Abstract
Leading theories propose that when remembering past events, medial temporal lobe (MTL) structures reinstate the neural patterns that were active when those events were initially encoded. Accurate reinstatement is hypothesized to support detailed recollection of memories, including their source. While several studies have linked cortical reinstatement to successful retrieval, indexing reinstatement within the MTL network and its relationship to memory performance has proved challenging. Here, we addressed this gap in knowledge by having participants perform an incidental encoding task, during which they visualized people, places, and objects in response to adjective cues. During a surprise memory test, participants saw studied and novel adjectives and indicated the imagery task they performed for each adjective. A multivariate pattern classifier was trained to discriminate the imagery tasks based on functional magnetic resonance imaging (fMRI) responses from hippocampus and MTL cortex at encoding. The classifier was then tested on MTL patterns during the source memory task. We found that MTL encoding patterns were reinstated during successful source retrieval. Moreover, when participants made source misattributions, errors were predicted by reinstatement of incorrect source content in MTL cortex. We further observed a gradient of content-specific reinstatement along the anterior-posterior axis of hippocampus and MTL cortex. Within anterior hippocampus, we found that reinstatement of person content was related to source memory accuracy, whereas reinstatement of place information across the entire hippocampal axis predicted correct source judgments. Content-specific reinstatement was also graded across MTL cortex, with PRc patterns evincing reactivation of people and more posterior regions, including PHc, showing evidence for reinstatement of places and objects. Collectively, these findings provide key evidence that source recollection relies on reinstatement of past experience within the MTL network.
Keywords: episodic memory, hippocampus, parahippocampal cortex, perirhinal cortex, retrieval
1. INTRODUCTION
The ability to recall details about prior experiences—such as their origin or source—is thought to rely on reinstatement of the neural patterns active during initial encoding (for a review see Davachi & Preston, 2015). Leading theories suggest that during recollection, the hippocampus and surrounding medial temporal lobe (MTL) cortex mediate reinstatement of memory representations in response to partial cues—a process known as pattern completion (McClelland, McNaughton, & O'Reilly, 1995; Norman & O'Reilly, 2003). In turn, reinstatement within the MTL network is thought to drive reinstatement of the corresponding cortical activation patterns associated with an original experience, allowing for recollection of event details. While several studies have shown that cortical reinstatement tracks source memory (Bird, Keidel, Ing, Horner, & Burgess, 2015; Bosch, Jehee, Fernandez, & Doeller, 2014; Gordon, Rissman, Kiani, & Wagner, 2014; Johnson, McDuff, Rugg, & Norman, 2009; Kuhl & Chun, 2014; Morcom, 2014; Thakral, Wang, & Rugg, 2015; Wheeler, Petersen, & Buckner, 2000; Wing, Ritchey, & Cabeza, 2015), a direct link between MTL reinstatement and successful source retrieval has been more elusive.
Electrophysiological work in humans has shown that individual MTL neurons active during encoding of short episodes fire again when those episodes are recalled (Gelbard-Sagiv, Mukamel, Harel, Malach, & Fried, 2008), with activity predicting both recognition strength and confidence (Rutishauser et al., 2015). Consistent with these human studies, physiological recordings in rodents have shown reactivation of hippocampal activity patterns during retrieval (for a review see Carr, Jadhav, & Frank, 2011). For instance, hippocampal cells representing a movement trajectory through a well-learned environment are replayed in sequence at remote time points (Karlsson & Frank, 2009). Interrupting such hippocampal replay impairs navigational ability in rodents (Jadhav, Kemere, German, & Frank, 2012). Collectively, these findings indicate that reinstatement of MTL memory representations plays an important role in guiding behavior and choice. However, these findings do not speak directly to the role of MTL reinstatement in the accurate retrieval of detailed source information.
In contrast to electrophysiological research, evidence for MTL reinstatement during retrieval as measured by functional magnetic resonance imaging (fMRI) has been limited. Recent work has shown that the magnitude of hippocampal activation during retrieval is associated with the speed (Gordon et al., 2014) and confidence (Leiker & Johnson, 2015; Thakral et al., 2015) of memory decisions. Several studies have further shown that hippocampal engagement during both encoding (Danker, Tompary, & Davachi, 2016) and retrieval is correlated with measures of cortical reinstatement at test (Bosch et al., 2014; Horner, Bisby, Bush, Lin, & Burgess, 2015; Leiker & Johnson, 2015; Ritchey, Wing, Labar, & Cabeza, 2012; Wing et al., 2015). Thus, while these studies suggest a link between MTL processing, cortical reinstatement, and successful retrieval, they do not provide evidence for retrieval-related MTL reinstatement per se.
Studies that have observed reinstatement of MTL encoding patterns during retrieval do not always see a link with memory behavior (Wimber, Alink, Charest, Kriegeskorte, & Anderson, 2015). Two notable exceptions have shown performance-related reinstatement of specific memory content in hippocampus, perirhinal cortex (PRc), and parahippocampal cortex (PHc) (Mack & Preston, 2016; Staresina, Henson, Kriegeskorte, & Alink, 2012). In one of these studies, PHc encoding patterns for word-scene associations were reinstated when participants recalled the correct scene in response to its associated word cue (Staresina et al., 2012). A more recent study (Mack & Preston, 2016) combined high-resolution fMRI with a multivariate decoding approach designed to index retrieval of specific items. Mack and colleagues provided evidence for reinstatement of specific faces in PRc and specific scenes in hippocampus during recall, with the fidelity of MTL reinstatement predicting the speed of memory decisions. However, memory performance in that study was near ceiling, limiting the connection between MTL reinstatement and the accuracy of source retrieval. A major goal of the present study is to test the prediction that MTL reinstatement should not only predict correct source retrieval, but also track the pattern of source memory errors. If there is a strong link between MTL reinstatement and source decisions, activation patterns reflecting reinstatement of incorrect source content should lead to source misattributions.
A second goal of the present study is to test the hypothesis that distinct MTL subregions support reinstatement of specific kinds of source content. Leading theories suggest that hippocampus, PRc, and PHc play unique roles in memory that depend on the content of experience (Bird & Burgess, 2008; Davachi, 2006; Diana, Yonelinas, & Ranganath, 2007; Ritchey, Libby, & Ranganath, 2015). One perspective suggests that PRc and PHc mediate encoding and retrieval of visual object (including faces) and visuospatial information respectively, while hippocampus plays a content-general role in memory (Davachi, 2006; Diana et al., 2007). An alternative account proposes that hippocampus may play a specialized role in visuospatial memory, as hippocampal lesions result in deficits in place, but not face memory (Bird & Burgess, 2008). Recent data further indicate that there may be functional differentiation within hippocampus, as anterior hippocampus shows preferential connectivity with PRc and posterior hippocampus with PHc (Libby, Ekstrom, Ragland, & Ranganath, 2012). This observation and others (Liang, Wagner, & Preston, 2013) suggest that while posterior hippocampus may mediate memory for visuospatial information, anterior hippocampus may be more sensitive to visual object content or show domain-general memory responses. Prior studies indexing MTL representation at encoding and retrieval have revealed content-based dissociations across MTL subregions (Diana, Yonelinas, & Ranganath, 2008; Huffman & Stark, 2014; Liang et al., 2013; Mack & Preston, 2016; Staresina et al., 2012). Here, we examine how such specialization relates to source memory, by indexing reinstatement of person, place, and object source information along the longitudinal axis of both hippocampus and MTL cortex.
