Abstract
Enteropathogenic Escherichia coli (EPEC) produces attaching and effacing (A/E) lesions and watery diarrhea, both of which are intimin and EspA dependent. In this work, we explored the mucosal immune response by detecting cytokine induction in rabbits with diarrhea caused by rabbit EPEC (REPEC). Orally inoculated rabbits exhibited weight loss and mucosal inflammation, developed watery diarrhea, and died (day 7). At day 6 postinoculation, animals were analyzed for the induction of proinflammatory cytokines in enterocytes. The role of lymphocyte-dependent immunity was determined through the expression of proinflammatory cytokines by lymphocytes from Peyer's patches (PP) and the spleen. EspA and intimin mutants were used to explore the role of A/E lesions in the expression of these cytokines. REPEC-infected rabbit enterocytes showed increased interleukin 1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) mRNA expression, but that of anti-inflammatory IL-10 was increased only slightly. In contrast, intimin mutant-infected rabbits were unable to produce this proinflammatory cytokine profile but did produce a remarkable increase in IL-10 expression. Bacteria lacking EspA increased the expression of IL-8 and TNF-α, but that of IL-10 was increased only slightly. PP lymphocytes also produced proinflammatory cytokines, which were dependent on EspA (except for TNF-α) and intimin, while IL-10 was induced by EspA and intimin mutants. In contrast, spleen lymphocytes (systemic compartment) were unable to produce IL-1β and TNF-α. These data show the importance of the proinflammatory cytokines secreted by enterocytes and those expressed locally by PP lymphocytes, which can activate effector mechanisms at the epithelium. Furthermore, this cytokine profile, including IL-6 and IL-1β, which may be involved in the diarrhea produced by EPEC, depends on intimin.
Infant intestinal infection by enteropathogenic Escherichia coli (EPEC) is one of the major causes of severe diarrhea disease and death in developing countries (35). EPEC is an extracellular pathogen that colonizes the small intestinal mucosa, damages intestinal epithelial cell function, and produces a characteristic histopathological feature known as attaching and effacing (A/E) lesions (26). A/E lesions are characterized by the localized destruction of brush border microvilli, the intimate attachment of the bacterium to the host cell membrane, and the formation of an underlying pedestal-like structure (21).
EPEC directly injects virulence factors into the target cell through its type III secretion system (TTSS) (9). In this way, the bacterial proteins are translocated to the cytoplasm, where they interact with host components and alter signaling pathways, resulting in disease (28). The EPEC TTSS is part of a chromosomal pathogenicity island, designated the locus for enterocyte effacement (LEE), which contains all of the genes required to produce A/E lesions (25).
LEE has been completely sequenced and contains diverse genes encoding secreted proteins for the TTSS, termed EPEC secreted proteins (Esp). These include EspA, EspB, EspD, and EspF as well as adhesins, such as intimin, and its own translocated receptor, Tir (39). EspA makes filamentous appendages surrounding the bacteria, and these filaments are present in a transient manner (21). These filamentous appendages form a translocation tube that acts as a channel to deliver proteins from the bacteria into the host (21). Intimate attachment of the bacteria to host enterocytes is mediated through the bacterial outer membrane protein intimin, which binds to bacterium-derived receptor Tir embedded in the host cell surface (17).
Even though inflammation is one of the pathogenic features induced by EPEC infection (13, 26, 37) and a well-known interaction between EPEC protein effectors and epithelial cells occurs during the initial processes of bacterium-host cell interactions (reviewed by Clarke et al. [8]), the innate or specific immune responses to EPEC are incipiently known in comparison with the pathogenic factors involved in the infection. The relationship between pathogenic mechanisms and the mucosal immune response is a delicate balance which determines the establishment of the disease.
Initial work related to histological specimens of intestines from EPEC-infected animals have shown extensive infiltration by inflammatory cells, mainly polymorphonuclear leukocytes, in the lamina propria. Polymorphonuclear leukocytes have also been seen crossing intact epithelial crypts and accumulating in the intestinal lumen (26, 37). However, the expression of proinflammatory cytokines in a natural model of infection by EPEC, such as rabbit EPEC (REPEC), which also contains the LEE pathogenicity island, has not been studied. Further, the scarce data are mainly related to interleukin 8 (IL-8) in cultured isolated cells or cell lines, such as epithelial cells, peripheral blood mononuclear cells, intestinal fibroblasts, or mucosal mononuclear cells (7, 18, 19). However, the interaction between the bacteria and the host cells invariably results in the release of more cytokines. The resulting cytokine network, of course, constitutes an important part of the immune response and represents the host's attempt to deal with a particular microorganism.
In this study, we analyzed the immune response to EPEC by detecting cytokine expression in enterocytes by using a model of experimental infection of rabbits by REPEC (strain E22) as well as the expression of proinflammatory cytokines by lymphocytes from Peyer's patches (PP) and the spleen (mucosal and systemic compartments). We also analyzed the proinflammatory cytokine profiles in rabbits infected with two nondiarrheagenic mutants involved in intimate adherence, the intimate adhesin mutant E22Δeae and the filamentous appendage mutant E22ΔespA.
MATERIALS AND METHODS
Rabbit infection experiment.
Two-month-old New Zealand White rabbits weighing between 1.8 and 2.2 kg were anesthetized and inoculated via an orogastric tube with 106 CFU of strain E22, E22Δeae, or E22ΔespA or an avirulent laboratory strain, E. coli HB101. Five rabbits were challenged with the wild-type strain or the mutants, while two control groups of five rabbits received either HB101 or only phosphate-buffered saline (PBS). All of the animals from the different groups were given water and fed with commercial feed. Each animal was checked for clinical symptoms, diarrhea, and mortality daily for 6 days. The animals were weighed twice, before the infection and before euthanasia at day 6 postinfection, when the diarrhea production was confirmed. For specific identification of the tested strains, fecal suspensions were spread on MacConkey plates with or without kanamycin.
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. Bacteria were grown in 10 ml of Luria-Bertani broth (with or without kanamycin at 50 μg/ml) overnight at 37°C with shaking at 200 rpm.
TABLE 1.
Strains used in this study
Tissue preparation for light microscopy.
A 5-cm piece of the distal small intestine was washed in PBS (pH 7.3), fixed in 4% neutral buffered formalin, and processed for paraffin embedding. Serial 5-μm-thick sections were cut, placed on glass slides, and stained for comparative studies with hematoxylin and eosin (H&E) and Alcian Blue. All intestinal samples were examined by light microscopy. Digital gel photographs of the stained tissues were analyzed for counting of infiltrate cells by using Image-Pro Plus software. The filter ranges selected for cell counting were area, density, diameter, and size of the cells.
Immunofluorescence staining.
For immunofluorescence labeling, 5-μm-thick tissue sections were stained simultaneously by using the fluorescence-actin staining assay with rhodamine-phalloidin to stain actin filaments (20) and the 4′,6′-diamidino-2-phenylindole (DAPI) technique to stain the DNA in the eukaryotic nuclei and the bacterial DNA (33). The tissue sections from the intestinal segments were deparaffinized, hydrated, permeabilized by adding 0.5% Triton X-100-PBS, and stained with 0.05 μg of tetramethyl rhodamine isothiocyanate-phalloidin (Sigma Chemical Co., St. Louis, Mo.)/ml and with 1 μg of DAPI (Sigma)/ml. Slides were mounted on Gelvatol (Sigma), covered with a glass coverslip, and examined by fluorescence microscopy (Olympus BX40S4).
