Abstract
Airway epithelial cells are among the first to encounter inhaled allergens and can initiate allergic responses by producing pro-Th2 innate cytokines. In this study, we investigated the role of epithelial-derived cytokines in sensitization to a clinically relevant allergen, cockroach allergen (CRA). Among the epithelial-derived cytokines, GM-CSF played a central role in the initiation of Th2 allergic responses to CRA. We show that initial exposure to CRA directly activated airway epithelial cells through a TLR4-MyD88-dependent pathway and MyD88 signaling in epithelial cells induced upregulation of GM-CSF during sensitization. Epithelial-derived GM-CSF was required for allergic sensitization and selectively restored Th2 responses in the absence of MyD88. Thus, we demonstrate that epithelial-derived GM-CSF is a critical early signal during allergic sensitization to CRA.
Introduction
Allergic asthma is a chronic lung disease driven by an immune response against allergens, which results in reversible airflow obstruction, mucus hypersecretion, and the recruitment of eosinophils and Th2 cells to the airway1. Airway epithelial cells are among the first to detect the presence of inhaled allergens through pattern recognition receptors (PRRs)2, and in response to allergens, produce cytokines that can initiate type 2 responses3,4. Although transgenic overexpression of epithelial-derived cytokines such as thymic stromal lymphopoietin (TSLP), IL-25, IL-33, and GM-CSF are each sufficient to induce a Th2 allergic response against an antigen5–8, mounting evidence suggests that the requirement for these cytokines in allergic responses to various allergens is distinct.
TSLP is induced in a large number of allergic responses9–14 and is required in ragweed-driven allergic inflammation. Yet TSLP has been shown to be dispensable for allergic sensitization to house dust mite (HDM), in which IL-33 appears to play a more central role10,15. GM-CSF, which can be produced by a number of different cell types including epithelial cells, has also emerged as an important regulator of allergic inflammation15. Patients with asthma have increased levels of GM-CSF expression; in mice, transgenic overexpression of GM-CSF serves as a potent adjuvant that permits sensitization to ovalbumin8 and sub-threshold levels of HDM16. Antibody blockade of GM-CSF during sensitization reduces allergic inflammation to HDM and Blomia tropicalis mite allergen15,17,18, suggesting an important role for GM-CSF early in the allergic response.
In this study, we focus on cockroach allergen (CRA), an important allergen associated with allergic asthma and asthma morbidity, especially in low-income urban populations19–21. CRA can activate protease-activated receptor 2 (PAR2)22–24 and toll-like receptors (TLRs)25 to induce the release of inflammatory cytokines. However, TSLP is not required for allergic responses to CRA26, while IL-25 is only required for chronic allergic responses to CRA27. Our studies demonstrate that MyD88-dependent induction of GM-CSF in epithelial cells was required for sensitization to inhaled CRA. Epithelial-derived GM-CSF specifically promoted Th2-mediated allergic responses, but not Th17-associated neutrophilia, downstream of the MyD88 signaling pathway. Interestingly, while induction of GM-CSF has been shown to occur downstream of IL-1 in response to HDM, CRA-induced GM-CSF was IL-1 independent. In contrast to the requirements for IL-33 in HDM-induced asthma, IL-33 only played a minimal role in response to CRA. Overall, our study demonstrates that epithelial-derived GM-CSF is a key mediator of sensitization to CRA, but that induction of GM-CSF by CRA is mediated by pathways distinct from HDM.
Results
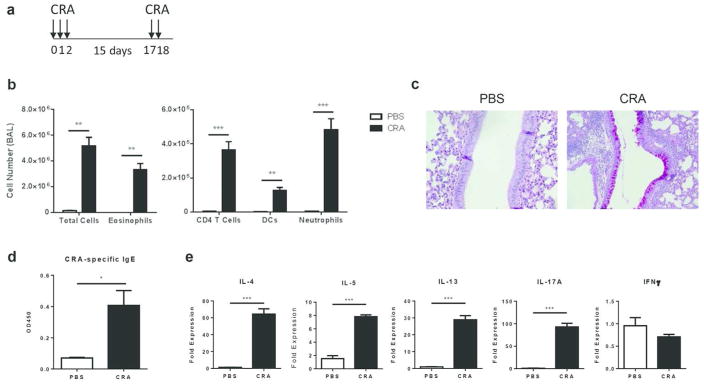
Intranasal sensitization and challenge with CRA results in airway inflammation with features of allergic asthma
We first established a model of CRA-mediated allergic airway disease in which mice were sensitized and challenged with the allergen in the absence of an adjuvant (Fig. 1a). Following CRA challenge, mice developed airway inflammation with eosinophils representing the main infiltrating cell type (Fig. 1b). There was also a significant increase in the recruitment of CD4 T cells, DCs, and neutrophils to the airway (Fig. 1b). Consistent with an allergic response, histological analysis of lung sections showed extensive goblet cell metaplasia and mucus production (Fig. 1c). In addition, we detected elevated levels of CRA-specific IgE antibodies in the serum (Fig. 1d) as well as increased lung mRNA expression of TH2 and TH17 cytokines, with no effect on IFNFγ (Fig. 1e). Depletion of CD4 T cells prior to sensitization and challenge protected mice from the development of allergic inflammation in response to CRA (Supplementary Fig. 1). These results show that sensitization and challenge with CRA leads to the development of airway inflammation that closely resembles allergic asthma.
Figure 1.
Intranasal sensitization and challenge with CRA results in airway inflammation with features of allergic asthma. (a) On days 0–2, C57BL/6 mice were intranasally sensitized with CRA and challenged with CRA on days 17–18. Allergic airway inflammation was assessed 72 hours after the last challenge. (b) BAL differential cell counts were determined by flow cytometry. (c) PAS staining of formalin-fixed and paraffin-embedded lung sections. PAS-positive cells (purple) represent mucus producing goblet cells. Original magnification, x40. (d) Serum levels of CRA-specific IgE were determined by ELISA. (e) RNA was isolated from the lungs of PBS- versus CRA-challenged mice and mRNA expression of IL-4, IL-5, IL-13, IL-17A, and IFNγ was determined by qPCR. All expression values are relative to PBS control samples and normalized to the housekeeping gene GAPDH. Data represent mean ± SEM (n = 4–8 mice per group). Comparisons were made using unpaired Student’s t test. * p < 0.05, ** p < 0.01, *** p < 0.001.