During incidental encoding, participants were cued to visualize a person, place, or object characterized by a presented adjective. During a surprise source memory task, participants saw studied and novel adjectives and indicated which imagery task they performed for each adjective or if an adjective was new. A neural classifier was trained to differentiate the three imagery tasks based on encoding data from each MTL subregion. The classifier was then applied to data from the source memory task to index content-specific reinstatement of source information within MTL subregions and its relationship to memory performance (see Polyn, Natu, Cohen, & Norman, 2005 for a similar approach). To address our central hypotheses, we (1) contrasted classifier performance for correct and incorrect source judgments and (2) assessed whether the classifier's output could predict participants’ patterns of source misattributions.
2. MATERIALS AND METHODS
2.1. Participants
Twenty-one healthy, right-handed volunteers participated in the experiment after giving informed consent in accordance with a protocol approved by the University of Texas at Austin Institutional Review Board. Participants received $25/hr for their involvement. Data from 15 participants were included in the analyses (age 19-33 years, mean = 23.9 ± 3.9 years; 12 females), with data from six total participants being excluded due to technical problems with the scanner (three participants), early termination of the experiment because of discomfort (one participant), and failure to perform the behavioral task (two participants).
2.2 Behavioral procedures
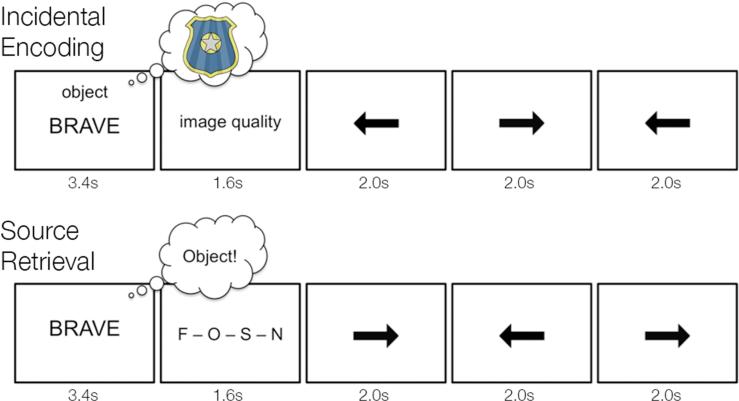
During functional scanning, participants performed a mental imagery task (Fig. 1) in response to visual word cues (black text on white background; Arial 36 point). In separate retrieval scans, participants performed a source recognition task in response to the same visual word cues. Stimuli were generated in MATLAB (The MathWorks, Inc., Natick, MA), using the Psychophysics Toolbox (Brainard, 1997), on an Apple MacBook laptop computer and back-projected via a magnet-compatible projector onto a screen that could be viewed through a mirror mounted above the participant's head. Participants responded with an optical button pad held in their right hand.
Figure 1.
Schematic of encoding and retrieval tasks. During incidental encoding, participants viewed adjectives and were cued to imagine a person, place, or object described by the adjective. After imagery, participants rated the quality of the generated images. Each imagery trial was followed by three trials of a baseline arrows task. After encoding, participants performed a surprise source memory task. During the source test, participants saw studied and novel lure adjectives. On each trial, participants were instructed to silently recall the mental image generated for the presented adjective and make a response indicating whether the retrieved image corresponded to a person, place, or object. To prevent confusion from overlapping letters during the retrieval prompt, “person” was represented on screen by an “F” (standing for face) and “place” was represented on screen by an “S” (standing for scene). At encoding the imagery cues for person and place trials were likewise denoted “face” and “scene” respectively. For adjectives not seen at encoding, participants were to respond “new.” Each source retrieval trial was followed by three trials of a baseline arrows task.
2.2.1. Stimuli
Two hundred adjectives selected from the MRC Psycholinguistic Database (http://websites.psychology.uwa.edu.au/MRCDatabase/uwa_mrc.htm) were used as stimuli. One hundred and fifty of these adjectives were presented during the incidental encoding task, with the adjectives split evenly across three imagery conditions. The remaining 50 adjectives were used as novel lures during the retrieval task. All adjectives were rated by an independent group of participants to ensure that they could be easily visualized across all three imagery conditions. Stimulus assignments were counterbalanced so that each adjective appeared in each condition (person imagery, place imagery, object imagery, and novel lure) across participants.
2.2.2. Incidental encoding
During five event-related encoding scans, participants were presented with written cues that designated whether they were to internally visualize a person, place, or object in response to a simultaneously presented adjective (Fig. 1). At the start of each imagery trial, the written cue and target adjective were presented for 3.6s. Following presentation of the imagery cue and adjective, a written prompt was presented for 1.4s during which time participants indicated with a key press whether their mental image was “vivid with strong details”, “vivid but lacking in detail”, “vague/unclear”, or “could not be visualized”.
For person imagery trials, participants were instructed to visualize the face of someone famous or familiar to them who could be described by the adjective. For place imagery trials, participants were instructed to visualize a spatial environment that could be described by the adjective and did not contain prominent foreground objects. For object imagery trials, participants were instructed to visualize a single non-living object in isolation that could be described by the adjective. Prior to being scanned, participants performed practice trials to ensure that they understood the imagery instructions and were executing them correctly. During each encoding scan, participants performed ten trials of the three imagery tasks (person, place, and object). Thus, each adjective – save the novel lures – was viewed once during the encoding phase of the experiment. Participants were not informed that their memory for the adjectives would later be tested.
To isolate the neural responses evoked during incidental encoding, each imagery trial was separated from the preceding and following trials by 6s of a baseline task (Fig. 1A). During this time, arrow stimuli were presented every 2s, and participants indicated with a key press whether each arrow pointed to the left or right. A 1s fixation crosshair indicated the start of the next imagery trial. The presentation order of person, place, and object imagery trials was generated using a sequencing algorithm to optimize efficiency in event-related designs and to ensure that within any scan, trials of each imagery task were equally likely to be followed by a person, place, or object trial (Dale 1999). Five presentation orders were generated as counterbalancing groups.
