Abstract
Objectives
The safety, painless and tolerability features of transcranial Direct Current Stimulation (tDCS) have prompted the research on the therapeutic effects of this neuromodulator technique in stroke; however, an in-depth and unarguable examination of the adverse effects of tDCS in stroke patients is still lacking. This review analyzes the reported adverse effects in stroke, looking for factors that may induce side-effects.
Materials and Methods
A comprehensive search of articles published from 1998 to 2015 describing tDCS application in stroke patients performed through data extraction from MEDLINE/PubMed database.
Results
Only 11.62% of published papers reported the occurrence of tDCS adverse effects in stroke patients. The most common was itching (70%), followed by burning sensation (40%), headache (40%), tingling (30%), sleepiness (20%), difficulty of concentration, mild fatigue, skin redness and dizziness (10%). No significant difference was found between studies ‘Reporting’ vs. ‘Non-reporting’ adverse effects regarding tDCS parameters (intensity, current density, duration of stimulation, and number of sessions).
Conclusion
In the majority of stroke patients, tDCS did not induce any severe adverse effect. Regrettably, many published papers did not provide a careful description of exclusion criteria, or a systematic report of side effects. Our work emphasizes the need of a more meticulous description of the adopted exclusion criteria and of the induced adverse effects, in order to optimize the therapeutic use of tDCS and to better delineate its safety parameters in stroke.
Keywords: tDCS, Safety, Adverse effects, Stroke, Patient selection
Introduction
Transcranial Direct Current Stimulation (tDCS) is a non-invasive brain stimulation (NIBS) technique which has experienced significant growth in recent years, as evidenced by the number of studies testing its behavioral and neurophysiological effects in healthy participants and patients affected by various diseases [1]. Such evidence shows that tDCS can effectively modulate cortical excitability, pointing out its potential therapeutic value for the treatment of different diseases, among which cognitive and motor disorders in stroke [2], neuropathic pain [3], migraine [4], and epilepsy [5]. However, evidence is still somewhat mixed and publication bias is also likely [6].
tDCS is simple, versatile and easy to apply by trained personnel. All those factors, together with its relative low cost and the reliability of the sham condition [7], have contributed to its popularity and its application in rehabilitative programs [8].
Through a simple 9-volt battery, tDCS delivers weak, direct electric currents (typically, ≈1–2 mA) over the scalp via two electrodes soaked with saline solution [9]. Generally, tDCS is considered to be safe. However, it should be noted that safety guidelines specific for tDCS are still not available, and both researchers and clinicians refer to those developed by Rossi and colleagues (2009), which are focused on Transcranial Magnetic Stimulation (TMS) [10]. Notwithstanding, as every intervention is intrinsically associated with the risk of harmful, the potential induction of adverse events and effects related to tDCS needs to be taken into careful consideration. Based on the available evidence, overall, the balance between beneficial and adverse effects using tDCS seems shifted toward the first. In fact, a number of investigations and review papers indicates that the adverse effects of tDCS are overall mild and highly subjective, mainly related to the application of the electrical current over the scalp. Adverse effects indeed range from itching, tingling and burning sensations to redness beneath the stimulated areas. More serious unwanted side-effects, such as headache, nausea and insomnia, are infrequent in both healthy adults and children, as well as in patients with neurological or neuropsychiatric diseases; whenever present, they are transitory and overall well-tolerated (for reviews, see [8,11–14]). Skin burn may occur, and few cases have been registered in major depression, tinnitus, neuropathic pain and in healthy individuals [15–18].
Despite the above-mentioned evidence regarding tDCS adverse effects, it is important to consider that safety of a certain device or drug is not similar across different diseases. Therefore, here we aimed to further explore safety issues focusing on the stroke population since tDCS has become increasingly popular in post-stroke motor and cognitive rehabilitation, and there is an increasing need to know to what extend this technique can be applied in safety with therapeutic purposes in this domain. Promising results come from studies assessing the effects of the tDCS (both in isolation and coupled with behavioral interventions) for the treatment of upper [19] and lower [20] limb motor disorders, as well as for enhancing motor learning [21]. Furthermore, tDCS has been used as an adjuvant therapy in post-stroke cognitive rehabilitation [22]. However, what seems lacking is a deeper evaluation of the adverse effects associated with its use in this specific population. Special concerns may arise when stimulating a brain that had suffered a lesion, targeting a neural system whose functioning has been disrupted by a cerebrovascular accident. In this condition, modeling studies showed that skull defect and stroke-related lesions may create distortions in the current flow, as injuries filled with cerebrovascular fluid may expose the tissue to a higher intensity of focused current [23]. In addition, stroke patients are usually older and their skin is more fragile, hence it is more likely for them to suffer from a further heating damage [24]. Thus, special attention should be given in the assessment and the evaluation of tDCS side-effects in stroke population. The present review faces this issue, exploring the adverse effects of tDCS in the stroke population as reported in the literature, also looking for their possible relationship with the parameters of stimulation (namely, current intensity and density, stimulation duration and number of applications) and the clinical (stroke duration) and demographic characteristics (age, gender) of the stroke patients. Finally, we have identified the used exclusion criteria applied in stroke studies, as this is an important parameter to take into account for assessing and predicting the safety of a tDCS intervention.
