Abstract
Cilia and eukaryotic flagella are threadlike cell extensions with motile and sensory functions. Their assembly requires intraflagellar transport (IFT), a bidirectional motor-driven transport of protein carriers along the axonemal microtubules. IFT moves ample amounts of structural proteins including tubulin into growing cilia likely explaining its critical role for assembly. IFT continues in non-growing cilia contributing to a variety of processes ranging from axonemal maintenance and the export of non-ciliary proteins to cell locomotion and ciliary signaling. Here, we discuss recent data on cues regulating the type, amount, and timing of cargo transported by IFT. A regulation of IFT-cargo interactions is critical to establish, maintain, and adjust ciliary length, protein composition, and function.
Keywords: flagella, microtubule, intraflagellar transport, diffusion
Introduction
Compartmentalization is a key feature of eukaryotic cells. Besides membrane-enclosed entities such as the mitochondria or Golgi, regions of the cytoplasm itself are often specialized by possessing a particular complement of proteins (and lipids and nucleic acids) enabling them to perform exclusive tasks. Examples include the leading edge of crawling cells, the mitotic spindle, and various cellular extensions such as microvilli and axons. Such cytoplasmic domains are established and maintained by self-assembly or self-organization, intracellular transport locally concentrating components, and barriers limiting the free flux of proteins. Here, we will focus on cilia and flagella (interchangeable terms), thin projections with a diameter of 200 nm that extend for several microns from the cell surface (Fig. 1A). While not membrane-bound organelles, cilia are partitioned from the cell body by the transition zone, a region at the base of the cilium which functions as a diffusion barrier.1 Proteomic studies indicate that of the ∼20,000 nuclear-encoded proteins ∼1,000 are present in cilia; many of them are highly enriched within the organelle.2 Since ribosomes are absent from cilia, all proteins required in the organelle have to be imported from the cell body.3 Most cilia are not assembled in the cell body and extruded, instead the axoneme, the microtubular scaffold of all cilia, grows by addition of subunits to its distal end.4,5 Intraflagellar transport (IFT) plays a major role in this process by picking up ciliary precursors in the cell body and delivering them via molecular motors into cilia and to the ciliary tip.6 Here, we will evaluate the role of IFT in establishing and maintaining the specialized protein content of cilia.
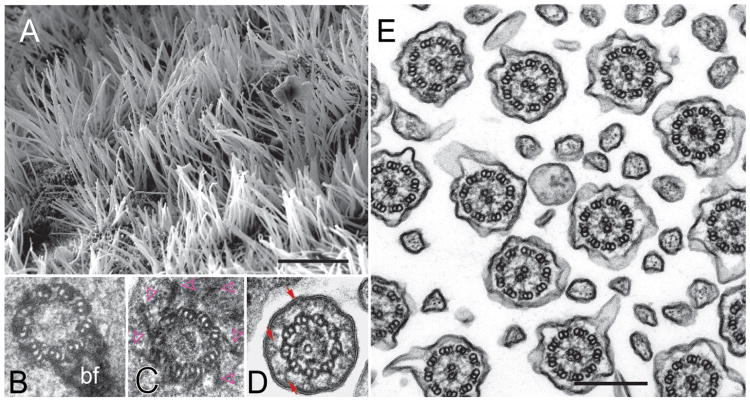
Figure 1. The structure of cilia and flagella.
A) Scanning micrograph showing the ciliated epithelium lining the ventricular system of the brain in mouse.
B-D) Thin sections showing the proximal region of the basal body (B) with the attached basal foot (bf), a more distal section with the paddle-wheel like transitional fibers (C, arrowheads), and the transition zone (D) in cross-sections. In D, note the Y-shaped connectors (arrows) linking the doublet microtubules to the ciliary membrane.
E) Micrograph showing airway cilia with typical 9+2 axonemes in cross-section. Bars = 10 μm (A) and 250 nm (E).
A briefing on cilia
Cilia are organized by basal bodies, barrel-shaped microtubule-based structures also termed centrioles (Fig. 1B). The A- and B-tubules of the centriolar triplets are continuous with the doublet microtubules of the axoneme (Fig. 1B-E). During ciliogenesis, basal bodies dock to the plasma membrane via the distal appendages or transitional fibers (TF) positioned at the distal end of the basal bodies (Fig. 1C).7 Between the basal body and the axoneme proper resides the transition zone (TZ), an ultrastructurally and biochemically specialized segment of the flagellum that functions as a diffusion barrier between the cell body and the cilium (Fig. 1D).8 However, large protein complexes such as the multimegadalton IFT trains move through the transition zone indicating that the TZ possesses a gating mechanism. In many IFT loss-of-function mutants, cilia terminate above the structurally intact transition zone indicating that the elongation of protruding cilia is IFT dependent.9 IFT-independent assembly of flagella has been described as well: Plasmodium (and other apicomplexa), for example, rapidly (∼10 min) assembles 12- 15 μm long axonemes within the cytoplasm which later will be surrounded by a membrane during exflagellation and remain active for ∼1 hour once protruding from the cell.10,11 Thus, IFT is not per se needed for cilia formation but standard cilia assembly in protrusions requires IFT.
Cilia have been long known as motile organelles which function in the locomotion of protists and spermatozoa or the transport of fluid across ciliated epithelia (Fig. 1A, E). The microtubules of motile cilia are densely decorated with protein complexes such as dynein arms and radial spokes (Fig. 1E). Many metazoans also possess non-motile cilia which function in sensing of the external and internal environment; sensing of light and odor, for example, involves receptors located inside ciliary membranes. Cilia are essential to mammalian development as the complete loss of cilia is embryonic lethal.12,13 A plethora of diseases, termed ciliopathies, are associated with defects in ciliary motility and sensation.14 Some of these conditions are caused by mutations impairing a cell- or tissue-specific function of cilia: The loss of an axonemal dynein, for example, will cause cilia paralysis but will not affect the sensory functions of non-motile cilia.15 In contrast, defects in general cilia assembly, length control, composition, or maintenance often result in multiorgan phenotypes because cilia are widely distributed in the mammalian body. Genes encoding TZ proteins are hotspots for disease-causing mutations in humans: TZ defects affect ciliary protein entry and retention typically affecting cilia performance in many cell types, tissues, and organs.16,17 Similarly, many IFT defects alter ciliary length and protein content affecting their sensory and signaling functions. In mammals, more or less subtle defects in IFT cause a wide range of diseases and developmental defects ranging from blindness and kidney anomalies to severe skeletal malformations and obesity.14 After a brief introduction to the IFT pathway, we will review data on protein transport by IFT and its regulation.