2.2.3. Source retrieval
In five separate retrieval scans, participants were given a surprise cued recall test on the adjectives studied in the encoding scans. Fifty adjectives not presented during encoding served as novel lures. At the start of each retrieval trial, one of the studied adjectives or lures appeared in the center of the screen for 3.6s (Fig. 1B), and participants were instructed to silently recall the mental image that they had created for that adjective during encoding. Following presentation of the adjective, a written prompt was presented (1.4s), during which time participants indicated with a key press whether the retrieved image associated with the adjective corresponded to a person, place, or object, or whether the adjective was not seen at encoding.
During each retrieval scan, participants viewed 30 studied adjectives (10 from each of the imagery conditions) and 10 lure adjectives. The presentation order of person-, place-, and object-related adjectives and novel lures was generated using the same sequencing algorithm as that used for the encoding scans (Dale, 1999). As with encoding, five distinct trial orders were created to counterbalance stimulus presentation order across participants. To isolate the neural responses evoked during cued recall, each retrieval trial was separated from the preceding and following trials by 6s of the same baseline task used during incidental encoding (Fig. 1B). A 1s fixation crosshair indicated the start of the next retrieval trial.
2.3. fMRI acquisition
Imaging data were acquired on a 3.0 T Signa whole-body MRI system (GE Medical Systems, Milwaukee, WI, USA) with a single-channel, custom-made transmit/receive head coil. Head movement was minimized using additional foam padding. For each participant, a high-resolution T1-weighted SPGR image (sagittal plane, 1mm3 resolution) was acquired. This image was used as the whole brain structural image to which all other participant-specific images were initially coregistered and was also used to calculate non-linear transformation parameters when normalizing each participant's structural and functional images to the MNI template. Prior to the incidental encoding task, a T2-weighted, flow-compensated spin-echo structural image (TR = 3000 ms; TE = 68 ms; 0.47 × 0.47 mm in-plane resolution) was then acquired, with 33 3-mm thick oblique axial slices (0.6 mm gap) oriented parallel to the main axis of the hippocampus and adjusted to maximize coverage of the whole brain; functional volumes from the incidental encoding task used the same slice locations as this T2 image. Prior to the retrieval scans, another T2-weighted structural volume was acquired with identical parameters to the first, but with a lower in-plane resolution (0.94 × 0.94 mm); functional volumes from the cued recall task used the same slice locations as this T2 image. This procedure enabled accurate and independent spatial coregistration of the encoding and retrieval scans to the high-resolution T1 image collected at the beginning of scanning.
Functional images collected during the encoding and retrieval scans were acquired using a T2*-sensitive gradient echo EPI sequence (TR = 2000 ms; TE = 30 ms; flip angle = 73°; FOV = 24 cm; 3.75 × 3.75 × 3.6 mm resolution, interleaved slice acquisition). Immediately prior to acquisition of the incidental encoding data, a high-order shimming procedure was utilized to reduce B0 heterogeneity. This high-order shimming procedure was repeated prior to the acquisition of the retrieval scans. For each participant, a total of 920 functional volumes were acquired over five encoding scans and 1220 volumes were acquired over five retrieval scans. In each functional scan, four EPI volumes (a total of 8 s) were collected prior to beginning the first trial of the experiment to allow for T1 stabilization. These initial four volumes were discarded prior to fMRI data analysis.
2.4. Preprocessing of fMRI data
Data were preprocessed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK) and custom Matlab routines. For each participant, the functional volumes from the encoding scans were realigned to the first volume in the timeseries to correct for motion. The first volume of the encoding timeseries was then coregistered to the T2-weighted structural image acquired prior to the encoding scans. The resulting coregistration parameters were then applied to the entire encoding timeseries. These steps were then separately performed for the retrieval scans, so that the entire retrieval timeseries was coregistered with the T2-weighted structural image taken immediately prior to retrieval. Both T2-weighted structural images were then coregistered with the high-resolution T1-weighted SPGR, and the resulting coregistration parameters were applied to the respective functional timeseries.
To enable group-level analyses, we used the Advanced Normalization Tools (Avants et al., 2011) to normalize individual participants’ brains to the MNI template. Specifically, each participant's high-resolution T1-weighted anatomical volume was normalized to the MNI template based on the shape of the gray- and white-matter boundaries, using non-linear diffeomorphic transformations. The transformation parameters were then applied to all of the participant's structural and functional volumes. Functional volumes were spatially smoothed (5mm FWHM), and then high-pass filtered to remove low frequency drift (longer than 128s). The resulting functional timeseries volumes were z-scored in preparation for multivoxel pattern analysis.
2.5. Identification of MTL regions-of-interest
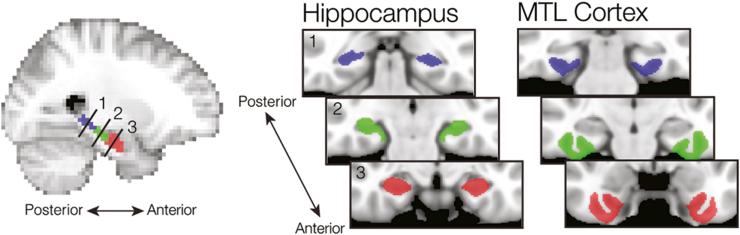
Anatomically defined regions-of-interest (ROIs) for hippocampus, PRc, and PHc were demarcated on the T1-weighted, high-resolution (1 mm3) standard MNI template, through automatic volumetric segmentation via the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu) (Fig. 2). The PRc mask was then manually edited according to guidelines derived from neurochemical and pathological studies of human PRc (Ding & Van Hoesen, 2010). Given previous studies that have raised the possibility that content representation may differ significantly along the anterior-posterior axis of both hippocampus and MTL cortex (Diana, Yonelinas, & Ranganath, 2010; Liang et al., 2013; Litman, Awipi, & Davachi, 2009; Staresina, Duncan, & Davachi, 2011), we further divided the hippocampal and MTL cortical masks into three ROIs of equal thickness.
Figure 2.
Anatomical regions of interest. Within hippocampus and MTL cortex, three regions of interest were demarcated along the longitudinal axis of each structure. Anterior hippocampus and PRc are depicted in red; middle hippocampus and the transitional zone of MTL cortex in green; posterior hippocampus and PHc in blue. The sagittal image in the left panel depicts the approximate locations of the hippocampal ROIs displayed on the right.
Within MTL cortex, we created three ROIs corresponding to PRc anteriorly (268 average voxels), PHc posteriorly (225 average voxels), and a middle segment corresponding to the transitional zone between PRc and PHc (299 average voxels). We defined the transitional zone based on recent data suggesting that encoding responses within this region are less selective than either PRc or PHc (Liang et al., 2013; Litman, Awipi, & Davachi, 2009; Staresina, Duncan, & Davachi, 2011). Within hippocampus, we created three subdivisions corresponding to anterior, middle, and posterior hippocampus (124, 87, and 106 average voxels respectively). Structural and functional differences have been observed across the hippocampal longitudinal axis, including differentiation of middle and posterior aspects (Liang et al., 2013; Libby et al., 2012). For this reason, we chose to consider three distinct hippocampal regions when examining content-specific reinstatement.