Methods
Literature search
We performed a comprehensive electronic search of articles published from 1998 to 2015 describing tDCS application in post stroke patients. Data were extracted from MEDLINE/PubMed database using the following key words: “transcranial Direct Current Stimulation”, “tDCS”, or “brain polarization” and “stroke” combined with “physical therapy modalities”, “occupational therapy”, “exercise” or “motor activity”.
Selection criteria
The research included articles that met the following criteria: (1) articles written in English, (2) studies conducted in humans only, (3) original research only, (4) assessing tDCS effects in stroke patients. In turn, we excluded: (1) review articles, (2) duplicated articles, studies conducted in (3) healthy individuals, (4) animals, (5) patients with other neurological degenerative diseases, (6) studies using other brain stimulation techniques such as TMS.
Data extraction
A structured checklist was elaborated to extract the following information: (1) Study design; (2) Demographic and clinical characteristics, such as: age (years), gender, and time since stroke (months); (3) Study characteristics: total sample, inclusion and exclusion criteria; (4) Stimulation parameters: intensity (mA), current density (mA/cm2), duration (minutes), and number of sessions; (5) Adverse events, considering the following: (i) absent when articles reported ‘all patients tolerated treatment well’ or ‘no side-effects were reported’; (ii) present when articles described any kind of adverse effects even if their description was not detailed (e.g., ‘no subjects reported adverse effects other than itching under the electrodes’); (iii) not applicable when articles did not mention the evaluation or the reporting of the adverse effects. Accordingly to the last criterion, studies were then classified as ‘Reporting’ and ‘Non-reporting’ adverse events. The group of articles as ‘not applicable’ was not considered for the main analysis.
Analysis
Firstly, normality of all data (i.e., tDCS parameters and clinical and demographic characteristics of the sample) was assessed by Kolmogorov-Smirnov test. Since the assumption was violated, we applied Mann-Whitney test to explore whether ‘Reporting’ studies differ from ‘Non-reporting’ ones, regarding tDCS parameters (intensity, current density, duration, and number of sessions), clinical (stroke duration) and demographic (age and gender) characteristics of the sample. P-values were corrected for multiple comparisons considering separately the four tDCS (namely, intensity, current density, duration, and number of sessions) and the two demographic (age and gender) parameters.
Then, we used the median values of our reviewed data, as shown in Table 1, to split the entire sample in two main groups (low and high values) with respect to the tDCS parameters: studies with values under the median to which we refer with “low” parameters, and studies with values above the median, considered “high”.
Table 1.
Median value for intensity of stimulation, current density, duration of stimulation and number of session obtained from the analyzed articles.