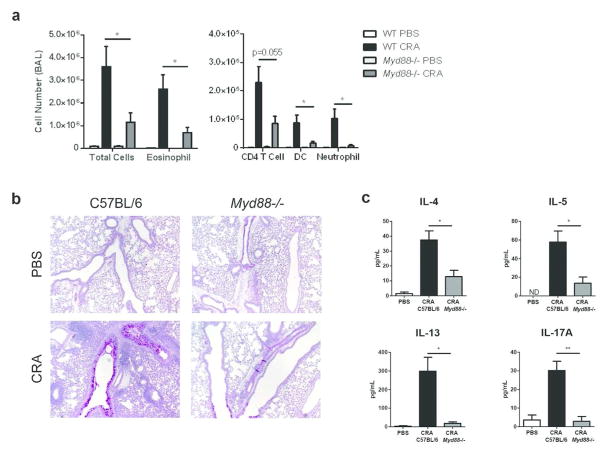
MyD88 is required for the development of allergic airway inflammation to CRA
A previous study showed that MyD88 is required in bone marrow-derived DCs for production of IL-6 and IL-23 after exposure to CRA, but it is unknown if MyD88 is required for allergic responses to CRA25. Myd88−/− mice sensitized and challenged with CRA developed markedly less airway inflammation compared to wild type (WT) mice; the number of eosinophils, neutrophils, and CD4 T cells were strongly reduced in the BAL (Fig. 2a). Histological analysis confirmed this reduction in immune cell infiltrates and revealed less mucus production in the lung (Fig. 2b). Furthermore, both TH2 and TH17 cytokine levels were significantly lower in the BAL fluid of Myd88−/− mice (Fig. 2c). These results reveal an important role for the MyD88 signaling pathway in the development of allergic airway inflammation to CRA.
Figure 2.
MyD88 is required for the development of allergic airway inflammation to CRA. C57BL/6 and Myd88−/− mice were sensitized and challenged with CRA as described in the Methods. (a) BAL differential cell counts were determined by flow cytometry 72 hours after the last challenge. (b) PAS staining of formalin-fixed and paraffin-embedded lung sections. Original magnification, x10. (c) Concentration of IL-4, IL-5, IL-13, and IL-17A from BAL fluid was determined by ELISA. Data represent combined data of two independent experiments, mean ± SEM (n = 4–9 mice per group). Comparisons were made using unpaired Student’s t test. * p < 0.05, ** p < 0.01.
Next, we investigated the role of individual TLRs in sensing CRA and found no significant differences in airway eosinophilia between WT and Tlr2−/− mice (Supplementary Fig. 2a). In contrast, TLR4-deficient mice exhibited significantly reduced airway eosinophilia, mucus production, and TH2 cytokine production (Supplementary Fig. 2b–d). Because LPS is a TLR4 agonist that can promote TH2 responses at low doses28, we measured the concentration of contaminating LPS in CRA. We found that each dose of CRA contained 11 ng of LPS (data not shown) and asked if this low level of LPS was sufficient to drive sensitization to an antigen. In contrast to CRA-treated mice, mice sensitized and challenged with 11 ng of LPS plus ovalbumin failed to develop airway eosinophilia (Supplementary Fig. 3). This suggests that, while LPS might contribute, it alone does not drive sensitization to CRA.
In addition to TLRs, MyD88 is also required for signaling pathways downstream of the IL-1 receptor family. Members of the IL-1 cytokine family, such as IL-1α and IL-33, have been shown to be important for sensitization to HDM15. When IL1R signaling was blocked with anakinra, an IL-1 receptor antagonist, levels of airway eosinophilia were similar to that of the control group (Supplementary Fig. 4a). These results demonstrate that IL-1α and IL1β are not required for allergic response to CRA. Consistent with this data, we also found that IL-1α was not upregulated in response to low or high dose of CRA (data not shown). Next, we compared WT and casp1−/− mice to determine the role of IL-18 and IL-1β. Caspase 1 is required for processing pro-IL-1β and pro-IL-18 into their active forms. Again, we found no significant differences in eosinophil numbers between WT and casp1−/− mice after CRA challenge (Supplementary Fig. 4b). However, when IL-33-deficient mice were sensitized and challenged with CRA, there was a significant decrease in airway eosinophilia compared to WT mice. Surprisingly, the numbers of CD4 T cells, DCs, and neutrophils remained elevated in IL-33-deficient mice (Supplementary Fig. 4c). These results show that among the IL-1 cytokines, IL-33 played a limited role in CRA-induced airway eosinophilia.
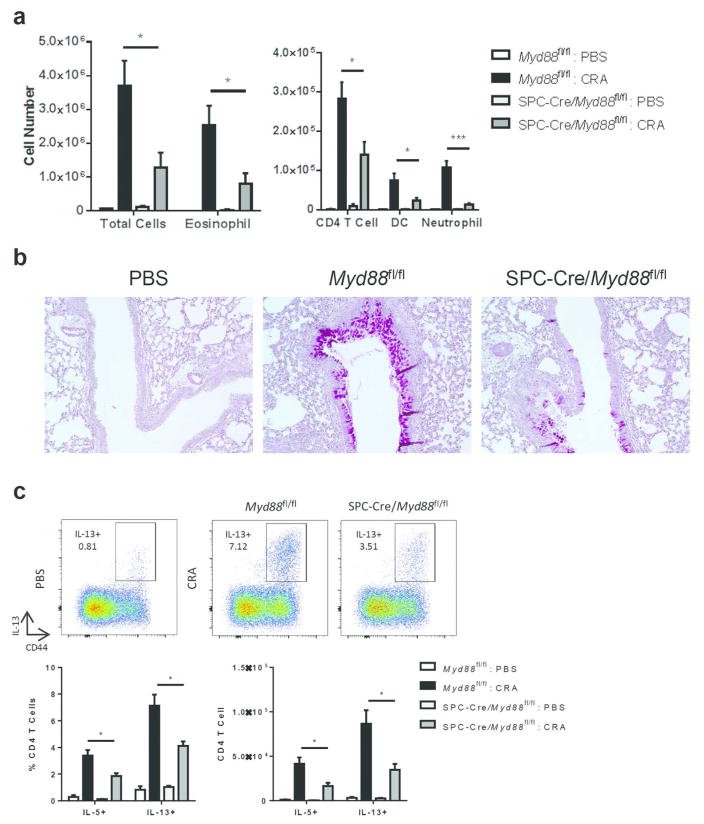
MyD88 is required in lung epithelial cells for allergic inflammation to CRA
MyD88 is expressed broadly in both immune and structural cells. To determine which of these cellular compartments were responsible for MyD88-dependent responses to CRA, we established reciprocal bone marrow (BM) chimeras in which WT or Myd88−/− BM cells were transferred into irradiated WT or Myd88−/− hosts. WT → WT BM chimeric mice developed a robust allergic response to CRA. In contrast, Myd88−/− Myd88−/− → BM chimeric mice developed reduced eosinophilia, which confirmed the critical role of MyD88 in CRA-induced allergic inflammation (Supplementary Fig. 5). A similar reduction in eosinophilia was observed when MyD88 was absent in structural cells (WT → Myd88−/−). In contrast, absence of MyD88 in the radiosensitive, hematopoietic compartment (Myd88−/− → WT) did not affect the degree of allergic inflammation to CRA (Supplementary Fig. 5). These results indicate that MyD88 expression is required in lung radioresistant cells for a TH2 allergic response against CRA.