IFT – the protein shuttle of the cilium
IFT is the bidirectional movement of large protein arrays (= IFT trains) along the axonemal microtubules (Fig. 2).18 The trains are strings of IFT particles, each consisting of at least 22 distinct proteins organized into IFT-A, IFT-B1, and IFT-B2 subcomplexes (Fig. 2A, C).19-22 IFT train assembly occurs near the TFs (Fig. 1C).23-25 In the first part of the journey, anterograde trains move from the ciliary base to the tip along the B-tubule of the doublets using the molecular motor kinesin-2 and carrying inactive IFT dynein, the retrograde motor, as a cargo (Fig. 2B, D).26,27 At the ciliary tip, anterograde IFT trains are remodeled for retrograde traffic and trains return to the cell body pulled by IFT dynein along the A-tubules.26,28 IFT trains function as protein carriers allowing non-IFT proteins such as axonemal precursors to hitch a ride into the cilium. Cell fusion experiments using Chlamydomonas mutants with defective axonemes and wild-type cells provided initial evidence for protein delivery into cilia by IFT: During the repair of the mutant cilia, the missing axonemal proteins or substructures introduced by the wild-type cell are first added at the tip (instead of near the base where the proteins will enter cilia). Assembly then progresses toward the base of the cilium; a pattern indicative for transport of these components via IFT to the ciliary tip.5,29,30 Direct imaging revealed that proteins of the ciliary matrix, membrane, and axoneme including tubulin, the major structural protein of cilia, move via IFT (Fig. 2E).31 In selected cases, unloading of cargoes from IFT and subsequent incorporation into the axoneme have been observed directly.32 The data confirm the role of IFT as the predominant protein transport pathway of cilia and flagella.
Figure 2. The intraflagellar transport machinery.
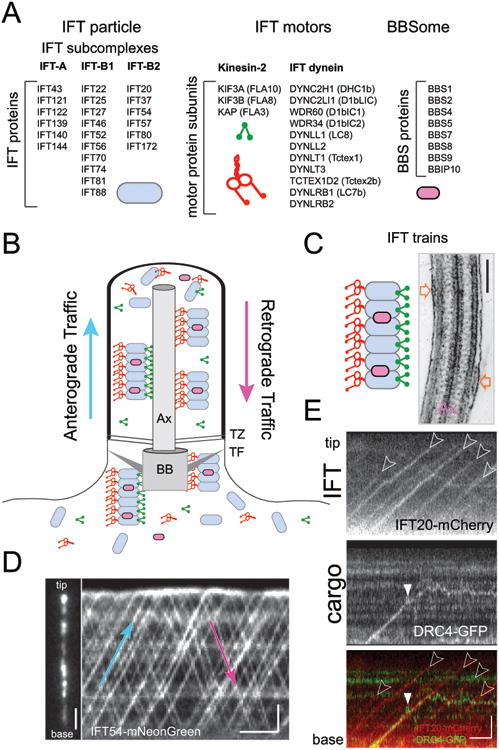
A) Composition of IFT particles, IFT motors, and the BBSome. For the motors, the mammalian protein names are shown; the Chlamydomonas protein names are listed in the brackets.
B) Schematic presentation of IFT. Ax, axoneme, TZ, transition zone, TF, transition fibers, BB, basal body.
C) Schematic presentation and electron micrograph depicting IFT trains (open arrows). Bar = 200 nm.
D) Still image (left) and kymogram (right) showing IFT54-NG inside a Chlamydomonas flagellum. In the kymogram, anterograde trains are indicated by trajectories running from the bottom left to the top tight (blue arrow); trajectories running from the top left to the bottom right represent retrograde trains (red arrow). Bars = 2s 2 μm.
E) Kymograms depicting transport by IFT and unloading of the axonemal protein DRC4-GFP. IFT20-mCherry was expressed to visualize IFT. IFT trajectories are marked by open arrowheads. DRC4-GFP initially co-migrates with an IFT train but is then unloaded (white arrowhead) as indicated by the transition of the trajectory from a linear diagonal to a back-and-forth motion indicative for diffusion. Note that most cargoes are unloaded in the vicinity of the ciliary tip. Bar = 1s 2 μm.
Post balance point: regulated loading of IFT trains contributes to ciliary length control
Ciliary length is typically tightly regulated in a cell type-specific manner and mutations that affect ciliary length reduce the swimming speed in protists and cause disease in mammals.33,34 Numerous factors have been shown to participate in ciliary length control.35 The supply of ciliary building blocks via the IFT pathway is likely to contribute to establishing and maintaining cilia of a defined length: Conceivably, reduced protein supply could result in shorter cilia; conversely, too much material might cause cilia to exceed their set length. A simple option to regulate the amount of protein transported into cilia would be an on demand system where IFT trains are only present or moving while cilia are assembled (Fig. 3A). While IFT is often abolished in mature sperm flagella, it continues in fully grown cilia in the vast majority of cells.36 When IFT is switched-off using conditional mutants, cilia shorten and tubulin exchange at the ciliary tip is reduced.37 These observations are the basis of the influential balance-point model which proposes that the capacity of IFT is restricting the length of cilia: As cilia elongate IFT trains will spend more and more time in transit and the frequency by which IFT trains reach the ciliary tip and drop off their cargoes will progressively decrease as the distance between the ciliary base and tip increases.37-40 At one point, the balance point, assembly fueled by IFT-dependent cargo delivery and the assumed length-independent disassembly of cilia will balance each other establishing the steady-state length (Fig. 3B).37 According to this model, cells could build cilia of a given length simply by limiting the number the IFT trains employed during assembly without invoking complex mechanisms to measure cilia length or regulate the size of the precursor pool. A prerequisite of this model is that the cargo load of IFT trains is constant and length-independent. IFT-cargo complexes are transient in nature largely impeding their isolation and biochemical analysis, and, for the longest time, it was unclear whether the amount of protein transported by an IFT train was regulated or not. Recent advances in direct imaging of protein flux inside cilia revealed that IFT trains are highly loaded with tubulin and other axonemal precursors while cilia grow but are largely devoid of these cargoes once cilia reach their set length.32,41 Thus, cells modulate the volume of structural proteins moved by IFT into cilia raising the question how IFT-cargo interactions are regulated.