Lymphocyte isolation.
Spleen samples and all of the small intestine PP samples were minced with scalpel blades, and clumps were pushed through wire mesh and washed in RPMI 1640 (GIBCO BRL, Grand Island, N.Y.) with 5% fetal calf serum. Only the PP cell suspension was passed through organza to remove the mucus. The whole-cell suspension was set over a Histopaque 1077 layer (Sigma) and centrifuged at 515 × g for 30 min at 25°C. Mononuclear cells were aspirated, washed in RPMI 1640, and centrifuged at 515 × g for 10 min at 4°C. The cell preparation was suspended in RPMI 1640. The isolated population was subjected to side and forward scatter analysis in a FACSort (Becton Dickinson) flow cytometer and compared with a lymphocyte cell line (Jurkat). The samples were 95 to 97% pure. Viability, as determined by trypan blue exclusion, was 95%.
Enterocyte isolation.
In order to examine cytokine production by the epithelium alone, we modified the method reported by Lundqvist et al. (23). Ten centimeters of the small intestine (ileum) was cut into small fragments and inverted. To remove the enterocytes from the tissue fragments, the fragments were incubated in RPMI 1640 with 1 mM dithiothreitol (Sigma) and 1.5 mM EDTA (Sigma) with shaking at 200 rpm for 30 min at 37°C. The cell suspension obtained was passed through organza to remove the mucus and centrifuged at 1,811 × g for 10 min at 4°C. The pellet was suspended in 15 ml of RPMI 1640, passed through organza, and washed two times in 15 ml of RPMI 1640 by centrifugation at 1,811 × g for 10 min at 4°C. The washed pellet was suspended in 10% Percoll and centrifuged over a discontinuous Percoll gradient at 515 × g for 30 min at 25°C. Enterocytes were recovered at the interphase between 20 and 44%. Enterocytes were washed in PBS and centrifuged as indicated above. The cell preparation was suspended in RPMI 1640. The purity of the samples was analyzed by light microscopy on the basis of the normal morphology for enterocytes; our samples contained up to 85% enterocytes. Viability, as determined by trypan blue exclusion, was 90%.
RNA isolation.
Total cellular RNA was obtained from enterocytes and spleen and PP lymphocytes (7.5 × 107) with TRIZol (GIBCO BRL) and incubation at room temperature for 5 min. RNA was extracted with chloroform (Sigma) and then centrifuged at 10,000 × g for 15 min at 4°C. The aqueous phase was precipitated with an equal volume of isopropanol and then centrifuged at 10,000 × g for 10 min at 4°C. The pellet was washed in 70% ethanol and suspended in 40 μl of diethyl pyrocarbonate-treated water. The total RNA concentration was determined by spectrometric analysis.
RT-PCR.
Specific cytokine mRNAs were examined by one-step reverse transcription (RT)-PCR following the manufacturer's instructions (GIBCO BRL). A total of 1 μg (for IL-10 and IL-6) or 0.5 μg (for IL-1β and TNF-α) of total RNA was reverse transcribed with Superscript II reverse transcriptase at 50°C for 30 min; this step was followed by amplification with the specific primers listed in Table 2. Thirty-five amplification cycles consisting of 30 s of denaturation at 94°C, 30 s of annealing (at the temperatures listed in Table 2), and 1 min of extension at 72°C were used. After amplification, RT-PCR products were analyzed on 2% agarose gels, and bands were visualized by ethidium bromide staining.
TABLE 2.
Primers used in this study
| Gene | GenBank accession no. | Product (bp) | Annealing temp (°C) | Oligonucleotide sequencesb |
|---|---|---|---|---|
| IL-1βa | M26295 | 354 | 60 | TACAACAAGAGCTTCCGGCA |
| GGCCACAGGTATCTTGTCGT | ||||
| TNF-αa | M12845 | 252 | 60 | AGCCCACGTAGTAGCAAACCC |
| TTGATGGCAGAGAGGAGGTTGA | ||||
| IL-6 | AF169176 | 399 | 60 | TCCGGATGTATCTCGAGCAC |
| CTAACGCTCATCTTCCTAGTTTCG | ||||
| IL-10 | AF068058 | 577 | 55 | TACTAGTCTCTGCTATGTTGCCTGGTCTT |
| ATGATCAACTGATGTCCTAGACTCTAGCCGA | ||||
| IL-8 | M58021 | 989 | 55 | GGCCGTAATGGAAGTAAACGT |
| GGAACTCCTTGCTAATAAGGCC | ||||
| GAPDHa | L23961 | 293 | 60 | TCACCATCTTCCAGGAGCGA |
| CACAATGCCGAAGTGGTCGT |
Data are from Reno et al. (32).
Sense and antisense primers.
Densitometry analysis.
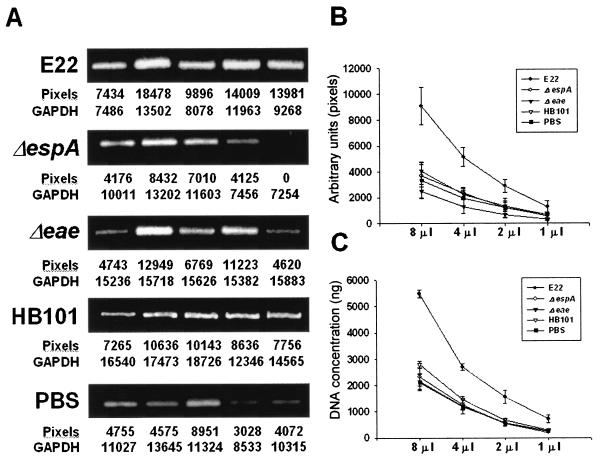
Digital gel photographs of the stained products were taken under UV exposure by using Kodak Digital Science 1D. Cytokine cDNA bands (8 μl of the RT-PCR products) were determined as the integrated area (pixels) of the band intensities by densitometry analysis with SIGMA-GEL software. The numerical values for cytokine cDNA band intensities were corrected with the values for the housekeeping glyceraldehyde 3-phosphate dehydrogenase (GAPDH) bands. Figure 1A shows an example for IL-1β from PP lymphocytes. To validate this quantitative method, titration of the RT-PCR products was performed by densitometric analysis (Fig. 1B) and DNA (Fig. 1C) quantitation; i.e., the initial 8 μl of the RT-PCR product was sequentially diluted 1:1, densitometric analysis was performed as previously described (Fig. 1B), and DNA was also measured at 260 nm (Fig. 1C). The quantitative data obtained showed linearity. For comparison between treatments, the data are presented as cytokine/GAPDH ratios.
FIG. 1.