| Variables | Median value |
|---|---|
| Intensity of Stimulation | 1mA |
| Current Density | 0.04 mA/cm2 |
| Duration of Stimulation | 20 min |
| Number of Sessions | 5 |
Abbreviations: mA, miliampère; cm2, square centimeter; min, minutes.
Then, we focused on the ‘Reporting’ studies group only, categorizing them as using “high” or “low” parameters, according to the median values of Table 1. Finally, we explored whether the studies of the ‘Reporting’ group differ according to each tDCS parameters (intensity, current density, duration, and number of sessions). However, it should be also noted that these analyses have an exploratory nature.
Analyses were performed using Statistical Package for Social Science for Windows (SPSS) version 23.0.
Results
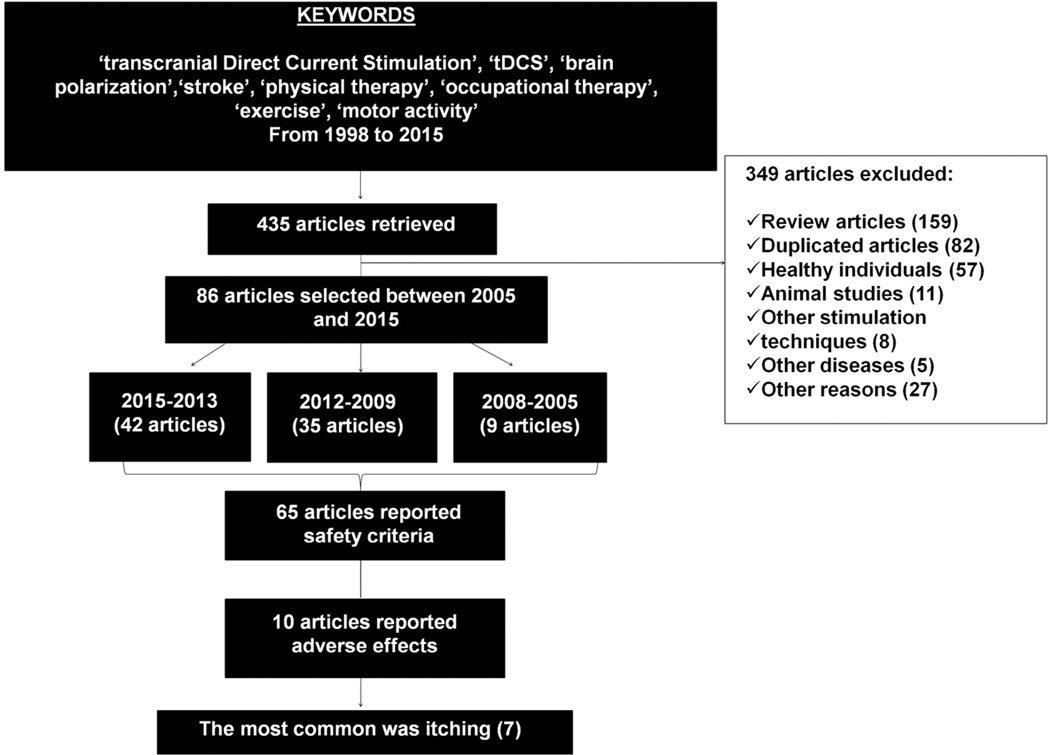
The initial search on the PubMed database retrieved 435 articles published between 1998 and 2015. Among these, 349 articles were excluded for the following reasons: 159 were review papers, 82 were duplicate studies, 57 were studies performed on healthy participants and 11 in animals, 8 applied NIBS techniques other than tDCS, 5 focused on other disease than stroke, 27 were not considered for other reasons, since they were out of scope of this review (e.g., computational models, developmental studies). Using the study criteria, we narrowed the list to 86 (Figure 1).
Figure 1.
Flow diagram of articles extraction and selection.
Demographic and clinical characteristics
In total, 1293 stroke patients (800 males and 451 females) with age ranging 7 to 92 years (mean= 59.38 ± Standard Deviation=9.19; median= 60.7) were including in the analyses. The 54.83% of the stroke patients were in a chronic stage of illness, with a mean time elapsed since the stroke of 43.81±33.96 months (range= 2–253 months). The remaining patients were in subacute (24.82%) or acute stage of illness (19.33%); 1.02% were children with congenital hemiplegia.
tDCS parameters
Concerning the tDCS parameters, a current intensity between 1 and 2 mA was usually used, with a median of 1 mA (52.95% of the articles applied ≤1mA). The median current density, defined as the mean of the stimulation current divided by the surface of the stimulation electrode, was of 0.04 mA/cm2 (50.63% of articles used ≤0.04 mA/cm2). The median duration of the stimulation was 20 min (80% of articles used durations less or equal). TDCS was applied for a median of 5 sessions in the 56.47% of the articles (see Table 1).
Adverse effects
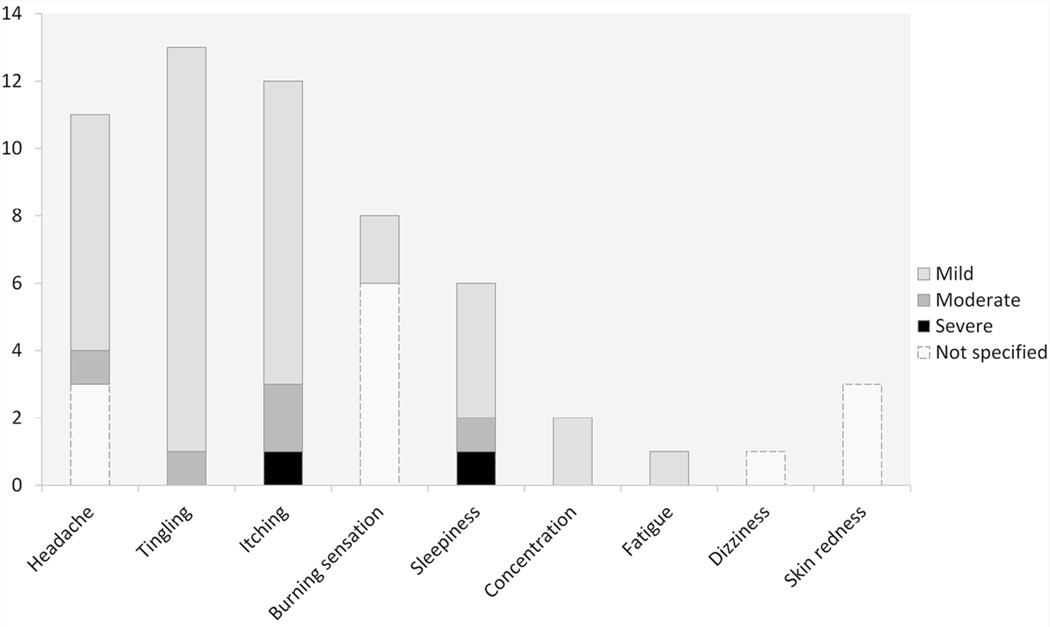
In the 11.62% of articles that reported the occurrence adverse events, (namely, ‘Reporting’ studies, N° 10 out of 86) at least one was described; the most common was itching (70%), followed by burning sensation (40%), headache (40%), tingling (30%), sleepiness (20%), difficulty of concentration (10%), mild fatigue (10%), skin redness (10%) and dizziness (10%). The main characteristics of these studies are detailed in Table 2. The number of patients who reported adverse effects and the related cumulative incidence are depicted in Figure 2.