Figure 3. Cargo transport by IFT during ciliary assembly and maintenance.
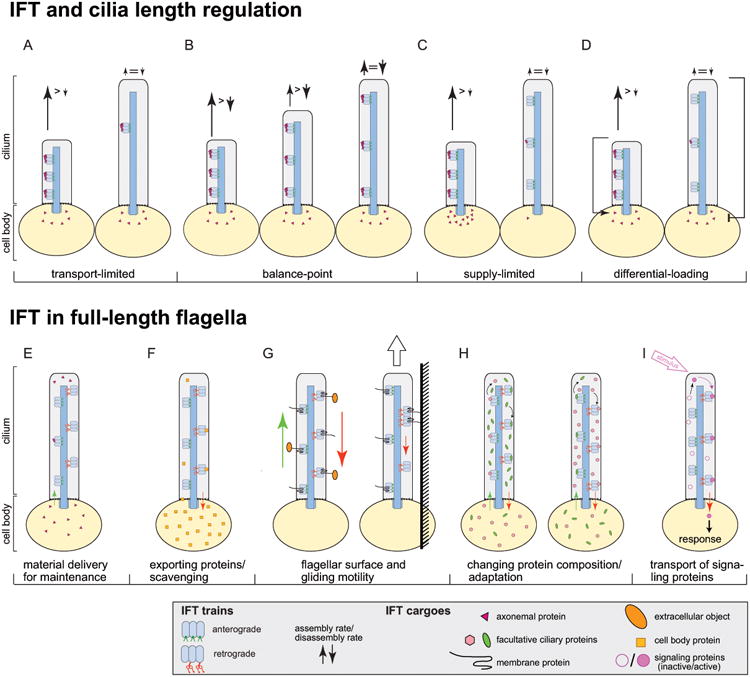
A-D) Models for ciliary length control.
A) The transport-limitation model suggests that cells will employ many IFT trains while cilia grow and reduce the number of trains in fully grown cilia. The cargo load per train is constant.
B) In the balance-point model, the ciliary assembly rate will decrease with increasing ciliary length because the time the trains spend in transit will increase. Neither the number of IFT trains nor the cargo load/train are regulated. Chlamydomonas flagella grow at a rate of up to 350 nm/min. In addition to delivering the building blocks accounting for this gain in length, IFT would have to provide those lost by ongoing ciliary disassembly. Our simulations showed that it is not possible to assemble cilia of 10 - 12 μm length in ∼60 min when assuming that the continuous length-independent disassembly of cilia is large enough to balance the large amount of building blocks provided ceaselessly by anterograde IFT32; rather cilia would need hours of slow growth to reach steady-state length.
C) The supply-limitation model predicts that cilia will grow until the cell body pool of precursors is exhausted. IFT cargo load is regulated passively by the availability of cargoes.
D) The differential-loading model suggests that cells alter the amount of cargo per IFT train in response to changes in ciliary length. The length of the arrows near the flagellar tip indicate the rates of material delivery and cilia disassembly.
E-I) Models of IFT function in fully assembled cilia.
E) Material delivery for cilia maintenance via a low but steady influx of ciliary proteins.
F) Removal of non-ciliary proteins entering cilia via diffusion from the cell body.
G) Back-and-forth movement of extracellular objects on the ciliary surface (left). In gliding motility, IFT dynein, immobilized via IFT particles and transmembrane proteins to the substrate, pulls the cell by moving toward the minus-end of the axonemal microtubules.
H) Import and export of proteins to change ciliary protein composition; e.g. during adaptation.
I) Conditional transport of activated signaling proteins. Signals such as ligand binding change the properties of a protein allowing it to adhere to IFT trains.
The load on the IFT trains could simply reflect the availability of ciliary precursors in the cell body and ciliary elongation will cease once the precursor pool has been drained (Fig. 3C).42 Classic experiments, however, revealed that even cells with full-length cilia still maintain a sizable precursor pool sufficient to rebuild half-length cilia in the absence of de novo protein synthesis.43,44 Thus, IFT does not simply shuttle all available precursors into the cilium but other factors control how much of the axonemal proteins present in the cell body will be transported and used for ciliary assembly. The study of so called long-short cells provided crucial insights into the regulation of cargo loading: Mechanical shear can be used to remove just one of the two flagella from Chlamydomonas. The cells will regenerate the missing flagellum while shortening the remaining flagellum allowing for the analysis of cargo transport when growing and non-growing cilia are present on the same cell body.44,45 While IFT traffic continues in both cilia, only those trains entering the growing cilium are highly loaded with tubulin.41 This suggests that cells regulate IFT loading in a cilium-autonomous and length-dependent manner. Cells apparently possess a system to recognize cilia of insufficient length and respond by increasing the cargo load of just those IFT trains entering short cilia (Fig. 3D). Once cilia approach full length, loading of IFT trains with structural proteins tapers off and, in cilia exceeding their desired length, shortening is triggered.46,47 These two feedback loops - increased cargo delivery with suppressed disassembly when cilia are too short, and suppressed IFT loading with increased disassembly when cilia are too long - could establish cilia of a defined length.
How to measure cilia length?
The molecular mechanisms by which cells measure the length of their cilia and respond to aberrant length by adjusting cargo influx into cilia via IFT remain essentially unknown. Mutations in several CDK-like and MAP kinases result in abnormally long cilia.48-50 Some of these length-regulating protein kinases have been shown to be present inside cilia and to move by IFT.51,52 Several such kinases phosphorylate the anterograde IFT motor kinesin-2 and phosphorylation of the Kif3b motor subunit by CAMK prevents the motor from associating with IFT particles and entry into the cilium.52,53 Some of these length-regulating protein kinases including the aurora-like kinase CALK reside exclusively or predominately in the cell body.50,54 The pattern of CALK phosphorylation changes in response to ciliary length and growth state (elongating, resorbing, or steady-state) providing evidence that cells register the condition of their cilia.55,56 Tubulin transport is dysregulated in lf2, a Chlamydomonas flagellar length mutant defective in a CDK-like kinase.41 These data establish a connection between length-regulating kinases, IFT, and cargo transport but how precisely cilia length is sensed and how such a ‘length signal’ is transmitted to the cell body and the IFT machinery has not yet been established. In a speculative ‘time-of-flight’ model the activity of a length-regulating kinase could change as it transits via IFT through the cilium. Because IFT trains move with an essentially constant velocity, the time a kinase requires to cycle through a cilium would be proportional to ciliary length and the activity of the kinase as it returns to the ciliary base provides a biochemical read-out reflecting cilia length. If correct, changes in IFT velocity should affect ciliary length. A mechanistically distinct model assumes that protein import into cilia depends on the presence of an ‘active’ import factor allowing ciliary proteins and loaded IFT trains to pass through the TZ. If the activation of such a freely diffusible factor occurs at the ciliary tip and its inactivation is time-dependent, a tip-to-base gradient of active factor will be established which could adjust ciliary protein influx in response to changes in organelle length.57 Several alternative models of how cells could sense ciliary length have been described.58 Real-time measurements of ciliary length fluctuations in steady-state could help to pinpoint the length-regulating pathway. At the molecular level, the identification of the substrates of length-regulating kinases is required.
