Summmary
Obstructive sleep apnea (OSA) is a complex and heterogeneous disorder and the apnea hypopnea index alone can not capture the diverse spectrum of the condition. Enhanced phenotyping can improve prognostication, patient selection for clinical trials, understanding of mechanisms, and personalized treatments. In OSA, multiple condition characteristics have been termed “phenotypes.” To help classify patients into relevant prognostic and therapeutic categories, an OSA phenotype can be operationally defined as: “A category of patients with OSA distinguished from others by a single or combination of disease features, in relation to clinically meaningful attributes (symptoms, response to therapy, health outcomes, quality of life).” We review approaches to clinical phenotyping in OSA, citing examples of increasing analytic complexity. Although clinical feature based OSA phenotypes with significant prognostic and treatment implications have been identified (e.g., excessive daytime sleepiness OSA), many current categorizations lack association with meaningful outcomes. Recent work focused on pathophysiologic risk factors for OSA (e.g., arousal threshold, craniofacial morphology, chemoreflex sensitivity) appears to capture heterogeneity in OSA, but requires clinical validation. Lastly, we discuss the use of machine learning as a promising phenotyping strategy that can integrate multiple types of data (genomic, molecular, cellular, clinical) to identify unique, meaningful OSA phenotypes.
Keywords: obstructive sleep apnea, phenotype, REM related, positional, cluster analysis, personalized medicine
Introduction
Obstructive sleep apnea (OSA) is increasingly recognized as a complex and heterogeneous disorder [1]. Recent work shows that this heterogeneity exists in the domains of the presenting symptoms [2], physiologic etiology [3], comorbid conditions [4], and important outcomes [5-7]. Despite this recognition, the diagnosis, assessment of severity, and management of OSA remains intimately linked to a single indicator, the apnea hypopnea index (AHI) [8]. An AHI-centered approach, with its lack of stratification by other syndrome characteristics likely contributes to the challenges of better understanding the genetic and biological underpinnings of the disorder [9] as well as to the modest treatment effects found in large randomized trials using continuous positive airway pressure (CPAP) [10-12].
One way to address these challenges is to classify the disorder into smaller, more homogeneous categories. Such classifications, sometimes referred to as “phenotypes,” can be based on clinical, pathophysiologic, cellular, or molecular characteristics [13]. Recent data suggest that patients may respond differently to non-positive airway pressure (non-PAP) treatments depending on their pathophysiologic characteristics such as arousal propensity or ventilatory sensitivity [14]. Successful therapeutic clinical trials can also be designed if patient categories more likely to respond to a given therapy are selected, such as those without complete concentric palatal collapse treated with upper airway neuro-stimulation [15]. These examples suggest that improved phenotyping approaches are an important step towards the goal of personalized medicine for OSA patients.
This manuscript provides an overview of the OSA phenotype literature in the context of various approaches to phenotyping. Specifically, the goals of this review are to:
Explore how phenotype identification can improve understanding of heterogeneous disorders (illustrated through other conditions) and propose a working definition of a phenotype in OSA for research and patient care
Summarize the OSA “phenotype literature” to-date, using key examples of OSA phenotyping approaches and their utility
Identify gaps in the current approaches and propose means to address them with a goal of personalizing care of patients with OSA
Role of phenotyping and lessons from other conditions
The aims of phenotyping include improving understanding of disease mechanisms, predicting response to therapy, risk for adverse events and reducing heterogeneity in clinical trials [16].
Similar to OSA, asthma and chronic obstructive pulmonary disease (COPD) are heterogeneous disorders whose diagnosis and management have been traditionally based on measures of physiological (expiratory volumes) and functional impairment. By better understanding the heterogeneity of these conditions, marked advances have been recently made in understanding mechanisms of disease [17], risk stratification [18] and developing novel treatments for these conditions [19, 20]. In asthma for example, cluster analysis (described later) identified two new categories of patients: 1) late-onset, inflammatory, obese and female, and 2) very severe, mixed inflammatory phenotypes, both with high morbidity and healthcare utilization [18]. Additionally, clinical and molecular based phenotyping methods have classified individuals based on levels of T helper cell (TH2) inflammation, with augmented corticosteroid responsiveness noted among TH2 high individuals [17]. This challenged the paradigm that all asthma is allergic, eosinophilic, and TH2 mediated. Phenotyping patients by markers of high TH2 inflammation resulted in clinical trials of new biological agents that demonstrated significant improvement in lung function and exacerbation frequency [20, 21]. These “positive” trials of better phenotyped patients stand in marked contrast to the early “negative” trials of such agents using unselected, more heterogeneous, asthma patients [13]. Another example from the COPD literature, characterizes patients according to a radiographic phenotype of emphysema identifying patients who benefit from lung volume reduction therapy (upper lobe predominant) and endobronchial valve placement (lack of collateral ventilation) [19].
Although improved phenotyping has clearly resulted in tangible improvements for patients with heterogeneous disorders, there are lessons to be learned from these approaches. Several of the patient phenotypes in asthma and COPD have been overlapping, not always reproducible, changed over time, and have not consistently pointed to greater mechanistic understanding or treatments [16, 22]. This may be due to disparate populations studied, lack of relevant features used for phenotyping, or a situation in which clinical phenotypes alone may not capture relevant biology. The absence of a consistent definition for the term “phenotype” in some cases may also lead to lack of clarity.
OSA phenotype, a definition
Ideally, the phenotype serves as an expression of an “endotype,” a subtype of disease defined by “a unifying and consistent natural history, clinical and physiological characteristics, an underlying pathobiology with identifiable biomarkers and genetics and a predictable response to general and specific therapies that impact relevant patient outcomes” [13]. Current categorizations of OSA patients, however, have not yet advanced to the status of endotype definition. At this point we believe that identifying meaningful phenotypes of OSA can be accomplished by anchoring them to relevant patient outcomes. Akin to the phenotype definition proposed for COPD [23], we propose the following operational definition for OSA phenotype:
“A category of patients with OSA distinguished from others by a single or combination of disease features, in relation to clinically meaningful attributes (symptoms, response to therapy, health outcomes, quality of life).”
Phenotypes lacking all of the qualities of an endotype can advance toward that status as supportive data (biologic markers, genomic, and genetic) are generated (Figure 1). This refinement may offer opportunities for even more targeted prognostication and treatments. The outcomes used in phenotype identification should be carefully defined, given that some features such as excessive daytime sleepiness, sleep fragmentation, or treatment-resistant hypertension may be viewed as either outcomes or phenotypes.
Figure 1. Heat map of key endotype and phenotype qualities illustrated through examples of possible phenotypes described in the literature.
