Abstract
Rationale
Thymic stromal lymphopoietin (TSLP) is known to be elevated and truncated in nasal polyps (NPs) of chronic rhinosinusitis and might play a significant role in type 2 inflammation in this disease. However, neither the structure nor the role of the truncated products of TSLP has been studied.
Objective
To investigate the mechanisms of truncation of TSLP in NPs and the function of the truncated products.
Methods
We incubated recombinant human TSLP with NP extracts, and determined the protein sequence of the truncated forms of TSLP using Edman protein sequencing and MALDI-TOF mass spectrometry (MS). We investigated the functional activity of truncated TSLP using a PBMC-based bioassay.
Results
Edman sequencing and MS results indicated that NP extracts generated two major truncated products, TSLP (residues 29-124) and TSLP (131-159). Interestingly, these two products remained linked with disulfide bonds and presented as a dimerized form, TSLP (29-124 + 131-159). We identified that members of the proprotein convertase were rate-limiting enzymes in the truncation of TSLP between residues 130 and 131 and generated a heterodimeric unstable metabolite TSLP (29-130 + 131-159). Carboxypeptidase N immediately digested 6 amino acids from the C-terminus of the longer subunit of TSLP to generate a stable dimerized form, TSLP (29-124 + 131-159), in NPs. These truncations were homeostatic but primate specific events. A metabolite TSLP (29-130 + 131-159) strongly activated myeloid dendritic cells and group 2 innate lymphoid cells compared to mature TSLP.
Conclusion
Post-translational modifications control functional activity of TSLP in humans and overproduction of TSLP may be a key trigger for the amplification of type 2 inflammation in diseases.
Capsule Summary
Post-translational modification of TSLP might control functional activity of TSLP in humans.
Keywords: Carboxypeptidase, Chronic rhinosinusitis, Dendritic cells, Group 2 innate lymphoid cells, Nasal polyps, Post-translational modification, Proprotein convertases, Thymic stromal lymphopoietin
Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the upper airways and sinuses that persists for more than 12 weeks. CRS affects nearly 30 million Americans, is responsible for over 500,000 surgeries annually, and produces significant morbidity.1-3 Although there is an ongoing debate about endotypes of disease, most investigators still accept a paradigm in which CRS is generally divided into two groups based on the presence or absence of nasal polyps (NPs): CRS with NPs (CRSwNP) and CRS without NPs (CRSsNP).4-7 Although CRSwNP is usually characterized by strong signs of type 2 inflammation, including pronounced eosinophilia and accumulation of T cells, dendritic cells (DCs), mast cells and group 2 innate lymphoid cells (ILC2s),7-12 the mechanisms underlying the amplification of type 2 inflammation in CRSwNP have not been clarified.
An epithelial cytokine, thymic stromal lymphopoietin (TSLP), is known to control type 2 inflammation via activation of DCs, mast cells and ILC2s.12-16 TSLP induces the recruitment of Th2 cells and the induction of Th2 differentiation in mDCs via production of chemokines, CCL17 and CCL22, and induction of OX40 ligand, respectively.17 Although TSLP alone is not sufficient to produce type 2 cytokines in mast cells and ILC2s, it strongly synergizes with IL-33 to do so.12, 18-21 TSLP is known to be highly expressed in several type 2 inflammatory diseases including atopic dermatitis and bronchial asthma.12-14, 22-24 We have recently found that TSLP is also upregulated in CRSwNP and that mRNA expression of TSLP positively correlates with markers of eosinophils and type 2 cytokines in NPs.25 Recently, a clinical trial showed that an anti-human TSLP monoclonal antibody (AMG 157) reduced allergen-induced allergic responses in patients with mild allergic asthma.26 This indicates that TSLP may be a key factor in allergic and type 2 inflammatory diseases including CRSwNP, and is an important therapeutic target in these diseases.
It is now recognized that post-translational modifications (PTMs) of cytokines and chemokines by endogenous proteases are important events in the fine-tuning of biological activity.27, 28 However, it is not known whether TSLP can be controlled by PTM after secretion from producing cells including epithelial cells. The human TSLP gene encodes a protein of 159 amino acids and contains a signal peptide (residues 1-28) in the N-terminus.29, 30 After removal of the signal peptide, cells release 14.9 KDa TSLP (residues 29-159) as a mature protein (referred to as TSLP (29-159) or mature TSLP). Interestingly, we have recently found that mature TSLP is unstable in NP tissues and we have detected truncated TSLP with a molecular weight of approximately 10-11 KDa, when we incubate NP extracts with recombinant human mature 15.1 KDa TSLP (Met (start codon) + residues 29-159, referred to as TSLP (M29-159)).25 Since a protease inhibitor cocktail (PIC) completely blocks NP extract-mediated truncation and degradation of TSLP, this event is controlled by endogenous proteases in NPs. Importantly, NP extract-treated TSLP, which is a mixture of mature and truncated products, has a higher activity than mature TSLP in mast cells.25 These results indicate that TSLP may be post-translationally modified by endogenous proteases in NPs and suggest that truncated TSLP may have enhanced activity when compared to the mature TSLP. Although this was the first evidence that TSLP may be controlled by PTM, the truncation enzymes, the structure of truncated TSLP and the function of the truncated products have not been identified.
In this study, we hypothesized that TSLP is post-translationally modified by endogenous proteases and that cleaved active TSLP metabolites are involved in the amplification of type 2 inflammation in NPs. We therefore set out to define the TSLP products, their activities and the enzymes that generate them in NP tissue.