Understanding IFT loading and protein entry into cilia
How cells regulate the import of structural proteins via IFT in a ciliary length-dependent manner is currently unknown. Several mechanisms could be involved including the regulation of IFT-cargo interactions, of the passage of loaded IFT trains through the TZ, and of cargo availability in the area of IFT train assembly and loading.
Our knowledge of how cargoes and IFT proteins interact is still rudimentary and only a few such binding sites have been identified including those for tubulin and the outer dynein arms. Tubulin dimers, for example, are bound by the N-terminal domains of IFT81 and IFT74.59-62 While many IFT proteins are phosphorylated, it is unknown whether posttranslational modifications or other structural changes in the trains modulate the binding capacity for tubulin and other cargoes.63
Protein entry into cilia is regulated at the TZ, which functions as a diffusion barrier and ciliary gate. Small soluble proteins (< 50kDa) and certain transmembrane proteins can move freely into cilia by diffusion64-66; such proteins could still require IFT to be enriched inside the ciliary compartment above cell body concentrations as we suggested for tubulin.41 For proteins with larger diameters ciliary entry by diffusion is largely prevented.65,67 In TZ mutants, some non-ciliary proteins are present in cilia while a subset of resident ciliary proteins are lost supporting its role as a bidirectional barrier.16,17,68 How large protein complexes such as outer dynein arms (>1.5 MDa) or IFT trains pass through the TZ remains to be determined. Certain nuclear pore proteins (NUPs) have been localized in the TZ which has led to the controversial model that the TZ functions similarly to the nuclear pore complex.69,70
Passage through the TZ could depend directly on features of the ciliary proteins themselves such as targeting sequences which open up the TZ. Ciliary localization sequences (CLS) that are required and sufficient for ciliary import have been identified for certain transmembrane and membrane-associated proteins.71-73 CLS are variable in position and frequently encompass residues that are modified by acylation. Proteins predicted to be myristoylated and dual fatty acid modified are enriched in the C. reinhardtii flagellar proteome and evidence from various systems indicates that fatty acid modifications function in ciliary targeting.2,74 Compared to the nuclear localization sequences (NLS), ciliary localization sequences are not conserved and more variable and many structural and soluble ciliary proteins lack recognizable import signals. Such proteins might bind to more universal import carriers. At least some cargoes require the activity of small G-proteins including Ran and Arl3 to enter cilia.75-77 IFT trains pass with apparent ease through the TZ and binding of proteins to IFT trains could be the entry ticket for proteins, which are unable to enter the cilia on their own. Then, the cargo selectivity of IFT would decisively control the protein composition of cilia at the posttranslational level.
To regulate protein influx into cilia, cells could also regulate the space and time available for IFT trains and cargoes to interact in the cell: IFT trains exposed to cargoes for extended periods of time might pick-up more cargo than IFT trains passing rapidly by. IFT proteins and axonemal proteins have been detected on intracellular vesicles suggesting that they might already interact well before reaching the basal bodies.78 In line with these observations, IFT20 is located at the Golgi in mammalian cells and aids in Golgi-to-cilium trafficking of certain membrane proteins.79 Our work using long-short cells of Chlamydomonas showed that the loading of IFT trains can differ considerably between the cilia of a given cell even while the cilia emerge from adjacent basal bodies. This suggests that the mechanism that determines how much cargo enters cilia by IFT is likely to be locally confined to the basal body region.
Protein transport in full-length cilia
In nearly all cell types, IFT continues as long as cilia are present. After the initial rapid phase of assembly, many cilia continue to elongated somewhat which is likely to require IFT.80 Further, IFT in steady-state cilia has diverse functions ranging from ciliary maintenance to cell locomotion and signaling. Most of these processes are likely to commence already during ciliary assembly and no principal difference between IFT in growing and full-length cilia is known, qualified by the observation that the transport of axonemal proteins is strongly reduced in the latter. In the following we will discuss IFT-based processes beyond cilia assembly.
Cilia maintenance
It has been reasoned that cilia and axonemes are intrinsically unstable and therefore require an ongoing supply of building materials to maintain their steady-state length.37 Indeed, pulse labeling experiments suggest a continuous exchange of certain proteins in steady-state cilia.81,82 Motile and primary cilia also lose material via the shedding of vesicles.83,84 The outer segment (a structurally specialized sensory cilium) of rod cells in the eye continuously releases membranous discs at the distal end and new discs are formed at the proximal end.85 This treadmilling of ciliary membranes requires a massive IFT-dependent transport of proteins such as opsin through the connecting cilium.86,87 Treadmilling has not been observed for axonemal microtubules in Chlamydomonas flagella37,88 and the exchange of tubulin subunits at the tip of steady-state cilia is rather slow suggesting that the axonemes are relatively durable.37,64
The degree to which ciliary maintenance depends on IFT varies considerably between species and cell types. In Chlamydomonas and Tetrahymena, cilia and flagella shorten slowly when anterograde IFT is abolished indicating a continual demand of an essential component or a failure of ciliary length regulation, which could hinge on IFT (Fig. 3E).89,90 Trypanosoma flagella maintain their length in the absence of IFT, but flagellar motility and protein distribution are increasingly affected.91 At the other end of the spectrum are sperm flagella that once assembled lack IFT but nevertheless maintain their length and functionality for extended periods of time.36 Apparently, the need for replacement proteins differs considerably between distinct types of cilia. More generally, IFT remains active in fully grown cilia of cycling cells, cells with sensory cilia etc., all of which require ongoing motor-based protein exchange between cilia and the cell-body.