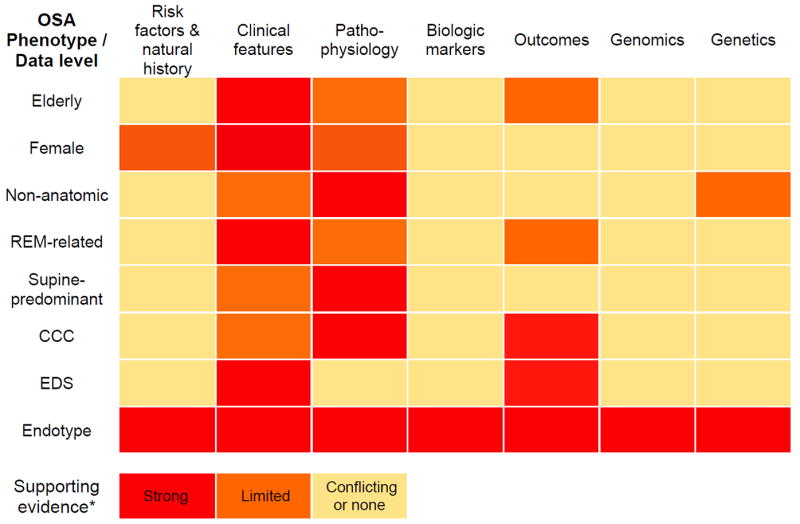
Phenotype illustrated above as having at least two data levels with strong supporting evidence (one must be “Outcomes”). Endotype illustrated above as having high degree of supporting evidence for each level (please see text for discussion).
CCC – Complete concentric palatal collapse, EDS – Excessive daytime sleepiness, Non-anatomic - (e.g. low arousal threshold or sensitive chemoreflex), OSA – Obstructive sleep apnea
* Degree of supporting evidence assigned by authors based on literature review (no formal definitions, for illustration purposes only).
An advantage of the proposed definition is that it offers the flexibility to identify phenotypes in situations where biological or genetic mechanisms of categorization may not yet be known but patient stratification may have prognostic or therapeutic utility. For example, complete concentric collapse of the palate in OSA patients predicts failure to respond to upper airway neuro-stimulation therapy [24]. Although the biological underpinnings of this OSA feature are not fully understood, meaningful benefits can accrue from using this phenotype to augment clinical trial design [15] and patient care [25]. The definition also provides a basis for deeper characterization of physiological or molecular mechanistic investigations. Those exhibiting similar clinical outcomes may behave so because of a similar underlying physiological or biological mechanism.
Phenotyping approaches
Phenotyping strategies can be grouped by features (e.g., clinical vs. molecular) and by experimental approaches (e.g., supervised vs. unsupervised) (Table 1) [16]. Clinical phenotyping focuses on identifying unique patient categories based on measures such as signs, symptoms, demographics, comorbidities, physiological and anatomic measures, or treatment responsiveness. Molecular phenotyping aims to classify individuals based on molecular features: DNA, RNA, mRNA, miRNA, proteins, metabolites and other biological products. Additionally, the number of features considered in the approach can vary from one (e.g., sex) to thousands (e.g., single nucleotide polymorphisms).
Table 1.
Phenotyping approaches*, associated analytic methods and examples of potential clinical phenotypes identified
| Approach | Features | Analysis method examples | Phenotyping examples |
|---|---|---|---|
| Supervised | Single |
|
|
| Multiple |
|
|
|
| Unsupervised | Multiple |
|
|
Supervised analytic methods evaluate a prespecified phenotype based on single or multiple features (Table 1). The premise is that the features differentiating subjects into specific subcategories represent biological/mechanistic differences between them [26]. In terms of complexity, these methods vary from standard regression analyses to machine learning algorithms incorporating complex, highly dimensional data [26].
Unsupervised analytic methods, such as cluster analysis, require no a priori classification of the data [27]. They are designed to organize information about features so that patients are classified into relatively homogeneous categories [28]. They have the advantage of generating new phenotypes based on unique associations between features not readily apparent in highly multidimensional data. As such, they are hypothesis generating rather than confirming. Ultimately, anchoring to outcomes using supervised analyses is necessary to establish the relevance of the newly identified phenotype.
Examples of these approaches and their corresponding methods are noted in Table 1, all of which have advantages and disadvantages related to underlying assumptions, hypotheses tested, and computational burden [29]. This schema will be used as a framework to review key examples of phenotyping in OSA in order of increasing analytical complexity. We start with phenotypes described using supervised, single feature analyses and end with multi-feature unsupervised and supervised approaches to OSA phenotypes. Given the relative paucity of publications in the molecular domain of OSA, this review focuses primarily on clinical phenotyping.
Potential, clinically relevant OSA phenotypes
Literature search
A literature search for key concepts related to sleep apnea and phenotypes was performed (see Supplement [Part A], for methods and article inclusion/exclusion criteria). Table of potential phenotypes and their characteristics can be viewed in the Supplement (Part B).
Single feature phenotypes (supervised analytic methods)
Symptom and demographics based features
The concept that differing presenting symptoms may have implications for pathogenesis and outcomes has been used to phenotype OSA individuals for decades. Up to sixty percent of OSA patients can be excessively sleepy [30] and report higher rates of impaired concentration, mood lability, and other neurocognitive difficulties [31]. Epidemiologic studies demonstrate that excessive daytime sleepiness (EDS) modifies the relationship between AHI and incidence of hypertension [32], glucose metabolism [33], and mortality [34]. This provided a ready target for treatment with CPAP and mandibular advancement devices which improve EDS [35]. Compared to those without sleepiness, treatment of patients with EDS has also been shown to reduce blood pressure, vascular risk [5, 10, 11], and improve quality of life [36]. Thus it has been proposed that OSA with EDS is a unique phenotype [37]. OSA with EDS phenotype appears to have clinical relevance but it is unlikely that it represents a true endotype given the multitude of contributors to EDS [38].
Age, gender, and race-ethnicity are increasingly recognized as factors impacting OSA presentation and implications [7, 39, 40]. For example, compared to younger patients with the same AHI, OSA in older patients is associated with less sleepiness [41] and often presents with enuresis, cognitive dysfunction, and mood impairments [42]. Additionally, with increasing age there is increased upper airway collapsibility as measured by pharyngeal critical closing pressure (Pcrit), decreased lung volume and ventilatory chemosensitivity. These observations have led some to suggest that OSA in the elderly may represent distinct physiological phenotype [43]. Supporting this claim, is a consistent lack of association between OSA and increased risk of hypertension [44] or atrial fibrillation [45] among older adults. Conflicting results exist, however, with regard to risk for coronary artery disease [46, 47], cognitive impairment [48-50] and death [47, 51]. The potential reasons for such variability are many, including differing definitions of OSA used in these studies and differing measures employed to characterize OSA severity (AHI, oxygen desaturation index [ODI], time spent below 90% oxygen saturation).