Validation of the quantitation of RT-PCR products. (A) Densitometry analysis. IL-1β RT-PCR products of PP lymphocytes from rabbits in all treatment groups were analyzed on 2% agarose gels, and bands were visualized by ethidium bromide staining. Cytokine cDNA bands (8 μl of the RT-PCR products) were determined as the integrated area (pixels) of the band intensities by densitometry. Numerical values for cytokine cDNA band intensities are shown below the gels, as are the values for the housekeeping GAPDH bands used to normalize those cytokine band values. (B) Titration of the RT-PCR products by densitometric analysis. Sequential dilutions of the RT-PCR products were quantified as described for panel A. (C) Titration of the RT-PCR products by DNA quantitation. Sequential dilutions of the RT-PCR products were measured at 260 nm. Error bars indicate SDs. See Fig. 4B for additional details.
Statistical analysis.
All data are presented as the mean and standard deviation (SD) from five rabbits per experimental condition. Comparisons of data were made by using the Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
RESULTS
REPEC infection in rabbits.
Before considering evaluation of the in vivo immune response induced by REPEC in infected rabbits, the optimal conditions for rabbit infection were assessed by testing different doses of bacteria. At day 2 after infection, rabbits infected with 1010 CFU of REPEC O103 (E22) developed mucoid bloody watery diarrhea and weight loss, and they died at day 3. Rabbits infected with either 108 or 107 CFU of E22 developed the same clinical symptoms, but at day 3, and they died at day 4 after infection. Finally, at 106 CFU of E22, rabbits developed diarrhea at day 5 after infection and died at day 7. Thus, in the following experiments, rabbits in all groups were infected with 106 CFU of the tested strains and euthanatized at day 6, since a goal of this study was to evaluate proinflammatory cytokine expression while diarrhea was present in REPEC-infected rabbits.
In the first group, five rabbits were infected with wild-type strain E22 and euthanatized at day 6. All of the rabbits showed mucoid bloody watery diarrhea and weight loss. To evaluate the role of intimate adherence, two groups were inoculated with the EspA mutant (E22ΔespA) and the intimin mutant (E22Δeae). In contrast to animals inoculated with the wild-type strain, these animals did not develop any clinical signs. Taking advantage of the fact that the E22ΔespA and E22Δeae mutants are kanamycin resistant, we measured the fecal shedding of these mutants at day 6 and found an average of 103 to 104 CFU per g of feces for both mutants. Another group of rabbits was inoculated with HB101, a nonpathogenic E. coli strain, and the negative control group was inoculated with PBS only. None of the rabbits in these two groups developed clinical symptoms.
Control rabbits showed an average weight gain of 160 g over the initial body weight during the 6-day study period. Rabbits inoculated with the E22ΔespA and E22Δeae mutants showed average weight gains of 210 and 160 g, respectively. In contrast, rabbits infected with strain E22 showed a mean weight loss of 228 g at the day of euthanasia.
Mucus secretion and inflammation in response to REPEC infection.
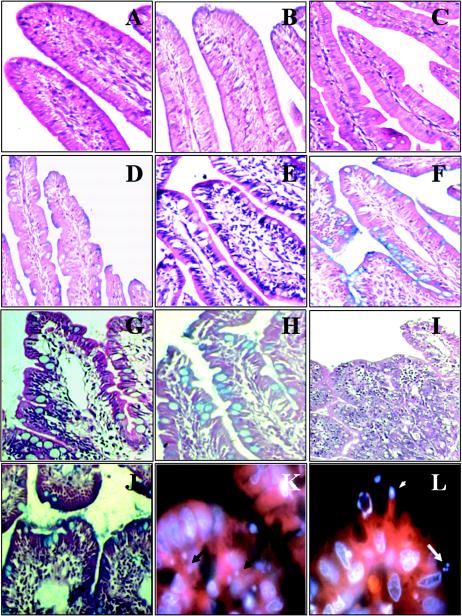
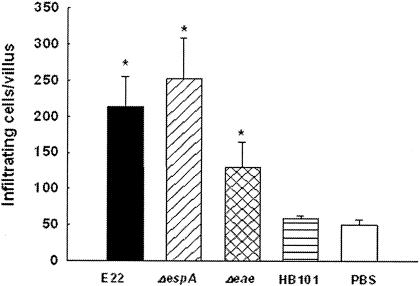
Rabbits were euthanatized at day 6 postinfection, and their small intestines were examined for signs of bacterial colonization and by histopathological analysis. Ileal tissue samples from rabbits inoculated with strain E22 displayed classical small intestinal atrophy; the villi were blunted, swollen, and fused (Fig. 2I). Staining of these samples with H&E showed the occurrence of extensive inflammation, i.e., mucosal infiltrates (Fig. 3), compared to the results for PBS- and E. coli HB101-treated rabbits (P < 0.01); these samples were characterized by a general increase in the numbers of round cells (Fig. 2I). The presence of bacterial microcolonies (Fig. 2K and L) as well as pedestal formation by REPEC (Fig. 2L) was evident. Alcian Blue staining was used to illustrate the physiological distribution of goblet cells and the occurrence of a continuous mucus layer covering the surface of the absorptive villi. High levels of mucus secretion occurred in REPEC-infected rabbits, and the presence of goblet cells was increased compared to that in noninfected rabbits (Fig. 2J versus Fig. 2B). The nondiarrheagenic intimin mutant induced intermediate lesions at the microscopic level (Fig. 2G). In animals infected with this mutant, the small intestinal villi were moderately atrophic and focally scalloped (Fig. 2G), and more mucus secretion and more goblet cells were seen in ileal tissue samples from these rabbits (Fig. 2H) than in those from E22-infected rabbits (Fig. 2J). Similarly, EspA mutant-infected rabbits had higher levels of mucus secretion but moderate numbers of goblet cells (Fig. 2F). The levels of cellular infiltrates were higher in these animals than in the control group animals (P < 0.01) but not significantly so in REPEC-infected rabbits (P = 0.1745) (Fig. 3); in addition, the intestinal villi showed pronounced tissue damage (Fig. 2E). Avirulent strain HB101 induced only small numbers of goblet cells (Fig. 2D). In contrast, uninfected tissue samples did not show any histopathological modifications (Fig. 2A and B).
FIG. 2.
Micrographs of rabbit distal ileum tissue sections at 6 days postinfection. Intestinal sections from a PBS-treated rabbit (A and B), from an HB101-infected rabbit (C and D), from an E22ΔespA-infected rabbit (E and F), from an E22Δeae-infected rabbit (G and H), and from an E22-infected rabbit (I, J, K, and L) are shown. Sections were stained with H&E (A, C, E, G, and I), Alcian Blue (B, D, F, H, and J), or rhodamine-phalloidin and DAPI (K and L). Magnifications: A to F, ×20; G, H, and J, ×40; I, ×10; and K and L, ×100. Arrows indicate microcolonies, and the arrowhead indicates a pedestal.
FIG. 3.
Cellular infiltrates in rabbit ileum tissue sections. At 6 days postinfection, cellular infiltrates in six intestinal villi from five rabbits infected with wild-type REPEC O103 (E22), an EspA or intimin mutant (E22ΔespA or E22Δeae), or E. coli HB101 or treated with PBS were counted as described in Materials and Methods. Error bars indicate SDs. An asterisk indicates a P value of <0.01 for a comparison with the control group (PBS).