Counteracting diffusional equilibration
Conceivably, the maintenance of cilia is unlikely to require the full-sized IFT system used for their assembly. Nevertheless, the size of the IFT machinery appears to be largely independent of the ciliary growth state.92,93 Motor proteins are often autoinhibited in the absence of cargoes preventing wasteful ATP consumption.94 However, the primary cargoes of the IFT motors are the IFT particles themselves. Thus, the IFT motors can be considered to be permanently engaged with “cargo” or “cargo adapters” while moving along cilia; it is unclear whether IFT trains also require a load of non-IFT proteins in order to enter and move along cilia.
What are the possible benefits offsetting the energy costs of continuously running IFT? Recent data link IFT-dependent protein transport in fully-assembled cilia to processes beyond structural maintenance. An example is the export of proteins from cilia. Just as certain proteins are highly concentrated inside cilia, many cytoplasmic proteins are efficiently excluded from the ciliary compartment. Small to midsize cell body proteins, however, are likely to diffuse across the transition zone continuously leaking into the cilium.65 The membrane-associated protein phospholipase D can enter C. reinhardtii cilia in an IFT-independent manner but depends on IFT and the BBSome, an IFT-associated eight-subunit protein complex (Fig. 2), to be removed from cilia.95,96 In mammals, loss of BBSome function causes an accumulation of cell body proteins in the outer segment of rod cells supporting the notion that the BBS/IFT system functions as a scavenger to export non-ciliary proteins from cilia.97 Similarly, abundant soluble ciliary proteins could escape by diffusion. Thus, IFT counteracts diffusional equilibration between the cell body and the cilium (Fig. 3F).
Surface motility
IFT also drives flagellar surface and gliding motility, which are best studied in Chlamydomonas and other protists (Fig. 3G).98,99 These on-and-off motilities are driven by transient interactions between IFT trains and ciliary transmembrane proteins. In contrast to IFT itself, the binding of the involved transmembrane proteins to IFT depend on extracellular calcium.100,101 In gliding motility, flagellar transmembrane proteins will adhere to a substrate immobilizing the associated IFT trains or capturing them after adhesion. Comparable to a filament gliding assay, the microtubule minus-end directed activity of IFT dynein will then pull on the axoneme dragging the entire cell in the direction of the cilium containing the adhesion.101 In surface motility, extracellular particles move up and down the cilium. In hindsight, the back-and-forth movement of particles adhered to the flagellar membrane provided early evidence for the existence of a bidirectional transport system inside flagella.99 Certain protists use their flagella to select and gather food probably involving this mechanism to move the pray to the ciliary base for endocytosis.102,103 Cilia could function as receivers for extracellular vesicles.104 In mammalian cilia, certain transmembrane proteins transiently associate with IFT while others appear to move essentially by diffusion once inside cilia.105,106 While primary cilia are likely to display surface motility, it is not yet known whether it has any functional relevance, e.g., during cell migration.
IFT and ectosomes (… and the cell cycle)
Cilia shed vesicles or ectosomes (50-200 nm in diameter) containing IFT and ciliary membrane proteins. Components of the ESRCT complex are present in cilia-derived vesicles and the topology of ectosome formation from cilia recapitulates other ESCRT-based events.107 Vesicle formation also involves the actin-based cytoskeleton.108,109 The role of IFT in vesicle formation is unclear but it could delivery vesicle-specific proteins to the ciliary tip.108 Due to their specific protein content, cilia-derived ectosomes possess biological functions in Chlamydomonas (during daughter cell release from sporangia and during mating) and likely elsewhere.83,110 GPCRs and other proteins are shed in vesicles from the ciliary tip either as their natural route of exit or because protein export via the BBS/IFT system is impaired.108,110 Further, IFT and other proteins are released from primary cilia prior to mitosis by shedding of the distal ciliary segment, a process termed decapitation.109 Decapitation precedes cilia resorption and entry into the cell cycle emphasizing the tight coordination of cilia growth and disassembly with the cell cycle. In many cell types, ciliary disassembly promotes G1-S transition (mammals) or entry into mitosis (Chlamydomonas). Evidence implying IFT in the export of proteins liberated by axonemal disassembly during cilia shortening is weak.111 IFT proteins reside at the mitotic spindle poles and loss of cilia or defects in IFT result in misoriented spindles and cell division planes.12,112 In other systems, cilia loss is the only known phenotype of IFT mutants and mitosis and cell growth appear to be normal.113 Clearly, the connections between IFT, cilia, ectosomes, and the cell cycle deserve further attention.
Adaptation and signaling
Cilia often undergo changes of composition or length in response to developmental or environmental cues (Fig. 3H). Examples include the import of cell adhesion molecules and the calcium channel PKD2 into Chlamydomonas flagella during gametic differentiation, the removal of certain GPCRs from cilia upon ligand binding, cyclic or pharmacologically induced changes in cilia length, light-dark adaptation of the outer segments, or the recruitment of Lis1-like into flagella to support axonemal dyneins under high-load conditions.114-120 Some of these changes are probably driven by diffusional entry and capture of proteins inside cilia.64,121 However, IFT could provide the means to rapidly execute such changes.
IFT not only installs and maintains the ciliary signaling machinery but evidence suggests that it also participates directly in signaling cascades. In Chlamydomonas, cilium-to-cilium contacts between gametes initiate fertilization signals and active IFT is required for successful cell fusion.122 The role of IFT is particularly well studied in the Hedgehog (Hh) pathway, which regulates a plethora of developmental processes.123-125 In vertebrates, Hh signaling involves the controlled translocation of signaling proteins in and out of cilia. After binding of the Hh ligand, its receptor Patched exits the cilium and Smoothened translocates into the cilium; when IFT is defective, both proteins are mislocalized compromising Hh signaling.123,126,127 Further down the cascade, Smoothened recruits β-arrestin in a process that depends on and might involve IFT.128 Then, β-arrestin binds activated Gpr161, an orphan GPCR suppressing Hh signaling, and mediates its removal from cilia probably by facilitating loading onto retrograde IFT trains.114,128 Evidence suggests further that Gli proteins, the transcription factors for Hh-responsive genes, are translocated from the cilium to the cell body in an IFT-dependent manner.129-131 While IFT27 is expendable for cilia assembly and IFT itself in mice, Ift27-/- animals display features typical of defective Hh signaling emphasizing that IFT might transport Hh signaling proteins.132 IFT-dependent transport of signaling proteins is likely to participate in other cilia-based signaling pathways.133 It is intriguing to consider that signals received by the cilium could modify a receptor or effector enabling them to associate with IFT trains, translocate to the cell body, and transmit a signal (Fig. 3I). Then, a perpetually running IFT system would keep the cilia on high alert ensuring that signals are rapidly transmitted to the cell body and vice versa.