Age related differences in OSA are also gender dependent. OSA is less prevalent among premenopausal women, but postmenopausal women have a similar risk to that of men. Women tend to have longer sleep latency and more slow wave sleep [40]. Overall, their AHIs are lower, especially during NREM (non-rapid eye movement) sleep and respiratory events tend to cluster in REM sleep [40]. Such findings suggest that OSA in women is a separate phenotype, further supported by differences in predisposing factors for OSA. Fat distribution may play a role, with truncal obesity measured by waste to hip ratio (lower in women) mediating some of the association between gender and AHI [40]. Men have a larger [52], but more collapsible airway then women [53] and retrusive movement of the mandible decreases cross-sectional area of the oropharynx-pharynx in men, but not in women [54]. Although it is clear that OSA prevalence and pathogenesis differ by gender, the mechanisms and implications of these differences are not well understood. Studies of known determinants of upper airway stability such as pharyngeal fat content, upper airway muscle responsiveness, ventilatory sensitivity to hypoxia or hypercapnia, and upper airway resistance all show conflicting results when comparing men to women [40, 53]. Perhaps more importantly, the clinical implications for the gender differences are uncertain, with investigators reporting conflicting risk differences of incident hypertension [55, 56], congestive heart failure [7, 46], stroke [57, 58], treatment responsiveness [59], neurocognitive outcomes [12] or death [51, 60].
Polysomnographic and physiological features
Anisotropy of the upper airway collapse observed with positional changes has resulted in categorization of some individuals as positional OSA. Reports suggest that over 50% of those referred for OSA evaluation exhibit supine AHI that is at least twice that in non-supine position [61]. Patients with positional (supine-predominant) OSA tend to be younger, have lower body mass indices (BMI), and lower AHIs compared to their non-positional counterparts [61]. The characteristics and prevalence vary depending on the definition used (i.e., ratio of supine to non-supine events, lower limit of non-supine AHI, etc…) [62]. A consistent finding in is that pharyngeal collapsibility (Pcrit), markedly improves in lateral position [63], suggesting that smaller lateral wall size, decreased fat content, and lower facial height are associated with velopharyngeal patency while lateral [64]. Despite the high prevalence and plausible physiological mechanisms, conflicting data exists on clinical presentation and treatment responsiveness. The two largest studies (425 and 630 patients) examining treatment success (defined as residual AHI < 10 and ≥ 50% reduction) with mandibular advancement devices (MAD) showed conflicting results [65, 66]. Short-term studies (1 month follow up) suggest that auto-set PAP use is associated with improved sleepiness and wakefulness among supine-predominant patients in comparison to CPAP [67, 68], lending credence to idea that fixed PAP may not be optimal in all OSA patients with similar AHIs. Multiple position altering devices are efficacious at reducing time spent in supine position and AHI, but devices vary significantly in patient comfort and adherence with only short-term data available [69].
In addition to position, distribution of respiratory disturbance within sleep stages has been used to categorize OSA, with REM-predominant OSA being most studied. Patients with REM-predominant OSA tend to be younger women and have reduced sleep time, sleep efficiency, and REM duration. Decreased genioglossal muscle activity in REM [70], lower respiratory drive [71], longer duration events and more severe hypoxia combined with increased sympathetic activity during REM sleep [72] have led to hypotheses that events during this stage may have unique clinical implications. Similar to supine predominant OSA, prevalence of REM-predominant OSA depends on the definition used [73]. Despite the physiological and polysomnographic differences, large cross-sectional analyses comparing REM-predominant to NREM (or stage independent) OSA have failed to show differences in sleepiness, functional outcomes, PAP adherence, or quality of life [74-76]. Although lower response rates to MAD have been noted in REM-predominant OSA, REM-predominance was not a predictor of MAD responsiveness when age, BMI, and AHI were considered [66]. Limited data exist on neurocognitive, metabolic, or cardiovascular outcomes, but findings from the Wisconsin cohort demonstrate that a REM AHI ≥ 15 was associated with both prevalent and incident risk of hypertension [6]. The increased risk was present even among those with an overall AHI < 5. This suggests that some patients not meeting the current criteria for diagnosis and treatment of OSA may stand to benefit from treatment.
Studies of other polysomnographic and physiological features such as severity measures of airway obstruction, event duration, type (i.e., central, mixed, or obstructive), NREM predominance, and hypoxic load have been differentially associated with outcomes (see Supplement, Part B). Overall, the features discussed above do seem to capture parts of symptomatology, pathophysiology, and outcome heterogeneity among OSA patients, but it remains to be seen whether they represent true phenotypes or features of latent phenotypes yet to be identified. Focusing on individual features in isolation likely probes the same latent structure from different perspectives, much like using a single lead of an electrocardiogram to assess cardiac conduction. Comprehensive studies simultaneously considering feature interactions and their impact on outcomes are lacking. To address these challenges, some investigators have focused on outcomes and measured multiple features simultaneously to predict salient phenotypes.
Outcome based “reverse engineering” phenotypes (supervised analytic methods)
An example of this approach, includes use of drug-induced sleep endoscopy (DISE) to identify patients who benefit from upper airway neuro-stimulation therapy (UAS). A DISE study of 1249 patients showed that the collapse pattern is heterogeneous with respect to the level within the airway (e.g., tongue base vs velopharynx), direction (anterior–posterior, lateral vs. concentric), and degree of collapse (complete vs. partial) [77]. In another study, those with complete concentric collapse (CCC) at level of the palate (~ 30%), showed no improvement in AHI with UAS therapy, compared to 81% of patients without CCC in whom success was achieved. [24]. A subsequent randomized trial of 126 patients without CCC showed marked improvement in AHI, sleepiness, and functional outcomes that persisted through 2 years of follow-up [15, 25]. This work serves as an example of “reverse engineering” by developing a targeted intervention and matching it with a corresponding clinically important phenotype.
Multiple feature phenotypes (with supervised methods)
Based on accumulating data suggesting that multiple pathophysiological processes are involved in genesis of sleep apnea [78], a framework for deeper phenotyping of sleep apnea based on physiology has been developed [3]. With this approach, simultaneous measurement of multiple causative traits (anatomical predisposition [passive Pcrit], arousal threshold [ventilation prior to arousal], respiratory controller stability [loop gain], and upper airway muscle responsiveness [upper airway gain], designated as “PALM”) is performed using a modified polysomnography technique [3]. Eckert and colleagues showed marked heterogeneity of these traits among 75 OSA patients, with non-anatomic features such as high loop gain, low arousal threshold, and low upper airway gain being abnormal in over 50% of the patients [3]. Utilizing this framework, investigators demonstrated that disparate groups of OSA patients (e.g., elderly, supine predominant) differentially express the PALM causative traits (or their combinations) [43, 63]. Small studies show that individual traits are modifiable. For example, esczopiclone increases arousal threshold, acetazolamide reduces loop gain, and weight loss or uvulopalatopharyngoplasty lower Pcrit, and all are associated with reductions in AHI [79-83]. An integrative model developed using the PALM framework features can classify patients as with or without OSA and determine how many and which patients may benefit from trait modification [14]. The sensitivity and specificity for OSA were 88% and 80% respectively. Importantly, modification of a single trait with above treatments showed that the proportion of patients that could be successfully treated (NREM AHI < 10) ranged between 19 and 38%, while honing in on the predominantly abnormal trait in each patient, improved the success to 48%. Notably, combination of trait modifications (e.g., loop gain, arousal threshold, and anatomical collapsibility) was predicted to alleviate OSA in 81%. These findings are notable because they suggest that OSA patients can be meaningfully categorized based on heterogeneity of their physiology, and targeted non-PAP treatments can alleviate respiratory events. Furthermore, this approach provides new targets for treatment and means to stratify patients for clinical trials.