Table 2.
Main characteristics of studies which encountered adverse effects
| Author (year) |
Population | Safety criteria | Intervention | Sessions | Intensity | Duration | Current density (mA/cm2) |
Adverse effects |
|---|---|---|---|---|---|---|---|---|
| Mortensen (2015) |
Intracerebral hemorrhage mean age:62.60 years ± 10.28 (SD) |
Traumatic ICH, epilepsy, metal implants in the head, other neurological diseases/cognitive disabilities |
(i)A-tDCS+occupational therapy; (ii)sham tDCS+occupational therapy |
5 |
1.5mA |
20 min |
0.04 |
Itching, tingling, burning sensation, headache and sleepiness |
|
Gillick (2015) |
Children with congenital hemiparesis+mean age:14 years ± 3.5 (SD) |
Epilepsy, pregnancy, indwelling metal or medical devices incompatible with NIBS, evidence of skin disease or skin abnormalities and botulinum toxin or phenol intramuscular block within 6 months preceding tDCS |
Bi-tDCS alone |
1 |
0.7mA |
10 min |
0.02 |
Itchining, burning, sleepiness and difficulty of concentration |
|
Kim (2014) |
Subacute poststroke +mean age:55.4 years ± 17.6 (SD) |
Contraindications for TMS or tDCS, previous seizure history, concurrent use of Na+ channel blocking agent or N-methyl-D-aspartate receptor antagonist |
(i)active wrist exercise alone; (ii)VR wrist exercise alone; (iii)active wrist exercise + atDCS; (iv)VR wrist exercise + atDCS |
4 |
1mA |
20 min |
0.028 |
Mild headache and itching |
|
Wang (2014) |
Subacute poststroke +mean age:52.9 years ± 11.9 (SD) |
Contraindications for TMS, history of alcohol/drug abuse, use of certain neuropsychotropic drugs, epilepsy, having already received methylphenidate and/or tDCS treatment, being pregnant, coexisten of advanced or terminal disease and use of certain neuropsychotropic drugs |
(i)Drug alone; (ii)tDCS alone; (iii)drug + tDCS |
1 |
1mA |
20 min |
0.028 |
Mild tingling and mild fatigue |
| Rosso (2014) |
Chronic poststroke +mean age:57 years ± 18 (SD) |
No contraindication for tDCS | (i)atDCS alone; (ii)ctDCS alone |
2 | 1mA | 15 min | 0.028 | Itching |
| Wu (2013) |
Chronic poststroke +mean age:45.9 years ± 11.2 (SD) |
Unstable, progressive, or severe neurologic disease and heart conditions |
(i)ctDCS+physical therapy; (ii)sham tDCS+physical therapy |
20 |
Not Specified |
20 min |
Not Specified |
Slight, itching and tingling |
| Cherney (2013) | Chronic poststroke+age not specified |
Not specified | ctDCS+speech therapy | 30 | 1mA | 13 min | 0.015 | Itching, burning sensation increasing over days |
|
Kim (2010) |
Subacute poststroke +mean age:55.3 years ± 16.4 (SD) |
Presence of a metallic foreign body implant, history of seizure or another unstable medical condition, depression history of severe alcohol or drug abuse; Patients who were taking Na+ or Ca2+ channel blockers or N-methyl-D-aspartate (NMDA) receptor antagonists were also excluded |
(i)atDCS+occupational therapy; (ii)ctDCS+occuptional therapy; (iii) sham tDCS+occupational therapy |
10 |
2mA |
20 min |
0.08 |
Headache and dizziness |
|
Jo (2009) |
Subacute poststroke +mean age: 44 years ± 16.43 (SD) |
Seizure disorder, intracranial metal insertion, cardiac pacemaker or history of other neuropsychiatric diseases |
(i)atDCS+working memory task; (ii) sham tDCS+working memory task |
2 |
2mA |
30 min |
0.08 |
Aching or burning and transient skin redness |
|
Hesse (2007) |
Subacute/ Chronic poststroke +mean age:63.3 years |
Preceding epileptic fits, an EEG suspect of elevated cortical excitability, a sensitive scalp skin, severe cognitive impairment, metallic implants within the brain, previous brain neurosurgery, medications altering the level of cortical excitability or with a presumed positive/negative effect on brain plasticity |
atDCS+robot assisted arm training |
30 | 1.5mA | 7 min | 0.042 |
Slight itching and bearable headache |
Abbreviations: ICH, Intracranial Hemorrhage; a-tDCS, anodal transcranial Direct Current Stimulation; c-tDCS, cathodal transcranial Direct Current Stimulation; bi-tDCS, bihemispheric transcranial Direct Current Stimulation; mA, miliampère, min, minutes; NIBS, Non Invasive Brain Stimulation; TMS, Transcranial Magnetic Stimulation; VR, Virtual Reality; EEG, electroencephalogram.