Summary
IFT trains are versatile with respect to the range of possible cargo proteins and the quantity of cargo bound to the carriers. While some aspects of the cellular circuits regulating the amount of structural proteins moved via IFT into cilia are emerging, it remains unclear how cells measure the length of their cilia and process this information to adjust the amount of cargo on the IFT trains. IFT also contributes in multiple ways to the maintenance and function of steady-state cilia. The ability of the cells to regulate when, which and how much protein is transported via IFT into cilia is likely to be a major determinant of cilia size, composition, and function and a major mechanism to adjust them in a controlled manner.
Synopsis.
Cilia and flagella are widely distributed cell organelles with motile and sensory functions. The intraflagellar transport (IFT) pathway moves proteins in and out of cilia and is required for ciliary assembly, maintenance, and signaling. Here, we discuss recent data revealing a complex regulation of IFT-cargo interactions. Continuously running IFT may prevent diffusional equilibration between the cell body and the cilium establishing and maintaining the specific protein content of cilia.
Acknowledgments
We apologize to our colleagues for not citing their work due to space limitations and the narrow focus of this article. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM110413. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Czarnecki PG, Shah JV. The ciliary transition zone: from morphology and molecules to medicine. Trends in cell biology. 2012;22(4):201–210. doi: 10.1016/j.tcb.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170(1):103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum JL, Child FM. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967;34(1):345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witman GB. The site of in vivo assembly of flagellar microtubules. Ann N Y Acad Sci. 1975;253:178–191. doi: 10.1111/j.1749-6632.1975.tb19199.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol. 1992;119(6):1605–1611. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 7.Lu Q, Insinna C, Ott C, et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. 2015;17(3):228–240. doi: 10.1038/ncb3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Gonzalo FR, Reiter JF. Open Sesame: How Transition Fibers and the Transition Zone Control Ciliary Composition. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazour GJ, Dickert BL, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151(3):709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billker O, Shaw MK, Jones IW, Ley SV, Mordue AJ, Sinden RE. Azadirachtin disrupts formation of organised microtubule arrays during microgametogenesis of Plasmodium berghei. J Eukaryot Microbiol. 2002;49(6):489–497. doi: 10.1111/j.1550-7408.2002.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Sinden RE, Croll NA. Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology. 1975;70(1):53–65. doi: 10.1017/s0031182000048861. [DOI] [PubMed] [Google Scholar]
- 12.Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183(3):377–384. doi: 10.1083/jcb.200808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18(5):1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 14.Brown JM, Witman GB. Cilia and Diseases. Bioscience. 2014;64(12):1126–1137. doi: 10.1093/biosci/biu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8(11):880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 16.Craige B, Tsao CC, Diener DR, et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190(5):927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CL, Li C, Kida K, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192(6):1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90(12):5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taschner M, Lorentzen E. The Intraflagellar Transport Machinery. Cold Spring Harb Perspect Biol. 2016;8(10) doi: 10.1101/cshperspect.a028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigino G, Geimer S, Lanzavecchia S, et al. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol. 2009;187(1):135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141(4):993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vannuccini E, Paccagnini E, Cantele F, et al. Two classes of short IFT trains with different 3D structure are present in Chlamydomonas flagella. Journal of cell science. 2016 doi: 10.1242/jcs.183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11(20):1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 24.Wei Q, Xu Q, Zhang Y, et al. Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nature communications. 2013;4:2750. doi: 10.1038/ncomms3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogowski M, Scholz D, Geimer S. Electron microscopy of flagella, primary cilia, and intraflagellar transport in flat-embedded cells. Methods Enzymol. 2013;524:243–263. doi: 10.1016/B978-0-12-397945-2.00014-7. [DOI] [PubMed] [Google Scholar]
- 26.Stepanek L, Pigino G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science. 2016;352(6286):721–724. doi: 10.1126/science.aaf4594. [DOI] [PubMed] [Google Scholar]
- 27.Reck J, Schauer AM, VanderWaal Mills K, et al. The role of the dynein light intermediate chain in retrograde IFT and flagellar function in Chlamydomonas. Mol Biol Cell. 2016;27(15):2404–2422. doi: 10.1091/mbc.E16-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buisson J, Chenouard N, Lagache T, Blisnick T, Olivo-Marin JC, Bastin P. Intraflagellar transport proteins cycle between the flagellum and its base. Journal of cell science. 2012 doi: 10.1242/jcs.117069. [DOI] [PubMed] [Google Scholar]
- 29.Lechtreck KF, Gould TJ, Witman GB. Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia. 2013;2(1):15. doi: 10.1186/2046-2530-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bower R, Tritschler D, Vanderwaal K, et al. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol Biol Cell. 2013;24(8):1134–1152. doi: 10.1091/mbc.E12-11-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechtreck KF. IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem Sci. 2015;40(12):765–778. doi: 10.1016/j.tibs.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wren KN, Craft JM, Tritschler D, et al. A Differential Cargo-Loading Model of Ciliary Length Regulation by IFT. Current biology : CB. 2013;23(24):2463–2471. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Developmental cell. 2010;18(2):237–247. doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barsel SE, Wexler DE, Lefebvre PA. Genetic analysis of long-flagella mutants of Chlamydomonas reinhardtii. Genetics. 1988;118(4):637–648. doi: 10.1093/genetics/118.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation. 2011 doi: 10.1016/j.diff.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.San Agustin JT, Pazour GJ, Witman GB. Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell. 2015;26(24):4358–4372. doi: 10.1091/mbc.E15-08-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155(3):405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall WF. Subcellular size. Cold Spring Harb Perspect Biol. 2015;7(6) doi: 10.1101/cshperspect.a019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall WF. How Cells Measure Length on Subcellular Scales. Trends in cell biology. 2015;25(12):760–768. doi: 10.1016/j.tcb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan YH, Marshall WF. How cells know the size of their organelles. Science. 2012;337(6099):1186–1189. doi: 10.1126/science.1223539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craft JM, Harris JA, Hyman S, Kner P, Lechtreck KF. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J Cell Biol. 2015;208(2):223–237. doi: 10.1083/jcb.201409036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goehring NW, Hyman AA. Organelle growth control through limiting pools of cytoplasmic components. Current biology : CB. 2012;22(9):R330–339. doi: 10.1016/j.cub.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 43.Lefebvre PA, Nordstrom SA, Moulder JE, Rosenbaum JL. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978;78(1):8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludington WB, Shi LZ, Zhu Q, Berns MW, Marshall WF. Organelle size equalization by a constitutive process. Curr Biol. 2012;22(22):2173–2179. doi: 10.1016/j.cub.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilton LK, Gunawardane K, Kim JW, Schwarz MC, Quarmby LM. The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Current biology : CB. 2013;23(22):2208–2214. doi: 10.1016/j.cub.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y, Meng D, Zhu B, Pan J. Mechanism of ciliary disassembly. Cell Mol Life Sci. 2016;73(9):1787–1802. doi: 10.1007/s00018-016-2148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefebvre PA, Harris EH, Witmann GB. The Chlamydomonas sourcebook Vol 3 Cell motility and behavior. 2.ed. Amsterdam [u.a]: Elsevier; 2009. Flagellar length Control; pp. 115–129. [Google Scholar]
- 49.Wilson NF, Iyer JK, Buchheim JA, Meek W. Regulation of flagellar length in Chlamydomonas. Semin Cell Dev Biol. 2008;19(6):494–501. doi: 10.1016/j.semcdb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam LW, Wilson NF, Lefebvre PA. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol. 2007;176(6):819–829. doi: 10.1083/jcb.200610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broekhuis JR, Verhey KJ, Jansen G. Regulation of cilium length and intraflagellar transport by the RCK-kinases ICK and MOK in renal epithelial cells. PLoS One. 2014;9(9):e108470. doi: 10.1371/journal.pone.0108470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaya T, Omori Y, Kuwahara R, Furukawa T. ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. EMBO J. 2014;33(11):1227–1242. doi: 10.1002/embj.201488175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Y, Pang Y, Wu Q, et al. FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Developmental cell. 2014;30(5):585–597. doi: 10.1016/j.devcel.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Developmental cell. 2004;6(3):445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 55.Luo M, Cao M, Kan Y, Li G, Snell W, Pan J. The phosphorylation state of an aurora-like kinase marks the length of growing flagella in Chlamydomonas. Current biology : CB. 2011;21(7):586–591. doi: 10.1016/j.cub.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao M, Meng D, Wang L, Bei S, Snell WJ, Pan J. Activation loop phosphorylation of a protein kinase is a molecular marker of organelle size that dynamically reports flagellar length. Proc Natl Acad Sci U S A. 2013;110(30):12337–12342. doi: 10.1073/pnas.1302364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ludington WB, Wemmer KA, Lechtreck KF, Witman GB, Marshall WF. Avalanche-like behavior in ciliary import. Proc Natl Acad Sci U S A. 2013;110(10):3925–3930. doi: 10.1073/pnas.1217354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludington WB, Ishikawa H, Serebrenik YV, et al. A systematic comparison of mathematical models for inherent measurement of ciliary length: how a cell can measure length and volume. Biophys J. 2015;108(6):1361–1379. doi: 10.1016/j.bpj.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhogaraju S, Cajanek L, Fort C, et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science. 2013;341(6149):1009–1012. doi: 10.1126/science.1240985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J Cell Biol. 2007;176(5):653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J Cell Biol. 2008;183(2):313–322. doi: 10.1083/jcb.200802025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubo T, Brown JM, Bellve K, et al. The IFT81 and IFT74 N-termini together form the major module for intraflagellar transport of tubulin. Journal of cell science. 2016 doi: 10.1242/jcs.187120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Gau B, Slade WO, Juergens M, Li P, Hicks LM. The global phosphoproteome of Chlamydomonas reinhardtii reveals complex organellar phosphorylation in the flagella and thylakoid membrane. Mol Cell Proteomics. 2014;13(9):2337–2353. doi: 10.1074/mcp.M114.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris JA, Liu Y, Yang P, Kner P, Lechtreck KF. Single-particle imaging reveals intraflagellar transport-independent transport and accumulation of EB1 in Chlamydomonas flagella. Mol Biol Cell. 2016;27(2):295–307. doi: 10.1091/mbc.E15-08-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nature cell biology. 2012;14(4):431–437. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belzile O, Hernandez-Lara CI, Wang Q, Snell WJ. Regulated Membrane Protein Entry into Flagella Is Facilitated by Cytoplasmic Microtubules and Does Not Require IFT. Current biology : CB. 2013;23(15):1460–1465. doi: 10.1016/j.cub.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197(6):697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Awata J, Takada S, Standley C, et al. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. Journal of cell science. 2014;127(21):4714–4727. doi: 10.1242/jcs.155275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del Viso F, Huang F, Myers J, et al. Congenital Heart Disease Genetics Uncovers Context-Dependent Organization and Function of Nucleoporins at Cilia. Dev Cell. 2016;38(5):478–492. doi: 10.1016/j.devcel.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol. 2013;203(1):129–147. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 72.Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. Journal of cell science. 2010;123(Pt 4):529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol. 2017 doi: 10.1083/jcb.201607095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maric D, McGwire BS, Buchanan KT, et al. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J Biol Chem. 2011;286(38):33109–33117. doi: 10.1074/jbc.M111.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kee HL, Verhey KJ. Molecular connections between nuclear and ciliary import processes. Cilia. 2013;2(1):11. doi: 10.1186/2046-2530-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gotthardt K, Lokaj M, Koerner C, Falk N, Giessl A, Wittinghofer AA. G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. Elife. 2015;4 doi: 10.7554/eLife.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright KJ, Baye LM, Olivier-Mason A, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011;25(22):2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood CR, Rosenbaum JL. Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Curr Biol. 2014;24(10):1114–1120. doi: 10.1016/j.cub.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17(9):3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wood CR, Wang Z, Diener D, Zones JM, Rosenbaum J, Umen JG. IFT proteins accumulate during cell division and localize to the cleavage furrow in Chlamydomonas. PLoS One. 2012;7(2):e30729. doi: 10.1371/journal.pone.0030729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephens RE. Tubulin and tektin in sea urchin embryonic cilia: pathways of protein incorporation during turnover and regeneration. Journal of cell science. 1994;107(Pt 2):683–692. [PubMed] [Google Scholar]
- 82.Song L, Dentler WL. Flagellar protein dynamics in Chlamydomonas. J Biol Chem. 2001;276(32):29754–29763. doi: 10.1074/jbc.M103184200. [DOI] [PubMed] [Google Scholar]
- 83.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Current biology : CB. 2013;23(10):906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176(4):483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25(1):8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17(2):117–133. [PubMed] [Google Scholar]
