample was hybridized to four microarrays. A type 2 experimental design was used, with fluorescently labeled genomic DNA as a reference channel in each experiment (84). For protocols, see http://www.ifr.bbsrc.ac.uk/Safety/Microarrays/protocols.
Data acquisition and microarray data analysis.
After hybridization, slides were washed and scanned by using a GenePix 4000A scanner (Axon Instruments, Inc.). Fluorescent spots and local background intensities were quantified by using Genepix Pro 3.0 software (Axon Instruments). The data were filtered such that spots with a reference signal lower than the background plus 3 standard deviations of the background were discarded. Signal intensities were corrected by subtracting the local background, and then the red/green (cyanine 5/cyanine 3) ratios were calculated. To compensate for unequal dye incorporation, data centering was performed by bringing the median ln(red/green ratio) for each block to zero (one block being defined as the group of spots printed by the same pin). The complete data set is available as supplemental material (http://www.ifr.ac.uk/safety/molmicro/pubs.html).
The choice of an appropriate comparator for the analysis of intracellular gene expression profiles is crucial. It was not possible to analyze S. flexneri gene expression in cell culture media, as the bacteria are unable to grow in this environment (data not shown). We selected a culture of S. flexneri growing at mid-exponential phase (optical density at 600 nm, 0.8) in LB as the reference for our experiments, as this represented a reproducible in vitro growth condition. Statistical verification of the data was done by using a parametric statistical test to adjust the individual P values with the Benjamini-Hochberg false discovery rate multiple-test correction (4). Changes in expression of threefold or greater were deemed to be significant for this study.
To compare the intracellular expression profiles for Shigella and Salmonella, we used the data from 2,429 pairs of orthologous genes that showed more than 60% identity at the level of their encoded proteins over at least 80% of their length. Taking into account chromosomal inversions, 98% of the selected genes showed the same sequential order on both chromosomes, suggesting that their functions may well be conserved across the two organisms.
RT-PCR.
Total RNA (10 ng) was converted to cDNA by using Stratascript (Stratagene) as described by the manufacturer. The cDNA then was used as a template for PCR (Taq DNA polymerase; Promega). The total number of PCR cycles ranged from 20 to 35. Reverse transcription (RT)-PCR products were analyzed by agarose gel electrophoresis. Total RNA was also used directly (without RT) as a template for PCR to ensure that there was no contaminating DNA. The following primers were used in RT-PCRs: SF0115-F (5′-GCCGTATTCCGCTGATTATCG) and SF0115-R (5′-CGCCACCAGACCAGAACC), predicted amplicon size, 522 bp; SF2715-F (5′-CGTTCGCTGGACATCAACC) and SF2715-R (5′-TGCCTGTTCCGCCAATACC), 407 bp; icsB-F (5′-ACTACAGGAACCAACTCATAC) and icsB-R (5′-GAACATTAAGTGCCATACCAG), 480 bp; icsA-F (5′-ACGGTAGCACTATTGTTGATCC) and icsA-R (5′-ATTATGAGCAGTCCAGCGTAT), 480 bp; virA-F (5′-AAGCCTGCACCAGAAGTTATT) and virA-R (5′-TGTGAAGAGATTGCCAGAATG), 407 bp; ospC2-F (5′-AGAGGTTGTGGATGAAGTTAG) and ospC2-R (5′-CTCCATCTCTGCGACATCTTG), 452 bp; ipgD-F (5′-GACGCCGACCAGTTTAACC) and ipgD-R (5′-GCATACCTGTTCTGTCCTTCC), 501 bp; and ipaH1.4-F (5′-CCTATGGAACTGACTCCATCTT) and ipaH1.4-R (5′-CGTGCCGAGAGTGATTGTAA), 417 bp.
LDH activity assay.
The lactate dehydrogenase (LDH) assay was performed by using a Cytotox 96 nonradioactive cytotoxicity assay system (Promega) according to the manufacturer's instructions. This assay measures the conversion of a tetrazolium salt to a formazan product, which is red in color and therefore detectable by measurement of the A490 by using a TherMo Max plate reader (Molecular Devices). Cytotoxicity is expressed as suggested by the manufacturer with the following equation: percent cytotoxicity = (A490 of the infected supernatant − A490 of the uninfected supernatant)/(A490 of the total lysate − A490 of the uninfected supernatant).
RESULTS AND DISCUSSION
DNA microarray, infection models, and bacterial RNA isolation.
We customized an E. coli DNA microarray that has already been successfully used for the transcriptional profiling of colicin-induced cell death in E. coli K-12 (81) and the phylogenomic analysis of pathogenic E. coli strains (2). This ShEcoli microarray allowed us to study the expression of 3,564 Shigella ORFs representing 93.3% of the 3,821 non-cross-hybridizing ORFs of S. flexneri Sf301 (see Materials and Methods).
HeLa epithelial and U937 macrophage-like human cells were chosen as model cells for infection because these two cell lines have been widely used for studying Shigella invasion and have produced consistent data in our experiments. The choice of U937 cells, rather than another widely used murine macrophage cell line, J774, reflects the fact that S. flexneri kills J774 cells very rapidly through apoptosis (90), preventing the recovery of sufficient intracellular bacteria for RNA isolation. In contrast, the development of apoptosis or oncosis following Shigella infection of U937 cells depends on the differentiation conditions (54). When treated with phorbol myristate acetate to induce differentiation, U937 cells become tolerant to Shigella infection; the onset of oncosis is delayed until many hours after infection, allowing the recovery of large numbers of intracellular bacteria (87). To obtain intracellular bacteria, HeLa and U937 cells were infected with S. flexneri strain Sf301 under the conditions described in Materials and Methods. At all time points, both cell lines were well attached to culture dishes, and no significant cell lysis was observed with a microscope.
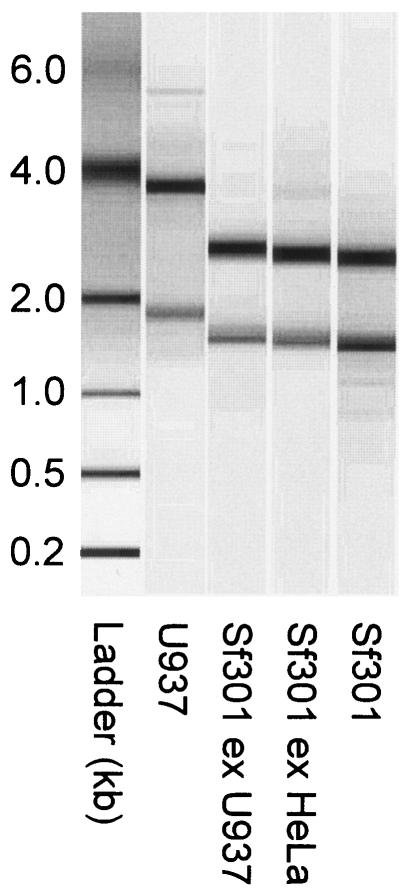
The isolation of high-quality bacterial RNA that accurately reflected S. flexneri gene expression during infection was critical, and the important factors were recently discussed (27). We essentially followed the cold phenol-ethanol protocol, which allows quick lysis of host cells and stabilization and preservation of bacterial RNA (18). The quality of RNA isolated from both cell lines at all time points was excellent, showing minimal degradation or host RNA contamination (Fig. 1). The amount of RNA isolated at each time point from both cell lines was at least 25 μg and was sufficient for hybridization to at least four microarrays with the labeling procedure outlined above.
FIG. 1.
Size chromatographic separation of RNAs. Total RNAs were extracted from intracellular S. flexneri bacteria as described in the text. Results for samples from the first time points of U937 cell (Sf301 ex U937) and HeLa cell (SF301 ex HeLa) infections are shown. Total RNAs extracted from a bacterial LB culture (Sf301) and uninfected U937 cells (U937) were used as controls to verify the purity of the intracellular bacterial RNAs.
Global transcription profiles.
