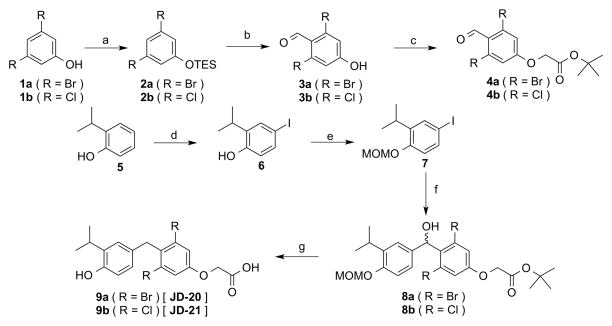
Figure 2.
Synthesis of JD-20 9a and JD-21 9b. Reagents and Conditions: (a) triethylsilyl chloride, imidazole, DCM, 0°C, 95%; (b) (i) nBuLi, DIA or TMP, THF, −78° C (ii) DMF, 56–67%; (c) tert-butylchloroacetate, NaI, Cs2CO3, acetone, 60–65° C, 84–88%; (d) NaI, NaOH, NaOCl, MeOH, H2O, 87%; (e) MOMCl, TBAI, NaOH, DCM, H2O, 81%; (f) (i) iPrMgCl, THF, 0° C to RT (ii) 4, −78° C, 54–79%; (g) TFA, triethylsilane, DCM, 0° C to RT, 58–69%.