MyD88 is expressed by various structural cells in the lung such as epithelial cells and fibroblasts29. Because epithelial cells are uniquely positioned to sense inhaled allergens and can initiate TH2 allergic responses, we hypothesized that MyD88 expression is required in epithelial cells to promote sensitization to CRA. To test this hypothesis, we generated mice that lacked MyD88 specifically in lung epithelial cells by crossing Myd88fl/fl mice with mice expressing Cre recombinase under the control of the 3.7-kb human surfactant protein C (SPC) promoter30,31. The SPC promoter is expressed in the primary lung buds starting at E10.5 and directs recombination in all airway and alveolar epithelial cells30,32–34. When SPC-Cre/Myd88fl/fl mice were sensitized and challenged with CRA, they developed significantly reduced airway eosinophilia and goblet cell metaplasia (Fig. 3a, b). In addition, restimulation of cells isolated from the lungs of these mice revealed a significant decrease in the percentage and number of IL-5+ and IL-13+ CD4 T cells (Fig. 3c). On the other hand, deletion of MyD88 in CD11c+ cells and CD4 T cells had no effect on the degree of airway inflammation following sensitization and challenge with CRA (Supplementary Fig. 6). Altogether, these results demonstrate that MyD88 signaling in lung epithelial cells, but not hematopoietic cells, is important for initiation of allergic responses against CRA.
Figure 3.
MyD88 is required in lung epithelial cells for allergic inflammation to CRA. SPC-Cre/Myd88fl/fl and Myd88fl/fl littermate control mice were sensitized and challenged with CRA. (a) BAL cell differentials were determined by flow cytometry. (b) PAS staining of formalin-fixed and paraffin-embedded lung sections. Original magnification, x20. (c) Percent and number of lung CD4 T cells positive for IL-5 and IL-13 after restimulation with PMA and ionomycin. Data represent combined data of two independent experiments, mean ± SEM (n = 4–9 mice per group). Comparisons were made using unpaired Student’s t test. * p < 0.05, ** p < 0.01.
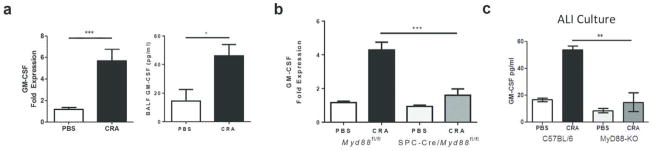
GM-CSF is produced by lung epithelial cells in a MyD88 dependent manner
Following allergen exposure, epithelial cells can initiate and regulate TH2 responses through production of prototypical cytokines such as TSLP, IL-25, and IL-332. However, we found no significant differences in CRA-induced airway eosinophilia between WT, Tslp−/−, and Il17rb−/− mice (Supplementary Fig. 7), while IL-33-deficient mice only displayed a reduction in eosinophilia (Supplementary Fig. 4c).
When we examined additional epithelial-derived cytokines induced by CRA, we found elevated levels of GM-CSF in the BAL as early as two hours post-sensitization (Fig. 4a). To assess if upregulation of GM-CSF was dependent on MyD88 signaling in lung epithelial cells, we analyzed the expression of GM-CSF in SPC-Cre/Myd88fl/fl mice after sensitization with CRA. SPC-Cre/Myd88fl/fl mice failed to upregulate GM-CSF in response to CRA, suggesting that MyD88 signaling in lung epithelial cells triggers the release of GM-CSF (Fig. 4b).
Figure 4.
GM-CSF is produced by lung epithelial cells in a MyD88 dependent manner. (a) C57BL/6 mice were intranasally treated with CRA. Two hours later, lung tissue was collected and mRNA expression of GM-CSF was determined by qPCR. Expression values are relative to PBS control samples and normalized to the housekeeping gene GAPDH. BAL fluid was also collected and GM-CSF protein level was measured by ELISA. Data represent combined data of two independent experiments, mean ± SEM (n = 7–15 mice per group). (b) SPC-Cre/Myd88fl/fl and Myd88fl/fl mice were intranasally treated with CRA. Two hours later, lung tissue was collected and mRNA expression of GM-CSF was determined by qPCR. Data represent combined data of two independent experiments, mean ± SEM (n = 4–10 mice per group). (c) Tracheas from C57BL/6 and Myd88−/− mice were harvested and primary epithelial cells were cultured at air-liquid interface. Cells were exposed to 15 μg of CRA or PBS for 24 hours and medium from the basal compartment was collected. GM-CSF levels were measured by ELISA. Data represent mean ± SEM of one experiment and are representative of two independent experiments. Comparisons were made using unpaired Student’s t test. * p < 0.05, ** p < 0.01, *** p < 0.001.
To confirm that epithelial cells produced GM-CSF in response to CRA, we established organotypic air-liquid interface (ALI) cultures of primary epithelial cells from WT and Myd88−/− tracheas. When WT ALI cultures were stimulated with CRA, there was a significant increase in GM-CSF levels in the culture medium on the basolateral side (Fig. 4c). In contrast, ALI cultures established from Myd88−/− mice failed to upregulate GM-CSF after stimulation with CRA (Fig. 4c). Altogether, these results demonstrate that airway epithelial cells produce GM-CSF in a MyD88-dependent manner following exposure to CRA.
GM-CSF is required during allergic sensitization, but not challenge, in CRA-driven allergic airway disease
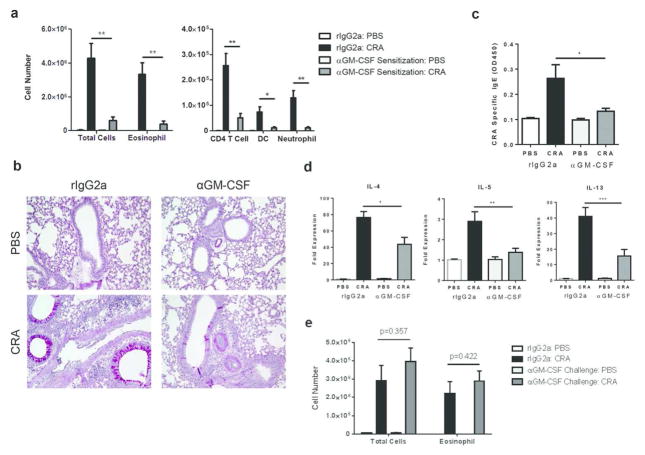
To investigate the role of epithelial-derived GM-CSF in regulating CRA-driven allergic airway disease, mice were treated with a neutralizing αGM-CSF antibody at either sensitization or challenge. Blocking GM-CSF at sensitization strongly reduced airway inflammation after CRA challenge, with eosinophil numbers in the BAL significantly lower compared to isotype control treatment (Fig. 5a). There was also a reduction in the number of CD4 T cells, DCs, and neutrophils (Fig. 5a). Furthermore, mRNA expression of type 2 cytokines and serum CRA-specific IgE levels were also significantly reduced (Fig. 5c, d). In contrast, blocking GM-CSF at challenge failed to reduce airway eosinophilia in mice sensitized and challenged with CRA (Fig. 5e). These results reveal a crucial role for GM-CSF during sensitization, but not challenge, in CRA-induced allergic airway disease.