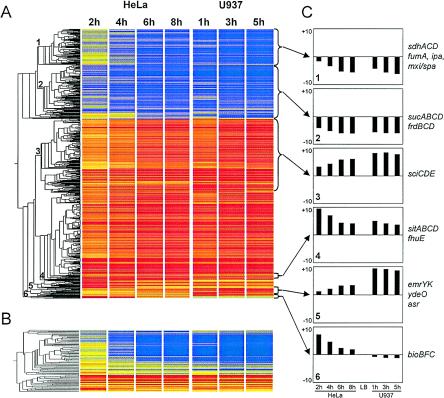
The gene expression profiles for intracellular S. flexneri bacteria were compared with the transcriptome of in vitro-grown bacteria (see Materials and Methods). In total, 597 S. flexneri genes were up-regulated and 332 genes were down-regulated more than threefold inside HeLa epithelial cells. The numbers of bacterial genes showing altered expression inside U937 cells were comparable, with 661 genes being up-regulated and 399 genes being down-regulated. Gene expression profiles for S. flexneri within HeLa and U937 cells were particularly similar toward the later stages of the infection, although epithelial cells and macrophages have radically different biological functions (Fig. 2). The relative changes in gene expression ranged from a 266-fold induction of the yfdW gene to a 63-fold down-regulation of the treB gene.
FIG. 2.
Gene expression profiles for Shigella during infection of HeLa and U937 cells. (A) Cluster diagram of the expression profile of 1,256 chromosomal genes showing statistically different levels of expression during infection of HeLa or U937 cells relative to the data for the reference LB culture. Each horizontal line represents one gene. Red indicates at least a twofold increase, yellow indicates no change, and blue indicates a minimum twofold decrease in expression. (B) Cluster diagram of the expression profile of 80 plasmid pCP301-borne genes. Hierarchical clustering was performed by using Genespring 6.2 with the Pearson correlation. (C) Average relative levels of expression of representative clusters. The vertical axis shows the fold change in expression for each gene on a logarithmic scale. Examples of genes corresponding to the various profiles are given.
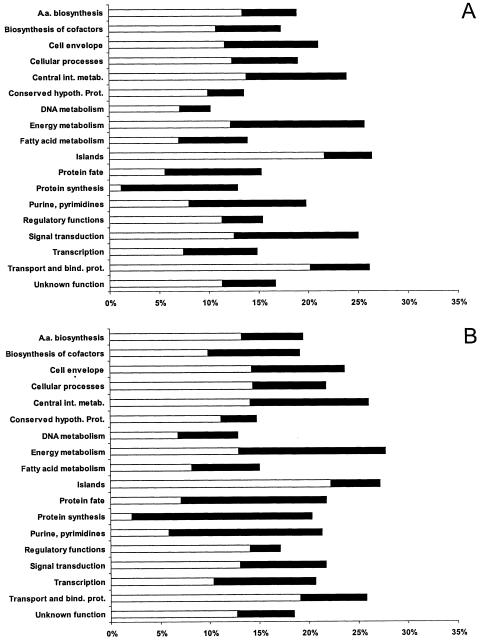
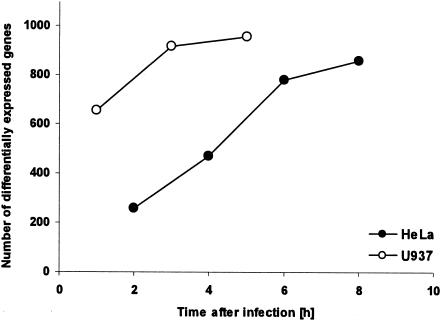
Examination of functional groups of intracellularly regulated bacterial genes confirmed the similarities of gene expression patterns in both cell lines (Fig. 3). In fact, no gene was found to be up-regulated in one cell line and down-regulated in the other, with the exception of hdeA, which was slightly down-regulated inside HeLa cells and up-regulated inside U937 cells. This gene is part of the YdeO regulon, which is discussed in more detail below. It appears that the adaptation of S. flexneri occurred more rapidly inside U937 cells; most of the changes occurred within 1 h of infection, whereas adaptation inside HeLa cells was a more gradual process (Fig. 4). These results may reflect the fact that differentiated phorbol myristate acetate-treated U937 cells possess macrophage characteristics (21) and therefore would be expected to have a greater effect on the gene expression of intracellular bacteria than HeLa cells.
FIG. 3.
Differential expression of S. flexneri genes divided into functional groups. Bars indicate percentages of genes in each group that showed significant changes in expression in HeLa (A) and U937 (B) cells. The white bars show the percentages of S. flexneri genes up-regulated and the black bars show the percentages of genes down-regulated during intracellular growth. Genes were divided into functional categories by using the Comprehensive Microbial Resource described by Peterson et al. (57). A.a., amino acid; int. metab., intermediary metabolism; hypoth. Prot., hypothetical protein; bind., prot., binding protein.
FIG. 4.
Time-dependent transcriptional adaptation of S. flexneri in U937 and HeLa cells. The graph shows the total number of Shigella genes which were significantly differentially expressed (more than threefold) at each time point for both U937 and HeLa cells.
It was important to ensure that this novel data set for S. flexneri in vivo-regulated genes was consistent with data from previous reports. Until now, the most comprehensive study relied on GFP-based technology to identify seven S. flexneri genes that were markedly up-regulated during infection of Henle epithelial cells (62). These genes included uhpT for hexose phosphate transport, bioA for biotin synthesis, lysA for lysine synthesis, fhuA and sitA for iron transport, pstS for high-affinity phosphate transport, and phoA, encoding an alkaline phosphatase. It is striking that the gene expression profiles presented here showed similar levels of induction of these genes during HeLa and U937 cell infections compared to those of the original GFP-based measurements in Henle cells (Table 1). Since different cell lines and bacterial strains were used in these two independent studies, the agreement of the data for six of these seven genes indicates that data from our DNA microarray analyses are reliable and consistent with data from previous reports.
Expression of essential invasion genes.
The expression of the plasmid-borne ipa-mxi-spa locus is known to be essential for Shigella entry into both epithelial cells and macrophages (see above). The expression of these genes was drastically down-regulated in both cell lines, although the reduction in the expression of these genes in HeLa cells was a gradual process (Fig. 5). The intracellular down-regulation of the ipa-mxi-spa genes in U937 cells was not unexpected, because bacteria would be free in the host cytosol by 1 h postinfection. Furthermore, there is no evidence that U937 cells form intermediate junctions, which are a prerequisite for the Ipa-dependent cell-to-cell spread of the bacteria (69). However, the down-regulation of the ipa-mxi-spa genes in HeLa cells is intriguing, as secreted Ipa proteins are required for intercellular bacterial spread within epithelial cell monolayers after entry (60, 72). We wanted to rule out the possibility that HeLa cells lost membrane integrity, exposing intracellular bacteria to gentamicin and altering the expression of particular genes.
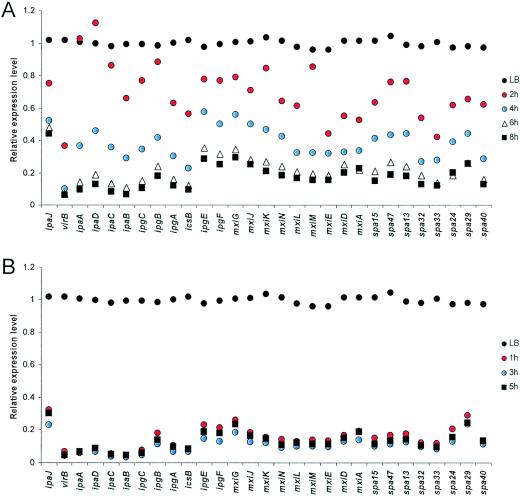
FIG. 5.
Time-dependent down-regulation of the ipa and mxi/spa virulence genes during infection of HeLa (A) and U937 (B) cells.
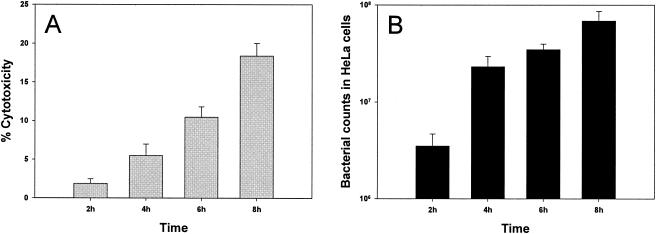
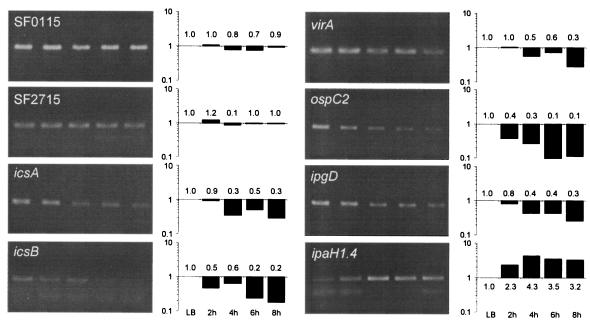
To investigate this possibility, we repeated HeLa cell infection under the same conditions as those used above, monitored intracellular bacterial growth, and determined cytotoxicity by testing the levels of LDH released into the cell culture medium. Figure 6 shows that as the level of cytotoxicity increased gradually, the intracellular bacteria grew steadily over time. Hence, Shigella infection is only cytotoxic to a small proportion of HeLa cells, and most intracellular bacteria are well protected from gentamicin killing. Total bacterial RNA was also isolated from this independent infection experiment for use in an RT-PCR analysis; the resulting data verified the microarray results and confirmed that the expression of icsA/virA and icsB/ipgD was significantly down-regulated during intracellular bacterial growth in HeLa cells (Fig. 7).