Although clearly an important step forward in personalizing approaches to OSA, important questions regarding this framework remain. Multiple traits can be measured simultaneously, yet it is unclear whether such traits change independently as ventilatory drive changes [84]. With some stimuli (e.g., hypoxia) for example, loop gain, upper airway gain, and arousal threshold are all affected [81]. Replication of this work is needed, as conflicting results of the traits’ association with OSA risk factors (gender [53, 85]) and treatments (trazodone) aimed at modifying individual traits (arousal threshold [86, 87]) have been found. Despite efforts to make measurements of these traits more accessible to the greater research community [88, 89], the technology and expertise required are complex and not yet available even in most academic centers. Finally, the use of this framework remains largely theoretical and the impact on OSA severity and most importantly relevant patient outcomes (e.g., cardiovascular, metabolic, neurocognitive, functional) is a needed area of future investigation.
Another approach to characterizing OSA physiology uses electrocardiography (ECG)-based measures of autonomic and respiratory interaction (cardiopulmonary coupling). An example of this approach integrates cardiac inter-beat (R-R interval) and respiratory (ECG-lead axis variation) dynamics to generate a map of coupled sleep oscillations, a sleep spectrogram [90]. The spectrographic measures correlate with electroencephalographic markers of sleep stability, not ordinarily captured by conventional sleep stage distributions [90]. Transitions occur between periods of high frequency coupling (HFC, putative “stable” sleep), low frequency coupling (LFC, “unstable” sleep) and very low frequency coupling (VLFC, wake or REM sleep). A subset of LFC, termed elevated narrow band LFC (e-LFCnb) was found to be a marker of increased chemoreflex activation. This is suggested by correlation with central apneas and increased risk of treatment emergent central sleep apnea (TE-CSA) [91]. e-LFCnb is heritable [92], and has been associated with prevalent hypertension and stroke in the sleep heart health study in adjusted analyses, including the AHI [93]. No associations with incident outcomes have been reported and treatment implications of this phenotype require investigation. In a single study e-LFCnb did not predict adaptive servo ventilator responsiveness among patients with TE-CSA [94]. It remains to be investigated how measures such as e-LFCnb are integrated into individual patient phenotyping and whether targeted interventions improve outcomes.
Complementary to the work on non-anatomical OSA risk factors, cephalometric measures have been used to characterize OSA in the domain of craniofacial skeletal and soft tissue morphology. Mandibular retrusion, maxillary deficiency, and inferior displacement of the hyoid bone and cranial base are commonly reported risk factors for OSA [95]. Cephalometric measures have been reported to explain some of the racial, ethnic, and gender variance in risk for sleep apnea [39]. Additionally, cephalometric analyses show that abnormalities in the soft tissue morphology predominate among obese OSA patients, in contrast to skeletal structure changes in non-obese OSA individuals [96]. Certain dimensions, including lower facial height, mandibular length and angles between sella-nasion and anterior points of maxilla/mandible are heritable among OSA patients [97]. The observed variability in cephalometric measures in OSA individuals has not, however, been translated into ability to predict clinically relevant outcomes. From treatment standpoint, most literature exists on MAD therapy. In one study using multivariate analyses and controlling for age, BMI and sex, only a long soft palate and large cranial base angulation [98] predicted failure of MAD (negative predictive value 98%). While some reports support these findings, others identify alternate correlations of treatment success, and most are limited by small and heterogeneous sample sizes without consideration of known important factors (BMI, sex, neck circumference) [99]. Similar conclusions have been made regarding cephalometric data on prediction of surgical outcomes [100].
Newer and less costly methods of capturing skeletal and adipose tissue morphologic heterogeneity from digital photography termed “facial phenotyping” have been recently reported. Shorter, retruded jaw, smaller mandibular enclosure area, wider and flatter mid-lower face predict OSA independent of BMI and sex [95]. Features of facial structure were highly correlated to magnetic resonance imaging cephalometric measures known to be risk factors for OSA. Some (maxillary-mandibular relationship, lower face height and mandibular length) were obesity independent, while others (tongue volume) were not, suggesting that the approach may be able to capture both skeletal and soft tissue risk factors for OSA [101]. No studies relating these measures to outcomes have been reported.
Characterization of OSA using features beyond those routinely measured in clinical practice and focusing on the putative causative mechanisms of OSA, may have important implications. Termed intermediate phenotypes, measures of physiological and anatomical mechanisms in pathogenesis of OSA highlighted above may help link genetic and biological pathways to the clinical expression of OSA (a domain where use of AHI and standard demographic data as phenotypes has led to limited success), and identify more robust phenotypes amenable to targeted interventions.
Multiple feature phenotypes (unsupervised methods with or without supervised outcome association)
Although approaches described above have resulted in identification of potentially clinically relevant OSA categories, they are based on a priori (usually clinical) observations, thus potentially missing more complex, and as yet unidentified phenotypes [102]. Recently, several groups began phenotyping OSA in a more statistically based manner with cluster analysis being most commonly employed technique.