Methods
Patients and biopsies
Patients with chronic rhinosinusitis (CRS) were recruited from the Otolaryngology clinic and the Northwestern Sinus Center of Northwestern Medicine. Nasal polyp tissue was obtained during routine endoscopic sinus surgery performed on patients with CRS. All patients met the criteria for CRS as defined by the European Position Paper on Rhinosinusitis and Nasal Polyps 2012 and the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force.5, 6 Patients with an established immunodeficiency, pregnancy, coagulation disorder or diagnosis of classic allergic fungal sinusitis, Churg-Strauss syndrome or cystic fibrosis were excluded from the study. Details can be found in the Methods section in the Online Repository.
TSLP cleavage assay
Recombinant mature TSLP (M29-159) was preincubated with 1 mg/ml tissue extracts for 6 or 24 hours. In some experiments, TSLP was incubated with NP extract in the presence of 1% DMSO (vehicle control) or protease inhibitors for 6 hours. Details can be found in the Methods section in the Online Repository.
Cell culture
Human PBMCs were stimulated with TSLP (M29-159), TSLP (M29-124), TSLP (131-159), PCSK-treated TSLP and 10 ng/ml IL-33 for 48 (CCL17) or 72 (IL-5) hours. The CCL17 and IL-5 concentrations in the supernatants were measured with DuoSet ELISA kits (R&D Systems). Details can be found in the Methods section in the Online Repository.
Reagents, Western blot analysis, PCR, protein sequencing and mass spectrometry
Details can be found in the Methods section in the Online Repository.
Results
Determination of cleavage products of TSLP by NP extracts
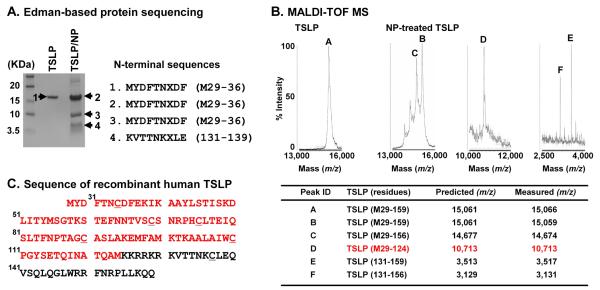
To determine the cleavage products of TSLP, we incubated TSLP (M29-159) with NP extracts, and assessed N-terminal protein sequences using an Edman-based sequencer. We found that the N-terminal sequence of the 10 KDa protein was identical to mature TSLP, indicating that the cleavage of TSLP by NP extracts was at a C-terminal site (Fig. 1A). Since there is no system to detect the protein sequence of the C-terminus, we also utilized MALDI-TOF mass spectrometry (MS) to assess the molecular weight of each product. The combination of these two methods allowed us to identify the structure of the truncation products. We initially sought 10 KDa truncated TSLP and found a spectral peak having mass-to-charge ratio (m/z) value of 10,713 (peak D) representing TSLP (M29-124) (Fig. 1B). We also detected two peaks (m/z 3,517 (peak E) and 3,131 (peak F)) representing TSLP (131-159) and TSLP (131-156), indicating that a major truncation site might be between residues 124 and 125 or between 130 and 131. In addition, proteases from NPs induced additional truncation between residues 156 and 157. Indeed, we also found a peak having an m/z value of 14,674 (peak C) representing TSLP (M29-156) after incubation with NP extracts (Fig. 1B).
Fig. 1. Identification of cleaved TSLP generated by nasal polyp extracts.
Recombinant mature TSLP was incubated with 1 mg/ml NP extracts for 6 hours and TSLP proteins were separated by SDS-PAGE. N-terminal protein sequences of each product were detected using an Edman-based sequencer (A). TSLP was incubated with 1 mg/ml NP extracts for 24 hours and truncated products were detected by MALDI-TOF MS. The x-axis of the mass spectra represents mass to charge ratio (m/z) (B). Summary table of identified truncated products assigned by comparison of measured with predicted m/z by MALDI-TOF MS and N-terminal sequencing (B). Protein sequence of recombinant human TSLP can be found in C and red color indicates the sequence of a potential major active metabolite, TSLP (M29-124), produced by NP extracts. Underlined letters indicate the cysteine residues (C). The results are representative of three separate experiments with separate donors (A and B).
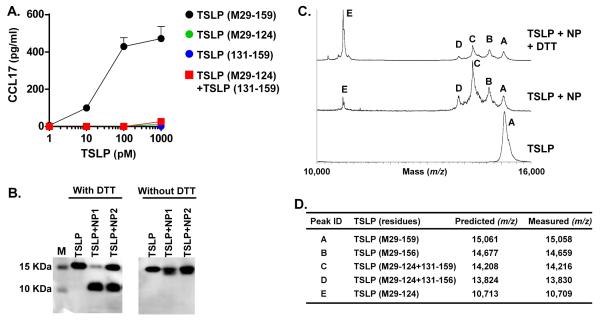
We generated a PBMC-based TSLP bioassay (Result section in the Online Repository and Supplementary Fig. S1), and then examined the activity of synthesized recombinant TSLP (M29-124) and TSLP (131-159) peptide (Supplementary Fig. S2 and not shown). Surprisingly, TSLP (M29-124), TSLP (131-159) and their mixture did not induce production of CCL17 in PBMCs (Fig. 2A). Since about 80% of synthesized recombinant TSLP (M29-124) was conjugated to glutathione (GSH) (Supplementary Fig. S2B), we also dissociated GSH using DTT and assayed activity using PBMCs. However, DTT-treated TSLP (M29-124) still did not stimulate PBMCs (Supplementary Fig. S2C).
Fig. 2. Cleaved TSLP is a heterodimeric protein.