Figure 5.
GM-CSF is required during allergic sensitization, but not challenge, in CRA-driven allergic airway disease. C57BL/6 mice were treated with rIgG2a or a neutralizing anti-GM-CSF antibody, five hours prior to sensitization. Mice were then sensitized and challenged with CRA and airway inflammation was analyzed 72 hours later. (a) BAL differential cell counts were determined by flow cytometry. (b) PAS staining of formalin-fixed and paraffin-embedded lung sections. Original magnification, x20. (c) Serum levels of CRA-specific IgE. (d) RNA was isolated from the lung and mRNA expression of IL-4, IL-5, and IL-13 was determined by qPCR. All expression values are relative to PBS control samples and normalized to the housekeeping gene GAPDH. Data represent combined data of two independent experiments, mean ± SEM (n = 3–10 mice per group). (e) C57BL/6 mice were treated with rIgG2a or a neutralizing anti-GM-CSF antibody, five hours prior to challenge. After allergen challenge, BAL differential cell counts were determined by flow cytometry. Data represent combined data of two independent experiments, mean ± SEM (n = 5–9 mice per group). Comparisons were made using unpaired Student’s t test. * p < 0.05, ** p < 0.01, *** p < 0.001.
GM-CSF is required for optimal dendritic cell function and Th2 priming
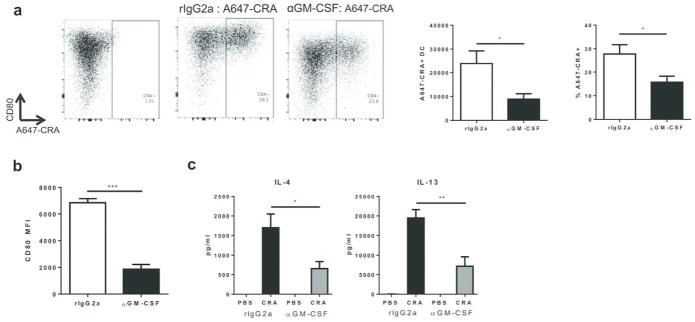
Given that the allergic response to CRA is CD4 T cell driven, we postulated that GM-CSF played a role in DC function during priming. To explore this possibility, we asked how neutralization of GM-CSF affected the maturation of DCs after exposure to CRA. Mice were sensitized with fluorescently-labeled CRA in the presence or absence of the αGM-CSF antibody, and the number of CRA+ DCs in the draining lymph node was determined by flow cytometry. Neutralization of GM-CSF significantly reduced the delivery of fluorescently labelled CRA to the draining lymph node. There was a reduction in both the percentage and total number of A647-CRA+ DCs in the lung-draining lymph node 24 hours after CRA administration (Fig. 6a). Additionally, surface expression of the costimulatory molecule CD80 was markedly decreased on A647+ DCs (Fig. 6b). These findings suggest that GM-CSF regulates the ability of DCs to acquire antigen, upregulate costimulatory molecules, and migrate to the draining lymph node during sensitization. To examine if GM-CSF blockade affected the ability of DCs to prime TH2 cells, we sensitized mice with CRA and collected lung-draining lymph node cells on day 3. When these cells were restimulated with CRA, there was a significant reduction in the production of TH2 cytokines from αGM-CSF treated mice (Fig. 6c). Overall, these results demonstrate that GM-CSF is required for optimal function of DCs and Th2 priming during CRA sensitization.
Figure 6.
GM-CSF is required for optimal dendritic cell function and Th2 priming. C57BL/6 mice were treated with rIgG2a or a neutralizing anti-GM-CSF antibody, five hours prior to intranasal administration with A647-CRA. The draining lymph node was collected 24 hours later and cells were analyzed by flow cytometry to assess the uptake of CRA and maturation of DCs. DCs were gated as CD11c+ and MHCII+. (a) Total number of A647+ DCs and percent of A647+ DCs within the DC population. (b) Mean fluorescent intensity (MFI) of costimulatory molecule CD80 on A647+ DCs. Data represent combined data of two independent experiments, mean ± SEM (n = 9 mice per group). (c) On days 0–2, C57BL/6 mice were sensitized with CRA. On day 3, the lung draining lymph node was collected and cells were restimulated with CRA. On day 7, culture supernatant was collected and concentration of IL-4 and IL-13 were determined by ELISA. Data represent mean ± SEM of one experiment (n = 3–5 mice per group) and are representative of two independent experiments. Comparisons were made using unpaired Student’s t test. * p < 0.05, ** p < 0.01, *** p < 0.001.
GM-CSF selectively rescues TH2 allergic airway response to CRA in Myd88−/− mice
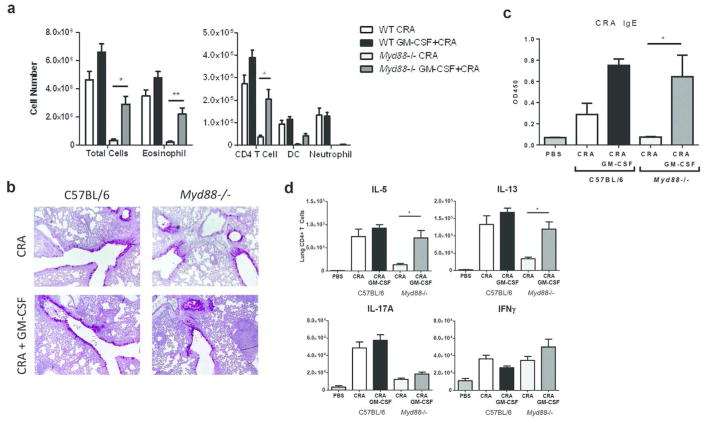
To determine the function of GM-CSF downstream of MyD88 signaling, GM-CSF was administered intranasally to Myd88−/− mice during CRA sensitization. Treatment with GM-CSF largely restored airway inflammation in Myd88−/− mice to levels observed in WT mice. This was due to a significant increase in the number of eosinophils and CD4 T cells, whereas recruitment of neutrophils to the BAL was not recovered by GM-CSF treatment (Fig. 7a, b). Furthermore, GM-CSF selectively restored TH2, but not TH17 responses, in Myd88−/− mice. In vitro restimulation of lung cells with PMA/ionomycin revealed a significant increase in the number of IL-5+ and IL-13+ CD4 T cells, but not IL-17A+ or IFNγ+ CD4 T cells (Fig. 7d). Consistent with the recovery of TH2 responses, CRA-specific IgE levels were also restored by GM-CSF treatment (Fig. 7c). These results demonstrate that epithelial-derived GM-CSF specifically promotes TH2-mediated eosinophilic inflammation following exposure to CRA.