Examples of this approach in OSA literature are few so far [2, 4, 103-106]. While most studies are relatively large (881 to 5983 patients), they vary widely by features and populations selected and only two used similar methodology. Ye et al., identified three clusters of patients in a predominantly male Iceland sleep apnea cohort: “excessive daytime sleepiness” 42%, “disturbed sleep/insomnia’’ 33% and ‘‘minimally symptomatic’’ 25% of those studied [2]. Although traditional risk and severity factors such as gender, BMI and AHI were equivalent in these clusters, measures of mental and physical health, as well as prevalence of cardiovascular disease (notably highest in the “minimally symptomatic” cluster) differed significantly. A more gender balanced study by Gagnadoux et al., [103] identified similar symptom based clusters with comparable distributions of comorbidities. Two additional patient categories were identified: 1) middle aged, obese women with marked insomnia, depression, and comorbidities, and 2) older, obese men with typical nocturnal and diurnal OSA symptoms, depression, and a marked comorbidity burden. Notably, the minimally symptomatic, the male disturbed sleep/insomnia (identified in both studies) and obese female OSA with insomnia clusters (identified only by Gagnadoux et al. [103]) exhibited 50 – 75% lower rates of successful CPAP treatment at six months in comparison to the “excessive daytime sleepiness” phenotype (noted in both studies). The differences were independent of socioeconomic status, sleepiness and AHI. These findings suggest that use of simple indicators such as AHI and sleepiness alone is insufficient to understand OSA’s clinical presentation, implications for treatment, and quality of life. They suggest for example, that in OSA patients with insomnia, combination therapy (e.g., cognitive behavioral therapy and CPAP) or non-PAP therapies rather than CPAP could be explored. Supporting this notion, are studies of insomnia timing subtypes in OSA, with both sleep initiation and late night insomnia patients being less sleepy, less adherent and responsive to CPAP therapy, in contrast to patients with middle of the night insomnia [107, 108]. In addition, these studies provide a basis for more rational patient selection in clinical trial design.
Vavougios et al., showed that a wide range of comorbidities can vary markedly within equivalent, AHI-based OSA severity categories [4]. A highly comorbid cluster and one without significant comorbidity were identified within both, moderate and severe OSA patient groups. Notably, both low comorbidity clusters were similar in age, BMI, sleepiness, and daytime oxygen saturation but exhibited a 2-3 fold difference in AHI, ODI, and arousal index [46]. Further work in this domain has the potential to explain factors responsible for “resiliency” to the increasing OSA severity.
Studies using the above approaches provide insights into symptomatic heterogeneity and identify potentially clinically relevant phenotypes. Further investigation however is needed. Most analyses are cross-sectional, lacking the ability to discern temporal relationships. It remains unknown when these phenotypes first manifest, whether they are stable over time, (single night or longer scales) and if/how individuals transition between them. Furthermore, many features capturing the known risk factors for and etiologic mechanisms in OSA are missing from the designs.
Summary and future directions
Evidence is accumulating and consensus is building that AHI alone is insufficient for diagnosis and management of individuals with OSA. Complementary to molecular phenotyping, clinical phenotyping may serve as an intermediate step towards personalized medicine in OSA. Emerging themes from current literature include needs for: 1) improved anchoring of phenotypes to clinically relevant outcomes and 2) addressing the interplay of features important in pathogenesis of OSA though advanced analytic methods.
Linking phenotypes to meaningful, longitudinal outcomes can facilitate their selection and refinement (e.g., REM-related OSA). Focus on outcomes distal to AHI reduction such as blood pressure or neurocognitive performance is prudent given examples in the literature that lowering AHI may not translate to benefit [5] or may even be harmful in some patients [109]. Expanding the outcome domain beyond “hard” endpoints such as mortality to include functional and patient-centered outcomes [110] may capture phenotypes with importance to our patients that would otherwise be missed.
Because of the inherent correlation of the features used to characterize OSA patients, applying machine learning approaches like cluster analysis or neural networks to simultaneously assess the clinical and the novel promising phenotype measures (e.g. PALM model, facial analysis and cardiopulmonary coupling features) will be important. Such approaches may not only provide phenotypic classification using a single level of data (e.g., clinical, biologic, genomic) but also show promise integrating data between levels to identify mechanistic distinctions [111] more closely resembling endotypes.
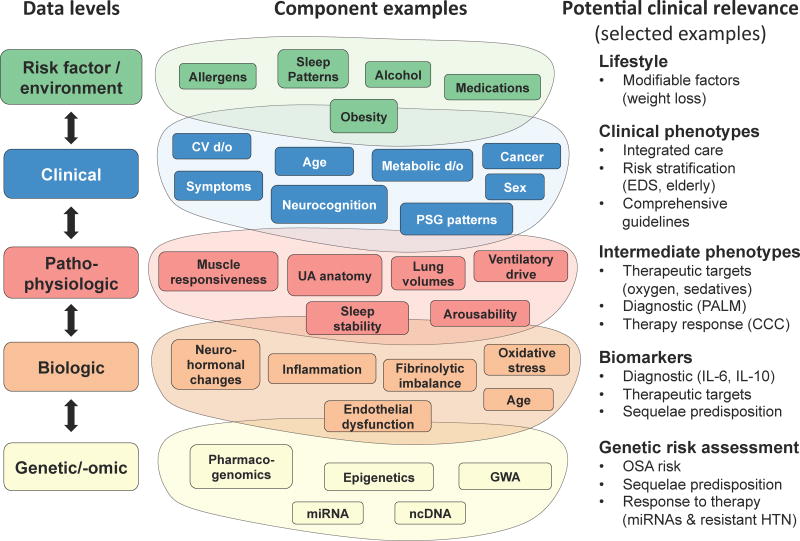
In the context of systems biology, clinical and pathophysiologic OSA features constitute just some levels of the data to be integrated to predictively model disease expression and response to therapy [112]. Figure 2 illustrates the levels of OSA characterization (risk factor/environmental, clinical, pathophysiologic, biologic, genomic/genetic) and the potential benefits of translating the new knowledge at each level for research and clinical practice. At the clinical level, for example, a better understanding of the relationships between comorbidities can improve strategies for integrated care. In addition, examples of clinical (EDS) and intermediate (CCC) phenotypes predicting response to CPAP exist [11, 15]. Similarly, better understanding of the genomic and biologic levels can help determine risk of future complications and response to treatment. For example, in OSA patients with resistant hypertension, a cluster of miRNAs discriminated between those with and without favorable blood pressure response to CPAP [113].
Figure 2. Data levels in obstructive sleep apnea (OSA) phenotyping and the potential benefits.
Illustration of phenotyping data levels (risk factor/environment, clinical, pathophysiologic, biological, gen-etic/omic) in OSA and the potential benefits (right-hand column). Each level shows only some examples of the potential components (not intended to be comprehensive). Arrows signify integration of the levels to better understand their relationship in OSA. CCC – complete concentric palatal collapse, CV d/o – cardiovascular disorders, EDS – excessive daytime sleepiness, GWA – genome-wide associations, HTN – hypertension, IL – interleukin, miRNA – microRNA, ncDNA – non-coding DNA, PALM - Passive Pcrit, Arousal threshold, Loop gain, and upper airway Muscle responsiveness model, PSG – polysomnographic, UA – upper airway. Structure adapted with permission from Agusti et al., 2011 [115], with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society.