PBMCs were stimulated with TSLP (M29-159), TSLP (M29-124) and TSLP (131-159) for 48 hours. Concentrations of CCL17 protein in the culture supernatant were measured by ELISA (A). TSLP (M29-159) was incubated with 1 mg/ml NP extracts for 24 hours and TSLP proteins were separated by SDS-PAGE in the presence or absence of DTT (B). TSLP proteins were detected by western blot using an anti-TSLP antibody. TSLP (M29-159) was incubated with 1 mg/ml NP extracts for 24 hours and truncated products were detected by MALDI-TOF MS in the presence or absence of 25 mM DTT (C). Summary table of identified truncated products assigned by comparison of measured with predicted m/z by MALDI-TOF MS (D).
Since GSH binds cysteine residues, we searched within the sequence of human TSLP protein and found 6 cysteine residues. Five were present in TSLP (M29-124) and one was in TSLP (131-159) (Fig. 1C). We therefore hypothesized that TSLP (M29-124) and TSLP (131-159) might still be dimerized via a disulfide bond after truncation. To test this hypothesis, we performed western blots in reducing and non-reducing conditions. We found that the 10 KDa protein was not detected in non-reducing conditions (Fig. 2B). MALDI-TOF MS showed that the level of peak E (TSLP (M29-124)) was strongly enhanced and peak C (14,216 m/z) and peak D (13,830 m/z) were largely reduced in the presence of DTT (Fig. 2C). These results suggest that the two cleaved products are dimerized with a disulfide bond and the dimerized form of TSLP (referred to as TSLP (M29-124 + 131-159)) is a major active metabolite while TSLP (M29-124 + 131-156) is a minor dimerized metabolite in NPs (Fig. 2D).
Proprotein convertases are rate limiting enzymes in the truncation of TSLP
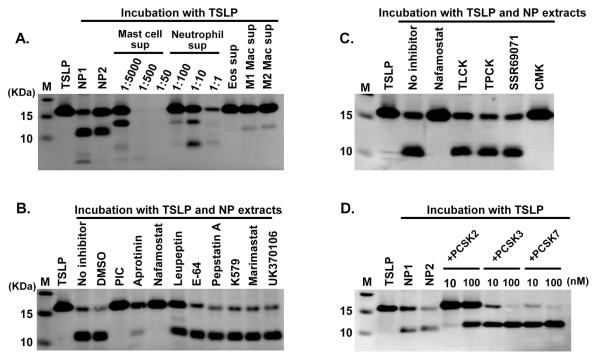
NPs are characterized by accumulation of inflammatory cells, including eosinophils, neutrophils, mast cells and macrophages, all of which contain several proteases. To examine the potential role of proteases released from inflammatory cells on the truncation of TSLP in NPs, we incubated TSLP (M29-159) with supernatants from activated cultures of these cells. However we could not detect the same 10 KDa cleavage product under reducing conditions when TSLP was incubated with those cell lysates (not shown) or supernatants (Fig. 3A), although macrophage supernatants made a 10 KDa protein very weakly (Fig. 3A). We also incubated TSLP (M29-159) with NP extracts in the presence of various specific protease inhibitors, and found that broad spectrum serine protease inhibitors, Aprotinin and Nafamostat, almost completely blocked NP-dependent degradation of TSLP (Fig. 3B). This indicates that tissue serine proteases rather than proteases from inflammatory cells might be important for the truncation of TSLP in NPs.
Fig. 3. PCSKs are key truncation enzymes in NPs.
TSLP (M29-159) was incubated with supernatants from activated mast cells, neutrophils, eosinophils (Eos), M1 macrophages (Mac) and M2 Mac for 24 hours (A). TSLP was incubated with 1 mg/ml NP extracts in the presence of 1% DMSO (vehicle control), 1% PIC (protease inhibitor cocktail), 1 μM Aprotinin, 10 μM Nafamostat mesylate, 10 μM Leupeptin, 10 μM E-64, 10 μM Pepstatin A, 10 μM K579, 20 μM Marimastat, 10 μM UK370106, 200 μM TLCK, 200 μM TPCK, 1 μM SSR69071 or 10 μM CMK for 24 hours (B, C). TSLP was incubated with 1 mg/ml NP extracts, recombinant PCSK2, PCSK3 or activated PCSK7 for 24 hours (D). TSLP proteins were detected by western blot using an anti-TSLP antibody. The results are representative of three separate experiments with separate donors (B, C).
Serine proteases can be classified into several clans.31, 32 The PA (proteases of mixed nucleophile, superfamily A) clan can be classified into three subfamilies, trypsin-like, chymotrypsin-like and elastase-like proteases and approximately 75% of human serine proteases belong to this clan.31 The SB (serine protease B) clan includes subtilisin-like proteases. Since the PA and SB clans are well studied and specific inhibitors are commercially available, we initially tested whether PA and SB clan specific inhibitors block NP extract-dependent truncation of TSLP. We found that truncation of TSLP by NP extracts was inhibited by decanoyl-Arg-Val-Lys-Arg-chloromethylketone (CMK, the proprotein convertase Subtilisin/Kexin (PCSK) inhibitor), but not by TLCK (trypsin-like protease inhibitor), TPCK (chymotrypsin-like protease inhibitor) or SSR69071 (elastase inhibitor) (Fig. 3C). This suggests that truncation of TSLP in NPs occurs in a PCSK family protease-dependent manner.