Figure 2.
Number of post-stroke patients who experienced at least one adverse effect and the related intensity, as reported in the articles.
On the other hand, the 47.67% of the studies reported the absence of adverse events (i.e., ‘Non-reporting’). To note, 40.69% of articles did not mention whether adverse effects did occur or not.
Exclusion Criteria
Data reporting eligibility criteria to tDCS referred to 65 articles: 21 out of 86 papers did not specify which exclusion criteria was adopted. The most common exclusion criterion used in tDCS stroke trials appears to be the presence of metallic implants in the patients, followed by history of epilepsy and of seizure (see Table 3).
Table 3.
Summarizes the list of exclusion criteria sorted by frequency used to exclude patients in the selected articles.
| Exclusion criteria | Frequency |
|---|---|
| Metallic implants | 50% |
| Epilepsy/History of epilepsy at family | 34.8% |
| Seizure/History of seizure | 34.8% |
| Neurologic disease | 31.8% |
| Cognitive/Consciousness disturbance | 31.8% |
| Psychiatric disease | 28.8% |
| Use of neuropsycoactive drugs | 28.8% |
| Pacemaker | 22.7% |
| Previous stroke | 18.2% |
| Uncontrolled medical condition | 16.7% |
| History of alcohol/drug abuse | 15.1% |
| Skin/Skull abnormalities | 13.7% |
| Cardiovascular disease | 12.1% |
| Depression/Major depression | 12.1% |
| Advance or terminal disease | 10.6% |
| History of brain neurosurgery | 10.6% |
| Multiple cerebral lesions | 9.1% |
| Cochlear implant | 7.6% |
| Pregnancy | 7.6% |
| Tumor | 4.5% |
| Dementia | 4.5% |
| Subarachnoid hemorrhage | 1.5% |
| Cerebral aneurysm | 1.5% |
| Organic neck disease | 1.5% |
| Traumatic Intracerebral hemorrhage | 1.5% |
| Contraindication to tDCS and/or TMS | 24.2% |
Abbreviations: tDCS, transcranial direct current stimulation; TMS, Transcranial Magnetic Stimulation.
Quantitative analysis
When comparing ‘Reporting’ vs. ‘Non-reporting’ no significant difference was found between this two categories of studies with respect to the adopted tDCS parameters: intensity (U=295.00, Z=−0.731, p=0.465), current density (U=274.500, Z=−1.067, p=0.286), duration of stimulation (U=293.500, Z=−1.216, p=0.224), and number of sessions (U=373.000, Z=−0.095, p=0.924). With respect to the clinical characteristics of the sample, we observed a significant difference of age, with ‘Reporting’ studies (mean age= 51.34 ± 14.72 years) having younger subjects than ‘Not-reporting’ (mean age= 60.47 ± 7.70 years, p<0.025. No difference between the two groups of studies was detected with respect to gender (number of male, U=300, Z=−0.969, p=0.332; number of female, U=333, Z=−0.514, p=0.607) and stroke duration (U=348, Z=−0.238, p=0.812).
The Mann-Whitney test failed to show differences in the incidence of adverse effects when studies were analyzed according to the tDCS current intensity (less or higher than 1mA) and density (less or higher than 0.04mA/cm2) and to the number of tDCS application (less or higher than 5 sessions) (all ps>0.2). To note, we did not perform any analysis of the incidence of side-effects with respect to the duration of stimulation because only one study used a duration above the median found [25]. All those exploratory calculations warrant further examination in future studies.
Discussion
The present work analyzes the incidence of adverse effects brought about by tDCS in stroke patients, as reported in the current literature. To this aim, studies were classified as ‘Reporting’ and ‘Non-Reporting’ adverse effects, and then possible relationship between stimulation parameters and protocol details was taken into consideration. The most relevant finding is that the vast majority of stroke patients who underwent tDCS sessions well tolerated this NIBS technique, without reporting any severe side-effect. Only the 11.6% of the studies (i.e., 10 articles) conducted in stroke patients reported at least one adverse effect, which although were modest and unserious. They in fact were limited to itching, tingling, burning sensation, headache and skin redness. These results are in line with previous findings in different clinical populations, as well in healthy individuals [8,13,14].
Itching, as well as tingling and burning sensation is a frequent report, given the application of a small current over the scalp. For this reason, operatively, it is important to take care of the electrodes’ montage, using appropriate sponges and saline solution to reduce possible discomforts. Given the physics and physiology of tDCS, the minor side-effects related to the application of electric current to the scalp seem virtually unavoidable [12].
Conversely, headache was rare (here reported by 4 studies of our sample), in line with the incidence of only 14.8% found in more than one hundred studies performed with humans [13]. Notwithstanding, when present, headache attacks are usually transient, lasting about 10 minutes and can be easily abolished by drug intake (acetaminophen or ibuprofen).