Determination of proinflammatory cytokines.
The cytokine profiles of small intestinal enterocytes, spleen lymphocytes, and PP lymphocytes in rabbits infected with E22, E22ΔespA, E22Δeae, or HB101 were determined by RT-PCR. The enterocytes and mononuclear cells were obtained from various tissues of rabbits infected for 6 days, and their total cellular RNAs were extracted.
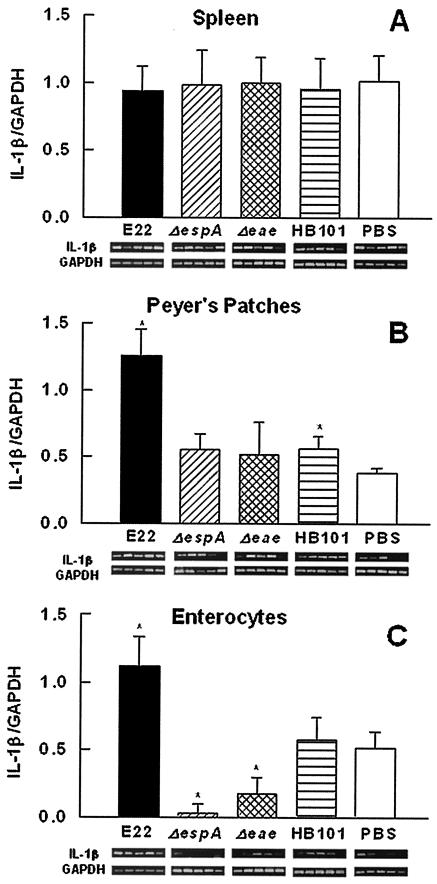
(i) IL-1β mRNA expression.
As shown in Fig. 4A, IL-1β mRNA from spleen lymphocytes was detected in all groups of treated rabbits, and there was not a significant difference in the expression of this cytokine among the groups. In PP lymphocytes, wild-type strain E22 induced a significant increase (P < 0.01) in IL-1β mRNA expression compared to the expression seen in rabbits infected with nonpathogenic E. coli HB101 (Fig. 4B). IL-1β/GAPDH ratios in PP lymphocytes for EspA mutant-, intimin mutant-, and PBS-treated rabbits were not significantly different (Fig. 4B). In contrast, in enterocytes from the small intestine, IL-1β mRNA expression was increased only with strain E22 (Fig. 4C); moreover, the EspA and intimin mutants induced a significant decrease in the level of this messenger compared to the levels seen in HB101-treated (P < 0.03) and PBS-treated (P < 0.02) control rabbits (Fig. 4C).
FIG. 4.
REPEC infection induces IL-1β mRNA expression in the intestinal mucosa but not in the spleen. Groups of rabbits were infected with wild-type REPEC O103 (E22), an EspA or intimin mutant (E22ΔespA or E22Δeae), or E. coli HB101 and euthanatized at 6 days postinfection. Total RNA was isolated from spleen lymphocytes (A), PP lymphocytes (B), and small intestinal enterocytes (C). IL-1β mRNA expression was assessed by RT-PCR. RT-PCR products were analyzed on 2% agarose gels, and bands were visualized by ethidium bromide staining. The changes in band intensities for each treatment (n = 5) were quantified by densitometric analysis as the integrated area (pixels) and are shown below the bars. Data are presented as the cytokine/GAPDH ratio; individual GAPDH bands (n = 5) are also shown below the bars. Comparisons between two values were made by using the Mann-Whitney U test. The results are expressed as the means and SDs from five rabbits per experimental condition. An asterisk indicates a P value of <0.02 for a comparison with the control group (PBS).
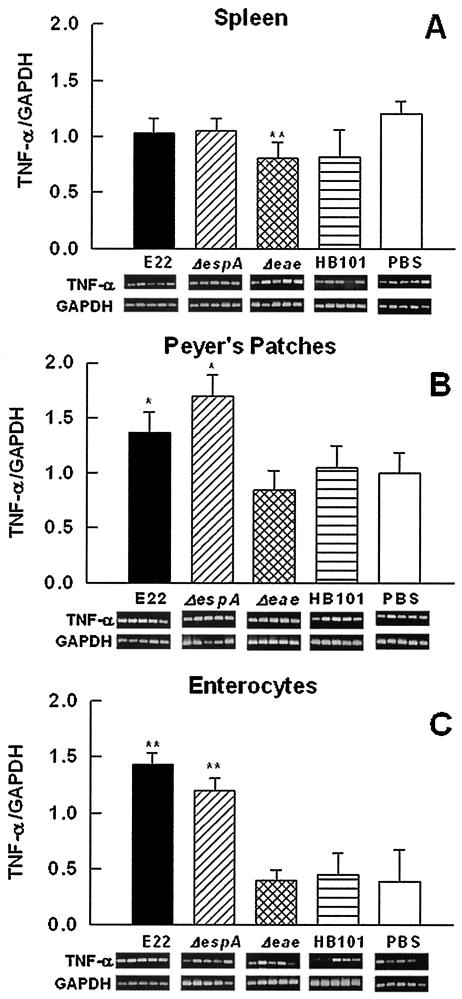
(ii) TNF-α mRNA expression.
In spleen lymphocytes, TNF-α mRNA expression in rabbits infected with the wild-type strain was not different from that in rabbits infected with the EspA mutant or in control rabbits (Fig. 5A). Whereas in rabbits infected with the intimin mutant there was a small but significant decrease (P < 0.02) in TNF-α mRNA expression (Fig. 5A) compared to that in PBS-treated rabbits, there was none (P = 0.75) compared to that in nonpathogenic E. coli HB101-infected rabbits. In PP lymphocytes, wild-type strain E22 or the EspA mutant induced an increase (P < 0.05) in TNF-α mRNA expression (Fig. 5B), but the level of this messenger in rabbits infected with the intimin mutant was similar to those in both rabbits infected with HB101 and rabbits treated with PBS (negative control) (Fig. 5B). There was also an increase (P < 0.03) in TNF-α mRNA expression in enterocytes from rabbits infected with strain E22 or the EspA mutant (Fig. 5C). Interestingly, as in PP lymphocytes, TNF-α mRNA expression in enterocytes from intimin mutant-infected rabbits was similar to that in control rabbits (Fig. 5C).
FIG. 5.
Infection with REPEC or the EspA mutant increases TNF-α mRNA expression in the intestinal mucosa but not in the spleen. Groups of rabbits were infected as described in the legend to Fig. 4. Total RNA was isolated from spleen lymphocytes (A), PP lymphocytes (B), and small intestinal enterocytes (C). TNF-α mRNA expression was assessed by RT-PCR. Data analysis was performed as described in the legend to Fig. 4. The results are expressed as the means and SDs from five rabbits per experimental condition. Gel bands are shown below the bars. Single and double asterisks indicate P values of <0.05 and <0.03, respectively, for a comparison with the control group (PBS).