2.6. Multivariate pattern analysis of fMRI data
To test whether different MTL subregions make content-specific contributions to episodic reinstatement, we used a multivoxel pattern classifier to measure reinstatement of encoding patterns during source retrieval for each kind of imagined content. Furthermore, we tested how reinstatement was related to source memory performance by assessing: 1) whether the degree of reinstatement differed for correct and incorrect source responses and 2) whether reinstatement predicted participants’ pattern of source errors.
2.6.1. MVPA classification of imagery-based encoding
As an initial step, we assessed whether MTL activation patterns evoked during incidental encoding discriminated between the three imagery conditions (i.e., imagined people, imagined places, and imagined objects). First, activation patterns for each encoding trial were created by averaging the three functional volumes corresponding to the peak of the hemodynamic response (i.e., the time points 4—8s after the trial onset). We then created a regressor matrix to label each trial's activation pattern according to the imagery condition to which it belonged (i.e., person imagery, place imagery, object imagery). Every encoding trial was included regardless of the vividness rating at encoding, so that there were an equal number of time points included for each condition (50 per condition).
Pattern classification analyses were implemented using the Princeton MVPA toolbox and custom code for Matlab. Within each anatomical ROI, classifier performance during imagery-based encoding for each participant was calculated using a 5-fold cross-validation procedure that implemented a regularized logistic regression algorithm to train the classifier. Data from four encoding scans were used for classifier training, and the remaining scan was used as test data to assess the generalization performance of the trained classifier. This process was iteratively repeated five times, one for each of the possible configurations of encoding scans. The classification performances from each fold of the cross-validation procedure were averaged to obtain the final pattern classification performances for every participant for each MTL subregion. Two-tailed Student's t-tests were conducted for each MTL ROI to assess whether classification accuracy across participants was significantly greater than the theoretical chance level of 33%.
2.6.2. Classification of source retrieval patterns
Next, we applied a classifier trained on the encoding data to the patterns from the source memory task to determine if content-specific activation patterns were reinstated during successful recall of source details. As with the encoding dataset, we first calculated an activation pattern for each of the cued recall trials by averaging the three functional volumes corresponding to the peak of the hemodynamic response (i.e., 4—8s after onset of the retrieval cue). We sorted these retrieval activation patterns according to the imagery task with which they were associated and according to memory performance (i.e., correct and incorrect retrieval trials). This procedure resulted in six conditions: correct source identification of person, place, and object detail, and incorrect source identification of person, place, and object detail.
For each MTL ROI, the classifier trained on the encoding data was tested on the retrieval activation patterns. Reinstatement for each of the six conditions was quantified by the mean classification accuracy, which was calculated as the proportion of trials for which the associated imagery task was correctly predicted by the classifier. To assess whether MTL subregional retrieval patterns were sensitive to imagery content as well as participants’ source memory performance, we subjected the mean classifier accuracies to repeated measures ANOVA. Specifically, we conducted separate 3-way ANOVAs for MTL cortex and hippocampus using ROI as a factor with three levels (i.e., anterior, middle, and posterior regions for both hippocampus and MTL cortex), imagery task as a factor with three levels (i.e., person, place, and object), and source memory performance as a factor with two levels (i.e., source correct and source incorrect).
We also conducted planned comparisons that assessed content-based differences in episodic reinstatement and its relationship to memory within the MTL. At the group-level, Student's t-tests assessed whether reinstatement during each of the six retrieval conditions significantly differed from the theoretical chance level of 33%, and whether mean classification accuracy for source correct trials was significantly greater than for source incorrect trials for each form of content. For the latter, we used a one-tailed significance threshold given our a priori directional predictions that reinstatement would be greater during correct source judgments.
2.6.3. Classification of participants’ source memory errors
In addition to assessing differences in reinstatement between correct and incorrect source memory trials, we further assessed whether the classifier could predict participants’ choices during source memory errors. To do so, we analyzed trials for which a participant identified the wrong imagery task (source error) and trials for which they identified a novel lure as being studied in one of the three imagery conditions (false alarms). Because trial numbers for source errors from each imagery condition (person mean: 6.7 SE: 1.0, object mean: 9.3 SE: 1.4, place mean: 9.4 SE: 1.0) and false alarms (mean: 11.0 SE: 1.4) were low for the individual conditions, they were combined to ensure sufficient power for statistical inference. Thus, for each trial we labeled the classifier output for the imagery task chosen by the participant as “selected task” and the combined (i.e., averaged) output for the imagery tasks that were not chosen as “alternate options”. In each MTL ROI, we calculated the mean classifier output for the selected imagery task and alternate options across all source error and false alarm trials. We performed paired sample t-test across participants to assess whether mean classifier output significantly differed between the selected imagery task and alternate options.
3. RESULTS
3.1. Behavioral performance
3.1.1. Vividness ratings during imagery-based encoding
To ensure that participants were able to visualize different forms of content during encoding, we first binned trials into vivid imagery trials (e.g., trials for which the participant indicated that imagery was “vivid with strong details” or “vivid but lacking detail”) and poor imagery trials (e.g., trials for which the participant indicated that imagery was “vague/unclear” or “could not be visualized”). Across participants, the proportion of vivid imagery trials was high for all content classes (person mean: 81% SE: 2.4%, place mean: 82% SE: 2.9%, object mean: 77% SE: 2.4%), and vividness ratings did not significantly differ by imagery condition (F(2,28) = 2.656, p = 0.09). Reaction time for imagery trials did significantly differ by imagery condition (F(2,28) = 8.199, p = 0.002). Pairwise comparisons showed that this effect was driven by reaction time for person trials (mean: 0.77s SE: 0.03s) being significantly faster than reaction time for object (mean: 0.84s SE: 0.03s, t(14) = 3.36, p = 0.005) and place (mean: 0.82 SE: 0.03s, t(14) = 3.07, p = 0.008) trials. Reaction time for object and place did not significantly differ (t(14) = 1.16, p = 0.27).
3.1.2. Source memory performance
At retrieval, participants were better than chance at identifying the information content for adjectives encoded in each of the imagery conditions (person mean: 71% SE: 3.1%, place mean: 64% SE: 3.2%, object mean: 63% SE: 3.7%, all t > 10.45, all p < 0.001). A repeated measures ANOVA of source accuracy across the three imagery conditions revealed a significant main effect of imagery content (F(2,28) = 3.394, p = 0.048) that reflected better source identification of person relative to object trials (t(14) = 2.47, p = 0.026). Source accuracy for the person condition did not differ from that for places, nor did source memory for the object condition differ from that for the place condition (all t < 1.90, all p > 0.05). Source reaction times also differed by imagery condition (F(2,28) = 3.86, p = 0.033). Pairwise comparisons showed that reaction time for person trials (mean: 0.62s SE: 0.03s) was significantly faster than that for place trials (mean: 0.67s SE: 0.03s, t(14) = 3.18, p = 0.007). Reaction time for object trials (mean: 0.65s SE: 0.03s) did not differ from any of the other imagery conditions (all t < 1.69, all p > 0.11).