Figure 7.
GM-CSF selectively rescues TH2 allergic airway response to CRA in Myd88−/− mice. C57BL/6 and Myd88−/− mice were intranasally treated with or without 50 ng of GM-CSF during sensitization, which was administered along with CRA. Mice were challenged with CRA as usual. (a) BAL differential cell counts were determined by flow cytometry (b) PAS staining of formalin-fixed and paraffin-embedded lung sections. Original magnification, x10. (c) Serum CRA-specific IgE levels were determined by ELISA. (d) Number of lung CD4 T cells positive for IL-5, IL-13, IL-17A, or IFNFγ after restimulation with PMA and ionomycin. Data represent combined data of two independent experiments, mean ± SEM (n = 7–11 mice per group). Comparisons were made using one-way ANOVA with Tukey posthoc test. * p < 0.05, ** p < 0.01.
Discussion
The airway epithelium coordinates allergic responses by producing pro-Th2 cytokines such as TSLP, IL-25, IL-33, and GM-CSF. Although studies using HDM allergen have provided valuable insights into the role of epithelial-derived cytokines in mucosal sensitization, understanding pathways of sensitization initiated by other clinically relevant allergens remain important given that allergens are structurally and functionally distinct. Here, we focused on cockroach allergen, an important factor in the development of asthma for inner-city children. We uncovered a critical role for epithelial-derived GM-CSF in allergic sensitization to CRA, in which GM-CSF specifically promoted Th2 priming by regulating the functional maturation of DCs. Interestingly, while IL-1α initiates the release of GM-CSF in response to HDM, IL-1α was not required for CRA-induced GM-CSF. Additionally, IL-33 only played a minor role in allergic response to CRA whereas IL-33 is critical for allergic sensitization to HDM. Thus, our study demonstrates that epithelial-derived GM-CSF is a critical early signal for the initiation of Th2 allergic responses to CRA.
Activation of PAR2 by CRA-derived proteases is important for initiation of allergic responses to CRA. However, in the absence of PAR2, allergic response to CRA is partially reduced but nonetheless intact, which suggests a role for other PRRs in the recognition of CRA35. In this study, we have identified TLR4-MyD88 as a major signaling pathway that promotes sensitization to CRA. We show that MyD88 signaling was required in lung epithelial cells for upregulation of GM-CSF during sensitization. Previous studies have shown that CRA-derived proteases can also induce GM-CSF production by epithelial cells through a PAR2 dependent mechanism36. Our findings suggest that in addition to PAR2, TLR4-MyD88 signaling pathway is another important signaling pathway that mediates production of GM-CSF in response to CRA.
TLR4 stimulation with a low dose of LPS can promote Th2 responses to inhaled antigens28. Here we show that the TLR4-mediated response to CRA extends beyond an immune response to LPS and antigen since the low level of contaminating LPS in CRA was not sufficient to induce Th2 allergic responses. Instead, it is possible that other components of CRA or the release of endogenous ligands mediates this TLR4-dependent response to CRA. Bla g 1, a major cockroach allergen, contains a hydrophobic cavity that binds to lipids known to activate TLR437. Therefore, Bla g 1 may lower the threshold of LPS needed to generate a TH2 allergic response by enhancing TLR4 binding. Alternatively, TLR4 may be activated by an endogenous ligand released in response to CRA such as high mobility group box 1 (HMGB1)38. This ability to trigger TLR4 is a characteristic shared by many allergens, and our findings further support the idea that TLR4 signaling is essential for the development of allergic inflammation.
Previous studies have shown that CRA contains a TLR2 agonist39. However, we found that TLR2 was not required for allergic responses to CRA. This finding is consistent with a study by Page et al. in which TLR2−/− mice displayed increased airway inflammation against CRA40. Although we did not find a protective role for TLR2, which could be due to differences in the route of sensitization and administration protocol, our data demonstrates that TLR2 is not required for allergic sensitization to CRA.
Activation of airway epithelial cells is a critical first step in the initiation of responses to aeroallergens. In this study, we demonstrate that MyD88 expression in radioresistant cells was necessary and sufficient for allergic airway response to CRA. Furthermore, we determined that MyD88 signaling in lung epithelial cells, but not in CD11c+ alveolar macrophages or DCs, was required for sensitization to CRA. Our findings indicate that MyD88 signaling in epithelial cells plays a predominant role in facilitating allergic responses to CRA. In contrast, a recent study in HDM showed that TLR4 signaling in both lung epithelial cells and hematopoietic cells contributes to the allergic response41. It is likely that this difference in the contribution of epithelial cells to CRA- versus HDM-driven allergic responses leads to further differences in the induction and requirement of epithelial-derived cytokines.
In this study, we found key differences in the requirement for epithelial-derived cytokines in allergic response to CRA, compared to other allergens. We show that TSLP and IL-25 were dispensable for the development of CRA-induced airway inflammation. Although TSLP has been considered a master regulator of Th2 responses, our data indicate a redundant role for TSLP and IL-25 in airway sensitization and is consistent with studies in HDM and peanut allergy10. Additionally, IL-33 only played a minor role in allergic responses to CRA; in stark contrast, IL-33 is essential for allergic sensitization to HDM10,15 and many other clinically relevant allergens such as Alternaria alternata, peanut allergen, cypress pollen, and bee venom10,42–44. Although the current paradigm considers TSLP, IL-25, and IL-33 to be critical drivers of Th2 allergic responses, we present a novel pathway in which all three epithelial-derived cytokines are not crucial for sensitization to CRA.
Inhaled allergens and other noxious stimuli such as viruses, cigarette smoke, and diesel exhaust fumes can upregulate the expression of GM-CSF in the airway45–48. Increased levels of GM-CSF amplifies the risk of allergic sensitization by lowering the threshold of allergen required for allergic responses16. Additionally, neutralizing GM-CSF at sensitization reduces allergic inflammation to HDM and Blomia tropicalis mite allergen15,17,18. However, in contrast to HDM in which both IL-33 and GM-CSF are required for allergic responses, we found that epithelial-derived GM-CSF was the primary driver of allergic sensitization to CRA. Furthermore, in the absence of MyD88, delivery of exogenous GM-CSF was sufficient to initiate an allergic response to CRA, which suggests that IL-33 is not required for GM-CSF mediated responses to CRA. Interestingly, we also show that GM-CSF selectively initiates the Th2, but not the Th17, arm of the immune response to CRA, which indicates a directed role for GM-CSF that extends beyond a proinflammatory action.
Although epithelial-derived GM-CSF plays an important role in sensitization to both HDM and CRA, there are key differences in the pathways that lead to production of GM-CSF by these distinct allergens. Willart et al. showed that sensitization to HDM is driven by epithelial-derived IL-1α, which acts upstream in the cytokine cascade to initiate the release of GM-CSF in an autocrine manner15. However, the requirement for IL-1α in HDM-driven allergic responses depends the allergen dose and administration protocol49. In contrast, IL-1α was not upregulated in response to CRA and was not required for allergic responses to CRA, suggesting that GM-CSF production is independent of IL-1α. Despite differences in the pathways of sensitization initiated by CRA versus HDM, these pathways converge on GM-CSF, which suggests a central role for GM-CSF in allergic sensitization.