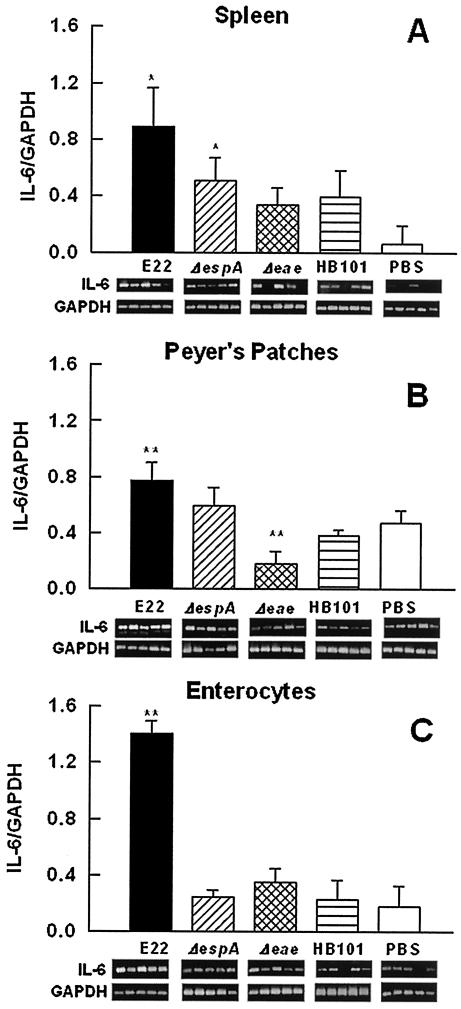
(iii) IL-6 mRNA expression.
For IL-6 mRNA expression, in contrast to that of IL-1β and TNF-α, it was possible to detect a difference at the systemic level among the different groups of rabbits. In spleen lymphocytes, wild-type strain E22 induced a significant (P < 0.05) induction of this cytokine compared to the results for rabbits infected with nonpathogenic E. coli HB101 (Fig. 6A). Rabbits infected with the EspA mutant also showed an increase in IL-6 mRNA expression, but it was similar to that produced by HB101-infected rabbits. The increase in IL-6 mRNA expression produced by the intimin mutant was not significantly different from that in HB101-infected rabbits or PBS-treated rabbits (negative control) (Fig. 6A). At the local level, in PP lymphocytes, only the wild-type strain increased IL-6 mRNA expression (Fig. 6B), while with the intimin mutant, it was significantly decreased (P < 0.01), compared to the results for HB101-infected and PBS-treated control rabbits (Fig. 6B). Furthermore, in enterocytes, only strain E22 induced a notable increase in the level of this messenger compared to the results for mutant-infected rabbits (P < 0.02) and control rabbits (P < 0.01), in which the IL-6 mRNA levels were similar (Fig. 6C).
FIG. 6.
IL-6 mRNA expression is increased in spleen lymphocytes and PP lymphocytes but reaches higher levels in enterocytes of REPEC-infected rabbits. Groups of rabbits were infected as described in the legend to Fig. 4. Total RNA was isolated from spleen lymphocytes (A), PP lymphocytes (B), and small intestinal enterocytes (C). IL-6 mRNA expression was assessed by RT-PCR. Data analysis was performed as described in the legend to Fig. 4. The results are expressed as the means and SDs from five rabbits per experimental condition. Gel bands are shown below the bars. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, for a comparison with the control group (PBS).
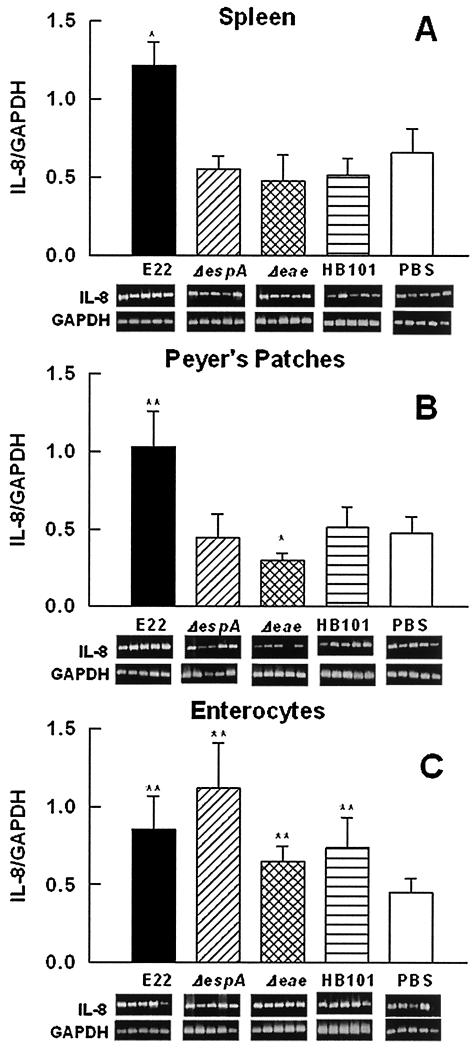
(iv) IL-8 mRNA expression.
IL-8 mRNA expression in spleen lymphocytes from rabbits infected with nonpathogenic E. coli HB101 or the REPEC mutants (E22ΔespA and E22Δeae) was similar to that in PBS-treated rabbits (negative control). However, wild-type strain E22 increased IL-8 mRNA expression more than twofold (Fig. 7A). IL-8 mRNA expression in PP lymphocytes was similar to that in spleen lymphocytes; an increase (P < 0.01) in IL-8 mRNA expression was induced only in rabbits infected with strain E22 (Fig. 7B). However, there was a decrease (P < 0.05) in the expression of this cytokine in PP lymphocytes from rabbits infected with the intimin mutant (Fig. 7B). At the local level, in enterocytes, the wild-type strain, the intimin mutant, and the EspA mutant induced increases (P < 0.01) in the expression of this cytokine over that seen in PBS-treated control rabbits, but these increases were not significantly different from that induced by HB101 (Fig. 7C).
FIG. 7.
REPEC increases IL-8 mRNA expression in PP lymphocytes and spleen lymphocytes but only slightly increases it in enterocytes. Groups of rabbits were infected as described in the legend to Fig. 4. Total RNA was isolated from spleen lymphocytes (A), PP lymphocytes (B), and small intestinal enterocytes (C). IL-8 mRNA expression was assessed by RT-PCR. Data analysis was performed as described in the legend to Fig. 4. The results are expressed as the means and SDs from five individual experiments. Gel bands are shown below the bars. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, for a comparison with the control group (PBS).
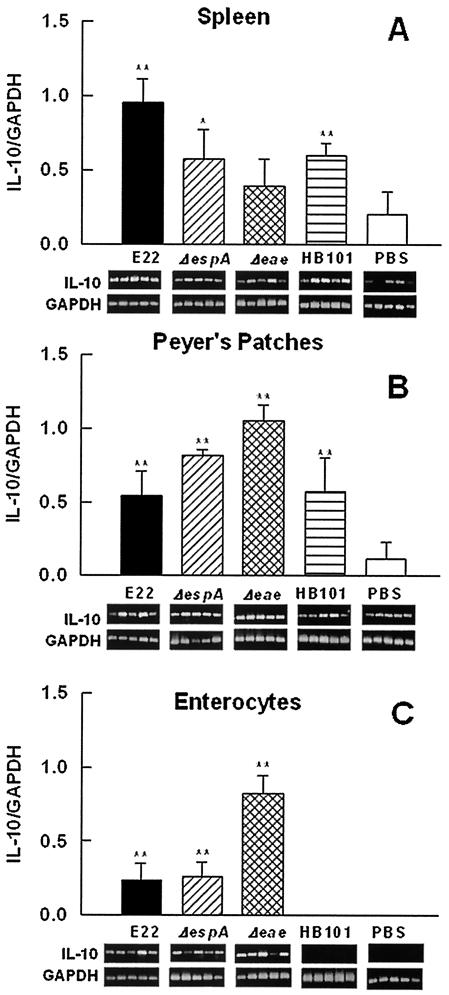
(v) IL-10 mRNA expression.