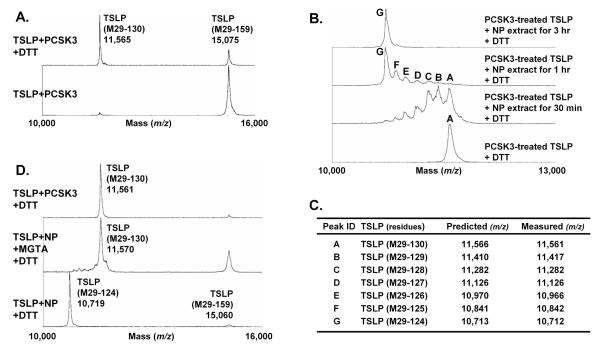
The PCSK family consists of 9 members, with the first seven members (PCSK1-7) recognizing shared motifs, (R/K)Xn(R/K)↓(n=0, 2, 4 or 6. The arrow indicates the cleaved site).33 Since CMK inhibits PCSK1-7 mediated truncations, we used commercially available recombinant PCSKs in our TSLP truncation assay. We found that recombinant PCSK2, PCSK3 and PCSK7 dose-dependently truncated TSLP and the cleaved product was similar in size to that generated by NP extracts (Fig. 3D). MS results under reducing conditions showed that recombinant PCSKs generated two truncated products, 11,565 m/z and 3,517 m/z, representing TSLP (M29-130) and TSLP (131-159) (Fig. 4A, Supplementary Fig. S3 and not shown). This indicates that the cleavage site for PCSKs is between residues 130 and 131, and PCSKs generate a heterodimeric metabolite TSLP (29-130 + 131-159).
Fig. 4. Carboxypeptidases generate a stable metabolite TSLP (M29-124 + 131-159).
TSLP (M29-159) was incubated with 0.5 μM recombinant PCSK3 for 24 hours (A). PCSK-treated TSLP was further incubated with 1 mg/ml NP extracts for 0.5-3 hours (B). TSLP was incubated with NP extract in the presence of 100 μM MGTA (carboxypeptidase N inhibitor) for 6 hours (D). Truncated products were detected by MALDI-TOF MS with or without the presence of 25 mM DTT. Summary table of identified truncated products assigned by comparison of measured with predicted m/z by MALDI-TOF MS (C).
Carboxypeptidase N generates a stable heterodimeric metabolite of TSLP
Since NP extracts generated TSLP (29-124 + 131-159), the C-terminus of the longer subunit of heterodimeric TSLP generated by PCSKs seemed likely to be further digested by other NP proteases. To investigate this mechanism, we treated mature TSLP with PCSK3 for 24 hours and then incubated PCSK3-treated TSLP with NP extracts for up to 3 hours. We detected seven peaks (m/z 11,561, 11,417, 11,282, 11,126, 10,966, 10,842 and 10,712), representing TSLP (M29-130) to TSLP (M29-124), appearing after 30 minutes incubation with NP extracts (Fig. 4BC). Interestingly, TSLP (M29-124) was time-dependently generated by NP extracts and was the major product after 1 hour incubation (Fig. 4B). In the absence of DTT, we found a major peak having m/z value of 14,224 representing TSLP (M29-124 + 131-159) by MS assay (not shown). Importantly, the C-terminal amino acid digestions occurred one by one and were not inhibited by Nafamostat or CMK indicating that these cleavages occur in a serine protease-independent but a carboxypeptidase-dependent manner (Fig. 4B and Supplementary Fig. S4).
Since NP extracts digested 6 amino acids representing KKRRKR from the C-terminus of a longer peptide, we hypothesized that carboxypeptidase N (CPN) which removes lysine and arginine from the C-terminus of many proteins, might be an enzyme responsible for this digestion. Interestingly, we found that NP extracts generated TSLP (M29-130 + 131-159), with an m/z value of 11,570 and 3,517 under reducing MS conditions, in the presence of a CPN inhibitor DL-2-mercaptomethyl-3-guanidinoethylthiopropanoic acid (MGTA) (Fig. 4D and not shown). These results indicate that PCSK family proteases are rate limiting enzymes in the truncation of mature TSLP (29-159) to generate the dimerized form TSLP (29-130 + 131-159) and then CPN further digests 6 amino acids from the C-terminus of the longer subunit to generate a stable and active heterodimeric metabolite, TSLP (29-124 + 131-159), in NPs.
Truncations of TSLP by PCSK and CPN may be homeostatic events in primates but not in rodents
Several PCSKs are known to be ubiquitously expressed, and CPN is known to be constitutively expressed in the liver and is secreted into the bloodstream.33, 34 We also found that levels of mRNA for most PCSKs in control sinus tissues were similar to levels in NPs, and were higher than in inflammatory cells (Supplementary Fig. S5). This suggests that truncation of TSLP may occur in control sinus tissue. Therefore we examined whether control sinus tissue also truncated TSLP. Interestingly, we found that tissue extracts from control uncinate sinus tissue and tonsil, as well as serum were able to truncate TSLP and the truncation products were the same size as those generated by NP extracts (Fig. 5). This suggests that truncation of TSLP may be a homeostatic but not a disease specific event. Surprisingly, skin tissue extracts almost completely digested TSLP protein at 1 mg/ml, and at lower concentrations, skin extracts did not generate TSLP (29-124 + 131-159) (Fig. 5 and not shown). This indicates that TSLP protein may not be stable in the skin, and that the balance of proteases and protease inhibitors might control the stability of TSLP protein in tissues. Further study will be required to identify the enzymes responsible for digestion of TSLP in skin.
Fig. 5. Truncation of TSLP by nasal polyp, control sinus, tonsil, skin and serum.
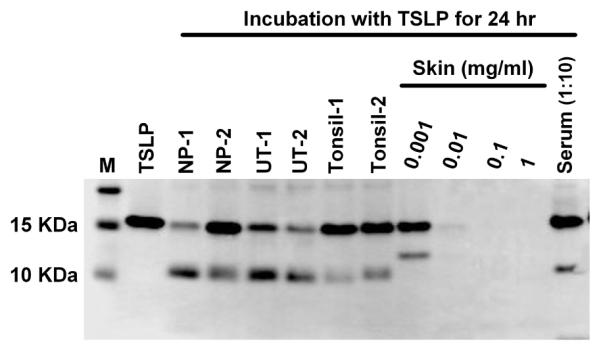
TSLP (M29-159) was incubated with 1 mg/ml NP extracts, 1 mg/ml control uncinate sinus tissue (UT) extracts, 1 mg/ml tonsil extracts, 0.001-1 mg/ml skin extracts or 10% control serum for 24 hours. Truncated products were determined by western blot under reducing conditions using anti-TSLP antibody.