Finally, skin redness under the electrode is another minor side-effect frequently registered [13,26]; it is due to the temperature increase by the current delivery to the scalp, which affects to local vasodilatation. In our sample, this adverse effect was explicitly reported by only one study that applied tDCS with “high” values, namely for 30 min with a current density of 0.08 mA/cm2 [25]. Instead, studies in healthy individuals have often reported an increase of skin redness over days in the scalp region where the electrodes were positioned [27]. Although the low incidence of skin redness in stroke patients is likely due to the fact the about 41% of articles did not exhaustively report (and likely monitor) the occurrence of tDCS side-effects in this clinical population, this finding may also suggest that tDCS should not induce additional skin damage in stroke subjects.
In order to characterize the incidence of adverse effects, the ‘Reporting’ studies were then contrasted to the ‘Non-reporting’ ones, in terms of stimulation parameters and clinical characteristics of the sample. Regarding tDCS parameters (namely intensity, current density, duration and number of sessions) we could not find any differences between the two groups. In particular, the intensity of stimulation, which was within the recommended limits (i.e., 1 and 2 mA) [28], did not influence the occurrence of adverse effects in stroke patients. Crucially, half of the ‘Reporting’ studies applied a current with an intensity less or equal to 1 mA. Depending on the size of the electrode (15–25 cm2), a current intensity between 0.04 and 0.08 mA/cm2 is considerably below the safety threshold [11]. Six out of the 10 ‘Reporting’ studies applied a low current density (referring to our median of 0.04 mA/cm2) and in only two studies, the current density was 0.08 mA/cm2. Similarly, the duration of the stimulation and the number of tDCS sessions seem unrelated to adverse effects: ‘Reporting’ and ‘Non-reporting’ studies do not differ with respect to the tDCS duration; only one case applied the tDCS for a period of time longer of 20 minutes. Instead, half of the ‘Reporting’ studies applied the stimulation for less than 5 sessions.
However, intriguing details arise when considering each study separately. Indeed, itching and burning sensations were detected in a single case study in a chronic stroke patient, who underwent tDCS to threat aphasia [29]. Specifically, the unpleasant sensations, which were localized around the reference electrode and persisted for the entire duration of the stimulation, emerged during the first week of treatment, and they increased over the days of treatment. Such side-effects were reduced after the substitution of the self-adhesive electrode with a saline solution electrode pad [29]. Similarly, 2 out of 10 patients enrolled in a 30 sessions rehabilitation study, combining tDCS and robot-assisted arm training in subacute stroke patients [30], reported a bearable headache in the first week immediately following the stimulation, that vanished with the ongoing of the trial.
Our analyses pointed out another interesting aspect: as compared to the studies not reporting adverse effects, ‘Reporting’ studies comprised younger patients. This finding is in accordance with a systematic analysis by Fertonani and colleagues (2015) showing that elderly participants overall perceived fewer tDCS-related sensations as compared to younger participants [26]. The authors proposed that the inverse correlation between age and induced sensations could be due to a physiological dysfunction in the peripheral and/or central nervous systems occurring in elderly [31], or it could be merely related to the psychological tendency to complain less by aged individuals.
There are a number of limitations of the present analyses, which are due to the insufficient information reported in many published studies in stroke patients. For example, we could not assess the different incidence of adverse effects brought about by the active and the sham tDCS, since among the reviewed articles, only two studies specified the allocation of participants with respect to type of stimulation [32,33]. In one study, itching (1 case) and burning (1 case) sensations under the electrodes were observed only in the group receiving the real tDCS, while sleepiness was detected in patients who underwent the sham stimulation; the incidence of difficulty concentrating was reported both in sham and real tDCS sessions (i.e., 1 case in each group) [32]. In another study, itching, tingling, burning sensation, headache and sleepiness occurred after real and sham tDCS, but apparently they were slightly prominent in the real condition [33]. Future studies needs to monitor and report more carefully the incidence of the side-effects by sham and real tDCS either to increase the current knowledge about the safety of this NIBS technique, and also for ensuring and measuring the effective blinding of participants. In fact, sham tDCS is considered reliable not only if the participants is unable to distinguish it from the real stimulation, but also if the sensations induced by these two stimulation modes are comparable [7].
Furthermore, it would have been of great interest to analyze the occurrence of dropouts, as they could be associated to adverse effects. To this aim, we have retrieved information regarding the withdrawal, in order to understand whether the reported cases were due to the tDCS. This information was not submitted to statistical analyses since only 3 out of 1293 stroke patients interrupted their participation to the tDCS study due to the stimulation. In particular, 2 dropouts, one for headache and one for dizziness, were reported in a rehabilitation study with 10 tDCS sessions (2mA, 20 minutes; total 0.08 mA/cm2 of current density; 10 daily sessions over 2 weeks) [34]. Interestingly, in such study, all the tDCS parameters were higher than the median values featuring the tDCS studies reviewed here (see Table 1). Unfortunately, the authors did not specify when the participants experienced their side-effects, nor in which group (anodal, cathodal or sham) they were allocated. Another drop-out, a child with congenital hemiparesis, was registered at the pretest, during the 30-second ramp-up, after reporting mild sleepiness; however this event was likely unrelated to the tDCS, being caused by the fact that the patient waked up early [32].