Wild-type strain E22 induced an increase (P < 0.02) in anti-inflammatory IL-10 mRNA expression in spleen lymphocytes compared to the results for nonpathogenic E. coli HB101-infected rabbits (Fig. 8A). The EspA mutant induced an important increase in IL-10 mRNA expression; however, this increase was similar to that induced by HB101. The levels of this cytokine in intimin mutant-infected rabbits were not significantly higher than those induced by PBS and HB101 (Fig. 8A). In contrast, in PP lymphocytes, strain E22 induced an increase in IL-10 mRNA expression similar to that produced by HB101 but higher (P < 0.02) than that seen in PBS-treated rabbits (negative control) (Fig. 8B). Interestingly, the EspA and intimin mutants induced a significant pronounced increase (P < 0.03) in the expression of this cytokine compared to the results obtained with strain E22 (Fig. 8B). In enterocytes from PBS- or HB101-treated rabbits, it was not possible to detect IL-10 mRNA. However, enterocytes from rabbits infected with strain E22 and the EspA mutant showed similar increases (P < 0.02) in the expression of this cytokine mRNA, but these increases were significantly (P < 0.03) lower (three times) than that induced by the intimin mutant (Fig. 8C).
FIG. 8.
IL-10 mRNA expression appears to be downregulated by intimin at the mucosal level. Groups of rabbits were infected as described in the legend to Fig. 4. Total RNA was isolated from spleen lymphocytes (A), PP lymphocytes (B), and small intestinal enterocytes (C). IL-10 mRNA expression was assessed by RT-PCR. Data analysis was performed as described in the legend to Fig. 4. The results are expressed as the means and SDs from five individual experiments. Gel bands are shown below the bars. Single and double asterisks indicate P values of <0.05 and <0.02, respectively, for a comparison with the control group (PBS).
DISCUSSION
In our model, wild-type REPEC strain E22 (106 CFU) was able to cause illness in 2-month-old rabbits (weighing about 2 kg) 5 days postinfection, producing a loss of body weight, diarrhea, A/E lesions, mucus secretion, infiltration of inflammatory cells, and death, as previously reported (13, 30, 31). Other authors have used infectious doses of REPEC O103 strain E22 (1, 13, 24) which were higher than those used in the present study. The use of our model for detecting cytokines produces results that are more reliable than those obtained with >109 CFU per cm of cultured cell lines; in addition, there is no cross talk among the different cell lineages present in the intestinal mucosa.
The intimate adhesin mutant (E22Δeae) and the filamentous appendage mutant (E22ΔespA) produced no diarrhea or A/E lesions but did produce mucus secretion which was similar to that seen with the wild type and more marked than that seen with E. coli HB101. The levels of inflammatory infiltrates caused by E22ΔespA were slightly higher than those caused by the wild type but not statistically significantly so, contrary to data from a previous study suggesting that the inflammatory response observed during REPEC O103 infection required, at least in part, the production of EspA and EspB (1).
We found differential cytokine expression by REPEC protein effectors related to adhesion to epithelial cells, such as the EspA filament and the outer membrane adhesin intimin. Cytokines are produced in response to microbes, and different cytokines stimulate diverse responses of cells involved in immunity and inflammation. Proinflammatory mediators produced by host cells in response to bacteria are known to determine the outcome of the inflammatory reaction occurring at the local site of infection in the host (for instance, the immune cell population residing in the lamina propria). However, in REPEC infection, only IL-8 has been studied extensively in cultured epithelial cells, even though it is not a unique factor during the inflammatory reaction.
In this study, we demonstrate that REPEC activates in rabbit enterocytes the expression of proinflammatory cytokines, such as IL-1β, TNF-α, IL-8, and IL-6, and the anti-inflammatory cytokine IL-10. Recently, another model of a pathogen causing A/E lesions was widely used to study the immune response: Citrobacter rodentium, a natural pathogen of mice. C. rodentium has many similarities to human EPEC or EHEC (11) but also has some important differences: (i) it is not E. coli, (ii) pathogenic characteristics (for instance, hyperplasia induced by C. rodentium versus diarrhea induced by EPEC and the presence of fimbria for adhesion), and (iii) the intestinal segment infected (small for EPEC versus large for C. rodentium). This latter feature may be of vital importance, since it has been reported that the mucosal immune responses in the small and large intestines may be different (2, 4, 5). Nevertheless, like C. rodentium infection, REPEC infection in rabbits appears to induce a mucosal Th1 cytokine response in purified enterocytes of the small intestine. In fact, the profiles for IL-1 and TNF-α induced by the wild type and the intimin mutant were similar to those reported for C. rodentium in colonic mucosal tissue (15). Interestingly, the expression of IL-6, a cytokine not previously tested in an infection with a pathogen causing A/E lesions, was remarkably increased in enterocytes from rabbits infected with REPEC only. Increased levels of IL-6 and the soluble IL-6 receptor have been demonstrated in both serum and intestinal tissues from patients with active Crohn's disease. In animal model studies, an anti-IL-6 receptor monoclonal antibody successfully prevented intestinal inflammation and systemic wasting disease by suppressing adhesion molecule expression by vascular endothelium. Moreover, the treatment displayed therapeutic efficacy against established colitis through the induction of lamina propria T-cell apoptosis (reviewed in reference 16).
Recently, it was found that early inflammation induced by subchronic treatment with IL-1β and IL-6 caused changes in mucosal ionic transport parameters, a reduction in the direct contractile response, and an alteration in neurotransmission (by an enhancing cholinergic component), which may affect the physiological pattern of colonic motility and the sensory reflex (27). Interestingly, in rabbits infected with wild-type REPEC and not in those infected with an intimin or an EspA mutant, there were increases in epithelial IL-1β and IL-6 levels, suggesting their participation in the diarrhea produced by REPEC and suggesting that their induction is related to A/E lesions (ΔespA and Δeae). TNF-α and IL-8 levels were increased in both wild-type- and EspA mutant-infected rabbits, suggesting that they probably do not participate in the diarrhea produced by REPEC because, as we showed above, an isogenic EspA mutant was unable to produce diarrhea but was able to produce an inflammatory infiltrate. Moreover, Goncalves et al. (12) explored the role of TNF-α in the immunopathology of infectious colitis caused by C. rodentium because the neutralization of TNF-α is of therapeutic benefit in patients with Crohn's disease. They found that TNF-α is not obligatory for protective immunity to C. rodentium in mice but appears to play some role in downregulating mucosal pathology and the Th1 immune response. On the other hand, the increase in the production of IL-8 was lower than that of the other proinflammatory cytokines. It is important to note the differences in IL-8 production by cultured epithelial cells because, in our model, the immune elements in the lamina propria interact with enterocytes, suggesting regulation of the cytokines produced by these immune elements and secreted locally. It is known that cytokines rarely, if ever, act in isolation but rather act to induce or inhibit other cytokines, creating a population or network of cytokines to which cells respond (40).