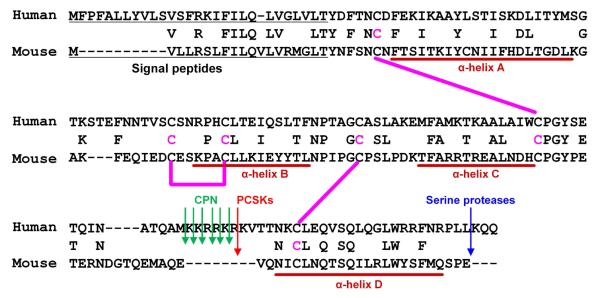
We next tested whether the identified truncation events can occur in animal models. We found that a PCSK and CPN cleavage site which contains seven basic amino acids (KKRRKRK) is only present in primate TSLP but not in rodent TSLP including mouse (Fig. 6), rat and rabbit.14, 35 This suggests that the identified truncation events of TSLP can only occur in primates.
Fig. 6. Sequence alignments for TSLP protein from human and mouse.
Pink lines indicate the putative disulfide bonds based on the structure of mouse TSLP.35 Arrows indicate identified cleavage sites.
Effect of truncation on the functional activity of TSLP
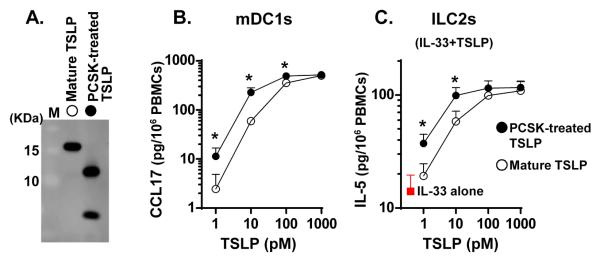
We have previously reported that NP extract-treated TSLP has a higher activity than mature TSLP which indicates that dimerized TSLP (29-124 + 131-159) is an active form.25 However, the degree of activity due to dimerized TSLP metabolites was not clear. Since the major metabolite made is a dimerized form and adding a linker between the two peptides may affect the functional activity, synthesis of a recombinant heterodimeric metabolite was risky. In addition, we were unable to assemble the desired recombinant dimerized protein without a linker (not shown). We therefore generated a completely truncated TSLP metabolite, TSLP (M29-130 + 131-159), using recombinant PCSKs (Fig. 7A and Supplementary Fig. S3) and investigated the functional activity on mDC1s using PBMCs. We found that PCSK-treated TSLP (M29-130 + 131-159) dose-dependently induced production of CCL17 and the potency was significantly higher (approximately three fold) than mature TSLP (Fig. 7B). Recently, Bartemes et al. reported that TSLP enhanced IL-33 mediated production of type 2 cytokines, IL-5 and IL-13, in PBMCs and this response was mediated via the activation of blood ILC2s.21 To test the effect of the truncation event on the activation of ILC2s, we stimulated PBMCs with TSLP in the presence of IL-33. We found that PCSK-treated TSLP (M29-130 + 131-159) dose-dependently enhanced IL-33 mediated production of IL-5 and the potency was also significantly higher than mature TSLP (Fig. 7C). These results indicate that the truncation event does not decrease, but rather enhances the functional activity of TSLP on TSLP targeting cells including mDC1s and ILC2s.
Fig. 7. TSLP (29-130 + 131-159) activates mDCs and ILC2s.
TSLP (M29-159) was incubated with 1 μM recombinant PCSK3 for 24 hours and truncation efficiency was determined by western blot in reducing conditions using anti-TSLP antibody (A). PBMCs were stimulated with 1-1000 pM mature TSLP or 1-1000 pM PCSK-treated TSLP (29-130 + 131-159) for 48 hours (B). PBMCs were stimulated with 1-1000 pM mature TSLP (M29-159) or 1-1000 pM PCSK-treated TSLP (29-130 + 131-159) in the presence of 10 ng/ml IL-33 for 72 hours (C). Concentrations of CCL17 and IL-5 proteins in the culture supernatant were measured by ELISA (B, C). Results shown are means ± SEMs of 5 (B) or 4 (C) independent experiments. Differences between TSLP and PCSK-treated TSLP were analyzed using the Paired t test and the Wilcoxon matched-pairs signed rank test. * p < .05.
Discussion
TSLP plays a critical role in type 2 inflammatory diseases as well as in host immunity. We initially discovered that TSLP was highly elevated in CRSwNP and that levels of TSLP positively correlated with markers of type 2 inflammation and eosinophilia in the US population.25 This suggests that the elevation of TSLP may control the accumulation of type 2 inflammation in NPs. However, we also have discovered that TSLP is truncated in NPs and truncated forms may retain activity.25 This indicates more complicated TSLP regulation in humans. However, the structure and activity of the truncated forms of TSLP in NPs or other human tissue was not clear. We report here that TSLP protein is post-translationally modified by two families of proteases in NPs and that a stable metabolite of TSLP demonstrates a heterodimerized structure and has increased biological activity compared to unmodified TSLP in TSLP receptor mediated reactions.