Finally, there is another important point that emerges from our revision. NIBS is considered to be safe as far as exclusion criteria are rigorously followed. To note, up to now tDCS has been applied following safety guidelines that were originally developed for TMS (for current international safety guidelines, see [10]). Hence, the overall lack of major adverse events, and thus the intrinsic safety features of tDCS, should be considered as the direct consequence of the current exclusion criteria for patients, which are transposed from TMS guidelines. Given the differences between the mechanisms of action the two techniques, basically tDCS is a neuro-modulation tool while TMS acts as neuro-stimulation, the current safety guidelines may be too conservative for tDCS. As consequence, safety guidelines for tDCS should be revisited following a more in-depth investigation of the specific adverse effects in stroke induced by this NIBS technique.
For instance, we found that almost 1/4 of the reviewed studies specifying the eligibility criteria for tDCS (24.2 %) are indicated in the papers as “contraindications to tDCS and/or TMS” (see Table 3). On the other hand, 24.42% of studies did not even specify the adopted exclusion criteria. Moreover, 33.85% of the published paper even did not exclude patients with history of seizures or epilepsy, as international guidelines recommend. With respect to epilepsy, which represent a major contraindication to NIBS, a study applying tDCS in patients with multifocal epilepsy and refractory seizures showed that the anodal stimulation did not increase epileptiform discharges in the area of stimulation [35]; moreover, there is no clear indication that tDCS can trigger seizures in patients with epilepsy. Overall, the issue of the potential adverse effects in stroke patients with epilepsy warrants a more systematic, ad-hoc, investigation. Studies specifically aimed at assessing the incidence of seizures brought about by tDCS in stroke patients with a history of seizure episodes seem now mandatory to redefine the inclusion/exclusion criteria of tDCS with respect to epilepsy in this clinical population. To this aim, an ad-hoc, individual examination of the potential risk of inducing seizures should be evaluated on a case-by-case basis, after an in-depth examination of each patient.
Conclusion
Current evidence shows that tDCS performed following the current safety guidelines [10] is well-tolerated by stroke patients; in few instances, adverse effects were reported, but they were largely minor and transient, as also observed in healthy individuals [12–14]. From the published data, we could not identify any specific stimulation parameter that may cause per se unwanted side-effects in stroke patients. It seems that there is a lack of either moderate or severe adverse effects in stroke individuals even with high intensities (up to 2mA) and long duration (up to 30 minutes, consecutive applications for up to 30 sessions) of the stimulation. Such findings are important and encouraging for the clinical use of tDCS in post-stroke rehabilitation.
However, even if tDCS is considered as a safe technique, this does not really assure that NIBS tool is completely free of undesirable effects, if not specifically assessed. In this regard, it is remarkable to note that a high percentage of the reviewed tDCS studies in stroke patients did not even mention whether adverse effects did occur or not, since in many cases the experimental protocol did not include any specific, detailed, assessment of the tDCS site-effects. It is now mandatory to increase our knowledge of the safety limit of tDCS in stroke. Useful questionnaires to monitor tDCS adverse effects have been published in the last years, which comprise even the collection of information about the risk attribution, and intensity scales to quantified and qualified the tDCS-related experiences, their onset and the location on the body (see [13]). The importance of conducting a systematic assessment of the tDCS adverse effects becomes obvious when considering the need to minimize the risk of depicting the intervention more safe (or more dangerous) than the reality.
In conclusion, whereas tDCS in stroke patients confirms its general safety features at least when the current guidelines for NIBS are rigorously followed, our review emphasizes the need of systematic and sharable procedure for the collection and description of tDCS adverse effects.
Acknowledgments
Source(s) of financial support: This review was supported by the Brazilian Funding Agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ grant 233372/2014-8 to M.C.); University of Milano-Bicocca (FAR grant 2015-ATE-0135 to N.B.) and National Institute of Health (NIH grant 5R21HD079048 to F.F.).
Footnotes
Authorship Statement: Cristina Russo and Maira Cameiro designed the study, including data collection, analysis and interpretation, and prepared the manuscript draft. Nadia Bolognini and Felipe Fregni contributed to the study conception and design, provided support in data analysis and interpretation, and revised the manuscript for intellectual content. All authors approved the final manuscript.
Conflict of Interest: The authors reported no conflict of interest.