Source errors, i.e., trials for which participants indicated the wrong imagery task for studied adjectives, did not significantly differ by imagery content (person mean: 14% SE: 2.1%, place mean: 19% SE: 1.9%, object mean: 19% SE: 2.8%, F(2,28) = 2.865, p = 0.074). Source misses, i.e., trials for which participants indicated a novel response for studied adjectives, did not significantly differ by content (person mean: 10% SE: 1.1%, place mean: 10% SE: 1.6%, object mean: 12% SE: 1.1%, F(2,28) = 0.530, p = 0.594). Participants were also able to correctly identify novel adjectives (correct rejection mean: 72% SE: 2.9%, t = 16.13, p < 0.001). False alarms rates for novel words did not significantly differ by content (person mean: 8.4% SE: 1.6%, place mean: 7.3% SE: 1.0%, object mean: 6.1% SE: 1.1%, F(2,28) = 1.17, p = 0.324).
3.2. Visualization of content-specific detail evokes distinct MTL responses
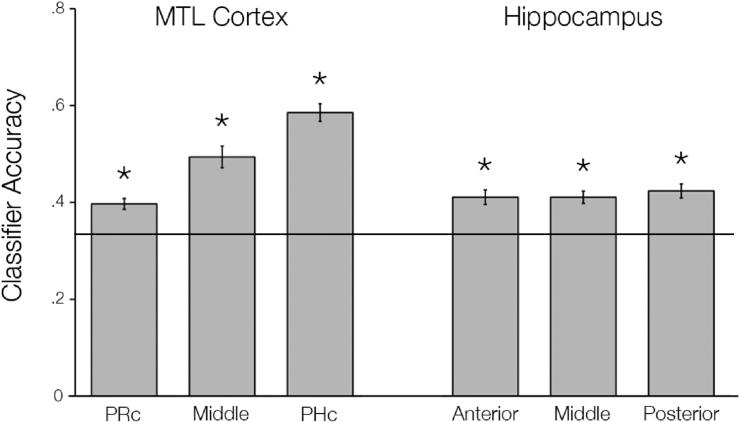
First, we confirmed that internal visualization of people, places, and objects during encoding evoked distinguishable patterns of activity in MTL subregions before examining content-specific reinstatement during source retrieval. All anatomical MTL ROIs demonstrated accurate classification of the three imagery conditions during encoding above the theoretical chance level of 33% (Fig. 3, all t > 5.332, all p < 0.001).
Figure 3.
Classification performance during incidental encoding. A pattern classifier was trained to discriminate the three imagery tasks using activation patterns from incidental encoding within each MTL subregion. We found that internal visualization of people, places, and objects evoked distinguishable patterns within all MTL subregions. The line represents chance level classifier performance; asterisks indicate significantly above chance classifier accuracy at p < 0.001.
3.3. Reinstatement in MTL is related to successful source memory
Successful performance in the cued recall task critically depends on retrieving source details specific to the original encoding event. We hypothesized that successful retrieval would therefore be reflected in the reinstatement of encoding patterns specific to the correct class of imagery content. To test this hypothesis, we measured reinstatement by training an MVPA classifier on encoding data from each anatomical ROI and testing whether the classifier could distinguish the imagery encoding condition from the activation patterns during source retrieval.
3.3.1. Content-sensitive reinstatement in MTL cortex
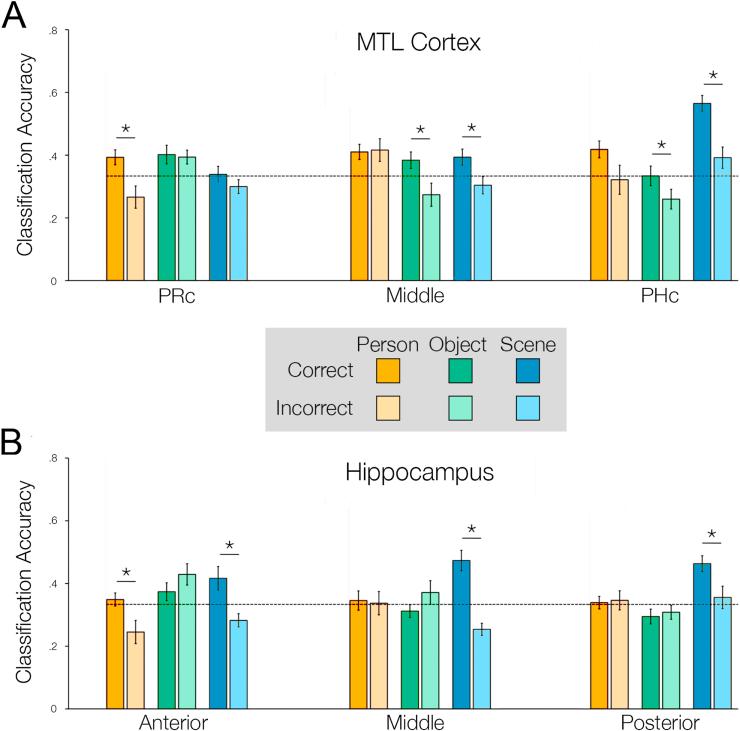
We first examined whether reinstatement of encoding patterns in PRc, PHc, and the transitional zone between PRc and PHc differed based on imagined content and source memory performance. A repeated measures ANOVA revealed a significant 3-way interaction between ROI, content, and source memory (Fig. 4A, F(4,56) = 3.357, p = 0.016).
Figure 4.
Classification performance during source retrieval. The classifier trained on the incidental encoding data was tested on MTL activation patterns from the source memory task. For both A) MTL cortex and B) hippocampus, we assessed classification accuracy as a function subregion, imagery condition (people in orange, places in blue, objects in green) and source memory accuracy (correct in dark colors, incorrect in light colors). The line represents chance level classifier performance. Asterisks indicate a significant difference between correct and incorrect source trials at p < 0.05.
In PRc, classification accuracy was greater for adjectives correctly identified as having been encoded in the person condition relative to incorrect trials (t(14) = 2.377, p = 0.016), but classification accuracy did not differ for correctly and incorrectly identified adjectives for the object or place conditions (all t < 1.135, all p > 0.05). Furthermore, classification accuracy was significantly above chance when adjectives were correctly identified as being studied in the person condition (t(14) = 2.513, p = 0.025), but was not significantly different from chance for incorrect trials (t(14) = −1.888, p = 0.08).