FIG. 6.
S. flexneri cytotoxicity for and growth inside epithelial cells. The bar graphs show the cytotoxic effect resulting from S. flexneri infection of HeLa cells (see Materials and Methods for details) (A) and the rate of S. flexneri replication inside HeLa cells (B). Values represent the means of measurements from three wells, and error bars indicate standard deviations.
FIG. 7.
Validation of microarray results by RT-PCR. The agarose gel analysis of the PCR fragments obtained after RT-PCR is shown along with the quantification of the corresponding bands (bar graphs). Numbers associated with the bars indicate fold differences relative to the data for the reference LB culture, which were established by densitometric analysis. Samples were isolated at 2, 4, 6, and 8 h after Shigella infection of HeLa cells, and data for these samples can be compared directly with data from the microarray experiment. The RNA used for RT-PCR was isolated from an infection independent from the infections used for gene expression profiling. One RNA sample was isolated from a mid-log LB culture and was used as a reference. Genes SF2715 and SF0115 were chosen as controls because they did not show any significant change in expression in the microarray experiment.
Headley and Payne reported that IpaBCD protein production was reduced significantly during the intracellular growth phase compared to that for bacteria grown in vitro and at the initial entry phase (23), and this report is consistent with our transcriptome analysis. Thus, it is possible that the levels of ipa-mxi-spa expression and icsA expression remained sufficient to meet the need of intercellular bacterial spread, even though the level of gene expression was much lower than that for bacteria grown in vitro. Alternatively, we hypothesize that bacteria may split into two pools once they are inside HeLa cells; one pool becomes “dormant,” as in U937 cells, and the other pool maintains the “invasive” status, which is associated with the production of protrusions and infection of adjacent cells. In this scenario, the gradual decrease in virulence gene expression that we observed for the ipa-mxi-spa genes would then be a reflection of the reduction of the subpopulation that is still able to spread from cell to cell. This hypothesis needs to be tested experimentally.
The expression of the virulence phenotype of S. flexneri involves a complex regulatory mechanism (15). Two plasmid-borne genes, virF and virB, encode essential regulatory proteins. The VirF protein, an AraC-like transcription factor, activates virB and icsA/virG, and the VirB protein in turn binds to the promoters of the entry genes and activates them (65). The chromosomally encoded DNA-binding proteins Fis, IHF, and H-NS are involved in regulating virF and virB. Fis and IHF positively regulate virF. H-NS negatively regulates both virF and virG by binding to the promoters at temperatures below 32°C but not at 37°C, the permissive temperature for virulence gene transcription (18). The H-NS repression complex is sensitive to DNA supercoiling. The microarray data revealed that both fis and virF were moderately but reproducibly down-regulated in both cell lines during the time course of the experiment. The expression of virB was decreased more markedly (up to 12-fold) in the two cell lines (Fig. 5). These results suggest that the down-regulation of the ipa-mxi-spa and icsA/virG virulence genes was initiated through a dialog between the chromosome and the plasmid that was mediated by the fis and virF regulatory genes. The contributions of H-NS and DNA topology to the down-regulation of virF and virB are likely to be minor, as cell invasion was carried out at 37°C.
Previously, Eriksson et al. found that the expression of the Salmonella SPI1 invasion genes is down-regulated inside macrophages but that SPI2, which is responsible for the survival of the bacteria in the Salmonella-containing vacuole (SCV), is up-regulated (18). Recently, it was reported that the expression of the locus of enterocyte effacement from enterohemorrhagic E. coli is also down-regulated after attachment of the bacteria to erythrocytes (11). Salmonella SPI1 genes and the enteropathogenic E. coli locus of enterocyte effacement show more sequence homology to Shigella mxi-spa-ipa genes than to Salmonella SPI2 genes (29). The consistency of these data suggests that the down-regulation of ipa-mxi-spa-like TTSS genes may be a conserved phenomenon that follows successful infection of mammalian cells by enteric pathogens.
Expression of the MxiE regulon.
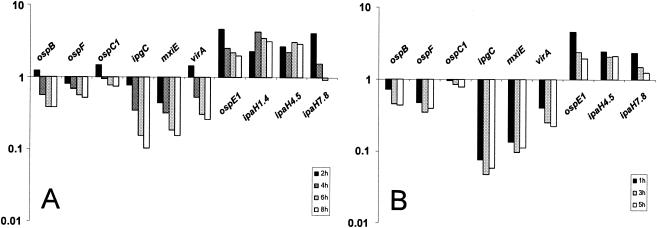
MxiE is a member of the AraC family of regulators which controls a set of late gene products that are secreted through the Mxi-Spa TTSS (12). The MxiE regulon has been reported to be transiently up-regulated after entry into epithelial cells (32); it includes virA (involved in the disruption of host microtubules and the formation of membrane ruffles that enhance bacterial entry) (85), ipaH7.8 (involved in the escape of Shigella into the host cytosol) (19), ipaH9.8 (the product of which is targeted to the host nucleus) (78), and the osp (outer Shigella protein) genes (whose functions are unknown) (8). MxiE works in concert with IpgC, the molecular chaperone for IpaBC, in Shigella and in E. coli (45). Activation by MxiE is mediated by binding to a 17-bp motif, coined the MxiE box, in the promoter region of the target genes (44). Strain Sf301 has 12 copies of ipaH genes—5 on the virulence plasmid and 7 on the chromosome. The MxiE box is present in each of the 12 ipaH promoter regions, although 3 of the chromosomal promoters have degenerate consensus sequences (30). Bioinformatic approaches had suggested that all of the ipaH genes could be targets for activation by Mxi-IpgC. Previously, Kane et al. (32) used flow cytometry to show that the expression of GFP transcriptional fusions of virA, ipaH9.8, ospE2, ospB, ospC1, and ospF was increased at 90 min after entry into L2 mouse fibroblasts. In an independent study, Demers et al. (12) used lacZ fusions to show that during HeLa cell invasion, the expression of virA and the plasmid-borne multiple ipaH genes peaked at 60 min and then dropped close to the levels seen in the in vitro culture after 150 min. The findings of Kane et al. (32) and Demers et al. (12) suggest that MxiE-IpgC-activated genes would be up-regulated during the early stages of HeLa cell infection but that their expression levels would subsequently decrease.
The microarray data show that the members of the MxiE regulon can be split into two groups according to their expression profiles. The expression of virA, ospB, and ospE2 gradually decreased from 2 h and continued to decline over time. In contrast, the expression of ipaH1.4, ipaH7.8, ipaH4.5, and ospE1 was consistently and significantly up-regulated at all time points (Fig. 8). It is worth mentioning that our data also showed that the chromosomal ipaH9.8 genes are induced intracellularly. However, various levels of potential cross-hybridization on our microarrays prevented us from making a definitive statement about the relative levels of expression of the seven ipaH9.8 genes, which share much sequence identity. The down-regulation of virA and the up-regulation of ipaH1.4 were independently confirmed by RT-PCR (Fig. 7). These data suggest that virA, ospB, and ospE2 are solely regulated by the decreasing levels of MxiE during infection (see Table S1 in the supplemental material [http://www.ifr.ac.uk/safety/molmicro/pubs.html]). It is likely that unidentified regulators other than MxiE-IpgC are responsible for the up-regulation of ipaH1.4, ipaH4.5, ipaH7.8, and ospE1 inside HeLa cells as well as inside U937 cells and that these may be important for the late stages of Shigella infection.
FIG. 8.
Expression of genes of the mxiE regulon in HeLa (A) and U937 (B) cells. The bar graphs show the levels of expression at all times relative to the data for the LB culture. The results for the ipaH1.4 gene are from RT-PCR (Fig. 7).