References
- 1.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain stimulation. 2012;5(3):175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marquez J, Vliet P, McElduff P, Lagopoulos J, Parsons M. Transcranial direct current stimulation (tDCS): does it have merit in stroke rehabilitation? A systematic review. Int. J. Stroke. 2015;10(3):306–316. doi: 10.1111/ijs.12169. [DOI] [PubMed] [Google Scholar]
- 3.DosSantos MF, Ferreira N, Toback RL, Carvalho AC, DaSilva AF. Potential Mechanisms Supporting the Value of Motor Cortex Stimulation to Treat Chronic Pain Syndromes. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenen J, Roberta B, Magis D, Coppola G. Noninvasive neurostimulation methods for migraine therapy: The available evidence. Cephalalgia. 2016 doi: 10.1177/0333102416636022. 0333102416636022. [DOI] [PubMed] [Google Scholar]
- 5.San-juan D, Morales-Quezada L, Garduño AJO, Alonso-Vanegas M, González-Aragón MF, López DAE, et al. Transcranial direct current stimulation in epilepsy. Brain Stimul. 2015;8(3):455–464. doi: 10.1016/j.brs.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Hummel F, Celnik P, Pascual-Leone A, Fregni F. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1(4):370–382. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Fregni F, Nitsche M, Loo C, Brunoni A, Marangolo P, Leite J, et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2015;32(1):22–35. doi: 10.3109/10601333.2015.980944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods A, Antal A, Bikson M, Boggio P, Brunoni A, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016;127(2):1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi S, Hallett M, Rossini PM, Pascual-Leone A Group SoTC. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulation. 2016 doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain stimulation. 2015;8(1):76–87. doi: 10.1016/j.brs.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011;14(8):1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 14.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007;72(4):208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Frank E, Wilfurth S, Landgrebe M, Eichhammer P, Hajak G, Langguth B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain stimulation. 2010;3(1):58–59. doi: 10.1016/j.brs.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Palm U, Keeser D, Schiller C, Fintescu Z, Reisinger E, Padberg F, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS) Brain stimulation. 2008;1(4):386–387. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez N, Opisso E, Pascual-Leone Á, Soler MD. Skin lesions induced by transcranial direct current stimulation (tDCS) Brain stimulation. 2014;7(5):765. doi: 10.1016/j.brs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Wei Y, Wen J, Li X. Skin burn after single session of transcranial direct current stimulation (tDCS) Brain stimulation. 2015;8(1):165. doi: 10.1016/j.brs.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Triccas LT, Burridge JH, Hughes A-M, Pickering RM, Desikan M, Rothwell JC, et al. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin. Neurophysiol. 2016;127(1):946–955. doi: 10.1016/j.clinph.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 20.Chang MC, Kim DY, Park DH. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain stimulation. 2015;8(3):561–566. doi: 10.1016/j.brs.2015.01.411. [DOI] [PubMed] [Google Scholar]
- 21.Kang N, Summers JJ, Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2016;87(4):345–355. doi: 10.1136/jnnp-2015-311242. [DOI] [PubMed] [Google Scholar]
- 22.Convento S, Russo C, Zigiotto L, Bolognini N. Transcranial Electrical Stimulation in Post-Stroke Cognitive Rehabilitation. Eur. Psychol. 2016 [Google Scholar]
- 23.Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain stimulation. 2011;4(3):169–174. doi: 10.1016/j.brs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Frontiers in bioscience: a journal and virtual library. 2010;15:718. doi: 10.2741/3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo JM, Kim Y-H, Ko M-H, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tDCS. Am. J. Phys. Med. Rehabil. 2009;88(5):404–409. doi: 10.1097/PHM.0b013e3181a0e4cb. [DOI] [PubMed] [Google Scholar]
- 26.Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015;126(11):2181–2188. doi: 10.1016/j.clinph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Durand S, Fromy B, Bouyé P, Saumet J, Abraham P. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. The Journal of physiology. 2002;540(1):261–269. doi: 10.1113/jphysiol.2001.013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 1990;37(10):996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 29.Cherney LR, Babbitt EM, Hurwitz R, Rogers LM, Stinear J, Wang X, et al. Transcranial direct current stimulation and aphasia: the case of Mr. C. Top. Stroke Rehabil. 2013;20(1):5–21. doi: 10.1310/tsr2001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hesse S, Werner C, Schonhardt E, Bardeleben A, Jenrich W, Kirker S. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor. Neurol. Neurosci. 2007;25(1):9–15. [PubMed] [Google Scholar]
- 31.Kemp J, Després O, Pebayle T, Dufour A. Age-related decrease in sensitivity to electrical stimulation is unrelated to skin conductance: an evoked potentials study. Clin. Neurophysiol. 2014;125(3):602–607. doi: 10.1016/j.clinph.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Gillick BT, Feyma T, Menk J, Usset M, Vaith A, Wood TJ, et al. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: randomized controlled preliminary study. Phys. Ther. 2015;95(3):337–349. doi: 10.2522/ptj.20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortensen J, Figlewski K, Andersen H. Combined transcranial direct current stimulation and home-based occupational therapy for upper limb motor impairment following intracerebral hemorrhage: a double-blind randomized controlled trial. Disabil. Rehabil. 2016;38(7):637–643. doi: 10.3109/09638288.2015.1055379. [DOI] [PubMed] [Google Scholar]
- 34.Kim D-Y, Lim J-Y, Kang EK, You DS, Oh M-K, Oh B-M, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am. J. Phys. Med. Rehabil. 2010;89(11):879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- 35.Fregni F, Thome-Souza S, Nitsche MA, Freedman SD, Valente KD, Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 2006;47(2):335–342. doi: 10.1111/j.1528-1167.2006.00426.x. [DOI] [PubMed] [Google Scholar]