In the transitional zone of MTL cortex, classifier accuracy was greater for correctly identified object (t(14) = 2.091, p = 0.027) and place (t(14) = 2.839, p = 0.006) trials relative to incorrect trials for each condition; classification accuracy did not differ for correct and incorrect source trials in the person condition (t(14) = −0.156, p = 0.561). Classifier accuracy was significantly above chance for correct place retrieval trials (t(14) = 2.32, p = 0.04), but was not significantly different from chance for correct object retrieval trials (t(14) = 1.92, p = 0.08) or for incorrect source trials from the object and place conditions (all t < −1.06, all p > 0.05).
In PHc, classifier accuracy was greater for correct relative to incorrect source identification for the object (t(14) = 1.798, p = 0.047) and place (t(14) = 4.70, p < 0.001) conditions, with a similar trend for the person condition (t(14) = 1.573, p = 0.069). Classifier accuracy was further above chance level during correct place trials (t(14) = 9.088, p < 0.001), but was not significantly different chance for correct object trials (t(14) = 0.018, p = 0.99). Moreover, the classifier did not perform differently from chance for incorrect source retrieval for the place condition (t(14) = 1.726, p = 0.11), and performed significantly below chance for incorrect source retrieval for the object condition (t(14) = −2.338, p = 0.035).
3.3.2. Content-sensitive reinstatement in hippocampus
We next examined whether reinstatement of encoding patterns in anterior, middle, and posterior hippocampus differed based on imagined content and source memory performance. A repeated measures ANOVA revealed a significant 3-way interaction between ROI, content, and source memory (Fig. 4B, F(4,56) = 2.807, p = 0.034).
In anterior hippocampus, classifier accuracy was greater for correct relative to incorrect source trials for the person (t(14) = 3.024, p = 0.004) and place (t(14) = 2.725, p = 0.008) conditions, but not for the object condition (t(14) = −0.125, p = 0.903). Classifier accuracy was above chance levels during correct source identification of place (t(14) = 2.213, p = 0.044) but not person trials (t(14) = 0.76, p = 0.460) in this region. Furthermore, the classifier performed below chance for incorrect source retrieval trials from the person (t(14) = −2.376, p = 0.032) and place conditions (t(14) = −2.376, p = 0.032).
In the middle segment of hippocampus, classifier accuracy was greater for correct relative to incorrect source trials for the place condition (t(14) = 5.221, p < 0.001), but not for the person and object conditions (all t < 0.154, all p > 0.05). Classifier accuracy was further above chance for the place condition (t(14) = 4.291, p < 0.001), but not for the person and object conditions (all t < 0.409, all p > 0.05). The classifier did not perform above chance for incorrect source retrieval for person and object conditions (all t < 1.014, all p > 0.05), and performed below chance level for incorrect source retrieval for place (t(14) = −4.10, p = 0.001).
Posterior hippocampus also showed condition specific classification performance, with greater accuracy for correct relative to incorrect source identification for the place condition (t(14) = 2.675, p = 0.009), but not the person and object conditions (all t < −0.228, all p > 0.05). Classifier accuracy was greater than chance for correctly identified adjectives from the place condition (t(14) = 5.161, p < 0.001), but not for the person and object conditions (all t < 0.296, all p > 0.05) or incorrect source trials from any of the imagery conditions (all t < 0.428, all p > 0.05).
3.4. Distributed patterns in MTL cortex predict participants’ source errors
If reinstated encoding patterns guide source judgments, the nature of incorrectly reinstated content should predict participants’ actual choices when they make errors. To test this hypothesis, we collapsed all trials for which participants made source errors (i.e., when they indicated the wrong imagery task for a studied adjective) or false alarms (i.e., when they incorrectly indicated a novel adjective as being studied in one of the imagery tasks). We then compared the mean classifier output for the imagery task selected by the participant to the mean classifier output for the alternate options for each trial.
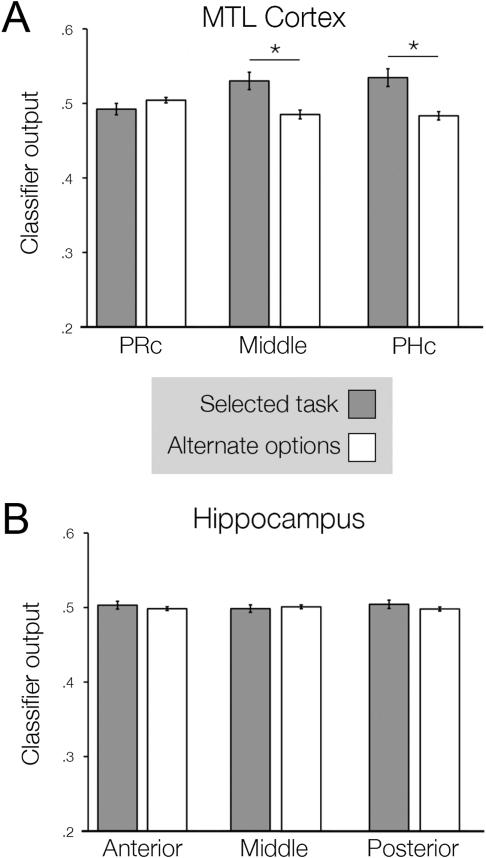
In PHc and the transitional zone of MTL cortex, classifier output for the selected imagery task was significantly greater than the mean output for the alternate options (Fig. 5A, all t > 2.580, all p < 0.05). By contrast, classifier output for PRc did not differ for the selected and the alternate options (t(14) = −1.062, p = 0.847), nor did classifier output for any of the hippocampal ROIs (Fig. 5B, all t < 0.762, all p > 0.05). We also assessed whether distributed activation patterns during novel lure trials, for which participants did not perform an imagery task, were predictive of participants’ choices. To test this possibility we repeated the same analysis but limited it to false alarm (lure) trials. This restricted analysis revealed that, in PHc, classifier output for the selected imagery task was greater than the output for the alternate options (t(14) = 2.382, p = 0.03).
Figure 5.
Reinstatement of encoding patterns and source memory errors. For each segment of A) MTL cortex and B) hippocampus, we assessed the relationship between classifier output and errors during the source memory task. During error trials (source confusions and false alarms), we compared classifier output for the response selected by the participant (dark gray bars) to the output for the options not selected (white bars). Asterisks indicate a significance difference between these conditions at p < 0.05.