Expression of the 64 SIs.
Strain Sf301 has 64 so-called Shigella islands (SIs) which have a size of >1 kb and which are thought to have been acquired by lateral gene transfer mediated by bacteriophages (30). Until now, it was unknown whether SI genes were up-regulated during infection. We found that a greater percentage of genes from SIs than from all other functional groups were up-regulated (30%) (Fig. 3), indicating that many of the SI genes are important following invasion. Apart from the ipaH genes mentioned above, a number of key SI genes are discussed below.
In SI-1, four genes (sciA and sciCDE) share homology with the Salmonella serovar Typhimurium genes STM0266 and STM0268 to STM0270, respectively. The sciC and sciE genes were up-regulated by 10- and 4-fold, respectively, in both cell lines, and the sciD gene was unchanged. Interestingly, the orthologous Salmonella serovar Typhimurium genes show a similar pattern of expression during macrophage infection; STM0268 and STM070 are induced, while STM0269 is unchanged. Because the functions of these genes are unknown (26), we cannot predict why the sciCE genes are expressed at a higher level in the cell cytosol. Nevertheless, they are good candidates for functional studies.
In SI-19 (ipaH island 2), there are four consecutive genes with homology to the Salmonella sitABCD genes and the Yersinia yfe operon (89). The Salmonella sitABCD genes are required for iron and manganese transport in the phagosome (33, 89) and are highly induced inside macrophages (Table S3). The Shigella sitA gene has already been reported to be up-regulated in the host cell cytosol (62), and SitA was recently demonstrated to transport iron for Shigella in vitro (63). We found up-regulation of sitAB by 10-fold and of sitCD by 3-fold in both cell lines throughout the infection (Table 1). SI-56 (also known as SHI-2) (80) harbors the iucABCD and iutA genes, which encode the Shigella aerobactin system (discussed below).
SI-3 harbors bacteriophage SfII, which mediates serotype conversion; the gtrII gene encodes a glucosyl transferase, and the gtrB gene encodes a bactoprenol glucosyl transferase (43). As both gtrII and gtrB were severely down-regulated in HeLa and U937 cells, type II antigen expression is probably not important for bacterial growth inside the cells. Consistent with this notion, the genes required for O-antigen synthesis, the rfbGFEC genes in SI-35, were down-regulated in macrophages and epithelial cells. SI-49 is the previously identified pathogenicity island SHI-1, which harbors the sigA and pic genes, encoding the autotransporter proteases, and the setAB genes, encoding Shigella enterotoxin 1 (59). The expression of these genes was unchanged or was down-regulated, a finding which correlates with their role in causing extracellular tissue destruction. It is likely that this new data set for SI genes that are up-regulated during infection (see Table S1 in the supplemental material [http://www.ifr.ac.uk/safety/molmicro/pubs.html]) will identify new gene targets for determining novel aspects of the Shigella survival strategy in the cell cytosol.
Maintenance of virulence plasmid pCP301.
Although it is known that the invasion plasmid is essential for Shigella virulence (68), very little is known about its maintenance during infection. The primary replication system of the virulence plasmid is that of plasmid R100, which belongs to the RepFIIA family of replicons (79). There are also multiple loci on pCP301 which are homologous to sequences known to be involved in plasmid segregation and stable maintenance: parA and parB of the bacteriophage P1 partitioning system, stbB and stbA of plasmid R100, ccdA and ccdB of plasmid F, and mvpA and mvpT. Among these, mvpA and mvpT have been demonstrated to form an effective system to maintain stability in vitro: the mvpT product is toxic to plasmidless daughter cells, whereas the mvpA product is an antidote protecting plasmid-carrying bacteria from killing by MvpT (71). While all of the other maintenance genes were either unchanged or down-regulated, the transcription of mvpA was found to be consistently up-regulated by 10- and 8-fold in HeLa and U937 cells, respectively, throughout infection (Table 1). Unfortunately, mvpT was not present on the microarray; therefore, expression data are not available. Interestingly, both of the Salmonella serovar Typhimurium virulence plasmid-borne mvpA and mvpT orthologs (PSLT106 and PSLT107) were also found to be strongly up-regulated during macrophage infection (18). Our findings suggest that an increase in mvpAT expression may ensure the stability of the virulence plasmid in the cell cytosol.
Two-component signal transduction systems, sigma factors, and stress responses.
Two-component signal transduction systems play important roles in regulating virulence in S. flexneri. CpxR is able to bind to the virF promoter region when it is phosphorylated by CpxA (52, 53). The well-characterized PhoP-PhoQ system is crucial for persistent infection and resistance to killing by cationic polypeptides derived from polymorphonuclear leukocytes and other sources (50). We observed that the transcription of these and other two-component systems changed little during intracellular growth. A considerable change was observed for the ompR-envZ osmosensor system, which was down-regulated twofold. Accordingly, the expression of its target genes, ompC and ompF, was significantly decreased in both cell lines (Table 1). OmpC is required for virulence in vitro and in vivo, probably through its role in cell-to-cell spread (5). Coupled with the decreased ipa-mxi-spa expression, the decreased transcription of ompC is another indication that the bacteria reduce their capacity for intercellular dissemination.
The expression of the S. flexneri rpoS gene, the master regulator of general stress and stationary phase (24), and its regulon was not significantly altered during infection of macrophages or epithelial cells. Therefore, as for Salmonella (18), the gene expression pattern for intracellular bacteria did not reflect the stationary phase of bacteria grown in vitro. In fact, the expression of most of the genes encoding sigma factors did not change, except for a moderate up-regulation of rpoH and rpoE, which encode σ32 and σE, respectively. However, the increase in the expression of rpoH was not accompanied by an induction of the known σ32-dependent genes, including dnaK and htpH (88). Similarly, the increase in the expression of rpoE had a limited impact on the expression of the σE regulon; only degP/htrA showed moderate up-regulation (by up to fivefold in both cell lines). The expression of other genes of the σE regulon, such as fkpA, surA, and imp, involved in envelope biogenesis, was unchanged. However, the spy gene (which encodes a periplasmic chaperone controlled by CpxRA), the psp gene (responsible for phage shock) (7), and the ibpAB nonspecific heat shock genes (31) were strongly induced, indicating that intracellular S. flexneri bacteria probably experienced a degree of envelope stress. The significance and mechanisms of these stress responses for the Shigella intracellular survival strategy require further investigation.
Bacterial metabolism in the cell.
Until now, there has been little information about the nature of S. flexneri metabolism during infection, and our intracellular gene expression data offer the first insight into this area. The intermediary metabolism and energy production systems were down-regulated during infection; genes encoding enzymes involved in glycolysis, the Krebs cycle, and oxidative phosphorylation were strongly decreased during intracellular growth in both cell lines. As both aceA and aceK are pseudogenes (29), S. flexneri is unable to utilize two-carbon compounds via the glyoxylate shunt. Taken together, these data suggest that intracellular S. flexneri exhibits reduced energy production, which accounts for the altered expression profiles of the genes encoding the aerobic respiratory chain. The bacteria appear to be rebalancing NAD+ regeneration, net electron efflux, and oxygen utilization to meet their new physiological needs. The nuo genes, encoding NADH dehydrogenase 1 (NDH-1), which can generate proton motive force, were strongly down-regulated, whereas ndh, encoding NADH dehydrogenase 2 (NDH-2), was strongly up-regulated. These data suggest that intracellular S. flexneri bacteria preferentially oxidizes NADH via the NDH-2 pathway, without the generation of proton motive force. The NDH-2 pathway would not primarily provide intracellular bacteria with energy but would recycle NADH into NAD+ to maintain the optimal redox balance. The strong up-regulation of the cyoABCD genes suggests that the reducing equivalents generated by NADH oxidation were preferentially passed to oxygen via cytochrome bo3, which has a lower affinity for oxygen than cytochrome bd, which is the alternative oxygen oxidase. Utilization of the NDH-2-cytochrome bo3 aerobic respiration pathway indicates that intracellular bacteria have low energy and oxygen requirements. None of the components of the anaerobic respiratory chain showed significant changes in expression, except for nitrate reductase A (narGHI). The significance of this finding is not yet clear. In addition, we noted that the rplJL-rpoBC operon, encoding the β subunit of RNA polymerase, was moderately down-regulated (two- to fivefold) inside both HeLa and U937 cells. This finding is consistent with the down-regulation of the catabolic genes and the reduced rate of growth of S. flexneri within the cell cytosol (doubling time, about 40 min) compared with the rate of growth in LB (doubling time, about 30 min).