Knowledge from each of the above levels can enable targeted patient selection in OSA clinical trials, reduce resource use and lessen the burden on patients unlikely to benefit from treatment. Phenotype identification and validation will likely require an iterative process (Figure 3), with multiple points of entry, as research is advancing in several domains simultaneously. One way to integrate phenotype information has been proposed in COPD [114] and an analogous approach may be applicable to OSA. It organizes the actionable information available for individualized treatment for a patient (e.g., symptom phenotype, polysomnographic phenotype, physiological trait measures, airway collapse pattern, co-morbid disease responsiveness to treatment, etc.) into domains of syndrome severity, causative factors, and impact. Biomarker and genomic data can be incorporated as our concepts of OSA phenotypes evolve, enabling development of personalized therapies for OSA.
Figure 3. Iterative process of phenotype identification and validation in obstructive sleep apnea (OSA).

Multiple points of entry into the process are possible. For example, phenotyping process may begin by differentiation of patient group based on a biomarker, later validated by similar clinical prognosis or response to treatment within that subgroup. Alternatively, patient groups may be identified by similar clinical outcomes potentially suggesting a physiologic target for focused treatment. Adapted from Han et al., 2010 [23], with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society.
Supplementary Material
Practice Points.
OSA phenotype can be defined operationally as: “A category of patients with OSA distinguished from others by a single or combination of disease features, in relation to clinically meaningful attributes (symptoms, response to therapy, health outcomes, quality of life)
The AHI is insufficient to capture clinical heterogeneity of patients with OSA and should not be used in isolation for management of patients
Symptoms other than sleepiness (e.g., insomnia) should be considered during evaluation of OSA patients.
Elevated AHI (≥ 15 events per hour) during rapid eye movement sleep, even in those with overall AHI < 5, should prompt a discussion regarding risks and benefits of treatment
The “PALM” physiological framework approach is promising for identifying patients who may benefit from non-CPAP therapies, but further clinical validation is required
Although cephalometry captures craniofacial structural differences among OSA patients, current data does not support its use in clinical practice
Unsupervised phenotyping approaches (e.g., cluster analysis) are promising for identifying unique categories amongst patients with OSA
Research Agenda.
Anchor clinical phenotypes to meaningful outcomes early during the phenotype identification process
Validate intermediate phenotypes (etiologic physiology, facial analysis, cardiopulmonary coupling measures) with clinical outcomes
Evaluate the putative phenotypes (and their features) using advanced analytic techniques (e.g. machine learning)
Assess temporal stability of identified phenotypes
Acknowledgments
This work was supported by Robert E. Leet and Clara Guthrie Patterson Trust Fellowship Program in Clinical Research, Bank of America, N.A., Trustee and by the National Institutes of Health training grant T32 HL007778-21.
Glossary of terms
- AHI
Apnea hypopnea index
- ASV
Adaptive servo ventilation
- Auto-PAP
Auto-set positive airway pressure
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CPAP
Continuous positive airway pressure
- DNA
Deoxyribonucleic acid
- DISE
Drug induced sleep endoscopy
- ECG
Electrocardiogram
- e-LFCnb
Elevated low frequency coupling in the narrow band as referred to in cardiopulmonary coupling
- ESS
Epworth sleepiness scale
- HFC
High frequency coupling as referred to in cardiopulmonary coupling
- LFC
Low frequency coupling as referred to in cardiopulmonary coupling
- MAD
Mandibular advancement device
- mRNA
Messenger ribonucleic acid
- miRNA
Mircro ribonucleic acid
- NREM
Non-rapid eye movement
- ODI
Oxygen desaturation index
- OSA
Obstructive sleep apnea
- Pcrit
Passive critical closing pressure
- PALM
Passive Pcrit, Arousal threshold, Loop gain, and upper airway Muscle responsiveness.
- REM
Rapid eye movement
- RNA
Ribonucleic acid
- TE-CSA
Treatment emergent central sleep apnea
- SDB
Sleep disordered breathing
- UAS
Upper airway neuro-stimulation
- VLFC
Very low frequency coupling as referred to in cardiopulmonary coupling
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayas NT, Owens RL, Kheirandish-Gozal L. Update in Sleep Medicine 2014. Am J Respir Crit Care Med. 2015;192(4):415–20. doi: 10.1164/rccm.201503-0647UP. [DOI] [PubMed] [Google Scholar]
- * 2.Ye L, Pien GW, Ratcliffe SJ, Bjornsdottir E, Arnardottir ES, Pack AI, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–7. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vavougios GD, Natsios G, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. 2015 doi: 10.1111/jsr.12344. [DOI] [PubMed] [Google Scholar]
- 5.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- * 6.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–67. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roca GQ, Redline S, Claggett B, Bello N, Ballantyne CM, Solomon SD, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort. Circulation. 2015;132(14):1329–37. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Sleep Medicine. International classification of sleep disorders. 3. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 9.Eastwood PR, Malhotra A, Palmer LJ, Kezirian EJ, Horner RL, Ip MS, et al. Obstructive Sleep Apnoea: From pathogenesis to treatment: Current controversies and future directions. Respirology. 2010;15(4):587–95. doi: 10.1111/j.1440-1843.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145(4):762–71. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–85. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35(12):1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 13.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- * 14.Owens RL, Edwards BA, Eckert DJ, Jordan AS, Sands SA, Malhotra A, et al. An Integrative Model of Physiological Traits Can be Used to Predict Obstructive Sleep Apnea and Response to Non Positive Airway Pressure Therapy. Sleep. 2015;38(6):961–70. doi: 10.5665/sleep.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 15.Strollo PJ, Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 16.Pillai RA, Calhoun WJ. Introduction to asthma and phenotyping. Adv Exp Med Biol. 2014;795:5–15. doi: 10.1007/978-1-4614-8603-9_1. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–62. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med. 2015;373(24):2325–35. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 21.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 22.Boudier A, Curjuric I, Basagana X, Hazgui H, Anto JM, Bousquet J, et al. Ten-year follow-up of cluster-based asthma phenotypes in adults. A pooled analysis of three cohorts. Am J Respir Crit Care Med. 2013;188(5):550–60. doi: 10.1164/rccm.201301-0156OC. [DOI] [PubMed] [Google Scholar]
- 23.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderveken OM, Maurer JT, Hohenhorst W, Hamans E, Lin HS, Vroegop AV, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9(5):433–8. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soose RJ, Woodson BT, Gillespie MB, Maurer JT, de Vries N, Steward DL, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Self-reported Outcomes at 24 Months. J Clin Sleep Med. 2015 doi: 10.5664/jcsm.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasier AR, Ju H. Analysis and predictive modeling of asthma phenotypes. Adv Exp Med Biol. 2014;795:273–88. doi: 10.1007/978-1-4614-8603-9_17. [DOI] [PubMed] [Google Scholar]