The intimin mutant was unable to induce the expression of TNF-α, IL-6, and IL-1β (the level of the latter was lower than that in PBS- and E. coli HB101-treated control rabbits but similar to that in rabbits infected with E22ΔespA). The findings that the level of IL-8 in E22Δeae-treated rabbits was higher (P < 0.01) than that in PBS-treated rabbits but similar (P > 0.5) to those in rabbits infected with E. coli HB101 and wild-type strain E22 (Fig. 7C) suggest that the increase was due mainly to lipopolysaccharide (LPS). Chakravortty and Kumar (7) found that purified LPS from EPEC increased the production of IL-8, other proinflammatory mediators, and adhesion molecules in human small intestinal lamina propria fibroblasts. All of these data suggest that intimin plays an important role in inducing proinflammatory cytokines, while EspA is not needed to induce TNF-α and IL-8.
Interestingly, the expression of IL-10, an anti-inflammatory cytokine, was slightly increased in rabbits infected with wild-type REPEC or the EspA mutant. Nevertheless, a remarkable increase was seen in rabbits infected with the intimin mutant, strongly suggesting that intimin is able to downregulate IL-10 expression and thereby to upregulate the expression of mucosal proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8. The important anti-inflammatory role of IL-10 in animal models of inflammatory bowel disease is well known and includes the earliest findings that IL-10−/− mice spontaneously develop chronic colitis (22) and that it is associated with aberrant cytokine production and CD4+ Th1-like responses (3). Recently, De Winter et al. (10) provided evidence for in vivo lymphoepithelial cross talk, in which IL-10 produced locally by epithelial cells can regulate immune responses in the intestine without systemic modifications.
The above information and recent data showing the important role of lymphocytes in immunity to C. rodentium (36, 38) led us to investigate cytokine expression by lymphocytes. Proinflammatory cytokine expression by lymphocytes may be an alternative pathway for reaching a protective immune response against pathogens causing A/E lesions, as suggested by Simmons et al. (36), since mucosal immunity is not clearly restored by B cells (or convalescent-phase sera) in μMT mice infected with C. rodentium. Simmons et al. (36) also suggested differential responses in the mucosal and systemic compartments. Therefore, in the present study, we used lymphocytes from the spleen and PP to test proinflammatory cytokines induced as a result of cytokine networks secreted by different cell lineages in the intestinal mucosa during REPEC infection. We found different cytokine profiles at the systemic level (in spleen lymphocytes) and at the mucosal level (in PP lymphocytes). This strategy allowed us to compare different responses to infection with the same organism, since local and systemic cytokine responses to gram-negative bacteria are differently regulated (14). The expression of IL-1β and TNF-α mRNAs at the systemic level was not different from the basal expression of these messengers, but TNF-α mRNA expression showed a small decrease that was dependent on intimin. Nevertheless, the formation of A/E lesions and other virulence factors are not inducers of these cytokines at this level. However, at the mucosal level, in PP lymphocytes, there was an increase in the induction of IL-1β dependent on the TTSS (no induction by ΔespA and Δeae bacteria). This latter profile for IL-1β was similar to that induced in enterocytes. In addition, at the mucosal level, the increase in TNF-α mRNA expression was dependent on intimin protein but not on EspA protein.
The increase in the expression of the multifunctional cytokine IL-6 was dependent on intimin expression at the systemic level and in PP but was dependent on the formation of A/E lesions in enterocytes. In PP lymphocytes, intimin is important in the induction of IL-6 mRNA, because its expression was significantly diminished in intimin mutant-infected animals compared to control animals. In PP, this cytokine might stimulate the growth of B lymphocytes that have been differentiated into antibody producers (i.e., anti-intimin secretory immunoglobulin A antibodies). Interestingly, the levels of IL-6 expression were higher in enterocytes than in spleen and PP lymphocytes (Fig. 6), suggesting that IL-6 locally expressed by epithelial cells may participate in intestinal inflammation rather than switching of B cells into secretory immunoglobulin A-producing plasma cells. Savkovic et al. (34) demonstrated the induction of transepithelial migration of polymorphonuclear leukocytes in intestinal cells (T84) infected with EPEC, due in part to the chemotactic activity of IL-8. In our rabbit model, at the systemic level and in PP, the increase in IL-8 mRNA expression was dependent on the formation of A/E lesions. However, in enterocytes, there was an increase in the induction of this cytokine independent of virulence factors of REPEC, because even nonpathogenic E. coli induced this cytokine. This latter finding could be related to results recently reported by Zhou et al. (41), who demonstrated that FliC of EPEC or of avirulent E. coli HB101 is sufficient to induce the release of IL-8 in T84 cells.
The suppression of proinflammatory cytokine expression may delay injury and sepsis, but reduced cytokine formation may favor microbial invasion. The anti-inflammatory cytokine IL-10 plays an important role in infection, since IL-10 inhibits cytokine production and T-cell activation and terminates cell-mediated immune reactions. At the systemic level, there was an increase in the transcription of IL-10 mRNA which depended on contact with bacteria (TTSS). At the PP level, IL-10 mRNA induction by REPEC was similar to that by nonpathogenic E. coli, but it is clear that intimin and EspA acted as repressors in the expression of IL-10 mRNA.
Even though this study was carried out to determine which proinflammatory cytokines are expressed while diarrhea is present in rabbits infected with REPEC (diarrhea was guaranteed at 6 days after infection with REPEC, and mutants that are unable to produce diarrhea were also tested), experiments should be performed to explore a time course which allows full appreciation of the molecular basis for the cytokine response. Additionally, the development of immunological tools for detecting rabbit cytokine proteins should allow us to confirm our expression data by measuring protein expression in situ.
Nevertheless, the profiles of proinflammatory cytokines expressed in rabbits infected with REPEC are similar to those characterized by Chakravortty and Kumar (7) for cultured human small intestinal lamina propria fibroblasts in response to LPS extracted from EPEC. These cells produced high levels of proinflammatory mediators, such as IL-1α, IL-1β, IL-6, IL-8, and TNF-α. These results, taken together, indicate that after EPEC colonization there is an immunological environment for a Th1 immune response, even when the bacterium is not intracellular. According to our findings and those reported for the C. rodentium model (36, 38), immunity appears to be dependent upon a population of CD4+ T cells that produce proinflammatory cytokines that activate effector mechanisms at the epithelium. This cytokine-mediated inflammatory response, which appears to be upregulated by the diminished expression of IL-10, which is downregulated by intimin, can also contribute to diarrhea caused by EPEC through IL-6 and IL-1β secretion.