4. DISCUSSION
The present findings indicate that memory reinstatement within MTL subregions plays a central role in the recollection of source details. We found that MTL activation patterns during incidental encoding discriminated whether participants were imagining people, places, or objects. In line with our central hypotheses, MTL encoding patterns were subsequently reinstated during source retrieval, with distinct patterns of content-specific reinstatement along the anterior-posterior axis of hippocampus and MTL cortex. Within hippocampus, reinstatement of place content was greater for correct relative to incorrect source retrieval across the entire longitudinal axis. Furthermore, we observed different levels of person-specific reinstatement for correct and incorrect source judgments in anterior hippocampus. Content-specific reinstatement during source retrieval was also graded across MTL cortex. Source memory for person imagery was related to reinstatement of person content in PRc, whereas memory for place and object source information was tracked by reinstatement of those content forms in PHc and the transitional zone between PRc and PHc. Moreover, across all forms of source content, reinstatement in PHc and the transitional zone of MTL cortex was predictive of participants’ source misattributions; source memory errors were reflected in reinstatement of incorrect source content in these regions.
4.1. Implications for models of MTL cortical function
An influential model of MTL function (Davachi, 2006; Diana et al., 2007) proposes that PRc and PHc play content-specific roles in memory. This model has support from several studies of episodic encoding, which have revealed content-specific dissociations in encoding activation across PRc and PHc (Davachi, Mitchell, & Wagner, 2003; Staresina et al., 2011). For instance, one study showed that PRc encoding activation predicted later source memory for objects, whereas PHc encoding activation predicted source memory for scenes (Staresina et al., 2011). Moreover, distributed activation patterns within MTL cortex have been shown to discriminate content forms during encoding (Diana et al., 2008; Huffman & Stark, 2014; Liang et al., 2013), with the degree of discrimination predicting subsequent memory (Kuhl, Rissman, & Wagner, 2012). The present data build upon this work by providing evidence that such dissociations in content-based processing within MTL cortex extend to retrieval. Consistent with work on encoding, our results indicate that PRc preferentially reinstates information about people. In contrast, PHc maintains distinct representations of multiple content forms, but also shows the greatest reinstatement effects for place content.
Notably, few neuroimaging studies to date have shown evidence for reinstatement of encoding activity patterns in human MTL cortex during retrieval. One such study showed successful recall of individual scenes was related to reinstatement of specific scene content in PHc (Staresina et al., 2012). Conversely, another study showed that the speed of memory decisions about people was linked to reinstatement of specific faces in PRc (Mack & Preston, 2016). Here, we demonstrate this dissociation between PRc and PHc processing of people and places within individual participants. Furthermore, our data provide a stronger test of the relationship between MTL cortical reinstatement and source memory performance than prior work. If MTL cortex drives source memory decisions, one would expect that reinstated content in MTL cortex would not only relate to correct source judgments, but also errors. Consistent with this prediction, we showed that content-specific reinstatement in MTL cortex tracks both successful source retrieval and individual participants’ pattern of source misattributions. When participants judged a source incorrectly or made a false alarm to a novel adjective, the nature of the source error was predicted by reinstatement of incorrect source content in the posterior aspects of MTL cortex. Thus, our findings indicate that reinstated content in MTL cortex supports correct source judgments as well as false recollection.
The present data further suggest that information about people and objects may be processed differently within MTL cortex. Person-specific reinstatement was limited to PRc, whereas object-specific reinstatement was observed in the transitional zone of MTL cortex and PHc. One possible interpretation of this pattern is that imagining and retrieving information about people evokes more emotional content than object imagery. In rodents, the amygdala is highly interconnected with PRc, but not with postrhinal cortex (the rodent homologue to PHc) (Agster, Tomas Pereira, Saddoris, & Burwell, 2016; Pereira, Agster, & Burwell, 2016). Increased emotional processing during person imagery could therefore have resulted in preferential recruitment of the PRc during source memory retrieval, as participants retrieved the emotional content they imagined during encoding. The distribution of object reinstatement in the present study is more posterior than prior reports of object-specific encoding responses, which have primarily been observed in PRc (Staresina et al., 2011). In the present study, object-specific reinstatement extended from the transitional zone of MTL cortex into PHc. Multivariate measures of content-specific encoding in MTL cortex have shown that PHc discriminates objects from other forms of information content (Liang et al., 2013). This finding, together with the present data, suggests that while PHc may show a preference for processing place information during encoding and retrieval, it is not selective for such information content. Additionally, it is possible that when generating specific items during object imagery, participants imagined objects that were highly contextual in nature thus promoting additional recruitment of PHc (Aminoff, Kveraga, & Bar, 2013).
4.2. Hippocampal reinstatement of content-specific information
Evidence for memory reinstatement in human hippocampus has largely been limited to electrophysiological work in patients (Gelbard-Sagiv et al., 2008). Neuroimaging studies that have observed reinstatement of encoding patterns within hippocampus during retrieval have provided only limited links to memory behavior (Mack & Preston, 2016; Wimber et al., 2015). Here, we provide evidence that reinstatement of encoded content in hippocampus supports accurate retrieval of source information. Notably, hippocampal reinstatement effects were strongest for place content; we observed place reinstatement effects that tracked source memory for places across the entire longitudinal axis of hippocampus. This finding is consistent with other recent work showing that during both encoding (Liang et al., 2013) and retrieval (Mack & Preston, 2016), hippocampal activation patterns are more reliable at discriminating place content relative to either person or object content. Collectively, these findings support the notion that hippocampus, and posterior hippocampus in particular, may play a specialized role in spatial processing (Bird & Burgess, 2008; Kumaran & Maguire, 2005).
However, in anterior hippocampus, we did see evidence that reinstatement of person content differed for correct and incorrect source retrieval, suggesting a more general role for hippocampus in memory beyond spatial processing. In the human brain, anterior hippocampus shows preferentially connectivity with PRc (Libby et al., 2012), a region that also showed selective reinstatement of person content in the present study. Furthermore, in rodents, gene expression in ventral hippocampus (which corresponds to anterior hippocampus in humans) and amygdala are correlated (Fanselow & Dong, 2010), consistent with an increased role for anterior hippocampus in emotional processing. As with PRc, the person imagery condition may have evoked anterior hippocampal processing due to the increased emotional content associated with people in comparison to objects and places. Thus, as a whole, the hippocampus may play an essential role in processing many forms of memory content (Davachi, 2006; Diana et al., 2007); however, our data suggest that there may be specialization of content representation along the anterior-posterior axis.
One notable aspect of the present data is that reinstatement in hippocampus was observed at the category level. Previous studies have often failed to find evidence for hippocampal reinstatement when using category-level decoding techniques, instead finding evidence only at the level of individual items (e.g., Mack & Preston, 2016). Based on such data, some researchers have proposed that hippocampus is “representationally agnostic” (Huffman & Stark, 2014), with a sparse coding strategy that does not differentiate stimuli based on information content. However, in prior work, category-level decoding has been performed on the basis of the visual properties of stimuli alone. Here, classifiers are trained to differentiate imagined rather than perceived content at encoding. When performing imagery and retrieving imagined details, participants likely evoke both visual and conceptual information when thinking about people, objects, and places. Our data suggest that the hippocampus may be more likely to discriminate categories based on differences between their conceptual, as opposed to visual, properties (Quian Quiroga, Kraskov, Koch, & Fried, 2009).