Although PTM is a well-known mechanism controlling the activity of cytokines, it had not been investigated whether TSLP protein is also regulated by PTM. This is the first evidence that TSLP is post-translationally modified by two families of enzymes in NPs. We first determined that PCSK family proteases are rate limiting enzymes in the truncation of mature TSLP and in the generation of a heterodimeric structure. The mammalian PCSK family comprises nine serine proteases. The first seven PCSKs have shared recognition motifs and are responsible for the activation of a large number of proteins including peptide hormones and growth factors.33, 36 Since CMK completely blocked NP extract mediated truncation of TSLP and recombinant PCSK2, 3 and 7 truncated TSLP (Fig. 3), it was deemed likely that PCSK1-7 must be responsible for the truncation of TSLP in NPs. PCSK1 and PCSK2 are known to be mainly localized within secretory granules of neural and endocrine cells.33 PCSK4 is exclusively expressed in testicular germ cells, placenta and ovary. In contrast, PCSK3, 5, 6 and 7 are widely expressed.33 We also found that expression of PCSK3, 5, 6 and 7 were higher than PCSK1, 2 and 4 in NPs (Supplementary Fig. S5). This indicates that PCSK3, 5, 6 and 7 are the main truncation enzymes likely to metabolize TSLP in NPs. We found the presence of mRNAs for PCSK3 and PCSK7 in mast cells by PCR, although we could not detect 10KDa protein after treatment with mast cell supernatants (Fig. 3 and S5). However, we found that other mast cell proteases, including chymase and cathepsin G, digested TSLP within 24 hours (Supplementary Fig. S6). These results indicate that mast cells may be able to truncate TSLP between residues 130 and 131 by PCSKs but other proteases immediately digest the derived products to smaller peptides. We also detected some PCSKs in macrophages and PCSK5 in neutrophils. However, the levels of PCSKs in inflammatory cells were lower than in NPs (Supplementary Fig. S5). This indicates that PCSKs from inflammatory cells might play a minor role in the truncation of TSLP in NPs. Future studies will be required to investigate whether all of these PCSKs contribute to the truncation of TSLP in NPs and to identify the local distribution of PCSKs in NP tissue.
We determined that the second step of TSLP metabolism in NPs was mediated by a 6 amino acid digestion from the C-terminus of the longer peptide, TSLP (29-130), by CPN within 1 hour (Fig. 4) and this generated a major heterodimeric metabolite TSLP (29-124 + 131-159). This indicates that TSLP (29-130 + 131-159) is an unstable intermediate metabolite having less than a 1 hour half-life and TSLP (29-124 + 131-159) is a stable metabolite in NPs. CPN is a tetrameric enzyme consisting of two catalytic subunits (CPN1) and two regulatory subunits (CPN2).34 We found that mRNAs for CPN1 and CPN2 were almost undetectable in NPs and sinus tissues compared to the liver (Supplementary Fig. S5). This indicates that CPN is not synthesized locally in NPs. NPs are known to be characterized by intense edema with accumulation of plasma proteins.37 This indicates that CPN may be coming from the bloodstream via vascular leakage. Future studies will be required to identify the source of CPN in NPs.
We determined the functional activity of TSLP (M29-130 + 131-159) compared with mature TSLP (M29-159) due to technical problems making recombinant TSLP (M29-124 + 131-159). We have found that truncation between residues 130 and 131 does not diminish, but rather enhances the functional activity, although TSLP (M29-124) did not activate the TSLPR complex (Fig. 2 and 7). This indicates that the C-terminal end of TSLP is necessary for the activation of the TSLPR complex. Since CPN is known to be present in the serum,34 TSLP (M29-130 + 131-159) might be very quickly further digested to TSLP (M29-124 + 131-159) during PBMC culture. Therefore the identified activity of TSLP (M29-130 + 131-159) might be from TSLP (M29-124 + 131-159). Further study will be required to identify the time-kinetics of 6 amino acids digestion from TSLP (M29-130 + 131-159) in serum containing culture medium.
In contrast to TSLP (M29-130 + 131-159), we could not find any activity in the mixture of TSLP (M29-124) and TSLP (131-159) (Fig. 2). Recently, Verstraete et al., identified the structure of the mouse TSLP-TSLPR-IL-7Rα complex by X-ray crystallography.35 TSLP adopts a short chain four-helix bundle (α-helix A to D). Helix A, helix D and the overhand AB loop contact the TSLPR. In contrast, helix A and helix C engage with IL-7Rα.35 Based on the protein sequence homology of TSLP between human and mouse, helix D of human TSLP exists on residues 134 to 153 which is located within the shorter subunit, TSLP (131-159) (Fig. 6).35 Since TSLP (M29-124) completely lacks helix D and mixing TSLP (M29-124) and TSLP (131-159) cannot form the 3D structure with correct disulfide bonds, TSLP (M29-124), TSLP (131-159) and their mixture may not be able to bind to the TSLPR complex. PCSKs and CPN remove 6 amino acid residues between 125 and 130 from human TSLP. This sequence is located in the long CD overhand loop, which does not contribute to any binding interface between TSLPR and IL-7Rα.35 This may be a reason why heterodimeric TSLP metabolites do not lose their bioactivity after the 6 amino acid removal from mature TSLP. This also indicates that cleavage of the CD overhand loop probably does not destroy the structure but may instead induce a small conformational change. This change may result in a higher binding affinity to the TSLPR complex. Further studies will be required to identify the crystal structure of the truncated human TSLP-TSLPR-IL-7Rα complex.