Interpretation of these changes during the adaptation of S. flexneri to the intracellular environment is challenging; regulation of the genes related to intermediary metabolism is complex, often involves many distinct pathways, and has not yet been studied in detail in S. flexneri. It will be important to determine whether the down-regulation of genes involved in glycolysis and other metabolic cycles that we observed is simply a consequence of growth in the intracellular milieu or is absolutely required for successful infection and whether the down-regulation is reflected at the level of enzyme activity.
Ionic composition of the cell cytosol.
One important function of animal cells is to maintain ion gradients across the plasma membrane to regulate cell volume and drive the transport of nutrients inside the cell. Therefore, the ionic composition of the cytosol is tightly regulated. For example, the Na+-K+ pump ensures the formation of an electrochemical gradient that maintains high K+ and low Na+ intracellular concentrations (55). Furthermore, the levels of Mg2+, Ca2+, and HCO3− are also tightly regulated (36). Therefore, Shigella bacteria will encounter during growth in vitro an ionic composition that is different from that in the cytosol; this property was expected to change the expression of certain genes after entry into host cells. Gene expression profiling is a powerful tool for reporting the type of environment being experienced by Shigella bacteria during infection; the bacteria effectively serve as environmental sensors. The observed up-regulation of the proVWX operon (by up to 57-fold in both cell lines) is likely to be a specific response to the cytosolic environment. Under high-osmolarity conditions, ProVWX actively transports a group of compounds termed neutral compatible solutes, including betaine and proline. These compatible solutes can substitute for other ions, such as K+ and glutamate, which inhibit enzyme function when present at high concentrations (37). However, the cytosolic osmolarity of mammalian cells does not differ greatly from that of in vitro LB. It is therefore possible that the induction of proVWX simply reflects differences in overall ionic compositions, such as high levels of K+, rather than differences in osmolarity between LB and the intracellular environment.
We observed that the phoRB regulon, responsible for phosphate uptake, was significantly up-regulated, suggesting that the cytosol has restricted levels of available phosphate; phoRB expression was increased by nearly 10-fold, and the expression of many genes of the phoBR regulon also was increased significantly. These included the phoA gene, encoding periplasmic phosphatase, and the pstS and phnC genes, encoding ABC phosphate transporters.
The cytosolic Mg2+ concentration is about 0.8 mM (36). This concentration appeared to impose a degree of stress upon S. flexneri, because the transcription of mgtA, which encodes a P-type ATPase involved in the uptake of Mg2+ (74), was increased by three- and eightfold in HeLa and U937 cells, respectively. These data are consistent with a limited availability of magnesium to S. flexneri during mammalian cell infection.
The hypothesis that iron availability is restricted inside the cytosol was confirmed by the observed induction of the sitABCD genes, encoding a putative iron transport system, and the iucABCD and iutA genes, encoding the aerobactin, in S. flexneri. In contrast to the sitABCD system, which was induced equally in HeLa and U937 cells, the aerobactin system was induced only in the early stages of HeLa cell infection and was unchanged throughout the infection of U937 cells. These data suggest that the Shigella sitABCD system is the main iron transport system during intracellular infection. However, a recent study suggested that the presence of either iuc or feoB [encoding a membrane protein involved in Fe(II) uptake] (40) is sufficient to support plaque formation on Henle cell monolayers, despite the low level of expression of these genes compared to that of the sitABCD genes during infection (63). It is clear that the cytosolic ionic composition could limit the intracellular growth of Shigella, but the gene expression data describe an effective adaptation by the bacteria for obtaining essential factors through the up-regulation of at least four transport systems.
Shigella genes differentially expressed between the two mammalian cell lines.
Epithelial cells and macrophages have radically different functions, with the latter possessing the ability to take up and kill microbes through the endocytic pathway. However, Shigella bacteria are thought to use a single strategy to escape into the cell cytosol in both cell types, and this notion is confirmed by the remarkably similar gene expression profiles reported here (see above). The most obvious difference between the two cell lines is the more gradual time-dependent change in expression during infection of HeLa cells than of U937 cells (Fig. 4 and 5). This finding is highlighted by considering the expression profiles for S. flexneri within HeLa and U937 cells at early and late time points. A comparison of the earliest time points between the two cell types reveals that 184 genes show a threefold difference in relative gene expression, while only 21 genes are differentially expressed at the final time points. Besides the ipa-mxi-spa virulence genes, the other genes that show a delayed response in HeLa cells encode proteins linked to energy production, such as NDH-1, cytochrome bo3, and succinate dehydrogenase. These data suggest that the HeLa cell cytosol is a more favorable environment for bacterial growth than the macrophage cytosol at the early stages of infection.
Only 18 S. flexneri genes were differentially expressed between HeLa and U937 cells throughout the infection process (Table 2). Among these, ydeP, yhiE, and ydeO play a role in the acid resistance of E. coli (40), and these genes are activated by the response regulator EvgA. The overexpression of YdeO is known to induce the expression of additional genes implicated in acid resistance (41). Most members of the YdeO regulon are strongly induced in U937 cells (by 26-fold, on average, over all time points) but are not induced or are only slightly induced in HeLa cells. Further evidence to suggest that U937 cells have a more acidic cytosol than HeLa cells is that the expression of asr (part of the phoB regulon, encoding an acid shock protein) is up-regulated by more than 10-fold in U937 cells but is increased by only 2-fold in HeLa cells. Taken together, these data suggest that Shigella is exposed to a lower pH in the macrophage cytosol than in the epithelial cell cytosol.
TABLE 2.
S. flexneri genes showing more than a 3-fold difference in intracellular expression level between HeLa and U937 cells
| Gene name
|
Gene expression levels in the following cells at the indicated time (h), relative to those in LB medium:
|
Function or comments | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Common | Systematic | HeLa
|
U937
|
||||||
| 2 | 4 | 6 | 8 | 1 | 3 | 5 | |||
| asr | SF1618 | 1.9 | 2.3 | 3.6 | 3.7 | 20.3 | 17.5 | 17.8 | Acid shock protein |
| bioB | SF0724 | 7.1 | 4.1 | 3.7 | 3.5 | 0.6 | 0.8 | 0.7 | Biotin synthesis |
| bioF | SF0726 | 4.9 | 2.9 | 2.4 | 2.4 | 0.5 | 0.6 | 0.6 | KAPA synthetaseb |
| emrKa | SF2435 | 1.6 | 2.1 | 3 | 3.4 | 23.6 | 38.5 | 36.1 | Multidrug resistance protein K |
| emrYa | SF2434 | 2.5 | 3.9 | 5.5 | 5.4 | 13 | 24.7 | 21.5 | Multidrug resistance protein Y |
| gadAa | SF3594 | 2.4 | 6.8 | 4.4 | 3.3 | 68.3 | 25.2 | 10.9 | Glutamate decarboxylase isozyme |
| gadBa | SF1734 | 2.5 | 7.4 | 4.6 | 3.5 | 71.3 | 25.6 | 11.2 | Glutamate decarboxylase isozyme |
| hdeAa | SF3544 | 0.5 | 0.9 | 0.5 | 0.3 | 6 | 2.4 | 1.2 | Conserved hypothetical protein |
| nrdI | SF2702 | 10.1 | 15.5 | 18.8 | 18.3 | 3.3 | 4.1 | 5.9 | Conserved hypothetical protein |
| SF2147 | SF2147 | 0.9 | 1 | 0.9 | 1 | 11.6 | 11.5 | 10.7 | Function unknown |
| SF2148 | SF2148 | 2 | 2 | 2.5 | 2.5 | 48.1 | 42.5 | 38.6 | Conserved hypothetical protein |
| ydeOa | SF1537 | 1.4 | 1.8 | 3.2 | 3.5 | 30 | 25 | 24.9 | AraC-type regulatory protein |
| ydePa | SF1538 | 2.9 | 4.4 | 5.1 | 5.2 | 130.8 | 93.9 | 70.5 | Acid resistance protein |
| yedX | SF2017 | 1.5 | 1.9 | 2.9 | 2.9 | 11.6 | 15.3 | 12.2 | Conserved hypothetical protein |
| yfdUa | SF2440 | 4 | 4.8 | 7 | 7.1 | 216.4 | 113 | 62.5 | Function unknown |
| yfdVa | SF2439 | 2.3 | 2.7 | 4.1 | 4.7 | 75.1 | 38.6 | 21.6 | Putative receptor protein |
| yfdWa | SF2441 | 5.1 | 5.6 | 8.1 | 8.2 | 266.1 | 173.7 | 101.4 | Function unknown |
| yhiEa | SF3546 | 1 | 1.3 | 1.7 | 1.6 | 20.2 | 11.8 | 5.9 | Acid resistance protein |
Member of the EvgA or YdeO regulon.