- * 27.Tarca AL, Carey VJ, Chen XW, Romero R, Draghici S. Machine learning and its applications to biology. PLoS Comput Biol. 2007;3(6):e116. doi: 10.1371/journal.pcbi.0030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhavnani SK, Drake J, Divekar R. The role of visual analytics in asthma phenotyping and biomarker discovery. Adv Exp Med Biol. 2014;795:289–305. doi: 10.1007/978-1-4614-8603-9_18. [DOI] [PubMed] [Google Scholar]
- 29.Kerr G, Ruskin HJ, Crane M, Doolan P. Techniques for clustering gene expression data. Comput Biol Med. 2008;38(3):283–93. doi: 10.1016/j.compbiomed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Bjorvatn B, Lehmann S, Gulati S, Aurlien H, Pallesen S, Saxvig IW. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath. 2015;19(4):1387–93. doi: 10.1007/s11325-015-1155-5. [DOI] [PubMed] [Google Scholar]
- 31.Vaessen TJ, Overeem S, Sitskoorn MM. Cognitive complaints in obstructive sleep apnea. Sleep Med Rev. 2015;19:51–8. doi: 10.1016/j.smrv.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Kapur VK, Resnick HE, Gottlieb DJ Sleep Heart Health Study G. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008;31(8):1127–32. [PMC free article] [PubMed] [Google Scholar]
- 33.Barcelo A, Barbe F, de la Pena M, Martinez P, Soriano JB, Pierola J, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63(11):946–50. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 34.Gooneratne NS, Richards KC, Joffe M, Lam RW, Pack F, Staley B, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34(4):435–42. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharples LD, Clutterbuck-James AL, Glover MJ, Bennett MS, Chadwick R, Pittman MA, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2015;27:108–24. doi: 10.1016/j.smrv.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186(7):677–83. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Zhang C, Jia P, Zhang J, Feng L, Wei S, et al. The association between the phenotype of excessive daytime sleepiness and blood pressure in patients with obstructive sleep apnea-hypopnea syndrome. Int J Med Sci. 2014;11(7):713–20. doi: 10.7150/ijms.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea--a review. Sleep. 2012;35(5):605–15. doi: 10.5665/sleep.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2015 doi: 10.1016/j.sleep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 40.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–96. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J. 2012;40(2):386–93. doi: 10.1183/09031936.00177411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014;30(3):591–627. doi: 10.1016/j.cger.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37(7):1227–36. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–21. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 45.Lin GM, Colangelo LA, Lloyd-Jones DM, Redline S, Yeboah J, Heckbert SR, et al. Association of Sleep Apnea and Snoring With Incident Atrial Fibrillation in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2015;182(1):49–57. doi: 10.1093/aje/kwv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, De la Cruz Moron I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909–16. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 48.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26(5):596–9. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 49.Zamora Molina L, Martinez Garcia MA, Chiner E, Hernández Blasco L, Cortes J, Catalan P, et al. Obstructive sleep apnea (OSA) in elderly patients. Role of continuos positive airway pressure (CPAP) treatment. A multicenter randomized controlled clinical trial. Eur Respir J. 2014;44(Suppl 58) [Google Scholar]
- 50.Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63(3):453–61. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perri RA, Kairaitis K, Wheatley JR, Amis TC. Anthropometric and craniofacial sexual dimorphism in obstructive sleep apnea patients: is there male-female phenotypical convergence? J Sleep Res. 2015;24(1):82–91. doi: 10.1111/jsr.12205. [DOI] [PubMed] [Google Scholar]
- 53.Jordan AS, Wellman A, Edwards JK, Schory K, Dover L, MacDonald M, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol (1985) 2005;99(5):2020–7. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohsenin V. Gender differences in the expression of sleep-disordered breathing : role of upper airway dimensions. Chest. 2001;120(5):1442–7. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–64. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 57.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189(12):1544–50. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 58.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye L, Pien GW, Ratcliffe SJ, Weaver TE. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J Clin Sleep Med. 2009;5(6):512–8. [PMC free article] [PubMed] [Google Scholar]
- 60.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 61.Gadoth N, Oksenberg A. Positional therapy in obstructive sleep apnea: For whom and for whom not. Positional Therapy in Obstructive Sleep Apnea. 2015:383–94. [Google Scholar]
- 62.Joosten SA, O’Donoghue FJ, Rochford PD, Barnes M, Hamza K, Churchward TJ, et al. Night-tonight repeatability of supine-related obstructive sleep apnea. Ann Am Thorac Soc. 2014;11(5):761–9. doi: 10.1513/AnnalsATS.201309-306OC. [DOI] [PubMed] [Google Scholar]
- 63.Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, et al. The Effect of Body Position on Physiological Factors that Contribute to Obstructive Sleep Apnea. Sleep. 2015;38(9):1469–78. doi: 10.5665/sleep.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saigusa H, Suzuki M, Higurashi N, Kodera K. Three-dimensional morphological analyses of positional dependence in patients with obstructive sleep apnea syndrome. Anesthesiology. 2009;110(4):885–90. doi: 10.1097/ALN.0b013e31819b5d57. [DOI] [PubMed] [Google Scholar]
- 65.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 66.Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J Clin Sleep Med. 2015;11(8):861–8. doi: 10.5664/jcsm.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oksenberg A, Silverberg DS, Arons E, Radwan H. The sleep supine position has a major effect on optimal nasal continuous positive airway pressure : relationship with rapid eye movements and non-rapid eye movements sleep, body mass index, respiratory disturbance index, and age. Chest. 1999;116(4):1000–6. doi: 10.1378/chest.116.4.1000. [DOI] [PubMed] [Google Scholar]
- 68.Series F, Marc I. Importance of sleep stage- and body position-dependence of sleep apnoea in determining benefits to auto-CPAP therapy. Eur Respir J. 2001;18(1):170–5. doi: 10.1183/09031936.01.98103501. [DOI] [PubMed] [Google Scholar]
- * 69.Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18(1):7–17. doi: 10.1016/j.smrv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 70.McSharry DG, Saboisky JP, Deyoung P, Jordan AS, Trinder J, Smales E, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37(3):561–9. doi: 10.5665/sleep.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982;126(5):758–62. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- 72.Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 73.Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16(2):519–26. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 74.Chami HA, Baldwin CM, Silverman A, Zhang Y, Rapoport D, Punjabi NM, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181(9):997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan A, Harrison SL, Kezirian EJ, Ancoli-Israel S, O’Hearn D, Orwoll E, et al. Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in Osteoporotic Fractures in Men (MrOS) Sleep Study. J Clin Sleep Med. 2013;9(3):191–8. doi: 10.5664/jcsm.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su CS, Liu KT, Panjapornpon K, Andrews N, Foldvary-Schaefer N. Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med. 2012;8(3):243–7. doi: 10.5664/jcsm.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vroegop AV, Vanderveken OM, Boudewyns AN, Scholman J, Saldien V, Wouters K, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope. 2014;124(3):797–802. doi: 10.1002/lary.24479. [DOI] [PubMed] [Google Scholar]