Although TSLP is involved in type 2 inflammation and disease in both mice and humans, the mechanism of induction of type 2 inflammation by TSLP might differ in the two species. For example, TSLP can directly promote Th2 differentiation in mice from naïve T cells in the absence of antigen presenting cells (APC).38, 39 In humans however, TSLP dependent-Th2 differentiation requires APC.17, 22 These data suggest that distribution of TSLP receptor and activation mechanisms by TSLP might be different in humans and mice, and therefore human studies are important to understand the complex biology of TSLP. Importantly, we report here that human TSLP protein is controlled by PTM. Although we initially hypothesized that PTM of TSLP is a disease specific event, our data clearly shows that it is a homeostatic event in primates (Figs. 5 and 6). This result suggests that the pathogenic role of TSLP might be controlled by over expression and over production rather than by PTM. However, understanding PTM of human TSLP may be very important in clinical situations. PTM affects conformational change and functional activity of TSLP protein and TSLP-targeting drugs will need to block both mature and truncated active TSLP metabolites. However, this mechanism cannot occur in rodent species that are used in preclinical studies for drug development. These data suggest that human studies will be necessary for clinical development of TSLP-targeting drugs. A recent clinical trial indicated that AMG 157, an anti-human TSLP antibody, reduced allergic responses.26 This suggests that AMG 157 may bind and inhibit both mature and truncated active TSLP metabolites. Future study will be required to determine whether AMG 157 inhibits TSLP metabolite-mediated responses.
In summary, we report here that TSLP is controlled by PTM in NP tissues, that PCSKs are rate-limiting enzymes in the truncation of TSLP, and that CPN immediately generates an active heterodimeric metabolite. Our findings indicate that the effects of TSLP overproduction in CRSwNP may be further amplified by PTMs. Future efforts to target TSLP in disease will need to account for this conformational variability and the mechanisms of PTM.
Supplementary Material
Supplementary Fig. S1. Myeloid DC mediated production of CCL17 by TSLP in PBMCs.
PBMCs, mDC1s, monocytes and CD1c+, CD14+ and CD19+ cell-depleted PBMCs were stimulated with medium control or 100 pg/ml recombinant mature TSLP for 48 hours (A). PBMCs were stimulated with 10-1000 pg/ml mature TSLP or NP-treated TSLP for 48 hours (B). Concentrations of CCL17 protein in the culture supernatant were measured by ELISA. Results shown are mean ± SEM of 3 (A) or 4 (B) independent experiments. Differences between TSLP and NP-treated TSLP were analyzed using the Paired t test and the Wilcoxon matched-pairs signed rank test (B). * p < .05.
Supplementary Fig. S2. TSLP (M29-124) does not activate mDC1s in PBMCs.
Recombinant TSLP (M29-124) was synthesized at BioLegend. The purity of recombinant TSLP (M29-124) was greater than 98% as analyzed by HPLC (A) and SDS-PAGE (not shown). Molecular mass was detected as 11,014 by ESI-TOF MS (B). 11,014 m/z protein converted to 10,713 m/z in the presence of DTT (B). PBMCs were incubated with TSLP (M29-159), TSLP (M29-124) and DTT-pretreated TSLP (M29-124) for 48 hours (C). Concentrations of CCL17 protein in the culture supernatant were measured by ELISA. Results shown are mean ± SEM of 3 independent experiments.
Supplementary Fig. S3. PCSKs generate TSLP (29-130 + 131-159) in NPs.
Recombinant mature TSLP (M29-159) was incubated with 1 μM PCSK3 for 24 hours and truncated products were detected by MALDI-TOF MS in the presence or absence of 25 mM DTT.
Supplementary Fig. S4. Serine protease inhibitor does not block 6 amino acid deletions from PCSK-treated TSLP by NP extracts
Recombinant mature TSLP (M29-159) and PCSK3-treated TSLP (TSLP/PCSK3) were incubated with NP extract in the presence of 1% PIC, 10 μM CMK or 10 μM Nafamostat for 3 hours. Truncated products were detected by MALDI-TOF MS in the presence of 25 mM DTT. The x-axis of the mass spectra represents mass to charge ratio (m/z).
Supplementary Fig. S5. Expression of PCSKs and CPN in NPs.
The gene expression of PCSK1-7 (also known as proprotein convertase 1 (PC1), PC2, furin, PC4, PC5, paired basic amino acid cleaving enzyme 4 (PACE4) and PC7, respectively), carboxypeptidase N subunit 1 (CPN1) and subunit 2 (CPN2) in normal ethmoid sinus tissues (ET) (n=8), NP tissues (n=8), lung (pooled), skin (pooled), liver (pooled), M1 macrophages (n=3), M2 macrophages (n=3), neutrophils (n=3) and mast cells (n=4) was measured by using real-time RT-PCR. Gene expression was normalized by a housekeeping gene, GUSB and expression levels were shown as % expression of GUSB. Pooled RNA from human liver was used as a positive control of the expression of CPN1 and CPN2. Differences between ET and NP were analyzed using the Mann Whitney test.
Supplementary Fig. S6. Digestion of TSLP by mast cell proteases
Recombinant mature TSLP (M29-159) was incubated with 1 mg/ml NP extracts, 40 U/ml tryptase, 23 U/ml chymase, 100 μg/m cathepsin D and 0.2 U/ml cathepsin G for 24 hours. Truncated products were determined by western blot under reducing conditions using anti-TSLP antibody.
Acknowledgments
This research was supported in part by NIH grants, R01 AI104733, R21 HL113913, U19 AI106683 and R37 HL068546 and by a grant from the Ernest S. Bazley Foundation.
We would like to gratefully acknowledge Dr. Lawrence J. Dangott and the Protein Chemistry Laboratory at Texas A&M University for their technical expertise, comments and suggestions.