KAPA, 7-keto-8-amino-perlargonic acid.
Comparison of Shigella and Salmonella transcriptional adaptations within macrophages.
S. enterica serovar Typhimurium is a pathogen radically different from S. flexneri, and this difference is reflected in the opposing strategies used during macrophage infection. In essence, both bacteria have effective tactics for avoiding contact with the toxic constituents of the lysosome, which are normally introduced into the phagosomal vacuole: S. flexneri escapes the vacuole, whereas Salmonella hijacks the cell by subverting the normal cellular trafficking processes, preventing lysosomal fusion, and creating the unique SCV (21, 46).
Eriksson et al. previously established the gene expression profile for Salmonella serovar Typhimurium during infection of murine macrophages (18), and we have used these data to compare the intramacrophage environments experienced by S. flexneri and Salmonella serovar Typhimurium. It should be remembered that this comparison involves bacterial growth in two distinct macrophage cell lines, one murine derived (J774A.1) and one human derived (U937). We compared the gene expression profile for each pathogen within macrophages with the gene expression profile observed for a mid-logarithmic-phase culture of each pathogen grown in LB in vitro. By normalizing the intramacrophage gene expression profile for each pathogen to the data obtained from the appropriate LB culture, we were able to perform direct comparisons. We identified which genes showed similar expression patterns and which ones showed differences between the two bacterial pathogens. These data are presented in Table S3 in the supplemental material (http://www.ifr.ac.uk/safety/molmicro/pubs.html). The relative levels of expression of some of the Salmonella genes are distinct from the earlier data (18), because the LB culture was used as a comparator instead of an RPMI medium cell culture. A total of 1,422 of 4,408 Salmonella serovar Typhimurium genes showed significant changes in expression, of greater than threefold, after 4 h within macrophages; 840 genes were up-regulated and 582 genes were down-regulated. We observed that 32% of the Salmonella serovar Typhimurium genes were in vivo regulated after 4 h in J774A.1 macrophages, whereas 27% of the S. flexneri genes were in vivo regulated after 3 h in U937 macrophages (see above for details). Overall, we could identify only 21 genes that showed opposite patterns of expression in macrophages for Shigella and Salmonella (see Table S3 in the supplemental material [http://www.ifr.ac.uk/safety/molmicro/pubs.html]). One sharp contrast that we observed was the level of proVWX expression; the proU operon was strongly up-regulated in Shigella (proV was up-regulated by 57-fold in U937 cells) but only slightly up-regulated in Salmonella. These data suggest that whereas Salmonella is protected in the SCV, Shigella is directly exposed to the ionic status of the cytosol. The fact that so few genes showed contrasting expression patterns is probably a reflection of the lack of conservation of the key virulence genes between these two bacterial pathogens.
Salmonella serovar Typhimurium experiences within macrophages a small degree of oxidative stress, which is reflected by a modest induction of the soxS, ibpA, ibpB, and ycfR genes; the same is observed in S. flexneri. Additionally, sbp is more highly induced in S. flexneri than in Salmonella, consistent with exposure to oxidative stress. Consequently, we focused on the expression of the OxyR-dependent genes involved in the thioredoxin and glutaredoxin systems, which are responsible for the repair of oxidized protein residues. We observed that trxC (a member of the glutaredoxin pathway) is down-regulated in S. flexneri but up-regulated in Salmonella. In contrast, msrA (a member of the thioredoxin pathway) shows the opposite pattern of expression, being up-regulated in S. flexneri. This pattern suggests that unlike Salmonella, S. flexneri favors the thioredoxin pathway over the glutaredoxin pathway for repairing damaged proteins during macrophage infection.
However, the katG and katE genes are more highly induced in Salmonella than in S. flexneri, indicating that Salmonella serovar Typhimurium is exposed to peroxide within macrophages and that S. flexneri is not. Peroxide is produced within macrophages by the Phox-mediated oxidative burst, which occurs after Salmonella infection. The other free radical encountered by Salmonella during macrophage infection is NO (17), which is thought to be detoxified by the flavohemoglobin encoded by hmpA, which was induced at later stages of infection (18). The S. flexneri data reveal no induction of hmpA, confirming that Shigella bacteria are not subject to NO stress within the cytosol.
Free radicals are likely to account for the moderate induction of the SOS response that we observed for Salmonella within macrophages (18). Low-level induction of the SOS response in S. flexneri is indicated by similar increases in the expression of uvrY, umuCD, sulA, recA, and lexA during macrophage infection. Taken together, these data suggest that Shigella experiences a certain degree of oxidative stress within the macrophage cytosol and show a surprising concordance of gene expression between S. flexneri and Salmonella serovar Typhimurium.
Conclusions.
This is the first report to consider the gene expression profile for an intracellular bacterial pathogen within two fundamentally different human cell types. Obviously, U937 cells and HeLa epithelial cells are quite distinct at the cell biological level. Nevertheless, the broadly similar patterns of intracellular S. flexneri gene expression that we observed in HeLa and U937 cells suggest that these cells have comparable cytosolic environments. Notable exceptions were some acid-induced genes, including those of the EvgA and YdeO regulons, which are more highly induced in U937 cells than in HeLa cells. These data suggest that S. flexneri infection causes acidification of the macrophage cytosol; this notion remains to be verified experimentally. Most strikingly, S. flexneri bacteria show a drastically reduced expression of genes encoding the Ipa-Mxi-Spa and other virulence determinants in the cytosol, indicating that these are not crucial for the growth of bacteria once inside host cells.
Other exciting findings are that the bacteria have to compete for iron, magnesium, and phosphate and have to cope with the unique ionic composition of the cytosol. These goals are achieved by up-regulation of the sitABCD, mgtA, phoRB regulon, proVWX, and other transport systems. In addition, we performed a novel comparison of the intracellular gene expression profiles for Salmonella serovar Typhimurium and S. flexneri and identified a surprising level of similarities in the ways in which these pathogens respond to the macrophage environment. These findings are a testament to the efficacy of the SPI2 and Spv-mediated systems in protecting Salmonella serovar Typhimurium from attack by the components of the phagolysosome, which Shigella manages to avoid by escaping the vacuole itself.
S. flexneri is exposed to numerous stresses within the cytosol of human cells, including starvation for essential minerals and oxidative damage. Nonetheless, S. flexneri is able to grow steadily in the cytosol, showing that it can readily adapt to intracellular life. The data that we have amassed are the first to describe this adaptation at the level of individual S. flexneri genes. The next challenge is to use these findings to develop an understanding of Shigella infection. Let us hope that this work lays the foundation for discovering the Achilles' heel of one of the medically important bacterial pathogens.
Acknowledgments
This work was financed by the High Technology Project (grant no. 2001AA223011) and the International Science and Technology Cooperation Project of the State Key Basic Research Program (grant no. 2001AA223116) from the Ministry of Science and Technology of China to Q.J., a BBSRC core strategic grant to J.C.D.H., and a Wellcome Trust (Cambridge, United Kingdom) grant to J.Y. (grant no. 047657/Z/96/Z).
We are grateful to Matt Rolfe and Arthur Thompson for help with microarray technology and to Isabelle Hautefort for assistance with the cell cultures. We thank Philippe Sansonetti for critical reading of the manuscript.