In conclusion, we have determined the role of epithelial-derived cytokines in CRA-driven allergic responses. We uncovered a novel pathway in which epithelial-derived GM-CSF, but not TSLP, IL-25, or IL-33, is essential for allergic sensitization to CRA. Upregulation of GM-CSF in lung epithelial cells occurs through a MyD88-dependent pathway that does not require IL-1α. Downstream of MyD88, GM-CSF selectively initiates a Th2 allergic response, but not Th17-mediated responses. Overall, our findings establish a central role for epithelial-derived GM-CSF in allergic sensitization to CRA and suggest that GM-CSF might be an important therapeutic target.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson or Charles River Laboratories. Tlr2−/− mice were provided by John Gebe (Benaroya Research Institute). Tlr4lps-del mice on the C57BL/6 background were obtained from The Jackson Laboratory; Myd88−/− mice were provided by Tobias Hohl (Memorial Sloan Kettering Cancer Center); Myd88flox/flox mice were provided by Mohamed Oukka (Seattle Children’s Hospital); casp1−/− mice were provided by Michael Gale (University of Washington); Il33−/− mice were provided by Dirk Smith (Amgen); SPC-Cre mice were provided by Brigid Hogan (Duke University); Il17rb−/− mice were provided by Xiaoxia Li (Cleveland Clinic Lerner Research Institute). TSLPR−/− mice were described previously50. All animals were housed in specific pathogen-free conditions in the Benaroya Research Institute animal facility and all experiments were approved by the Benaroya Research Institute Animal Care and Use Committee.
Reagents
Lyophilized whole body German cockroach extract was purchased from Greer laboratories (XPB46D3A4, Lot # 259066) and resuspended in PBS to a protein concentration of 2 mg/ml. Low endotoxin ovalbumin was purchased from Worthington Biochemical Corporation (LS003061). LPS from E. coli 026:B6 was purchased from Sigma (L2654). Human IL-1ra (anakinra) was purchased from the Virginia Mason hospital pharmacy. Recombinant GM-CSF was purchased from Peprotech (315-03).
Cockroach allergen induced allergic airway model
On days 0–2, mice were anesthetized with isoflurane and sensitized intranasally with 40 μg of CRA (Greer, B46) in a total volume of 20 μl PBS. After 15 days, mice were challenged intranasally with 40 μg of CRA on two consecutive days and airway inflammation was assessed 72 hours later. Control mice were treated with PBS under the same protocol. For neutralization experiments, anti-GM-CSF antibody (Clone MP1-22E9, Biolegend) was injected 5 hours before the first sensitization or challenge dose. 120 μg of antibody was administered i.p. and 30 μg of antibody was administered intranasally. Anakinra, IL-1ra, was i.p. administered at 25 mg/kg with each dose of CRA for a total of five times. To deplete CD4 T cells, 150 μg of anti-CD4 antibody (GK1.5, Bio X Cell) was i.p. administered on days -1, 2, and 16. To determine if GM-CSF can recover TH2 allergic inflammation in Myd88−/− mice, mice were intranasally treated with 50 ng of GM-CSF plus CRA during sensitization and were only challenged with CRA.
Evaluation of airway inflammation
Mice were euthanized by i.p. injection with 1 ml of 2.5% Avertin. Bronchoalveolar lavage (BAL) was performed four times, each time with 1 ml of PBS. Next, BAL was centrifuged at 250 x g for 5 min to collect the cell pellet. Cells were resuspended in FACS buffer and counted using a hemocytometer. Differential cell counts were performed by flow cytometry. The first ml of BAL fluid collected for ELISA.
After BAL, lungs were perfused by injecting 3 ml of PBS into the right ventricle of the heart until the lungs turned white. The right upper lobe of the lung was placed in 1 ml of RNALater and stored at −80°C for later analysis by qPCR. The remaining lung tissue was fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin, sectioned, and stained with H&E and periodic acid-Schiff. Serum was collected and analyzed for CRA-specific antibodies by ELISA.
To determine differential cell counts in the lung, single cell suspensions were prepared by cutting the lung into fine pieces using a scissor. Next, lung tissue was digested in 50 μg/ml of Liberase TM (Roche) and 10 U/ml of DNase I (Sigma) in RPMI-1640 at 37°C for 30 min. Digested lung samples were filtered through a 100-μm cell strainer and washed with equal volumes of RPMI plus 20% FBS. Red blood cells were lysed with ACK lysis buffer and lung cells were centrifuged at 250 x g for 5 min. Cells were resuspended in FACS buffer and counted using a hemocytometer. Differential cell counts were performed by flow cytometry. Lung cells were stimulated for 4 h with PMA (50 ng/ml), ionomycin (500 ng/ml), and GolgiPlug™ (BD Biosciences) at the recommended concentration.
A647-CRA and Migration of DCs
A647 was conjugated to CRA using the Alexa Fluor 647 microscale protein labeling kit per manufacturer’s instructions (Life Technologies). DC uptake of A647-CRA and migration to the draining lymph node was assessed by administering 11 μg of A647-CRA plus 39 μg of unconjugated CRA intranasally. Twenty four hours later, lung draining lymph node was collected and single cell suspension was prepared. Lymph nodes were teased apart using needles and digested in 1 ml of digest solution containing 50 μg/ml of Liberase TM (Roche) and 10 U/ml of DNase I in RPMI-1640. After digesting for 25 minutes at 37°C, 100 μl of 0.1 M EDTA was added to each sample and digested for five additional minutes at 37°C. Next, digested lymph nodes were crushed with a 1 ml syringe and filtered through a 100-μM cell filter. Cells were resuspended in FACS buffer and analyzed by flow cytometry.
Evaluation of sensitization to CRA
On days 0–2, mice were sensitized with 40 μg of CRA intranasally. On day 3, lung draining lymph nodes were collected and single cell suspensions were prepared as described above. Cells were plated in a 96-well flat bottom plate at 5×106 cells/ml and restimulated with 0 or 10 μg/ml of CRA. The cell culture supernatant was collected on day 7 and cytokine levels were analyzed by ELISA.
Bone marrow chimeras
Mice were irradiated twice with 4.5 Gy and at least 2 × 106 bone marrow cells were transferred i.v. by retro-orbital injection. Mice were used for experiments after 8–10 weeks to allow for bone marrow reconstitution.