4.3. MTL subregions and subjective memory decisions
A central question in memory research is how MTL regions contribute to subjective aspects of memory decisions. An early study (Cabeza, Rao, Wagner, Mayer, & Schacter, 2001) suggested a dissociation between hippocampus and PHc during memory retrieval, in which hippocampal activation tracked subjective judgments of memory status, while PHc activation accurately predicted the objective status of memory probes. Recent work demonstrates that hippocampal engagement tracks memory confidence during retrieval (Gordon et al., 2014; Leiker & Johnson, 2015; Rutishauser et al., 2015; Thakral et al., 2015), further linking hippocampal processing to subjective decisions. The present analyses provide a different window into the relationship between MTL retrieval processes and subjective memory. Instead of relating MTL engagement to subjective confidence, we assessed how reinstatement within the MTL network predicted true and false source decisions. In contrast to prior work, we found that MTL cortex, and PHc in particular, tracked subjective choices about source content during memory misattributions.
5. CONCLUSIONS
The present study provides a fundamental demonstration that recollection of source details requires reinstatement of MTL encoding patterns. The findings go beyond prior work by demonstrating that MTL reinstatement of encoded content not only supports successful source memory, but also underlies individuals’ source misattributions. Our results thus indicate that the same mechanism—reinstatement of previously experienced content—underlies both true and false memories. Moreover, the present data indicate that different MTL subregions support retrieval of distinct forms of memory content, thereby informing current models of MTL subregional function. The results suggest an anterior-posterior distribution of content representation within hippocampus and MTL cortex, with information about people, objects, and places being processed in the anterior, middle, and posterior aspects of the MTL respectively.
ACKNOWLEDGEMENTS
The authors thank Amelia Wattenberger and Christopher Conser for assistance with data collection. This work was supported by the National Institute of Mental Health (F31MH097441 and R01MH100121) and by the National Science Foundation (1056019).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agster KL, Tomas Pereira I, Saddoris MP, Burwell RD. Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. II. efferents. Hippocampus. 2016 doi: 10.1002/hipo.22600. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9(3):182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bird CM, Keidel JL, Ing LP, Horner AJ, Burgess N. Consolidation of Complex Events via Reinstatement in Posterior Cingulate Cortex. J Neurosci. 2015;35(43):14426–14434. doi: 10.1523/JNEUROSCI.1774-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch SE, Jehee JF, Fernandez G, Doeller CF. Reinstatement of associative memories in early visual cortex is signaled by the hippocampus. J Neurosci. 2014;34(22):7493–7500. doi: 10.1523/JNEUROSCI.0805-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci U S A. 2001;98(8):4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14(2):147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Tompary A, Davachi L. Trial-by-Trial Hippocampal Encoding Activation Predicts the Fidelity of Cortical Reinstatement During Subsequent Retrieval. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw146. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell J, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences. USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Preston AR. The medial temporal lobe and memory. In: Gazzaniga MS, editor. The Cognitive Neurosciences. 5th ed. MIT Press; Cambridge, MA: 2015. pp. 539–546. [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18(6):536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial temporal lobe activity during source retrieval reflects information type, not memory strength. J Cogn Neurosci. 2010;22(8):1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW. Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Hum Brain Mapp. 2010;31(9):1359–1379. doi: 10.1002/hbm.20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322(5898):96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD. Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cereb Cortex. 2014;24(12):3350–3364. doi: 10.1093/cercor/bht194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Bisby JA, Bush D, Lin WJ, Burgess N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat Commun. 2015;6:7462. doi: 10.1038/ncomms8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman DJ, Stark CE. Multivariate pattern analysis of the human medial temporal lobe revealed representationally categorical cortex and representationally agnostic hippocampus. Hippocampus. 2014;24(11):1394–1403. doi: 10.1002/hipo.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, McDuff SG, Rugg MD, Norman KA. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron. 2009;63(5):697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12(7):913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Chun MM. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. J Neurosci. 2014;34(23):8051–8060. doi: 10.1523/JNEUROSCI.4328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Wagner AD. Multi-voxel patterns of visual category representation during episodic encoding are predictive of subsequent memory. Neuropsychologia. 2012;50(4):458–469. doi: 10.1016/j.neuropsychologia.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The human hippocampus: cognitive maps or relational memory? J Neurosci. 2005;25(31):7254–7259. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiker EK, Johnson JD. Pattern reactivation co-varies with activity in the core recollection network during source memory. Neuropsychologia. 2015;75:88–98. doi: 10.1016/j.neuropsychologia.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cereb Cortex. 2013;23(1):80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci. 2012;32(19):6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19(3):308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Preston AR. Decisions about the past are guided by reinstatement of specific memories in the hippocampus and perirhinal cortex. Neuroimage. 2016;127:144–157. doi: 10.1016/j.neuroimage.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Morcom AM. Re-engaging with the past: recapitulation of encoding operations during episodic retrieval. Front Hum Neurosci. 2014;8:351. doi: 10.3389/fnhum.2014.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Pereira IT, Agster KL, Burwell RD. Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. I. afferents. Hippocampus. 2016 doi: 10.1002/hipo.22603. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310(5756):1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Kraskov A, Koch C, Fried I. Explicit encoding of multimodal percepts by single neurons in the human brain. Curr Biol. 2009;19(15):1308–1313. doi: 10.1016/j.cub.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Prog Brain Res. 2015;219:45–64. doi: 10.1016/bs.pbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, Labar KS, Cabeza R. Neural Similarity Between Encoding and Retrieval is Related to Memory Via Hippocampal Interactions. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Ye S, Koroma M, Tudusciuc O, Ross IB, Chung JM, Mamelak AN. Representation of retrieval confidence by single neurons in the human medial temporal lobe. Nat Neurosci. 2015;18(7):1041–1050. doi: 10.1038/nn.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31(24):8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Henson RN, Kriegeskorte N, Alink A. Episodic reinstatement in the medial temporal lobe. J Neurosci. 2012;32(50):18150–18156. doi: 10.1523/JNEUROSCI.4156-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD. Cortical reinstatement and the confidence and accuracy of source memory. Neuroimage. 2015;109:118–129. doi: 10.1016/j.neuroimage.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: Vivid remembering reactivates sensory-specific cortex. Proceedings of the National Academy of Science, USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimber M, Alink A, Charest I, Kriegeskorte N, Anderson MC. Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat Neurosci. 2015;18(4):582–589. doi: 10.1038/nn.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R. Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J Cogn Neurosci. 2015;27(4):679–691. doi: 10.1162/jocn_a_00740. [DOI] [PMC free article] [PubMed] [Google Scholar]