- 87.Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell. 2011;22(7):921–930. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watanabe Y, Hayashi M, Yagi T, Kamiya R. Turnover of actin in Chlamydomonas flagella detected by fluorescence recovery after photobleaching (FRAP) Cell structure and function. 2004;29(3):67–72. doi: 10.1247/csf.29.67. [DOI] [PubMed] [Google Scholar]
- 89.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131(6 Pt 1):1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown JM, Marsala C, Kosoy R, Gaertig J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol Biol Cell. 1999;10(10):3081–3096. doi: 10.1091/mbc.10.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fort C, Bonnefoy S, Kohl L, Bastin P. Intraflagellar transport is required for the maintenance of the trypanosome flagellum composition but not its length. Journal of cell science. 2016;129(15):3026–3041. doi: 10.1242/jcs.188227. [DOI] [PubMed] [Google Scholar]
- 92.Dentler W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J Cell Biol. 2005;170(4):649–659. doi: 10.1083/jcb.200412021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16(1):270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 95.Lechtreck KF, Brown JM, Sampaio JL, et al. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J Cell Biol. 2013;201(2):249–261. doi: 10.1083/jcb.201207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin H, Nachury MV. The BBSome. Current biology : CB. 2009;19(12):R472–473. doi: 10.1016/j.cub.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 97.Datta P, Allamargot C, Hudson JS, et al. Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2015;112(32):E4400–4409. doi: 10.1073/pnas.1510111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saito A, Suetomo Y, Arikawa M, et al. Gliding movement in Peranema trichophorum is powered by flagellar surface motility. Cell Motil Cytoskeleton. 2003;55(4):244–253. doi: 10.1002/cm.10127. [DOI] [PubMed] [Google Scholar]
- 99.Bloodgood RA. Motility occurring in association with the surface of the Chlamydomonas flagellum. J Cell Biol. 1977;75(3):983–989. doi: 10.1083/jcb.75.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collingridge P, Brownlee C, Wheeler GL. Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Current biology : CB. 2013;23(22):2311–2318. doi: 10.1016/j.cub.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 101.Shih SM, Engel BD, Kocabas F, et al. Intraflagellar transport drives flagellar surface motility. Elife. 2013;2:e00744. doi: 10.7554/eLife.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Breglia SA, Yubuki N, Leander BS. Ultrastructure and molecular phylogenetic position of Heteronema scaphurum: a eukaryovorous euglenid with a cytoproct. J Eukaryot Microbiol. 2013;60(2):107–120. doi: 10.1111/jeu.12014. [DOI] [PubMed] [Google Scholar]
- 103.Wetherbee R, Anderson RA. Flagella of a chrysophycean alga play an active role in prey capture and selection. Direct observations of Epipyxia pulchra using enhanced video microscopy. Protoplasma. 1992;166:1–7. [Google Scholar]
- 104.Wang J, Barr MM. Ciliary Extracellular Vesicles: Txt Msg Organelles. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams CL, McIntyre JC, Norris SR, et al. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nature communications. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye F, Breslow DK, Koslover EF, Spakowitz AJ, Nelson WJ, Nachury MV. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife. 2013;2:e00654. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long H, Zhang F, Xu N, et al. Comparative Analysis of Ciliary Membranes and Ectosomes. Curr Biol. 2016;26(24):3327–3335. doi: 10.1016/j.cub.2016.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nager AR, Goldstein JS, Herranz-Perez V, et al. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell. 2017;168(1-2):252–263 e214. doi: 10.1016/j.cell.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phua SC, Chiba S, Suzuki M, et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell. 2017;168(1-2):264–279 e215. doi: 10.1016/j.cell.2016.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao M, Ning J, Hernandez-Lara CI, et al. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife. 2015;4 doi: 10.7554/eLife.05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engel BD, Ishikawa H, Wemmer KA, et al. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. J Cell Biol. 2012;199(1):151–167. doi: 10.1083/jcb.201206068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol. 2011;13(4):461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hou Y, Pazour GJ, Witman GB. A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Mol Biol Cell. 2004;15(10):4382–4394. doi: 10.1091/mbc.E04-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pal K, Hwang SH, Somatilaka B, et al. Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol. 2016;212(7):861–875. doi: 10.1083/jcb.201506132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hunnicutt GR, Snell WJ. Rapid and slow mechanisms for loss of cell adhesiveness during fertilization in Chlamydomonas. Dev Biol. 1991;147(1):216–224. doi: 10.1016/s0012-1606(05)80019-3. [DOI] [PubMed] [Google Scholar]
- 116.Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009;388(4):757–762. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- 117.Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot Cell. 2004;3(5):1307–1319. doi: 10.1128/EC.3.5.1307-1319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rompolas P, Patel-King RS, King SM. Association of Lis1 with outer arm dynein is modulated in response to alterations in flagellar motility. Mol Biol Cell. 2012;23(18):3554–3565. doi: 10.1091/mbc.E12-04-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liew GM, Ye F, Nager AR, et al. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell. 2014;31(3):265–278. doi: 10.1016/j.devcel.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179(3):501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends in cell biology. 2006;16(11):560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125(3):549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 123.Eguether T, San Agustin JT, Keady BT, et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Developmental cell. 2014;31(3):279–290. doi: 10.1016/j.devcel.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seo S, Zhang Q, Bugge K, et al. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011;7(11):e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426(6962):83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 126.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 127.May SR, Ashique AM, Karlen M, et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287(2):378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 128.Green JA, Schmid CL, Bley E, et al. Recruitment of beta-Arrestin into Neuronal Cilia Modulates Somatostatin Receptor Subtype 3 Ciliary Localization. Mol Cell Biol. 2016;36(1):223–235. doi: 10.1128/MCB.00765-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Firestone AJ, Weinger JS, Maldonado M, et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484(7392):125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106(51):21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237(8):2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang N, Li L, Eguether T, Sundberg JP, Pazour GJ, Chen J. Intraflagellar transport 27 is essential for hedgehog signaling but dispensable for ciliogenesis during hair follicle morphogenesis. Development. 2015;142(16):2860. doi: 10.1242/dev.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mourao A, Christensen ST, Lorentzen E. The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol. 2016;41:98–108. doi: 10.1016/j.sbi.2016.06.009. [DOI] [PubMed] [Google Scholar]