Flow Cytometry
To prevent nonspecific binding of antibodies, cells were incubated with anti-CD16/CD32 (Clone: 2.4G2) in FACS buffer for 15 minutes at room temperature. The following antibodies were purchased: anti-Ly6G FITC (1A8), anti-IFNFγ FITC (XMG1.2), anti-Ly6C PerCpCy5.5 (HK1.4), anti-CD103 Pacific Blue (2E7), anti-Foxp3 e450 (FJK-16s), anti-CD4 BV605 (RM4-5), anti-CD80 BV605 (16-10A1), anti-CD45R BV650 (RA3-6B2), anti-IL-5 APC (TRFK5), anti-MHCII A700 (M5/114.15.2), anti-CD44 A700 (IM7), anti-CD11b APC-e780 (M1/70), anti-TCRβ APC-e780 (H57-597), anti-Siglec F PE (E50-2440), anti-IL-13 PE (eBIo13A), anti-CD3 PECy5 (145-2C11), anti-CD11c PE-Cy7 (N418), anti-IL-4 PECy7 (BVD6-24G2). Cells were stained with surface antibodies for 12 minutes at room temperature. For intracellular staining of cytokines, cells were fixed, permeabilized, and stained using the Foxp3 fix/perm buffer set (Biolegend) according to manufacturer’s instructions. Samples were analyzed with BD LSR II flow cytometer (BD Biosciences).
Real-time PCR
Lung tissues were homogenized in RNA lysis buffer and RNA was isolated using the Nucleospin RNA kit (Clontech). RNA was reverse-transcribed into cDNA using PrimeScript Reverse Transcriptase (Takara) according to the manufacturer’s instructions. cDNA was amplified in a 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) using SYBR Premix Ex Taq II (Takara). The following primer pairs were used: csf2 forward, 5′-GGCTAAGGTCCTGAGGAGGAT-3′; csf2 reverse, 5′-ACCTCTTCATTCAACGTGA CAGG-3′; Il4 forward, 5′-TCATCGGCATTTTGAACGAG-3′; Il4 reverse, 5′-TTTGGCACATCCA TCTCCG-3′; Il5 forward, 5′-TGCCTGGAGCAGCTGGAT-3′; Il5 reverse, 5′-TGGCTGGCTCTC ATTCACACT-3′; Il13 forward, 5′-ATTCCCTGACCAACATCTCCAA-3′; Il13 reverse, 5′-CGGTT ACAGAGGCCATGCAA-3′; Il17a forward, 5′-ATCAGGACGCGCAAACATGAGT-3′; Il17a reverse, 5′-ACGCTGAGCTTTGAGGGATGAT-3′; Ifng forward, 5′-CCTGCGGCCTAGCTCTGA G-3′; Ifng reverse, 5′-GCCATGAGGAAGAGCTGCA-3′; Gapdh forward, 5′-TCCATGACAACTT TGGCATTG-3′; Gapdh reverse, 5′-CAGTCTTCTGGGTGGCAGTGA-3′.
Air-liquid interface cultures
Primary airway epithelial cells were cultured as previously described 51. Mice were euthanized by CO2 and the trachea was collected and digested in 0.2% pronase at 4°C overnight. Mouse tracheal epithelial cells (MTEC) were isolated and cultured on collagen-coated transwells. After one week, MTEC cultures were transitioned to air-liquid interface (ALI) by removing medium from the upper compartment and maintained at ALI for 2 weeks. Cells were exposed to 15 μg of CRA for 24 hours and medium from the basal compartment was collected for ELISA.
ELISA
To detect CRA-specific IgE, plates were coated overnight with 6.25 μg/ml CRA in PBS at 4°C. Plates were washed and blocked with 1% BSA in PBS for 2 hours at room temperature. Serum samples were added to the plates and incubated for 2 hours at room temperature. Plates were washed and biotin-conjugated anti-mouse IgE (R35-118) was added at 1:250 dilution and incubated for 2 hours at room temperature. Plates were washed and incubated with streptavidin-HRP for 30 minutes. 1-Step Ultra TMB-ELISA substrate (Pierce) was added and incubated at room temperature in the dark. The reaction was stopped with 2 N H2SO4 and OD was measured at 450 nm.
To detect cytokines, the following capture and detection antibody pairs were used: anti-IL-4 Purified (11B11) and anti-IL-4 Biotin (BVD6-24G2), anti-IL-5 Purified (TRFK5) and anti-IL-5 Biotin (TRFK4), anti-IL-13 Purified (eBio13A) and anti-IL-13 Biotin (eBio1316H), anti-IL-17A Purified (eBio17CK15A5) and anti-IL-17A Biotin (eBio17B7), anti-GM-CSF Purified (MP1-22E9) and anti-GM-CSF Biotin (MP1-3G6). The concentration of cytokines in the BAL fluid were detected using these antibodies and the protocol described above.
Statistics
Statistical analysis was performed using GraphPad Prism 5. Comparisons were made using either unpaired Student’s t test or one-way ANOVA with a Tukey posthoc test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Supplementary Material
Acknowledgments
We thank Drs. Jessica Hamerman, Daniel Campbell, Florence Roan, and Jen-Feng Lai for critical discussion of the manuscript. The work was supported in part by NIH grants RO1 AI068731 and PO1 HL098067 (S.F.Z). A.S. is supported by the National Science Foundation Graduate Research Fellowship Grant DGE-1256082.
Footnotes
Author Contribution
A.S., W.C.P., and S.F.Z. designed experiments. A.S. performed all experiments. A.S., W.C.P., and S.F.Z. wrote the manuscript.
Disclosure
The authors declared no conflict of interest.