Abbreviations
- CCL
CC chemokine ligand
- CPN
Carboxypeptidase N
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- DCs
Dendritic cells
- ILC2
Group 2 innate lymphoid cells
- mDCs
Myeloid DCs
- MS
Mass spectrometry
- m/z
Mass-to-charge ratio
- NP
Nasal polyp
- PCSK
Proprotein convertase subtilisin/kexin
- PTM
post-translational modification
- TSLP
Thymic stromal lymphopoietin
Footnotes
Competing interests: The authors declare no conflict of interest as to the interpretation and presentation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144:440–5. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–7. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–56. e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld RM, Andes D, Bhattacharyya N, Cheung D, Eisenberg S, Ganiats TG, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 6.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;3:1–298. [PubMed] [Google Scholar]
- 7.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachert C, Van Bruaene N, Toskala E, Zhang N, Olze H, Scadding G, et al. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis - a GALEN study. Allergy. 2009;64:520–33. doi: 10.1111/j.1398-9995.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 9.Poposki JA, Peterson S, Welch K, Schleimer RP, Hulse KE, Peters AT, et al. Elevated presence of myeloid dendritic cells in nasal polyps of patients with chronic rhinosinusitis. Clin Exp Allergy. 2015;45:384–93. doi: 10.1111/cea.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–20. e5. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 12.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015;64:121–30. doi: 10.1016/j.alit.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–55. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–47. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 16.Kato A, Favoreto S, Jr., Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–32. e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–8. e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 23.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 24.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600. e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 27.Afonina IS, Muller C, Martin SJ, Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42:991–1004. doi: 10.1016/j.immuni.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Mortier A, Gouwy M, Van Damme J, Proost P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp Cell Res. 2011;317:642–54. doi: 10.1016/j.yexcr.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 30.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–92. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 31.Heutinck KM, ten Berge IJ, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010;47:1943–55. doi: 10.1016/j.molimm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Di Cera E. Serine proteases. IUBMB Life. 2009;61:510–5. doi: 10.1002/iub.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–83. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 34.Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004;40:785–93. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Verstraete K, van Schie L, Vyncke L, Bloch Y, Tavernier J, Pauwels E, et al. Structural basis of the proinflammatory signaling complex mediated by TSLP. Nat Struct Mol Biol. 2014;21:375–82. doi: 10.1038/nsmb.2794. [DOI] [PubMed] [Google Scholar]
- 36.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–66. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 39.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–80. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Myeloid DC mediated production of CCL17 by TSLP in PBMCs.
PBMCs, mDC1s, monocytes and CD1c+, CD14+ and CD19+ cell-depleted PBMCs were stimulated with medium control or 100 pg/ml recombinant mature TSLP for 48 hours (A). PBMCs were stimulated with 10-1000 pg/ml mature TSLP or NP-treated TSLP for 48 hours (B). Concentrations of CCL17 protein in the culture supernatant were measured by ELISA. Results shown are mean ± SEM of 3 (A) or 4 (B) independent experiments. Differences between TSLP and NP-treated TSLP were analyzed using the Paired t test and the Wilcoxon matched-pairs signed rank test (B). * p < .05.
Supplementary Fig. S2. TSLP (M29-124) does not activate mDC1s in PBMCs.
Recombinant TSLP (M29-124) was synthesized at BioLegend. The purity of recombinant TSLP (M29-124) was greater than 98% as analyzed by HPLC (A) and SDS-PAGE (not shown). Molecular mass was detected as 11,014 by ESI-TOF MS (B). 11,014 m/z protein converted to 10,713 m/z in the presence of DTT (B). PBMCs were incubated with TSLP (M29-159), TSLP (M29-124) and DTT-pretreated TSLP (M29-124) for 48 hours (C). Concentrations of CCL17 protein in the culture supernatant were measured by ELISA. Results shown are mean ± SEM of 3 independent experiments.
Supplementary Fig. S3. PCSKs generate TSLP (29-130 + 131-159) in NPs.
Recombinant mature TSLP (M29-159) was incubated with 1 μM PCSK3 for 24 hours and truncated products were detected by MALDI-TOF MS in the presence or absence of 25 mM DTT.
Supplementary Fig. S4. Serine protease inhibitor does not block 6 amino acid deletions from PCSK-treated TSLP by NP extracts
Recombinant mature TSLP (M29-159) and PCSK3-treated TSLP (TSLP/PCSK3) were incubated with NP extract in the presence of 1% PIC, 10 μM CMK or 10 μM Nafamostat for 3 hours. Truncated products were detected by MALDI-TOF MS in the presence of 25 mM DTT. The x-axis of the mass spectra represents mass to charge ratio (m/z).
Supplementary Fig. S5. Expression of PCSKs and CPN in NPs.
The gene expression of PCSK1-7 (also known as proprotein convertase 1 (PC1), PC2, furin, PC4, PC5, paired basic amino acid cleaving enzyme 4 (PACE4) and PC7, respectively), carboxypeptidase N subunit 1 (CPN1) and subunit 2 (CPN2) in normal ethmoid sinus tissues (ET) (n=8), NP tissues (n=8), lung (pooled), skin (pooled), liver (pooled), M1 macrophages (n=3), M2 macrophages (n=3), neutrophils (n=3) and mast cells (n=4) was measured by using real-time RT-PCR. Gene expression was normalized by a housekeeping gene, GUSB and expression levels were shown as % expression of GUSB. Pooled RNA from human liver was used as a positive control of the expression of CPN1 and CPN2. Differences between ET and NP were analyzed using the Mann Whitney test.
Supplementary Fig. S6. Digestion of TSLP by mast cell proteases
Recombinant mature TSLP (M29-159) was incubated with 1 mg/ml NP extracts, 40 U/ml tryptase, 23 U/ml chymase, 100 μg/m cathepsin D and 0.2 U/ml cathepsin G for 24 hours. Truncated products were determined by western blot under reducing conditions using anti-TSLP antibody.