Editor: J. T. Barbieri
REFERENCES
- 1.Andrews, G. P., A. E. Hromockyj, C. Coker, and A. T. Maurelli. 1991. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect. Immun. 59:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum, M. F., S. Lucchini, A. Thompson, J. C. Hinton, and M. J. Woodward. 2003. Comparative genomic indexing reveals the phylogenomics of Escherichia coli pathogens. Infect. Immun. 71:4674-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoleschi, C., M. C. Pardini, C. Scaringi, M. C. Martino, C. Pazzani, and M. L. Bernardini. 2002. Selection of Shigella flexneri candidate virulence genes specifically induced in bacteria resident in host cell cytoplasm. Cell. Microbiol. 4:613-626. [DOI] [PubMed] [Google Scholar]
- 4.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57: 289-300. [Google Scholar]
- 5.Bernardini, M. L., M. G. Sanna, A. Fontaine, and P. J. Sansonetti. 1993. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect. Immun. 61:3625-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Brissette, J. L., M. Russel, L. Weiner, and P. Model. 1990. Phage shock protein, a stress protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:862-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchrieser, C., P. Glaser, C. Rusniok, H. Nedjari, H. D'Hauteville, F. Kunst, P. Sansonetti, and C. Parsot. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38:760-771. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, Z. F., Y. Zuo, Z. Li, K. E. Rudd, and M. P. Deutscher. 1998. The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J. Biol. Chem. 273:14077-14080. [DOI] [PubMed] [Google Scholar]
- 10.Clements, M. O., S. Eriksson, A. Thompson, S. Lucchini, J. C. Hinton, S. Normark, and M. Rhen. 2002. Polynucleotide phosphorylase is a global regulator of virulence and persistency in Salmonella enterica. Proc. Natl. Acad. Sci. USA 99:8784-8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahan, S., S. Knutton, R. K. Shaw, V. F. Crepin, G. Dougan, and G. Frankel. 2004. Transcriptome of enterohemorrhagic Escherichia coli O157 adhering to eukaryotic plasma membranes. Infect. Immun. 72:5452-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demers, B., P. J. Sansonetti, and C. Parsot. 1998. Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. EMBO J. 17:2894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Hauteville, H., S. Khan, D. J. Maskell, A. Kussak, A. Weintraub, J. Mathison, R. J. Ulevitch, N. Wuscher, C. Parsot, and P. J. Sansonetti. 2002. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 168:5240-5251. [DOI] [PubMed] [Google Scholar]
- 14.Diehl, F., S. Grahlmann, M. Beier, and J. D. Hoheisel. 2001. Manufacturing DNA microarrays of high spot homogeneity and reduced background signal. Nucleic Acids Res. 29:E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorman, C. J., S. McKenna, and C. Beloin. 2001. Regulation of virulence gene expression in Shigella flexneri, a facultative intracellular pathogen. Int. J. Med. Microbiol. 291:89-96. [DOI] [PubMed] [Google Scholar]
- 16.Edgeworth, J. D., J. Spencer, A. Phalipon, G. E. Griffin, and P. J. Sansonetti. 2002. Cytotoxicity and interleukin-1beta processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur. J. Immunol. 32:1464-1471. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson, S., J. Bjorkman, S. Borg, A. Syk, S. Pettersson, D. I. Andersson, and M. Rhen. 2000. Salmonella typhimurium mutants that downregulate phagocyte nitric oxide production. Cell. Microbiol. 2:239-250. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Prada, C. M., D. L. Hoover, B. D. Tall, A. B. Hartman, J. Kopelowitz, and M. M. Venkatesan. 2000. Shigella flexneri IpaH(7.8) facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect. Immun. 68:3608-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzon, V. L., J. Arondel, and P. J. Sansonetti. 1990. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect. Immun. 58:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-del Portillo, F. 2001. Salmonella intracellular proliferation: where, when and how? Microbes Infect. 3:1305-1311. [DOI] [PubMed] [Google Scholar]
- 22.Harris, P. E. 1996. Human myeloid cell lines, p. 1-16. In L. A. Herzenberg, D. M. Wei, and C. Blackwell (ed.), Weir's handbook of experimental immunology, vol. 4. Blackwell Scientific Publications Ltd., Oxford, England.
- 23.Headley, V. L., and S. M. Payne. 1990. Differential protein expression by Shigella flexneri in intracellular and extracellular environments. Proc. Natl. Acad. Sci. USA 87:4179-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hengge-Aronsis, R. 2000. The general stress response in Escherichia coli, p. 161-178. In G. Storz and G. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 25.High, N., J. Mounier, M. C. Prevost, and P. J. Sansonetti. 1992. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 11:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinton, J. C. 1997. The Escherichia coli genome sequence: the end of an era or the start of the FUN? Mol. Microbiol. 26:417-422. [DOI] [PubMed] [Google Scholar]
- 27.Hinton, J. C., I. Hautefort, S. Eriksson, A. Thompson, and M. Rhen. 2004. Benefits and pitfalls of using microarrays to monitor bacterial gene expression during infection. Curr. Opin. Microbiol. 7:277-282. [DOI] [PubMed] [Google Scholar]
- 28.Hong, M., Y. Gleason, E. E. Wyckoff, and S. M. Payne. 1998. Identification of two Shigella flexneri chromosomal loci involved in intercellular spreading. Infect. Immun. 66:4700-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin, Q., Z. Yuan, J. Xu, Y. Wang, Y. Shen, W. Lu, J. Wang, H. Liu, J. Yang, F. Yang, X. Zhang, J. Zhang, G. Yang, H. Wu, D. Qu, J. Dong, L. Sun, Y. Xue, A. Zhao, Y. Gao, J. Zhu, B. Kan, K. Ding, S. Chen, H. Cheng, Z. Yao, B. He, R. Chen, D. Ma, B. Qiang, Y. Wen, Y. Hou, and J. Yu. 2002. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res. 30:4432-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaldalu, N., R. Mei, and K. Lewis. 2004. Killing by ampicillin and ofloxacin induces overlapping changes in Escherichia coli transcription profile. Antimicrob. Agents Chemother. 48:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane, C. D., R. Schuch, W. A. Day, Jr., and A. T. Maurelli. 2002. MxiE regulates intracellular expression of factors secreted by the Shigella flexneri 2a type III secretion system. J. Bacteriol. 184:4409-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwae, A., S. Yoshida, K. Tamano, H. Mimuro, T. Suzuki, and C. Sasakawa. 2001. Shigella invasion of macrophage requires the insertion of IpaC into the host plasma membrane. Functional analysis of IpaC. J. Biol. Chem. 276:32230-32239. [DOI] [PubMed] [Google Scholar]
- 35.Lett, M. C., C. Sasakawa, N. Okada, T. Sakai, S. Makino, M. Yamada, K. Komatsu, and M. Yoshikawa. 1989. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J. Bacteriol. 171:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lodish, H., D. Baltimore, A. Berk, S. L. Zipursky, P. Matsudira, and J. Darnell. 1995. Transport across cell membranes, p. 633-668. In Molecular cell biology. Scientific American Books, New York, N.Y.