References
- 1.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134(3):499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Saenz Sa, Taylor BC, Artis D. Welcome to the neighborhood: Epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler S, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11(4):289–293. doi: 10.1038/ni.1852.Sensing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6(10):1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, Owyang A, Oldham E, et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Tamachi T, Maezawa Y, Ikeda K. IL-25 enhances allergic airway inflammation by amplifying a TH 2 cell – dependent pathway in mice. :606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 8.Stämpfli MR, Wiley RE, Neigh GS, et al. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest. 1998;102(9):1704–1714. doi: 10.1172/JCI4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashrin MN, Arakaki R, Yamada A, et al. A Critical Role for Thymic Stromal Lymphopoietin in Nickel-Induced Allergy in Mice. J Immunol. 2014 Mar; doi: 10.4049/jimmunol.1300276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu DK, Llop-Guevara A, Walker TD, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2012:25. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Bleck B, Grunig G, Chiu A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol. 2013;190(7):3757–3763. doi: 10.4049/jimmunol.1201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183(2):1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li DQ, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128(6):1318–1325. e2. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dyken SJ, Mohapatra A, Nussbaum JC, et al. Chitin Activates Parallel Immune Modules that Direct Distinct Inflammatory Responses via Innate Lymphoid Type 2 and γδ T Cells. Immunity. 2014 Mar;:1–11. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willart MaM, Deswarte K, Pouliot P, et al. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012 Jul; doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llop-Guevara A, Chu DK, Walker TD, et al. A GM-CSF/IL-33 pathway facilitates allergic airway responses to sub-threshold house dust mite exposure. PLoS One. 2014;9(2):e88714. doi: 10.1371/journal.pone.0088714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Ho AWS, Schlitzer A, et al. GM-CSF-Licensed CD11b+ Lung Dendritic Cells Orchestrate Th2 Immunity to Blomia tropicalis. J Immunol. 2014 Jun; doi: 10.4049/jimmunol.1303138. [DOI] [PubMed] [Google Scholar]
- 18.Cates E, Fattouh R, Wattie J. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. [Accessed June 29, 2012];J Immunol. 2004 173(10):6384–6392. doi: 10.4049/jimmunol.173.10.6384. http://www.jimmunol.org/content/173/10/6384.short. [DOI] [PubMed] [Google Scholar]
- 19.Arruda LK, Pomés A. Every Cockroach Is Beautiful to Its Mother. Int Arch Allergy Immunol. 2013;161(4):289–292. doi: 10.1159/000350207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 21.Eggleston Pa, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102(4 I):563–570. doi: 10.1016/S0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 22.Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J Allergy Clin Immunol. 2003;112(6):1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Sohn JH, Choi J-M, et al. Alveolar Macrophages Play a Key Role in Cockroach-Induced Allergic Inflammation via TNF-α Pathway. PLoS One. 2012;7(10):e47971. doi: 10.1371/journal.pone.0047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH. Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp Allergy. 2007;37(9):1364–1373. doi: 10.1111/j.1365-2222.2007.02797.x. [DOI] [PubMed] [Google Scholar]
- 25.Page K, Zhou P, Ledford JR, et al. Early immunological response to German cockroach frass exposure induces a Th2/Th17 environment. J Innate Immun. 2011;3(2):167–179. doi: 10.1159/000320718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang S, Morris S, Lukacs NW. TSLP Promotes Induction of Th2 Differentiation but Is Not Necessary during Established Allergen-Induced Pulmonary Disease. PLoS One. 2013;8(2):e56433. doi: 10.1371/journal.pone.0056433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen BC, Budelsky AL, Baptist AP, Schaller Ma, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med. 2012;18(5):751–758. doi: 10.1038/nm.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenbarth SC, Piggott Da, Huleatt JW, Visintin I, Herrick Ca, Bottomly K. Lipopolysaccharide-enhanced, Toll-like Receptor 4-dependent T Helper Cell Type 2 Responses to Inhaled Antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Hori K, Ding J, et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol. 2011;226(5):1265–1273. doi: 10.1002/jcp.22454. [DOI] [PubMed] [Google Scholar]
- 30.Okubo T, Hogan BLM. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3(3):11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132(6):1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 32.Didon L, Barton JL, Roos AB, et al. Lung epithelial CCAAT/enhancer-binding protein-beta is necessary for the integrity of inflammatory responses to cigarette smoke. Am J Respir Crit Care Med. 2011;184(2):233–242. doi: 10.1164/rccm.201007-1113OC. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Nazario N, Rangel-Moreno J, O’Reilly Ma, Pasparakis M, Gigliotti F, Wright TW. Selective ablation of lung epithelial IKK2 impairs pulmonary Th17 responses and delays the clearance of Pneumocystis. J Immunol. 2013;191(9):4720–4730. doi: 10.4049/jimmunol.1301679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzo ND, Foster WM, Stripp BR. Amphiregulin-dependent mucous cell metaplasia in a model of nonallergic lung injury. Am J Respir Cell Mol Biol. 2012;47(3):349–357. doi: 10.1165/rcmb.2011-0257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arizmendi NG, Abel M, Mihara K, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011;186(5):3164–3172. doi: 10.4049/jimmunol.0903812. [DOI] [PubMed] [Google Scholar]
- 36.Day SB, Ledford JR, Zhou P, Lewkowich IP, Page K. German cockroach proteases and protease-activated receptor-2 regulate chemokine production and dendritic cell recruitment. J Innate Immun. 2012;4(1):100–110. doi: 10.1159/000329132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller Ga, Pedersen LC, Lih FB, et al. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol. 2013;132(6):1420–1426. doi: 10.1016/j.jaci.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullah MA, Loh Z, Gan WJ, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014;134(2):440–450. e3. doi: 10.1016/j.jaci.2013.12.1035. [DOI] [PubMed] [Google Scholar]
- 39.Page K, Lierl KM, Hughes VS, Zhou P, Ledford JR, Wills-Karp M. TLR2-mediated activation of neutrophils in response to German cockroach frass. J Immunol. 2008;180(9):6317–6324. doi: 10.4049/jimmunol.180.9.6317. http://www.ncbi.nlm.nih.gov/pubmed/18424755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page K, Ledford JR, Zhou P, Wills-Karp M. A TLR2 agonist in German cockroach frass activates MMP-9 release and is protective against allergic inflammation in mice. J Immunol. 2009;183(5):3400–3408. doi: 10.4049/jimmunol.0900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAlees JW, Whitehead GS, Harley IT, et al. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 2015;8(4):863–873. doi: 10.1038/mi.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snelgrove RJ, Gregory LG, Peiró T, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134(3) doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee Venom Phospholipase A2 Induces a Primary Type 2 Response that Is Dependent on the Receptor ST2 and Confers Protective Immunity. Immunity. 2013;39(5):976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabriele L, Schiavoni G, Mattei F, et al. Novel allergic asthma model demonstrates ST2-dependent dendritic cell targeting by cypress pollen. J Allergy Clin Immunol. 2013;132(3) doi: 10.1016/j.jaci.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 45.Subauste MCEA. Infection of a HUman Respiratory Epithelial cell line with rhinovirus-Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. Am Soc Clin Investig. 1995;96(July):549–557. doi: 10.1172/JCI118067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schelenz S, Smith DA, Bancroft GJ. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med Mycol. 1999;37(3):183–194. doi: 10.1046/j.1365-280x.1999.00219.x. <Go to ISI>://000081444400006. [DOI] [PubMed] [Google Scholar]
- 47.Ohta K, Yamashita N, Tajima M, et al. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: Essential role of GM-CSF. J Allergy Clin Immunol. 1999;104(5):1024–1030. doi: 10.1016/S0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- 48.Trimble NJ, Botelho FM, Bauer CMT, Fattouh R, Stämpfli MR. Adjuvant and Anti-Inflammatory Properties of Cigarette Smoke in Murine Allergic Airway Inflammation. Am J Respir Cell Mol Biol. 2009;40(1):38–46. doi: 10.1165/rcmb.2008-0107OC. [DOI] [PubMed] [Google Scholar]
- 49.Nikota JK, Botelho FM, Bauer CMT, et al. Differential expression and function of breast regression protein 39 (BRP-39) in murine models of subacute cigarette smoke exposure and allergic airway inflammation. Respir Res. 2011;12:12. doi: 10.1186/1465-9921-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpino N, Thierfelder W. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004 doi: 10.1128/MCB.24.6.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.