- 78.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120(12):505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590(Pt 5):1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edwards BA, Sands SA, Owens RL, White DP, Genta PR, Butler JP, et al. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol. 2014;592(Pt 20):4523–35. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144(3 Pt 1):494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz AR, Schubert N, Rothman W, Godley F, Marsh B, Eisele D, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145(3):527–32. doi: 10.1164/ajrccm/145.3.527. [DOI] [PubMed] [Google Scholar]
- 84.Kirkness JP. Obesity-related ventilatory phenotypes of sleep-disordered breathing. Am J Respir Crit Care Med. 2014;190(8):853–4. doi: 10.1164/rccm.201409-1674ED. [DOI] [PubMed] [Google Scholar]
- 85.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999;159(1):149–57. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 86.Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811–9. doi: 10.5665/sleep.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smales ET, Edwards BA, Deyoung PN, McSharry DG, Wellman A, Velasquez A, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758–64. doi: 10.1513/AnnalsATS.201408-399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45(2):408–18. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28(9):1151–61. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 91.Thomas RJ, Mietus JE, Peng CK, Gilmartin G, Daly RW, Goldberger AL, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30(12):1756–69. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ibrahim LH, Jacono FJ, Patel SR, Thomas RJ, Larkin EK, Mietus JE, et al. Heritability of abnormalities in cardiopulmonary coupling in sleep apnea: use of an electrocardiogram-based technique. Sleep. 2010;33(5):643–6. doi: 10.1093/sleep/33.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas RJ, Weiss MD, Mietus JE, Peng CK, Goldberger AL, Gottlieb DJ. Prevalent hypertension and stroke in the Sleep Heart Health Study: association with an ECG-derived spectrographic marker of cardiopulmonary coupling. Sleep. 2009;32(7):897–904. [PMC free article] [PubMed] [Google Scholar]
- 94.Ramar K, Desrues B, Ramar P, Morgenthaler TI. Analysis of cardiopulmonary coupling to assess adaptive servo-ventilation success in complex sleep apnea management. Sleep Breath. 2013;17(2):861–6. doi: 10.1007/s11325-012-0780-5. [DOI] [PubMed] [Google Scholar]
- 95.Lee RWW, Chan ASL, Grunstein RR, Cistulli PA. Craniofacial phenotyping in obstructive sleep apnea - A novel quantitative photographic approach. Sleep. 2009;32(1):37–45. [PMC free article] [PubMed] [Google Scholar]
- 96.Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnoea: multiple comparisons of cephalometric variables of obese and non-obese patients. J Craniomaxillofac Surg. 2000;28(4):204–12. doi: 10.1054/jcms.2000.0147. [DOI] [PubMed] [Google Scholar]
- 97.Chi L, Comyn FL, Keenan BT, Cater J, Maislin G, Pack AI, et al. Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep. 2014;37(10):1689–98. doi: 10.5665/sleep.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Cephalometry and prediction of oral appliance treatment outcome. Sleep Breath. 2012;16(1):47–58. doi: 10.1007/s11325-011-0484-2. [DOI] [PubMed] [Google Scholar]
- * 99.Guarda-Nardini L, Manfredini D, Mion M, Heir G, Marchese-Ragona R. Anatomically Based Outcome Predictors of Treatment for Obstructive Sleep Apnea with Intraoral Splint Devices: A Systematic Review of Cephalometric Studies. J Clin Sleep Med. 2015;11(11):1327–34. doi: 10.5664/jcsm.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Denolf PL, Vanderveken OM, Marklund ME, Braem MJ. The status of cephalometry in the prediction of non-CPAP treatment outcome in obstructive sleep apnea patients. Sleep Med Rev. 2016;27:56–73. doi: 10.1016/j.smrv.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 101.Sutherland K, Schwab RJ, Maislin G, Lee RW, Benedikstdsottir B, Pack AI, et al. Facial phenotyping by quantitative photography reflects craniofacial morphology measured on magnetic resonance imaging in Icelandic sleep apnea patients. Sleep. 2014;37(5):959–68. doi: 10.5665/sleep.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burgel PR, Paillasseur JL, Roche N. Identification of clinical phenotypes using cluster analyses in COPD patients with multiple comorbidities. Biomed Res Int. 2014;2014:420134. doi: 10.1155/2014/420134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 103.Gagnadoux F, Le Vaillant M, Paris A, Pigeanne T, Leclair-Visonneau L, Bizieux-Thaminy A, et al. Relationship Between OSA Clinical Phenotypes and CPAP Treatment Outcomes. Chest. 2016;149(1):288–90. doi: 10.1016/j.chest.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 104.Hierl T, Humpfner-Hierl H, Frerich B, Heisgen U, Bosse-Henck A, Hemprich A. Obstructive sleep apnoea syndrome: results and conclusions of a principal component analysis. J Craniomaxillofac Surg. 1997;25(4):181–5. doi: 10.1016/s1010-5182(97)80073-x. [DOI] [PubMed] [Google Scholar]
- 105.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17(1):99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 106.Tsuchiya M, Lowe AA, Pae EK, Fleetham JA. Obstructive sleep apnea subtypes by cluster analysis. Am J Orthod Dentofacial Orthop. 1992;101(6):533–42. doi: 10.1016/0889-5406(92)70128-W. [DOI] [PubMed] [Google Scholar]
- 107.Bjornsdottir E, Janson C, Sigurdsson JF, Gehrman P, Perlis M, Juliusson S, et al. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901–9. doi: 10.5665/sleep.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subramanian S, Guntupalli B, Murugan T, Bopparaju S, Chanamolu S, Casturi L, et al. Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep Breath. 2011;15(4):711–5. doi: 10.1007/s11325-010-0426-4. [DOI] [PubMed] [Google Scholar]
- 109.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med. 2015;373(12):1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Billings ME, Rosen CL, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, et al. Psychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: the HomePAP study. Sleep. 2014;37(12):2017–24. doi: 10.5665/sleep.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S, Herazo-Maya JD, Kang DD, Juan-Guardela BM, Tedrow J, Martinez FJ, et al. Integrative phenotyping framework (iPF): integrative clustering of multiple omics data identifies novel lung disease subphenotypes. BMC Genomics. 2015;16:924. doi: 10.1186/s12864-015-2170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12(1):56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, Martinez-Alonso M, Martinez-Garcia MA, Barcelo A, et al. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. J Am Coll Cardiol. 2015;66(9):1023–32. doi: 10.1016/j.jacc.2015.06.1315. [DOI] [PubMed] [Google Scholar]
- 114.Agusti A, Gea J, Faner R. Biomarkers, the control panel and personalized COPD medicine. Respirology. 2016;21(1):24–33. doi: 10.1111/resp.12585. [DOI] [PubMed] [Google Scholar]
- 115.Agusti A, Vestbo J. Current controversies and future perspectives in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(5):507–13. doi: 10.1164/rccm.201103-0405PP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.