- 37.Lucht, J. M., and E. Bremer. 1994. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system proU. FEMS Microbiol. Rev. 14:3-20. [DOI] [PubMed] [Google Scholar]
- 38.Mac Siomoin, R. A., N. Nakata, T. Murai, M. Yoshikawa, H. Tsuji, and C. Sasakawa. 1996. Identification and characterization of ispA, a Shigella flexneri chromosomal gene essential for normal in vivo cell division and intracellular spreading. Mol. Microbiol. 19:599-609. [DOI] [PubMed] [Google Scholar]
- 39.Mantis, N., M. C. Prevost, and P. Sansonetti. 1996. Analysis of epithelial cell stress response during infection by Shigella flexneri. Infect. Immun. 64:2474-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuda, N., and G. M. Church. 2002. Escherichia coli gene expression responsive to levels of the response regulator EvgA. J. Bacteriol. 184:6225-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuda, N., and G. M. Church. 2003. Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 48:699-712. [DOI] [PubMed] [Google Scholar]
- 42.Mathan, M. M., and V. I. Mathan. 1991. Morphology of rectal mucosa of patients with shigellosis. Rev. Infect. Dis. 13(Suppl. 4):S314-S318. [DOI] [PubMed] [Google Scholar]
- 43.Mavris, M., P. A. Manning, and R. Morona. 1997. Mechanism of bacteriophage SfII-mediated serotype conversion in Shigella flexneri. Mol. Microbiol. 26:939-950. [DOI] [PubMed] [Google Scholar]
- 44.Mavris, M., A. L. Page, R. Tournebize, B. Demers, P. Sansonetti, and C. Parsot. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543-1553. [DOI] [PubMed] [Google Scholar]
- 45.Mavris, M., P. J. Sansonetti, and C. Parsot. 2002. Identification of the cis-acting site involved in activation of promoters regulated by activity of the type III secretion apparatus in Shigella flexneri. J. Bacteriol. 184:6751-6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills, S. D., and B. B. Finlay. 1998. Isolation and characterization of Salmonella typhimurium and Yersinia pseudotuberculosis-containing phagosomes from infected mouse macrophages: Y. pseudotuberculosis traffics to terminal lysosomes where they are degraded. Eur. J. Cell Biol. 77:35-47. [DOI] [PubMed] [Google Scholar]
- 47.Mogull, S. A., L. J. Runyen-Janecky, M. Hong, and S. M. Payne. 2001. dksA is required for intercellular spread of Shigella flexneri via an RpoS-independent mechanism. Infect. Immun. 69:5742-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morona, R., D. F. Macpherson, L. Van Den Bosch, N. I. Carlin, and P. A. Manning. 1995. Lipopolysaccharide with an altered O-antigen produced in Escherichia coli K-12 harbouring mutated, cloned Shigella flexneri rfb genes. Mol. Microbiol. 18:209-223. [DOI] [PubMed] [Google Scholar]
- 49.Morona, R., and L. Van Den Bosch. 2003. Lipopolysaccharide O antigen chains mask IcsA (VirG) in Shigella flexneri. FEMS Microbiol. Lett. 221:173-180. [DOI] [PubMed] [Google Scholar]
- 50.Moss, J. E., P. E. Fisher, B. Vick, E. A. Groisman, and A. Zychlinsky. 2000. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell. Microbiol. 2:443-452. [DOI] [PubMed] [Google Scholar]
- 51.Nakata, N., C. Sasakawa, N. Okada, T. Tobe, I. Fukuda, T. Suzuki, K. Komatsu, and M. Yoshikawa. 1992. Identification and characterization of virK, a virulence-associated large plasmid gene essential for intercellular spreading of Shigella flexneri. Mol. Microbiol. 6:2387-2395. [DOI] [PubMed] [Google Scholar]
- 52.Nakayama, S., and H. Watanabe. 1998. Identification of CpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J. Bacteriol. 180:3522-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakayama, S., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nonaka, T., A. Kuwae, C. Sasakawa, and S. Imajoh-Ohmi. 1999. Shigella flexneri YSH6000 induces two types of cell death, apoptosis and oncosis, in the differentiated human monoblastic cell line U937. FEMS Microbiol. Lett. 174:89-95. [DOI] [PubMed] [Google Scholar]
- 55.Offner, F. F. 1991. Ion flow through membranes and the resting potential of cells. J. Membr. Biol. 123:171-182. [DOI] [PubMed] [Google Scholar]
- 56.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 57.Peterson, J. D., L. A. Umayam, T. Dickinson, E. K. Hickey, and O. White. 2001. The Comprehensive Microbial Resource. Nucleic Acids Res. 29:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purdy, G. E., M. Hong, and S. M. Payne. 2002. Shigella flexneri DegP facilitates IcsA surface expression and is required for efficient intercellular spread. Infect. Immun. 70:6355-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajakumar, K., C. Sasakawa, and B. Adler. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 65:4606-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rathman, M., N. Jouirhi, A. Allaoui, P. Sansonetti, C. Parsot, and G. Tran Van Nhieu. 2000. The development of a FACS-based strategy for the isolation of Shigella flexneri mutants that are deficient in intercellular spread. Mol. Microbiol. 35:974-990. [DOI] [PubMed] [Google Scholar]
- 61.Reeves, S. A., A. G. Torres, and S. M. Payne. 2000. TonB is required for intracellular growth and virulence of Shigella dysenteriae. Infect. Immun. 68:6329-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Runyen-Janecky, L. J., and S. M. Payne. 2002. Identification of chromosomal Shigella flexneri genes induced by the eukaryotic intracellular environment. Infect. Immun. 70:4379-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Runyen-Janecky, L. J., S. A. Reeves, E. G. Gonzales, and S. M. Payne. 2003. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71:1919-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandlin, R. C., M. B. Goldberg, and A. T. Maurelli. 1996. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol. Microbiol. 22:63-73. [DOI] [PubMed] [Google Scholar]
- 65.Sandlin, R. C., K. A. Lampel, S. P. Keasler, M. B. Goldberg, A. L. Stolzer, and A. T. Maurelli. 1995. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect. Immun. 63:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sansonetti, P. 2002. Host-pathogen interactions: the seduction of molecular cross talk. Gut 50(Suppl. 3):III2-III8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sansonetti, P. J., J. Arondel, J. R. Cantey, M. C. Prevost, and M. Huerre. 1996. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect. Immun. 64:2752-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sansonetti, P. J., D. J. Kopecko, and S. B. Formal. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sansonetti, P. J., J. Mounier, M. C. Prevost, and R. M. Mege. 1994. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell 76:829-839. [DOI] [PubMed] [Google Scholar]
- 70.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 71.Sayeed, S., L. Reaves, L. Radnedge, and S. Austin. 2000. The stability region of the large virulence plasmid of Shigella flexneri encodes an efficient postsegregational killing system. J. Bacteriol. 182:2416-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuch, R., R. C. Sandlin, and A. T. Maurelli. 1999. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol. Microbiol. 34:675-689. [DOI] [PubMed] [Google Scholar]
- 73.Skoudy, A., J. Mounier, A. Aruffo, H. Ohayon, P. Gounon, P. Sansonetti, and G. T. Nhieu. 1999. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell. Microbiol. 2:19-33. [DOI] [PubMed] [Google Scholar]
- 74.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki, T., T. Murai, I. Fukuda, T. Tobe, M. Yoshikawa, and C. Sasakawa. 1994. Identification and characterization of a chromosomal virulence gene, vacJ, required for intercellular spreading of Shigella flexneri. Mol. Microbiol. 11:31-41. [DOI] [PubMed] [Google Scholar]
- 76.Tedin, K., and U. Blasi. 1996. The RNA chain elongation rate of the lambda late mRNA is unaffected by high levels of ppGpp in the absence of amino acid starvation. J. Biol. Chem. 271:17675-17686. [DOI] [PubMed] [Google Scholar]
- 77.Thompson, A., S. Lucchini, and J. C. Hinton. 2001. It's easy to build your own microarrayer! Trends Microbiol. 9:154-156. [DOI] [PubMed] [Google Scholar]
- 78.Toyotome, T., T. Suzuki, A. Kuwae, T. Nonaka, H. Fukuda, S. Imajoh-Ohmi, T. Toyofuku, M. Hori, and C. Sasakawa. 2001. Shigella protein IpaH(9.8) is secreted from bacteria within mammalian cells and transported to the nucleus. J. Biol. Chem. 276:32071-32079. [DOI] [PubMed] [Google Scholar]
- 79.Venkatesan, M. M., M. B. Goldberg, D. J. Rose, E. J. Grotbeck, V. Burland, and F. R. Blattner. 2001. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect. Immun. 69:3271-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vokes, S. A., S. A. Reeves, A. G. Torres, and S. M. Payne. 1999. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol. Microbiol. 33:63-73. [DOI] [PubMed] [Google Scholar]
- 81.Walker, D., M. Rolfe, A. Thompson, G. R. Moore, R. James, J. C. Hinton, and C. Kleanthous. 2004. Transcriptional profiling of colicin-induced cell death of Escherichia coli MG1655 identifies potential mechanisms by which bacteriocins promote bacterial diversity. J. Bacteriol. 186:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watarai, M., S. Funato, and C. Sasakawa. 1996. Interaction of Ipa proteins of Shigella flexneri with alpha5beta1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 183:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Way, S. S., S. Sallustio, R. S. Magliozzo, and M. B. Goldberg. 1999. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181:1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang, Y. H., and T. Speed. 2002. Design issues for cDNA microarray experiments. Nat. Rev. Genet. 3:579-588. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida, S., E. Katayama, A. Kuwae, H. Mimuro, T. Suzuki, and C. Sasakawa. 2002. Shigella deliver an effector protein to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization. EMBO J. 21:2923-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu, J. 1998. Inactivation of DsbA, but not DsbC and DsbD, affects the intracellular survival and virulence of Shigella flexneri. Infect. Immun. 66:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu, J., E. E. Oragui, A. Stephens, J. S. Kroll, and M. M. Venkatesan. 2001. Inactivation of DsbA alters the behaviour of Shigella flexneri toward murine and human-derived macrophage-like cells. FEMS Microbiol. Lett. 204:81-88. [DOI] [PubMed] [Google Scholar]
- 88.Yura, T., H. Nagai, and H. Mori. 1993. Regulation of the heat-shock response in bacteria. Annu. Rev. Microbiol. 47:321-350. [DOI] [PubMed] [Google Scholar]
- 89.Zhou, D., W. D. Hardt, and J. E. Galan. 1999. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect. Immun. 67:1974-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]