Acknowledgments
This work was supported by a grant from Consejo Nacional de Ciencia y Tecnología de México (CONACYT; 44660) to F.N.-G. K.R. received a scholarship from CONACYT (129409). E.O. was supported by a grant from the European Union Fifth Framework Quality of Life Program (QLK2-2000-00600), and C.G.T. was supported by a grant from PAPIIT (IN202301).
We thank Manuel Flores and Germán Garrido-Fariña (FES-Cuatitlán) for technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannai, M., T. Kawamura, T. Naito, H. Kameyama, T. Abe, H. Kawamura, C. Tsukada, H. Watanabe, K. Hatakeyama, H. Hamada, Y. Nishiyama, H. Ishikawa, K. Takeda, K. Okumura, M. Taniguchi, and T. Abo. 2001. Abundance of unconventional CD8(+) natural killer T cells in the large intestine. Eur. J. Immunol. 31:3361-3369. [DOI] [PubMed] [Google Scholar]
- 3.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boll, G., and J. Reimann. 1995. Lamina propria T cell subsets in the small and large intestine of euthymic and athymic mice. Scand. J. Immunol. 42:191-201. [DOI] [PubMed] [Google Scholar]
- 5.Camerini, V., C. Panwala, and M. Kronenberg. 1993. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J. Immunol. 151:1765-1776. [PubMed] [Google Scholar]
- 6.Camguilhem, R., and A. Milon. 1989. Biotypes and O serogroups of Escherichia coli involved in intestinal infections of weaned rabbits: clues to diagnosis of pathogenic strains. J. Clin. Microbiol. 27:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravortty, D., and K. S. Kumar. 1999. Interaction of lipopolysaccharide with human small intestinal lamina propria fibroblasts favors neutrophil migration and peripheral blood mononuclear cell adhesion by the production of proinflammatory mediators and adhesion molecules. Biochim. Biophys. Acta 1453:261-272. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, S. C., R. D. Haigh, P. P. Freestone, and P. H. Williams. 2003. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin. Microbiol. Rev. 16:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVinney, R., A. Gauthier, A. Abe, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells. Cell. Mol. Life Sci. 55:961-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Winter, H., D. Elewaut, O. Turovskaya, M. Huflejt, C. Shimeld, A. Hagenbaugh, S. Binder, I. Takahashi, M. Kronenberg, and H. Cheroutre. 2002. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology 122:1829-1841. [DOI] [PubMed] [Google Scholar]
- 11.Frankel, G., D. C. Candy, P. Everest, and G. Dougan. 1994. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect. Immun. 62:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalves, N. S., M. Ghaem-Maghami, G. Monteleone, G. Frankel, G. Dougan, D. J. Lewis, C. P. Simmons, and T. T. MacDonald. 2001. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect. Immun. 69:6651-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heczko, U., A. Abe, and B. B. Finlay. 2000. In vivo interactions of rabbit enteropathogenic Escherichia coli O103 with its host: an electron microscopic and histopathologic study. Microbes Infect. 2:5-16. [DOI] [PubMed] [Google Scholar]
- 14.Hedges, S., W. Agace, M. Svensson, A. C. Sjogren, M. Ceska, and C. Svanborg. 1994. Uroepithelial cells are part of a mucosal cytokine network. Infect. Immun. 62:2315-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 16.Ito, H. 2003. IL-6 and Crohn's disease. Curr. Drug Targets Inflamm. Allergy 2:125-130. [DOI] [PubMed] [Google Scholar]
- 17.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 18.Klapproth, J. M., M. S. Donnenberg, J. M. Abraham, and S. P. James. 1996. Products of enteropathogenic E. coli inhibit lymphokine production by gastrointestinal lymphocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 271:G841-G848. [DOI] [PubMed] [Google Scholar]
- 19.Klapproth, J. M., M. S. Donnenberg, J. M. Abraham, H. L. Mobley, and S. P. James. 1995. Products of enteropathogenic Escherichia coli inhibit lymphocyte activation and lymphokine production. Infect. Immun. 63:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 23.Lundqvist, C., M. L. Hammarstrom, L. Athlin, and S. Hammarstrom. 1992. Isolation of functionally active intraepithelial lymphocytes and enterocytes from human small and large intestine. J. Immunol. Methods 152:253-263. [DOI] [PubMed] [Google Scholar]
- 24.Marches, O., J. P. Nougayrede, S. Boullier, J. Mainil, G. Charlier, I. Raymond, P. Pohl, M. Boury, J. De Rycke, A. Milon, and E. Oswald. 2000. Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natale, L., A. L. Piepoli, M. A. De Salvia, G. De Salvatore, C. I. Mitolo, A. Marzullo, P. Portincasa, A. Moschetta, G. Palasciano, and D. Mitolo-Chieppa. 2003. Interleukins 1 beta and 6 induce functional alteration of rat colonic motility: an in vitro study. Eur. J. Clin. Investig. 33:704-712. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nougayrede, J. P., O. Marches, M. Boury, J. Mainil, G. Charlier, P. Pohl, J. De Rycke, A. Milon, and E. Oswald. 1999. The long-term cytoskeletal rearrangement induced by rabbit enteropathogenic Escherichia coli is Esp dependent but intimin independent. Mol. Microbiol. 31:19-30. [DOI] [PubMed] [Google Scholar]
- 30.Peeters, J. E., R. Geeroms, and F. Orskov. 1988. Biotype, serotype, and pathogenicity of attaching and effacing enteropathogenic Escherichia coli strains isolated from diarrheic commercial rabbits. Infect. Immun. 56:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillien, F., C. Chalareng, M. Boury, C. Tasca, J. de Rycke, and A. Milon. 1996. Role of adhesive factor/rabbit 2 in experimental enteropathogenic Escherichia coli O103 diarrhea of weaned rabbit. Vet. Microbiol. 50:105-115. [DOI] [PubMed] [Google Scholar]
- 32.Reno, C., R. Boykiw, M. L. Martinez, and D. A. Hart. 1998. Temporal alterations in mRNA levels for proteinases and inhibitors and their potential regulators in the healing medial collateral ligament. Biochem. Biophys. Res. Commun. 252:757-763. [DOI] [PubMed] [Google Scholar]
- 33.Salari, S. H., and M. E. Ward. 1979. Early detection of Chlamydia trachomatis using fluorescent, DNA binding dyes. J. Clin. Pathol. 32:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senerwa, D., O. Olsvik, L. N. Mutanda, K. J. Lindqvist, J. M. Gathuma, K. Fossum, and K. Wachsmuth. 1989. Enteropathogenic Escherichia coli serotype O111:HNT isolated from preterm neonates in Nairobi, Kenya. J. Clin. Microbiol. 27:1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons, C. P., S. Clare, M. Ghaem-Maghami, T. K. Uren, J. Rankin, A. Huett, R. Goldin, D. J. Lewis, T. T. MacDonald, R. A. Strugnell, G. Frankel, and G. Dougan. 2003. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzipori, S., R. Gibson, and J. Montanaro. 1989. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect. Immun. 57:1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallance, B. A., W. Deng, L. A. Knodler, and B. B. Finlay. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson, M., R. Seymour, and B. Henderson. 1998. Bacterial perturbation of cytokine networks. Infect. Immun. 66:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, X., J. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]