Abstract
Objectives
Recent evidences suggest that mitochondrial dysfunction maybe involved in the pathophysiology of major depressive disorder (MDD); however, the role of mitochondrial genes in this disorder has not been studied systematically. In the present study, we profiled expression of mitochondrial genes in dorsolateral prefrontal cortex (dlPFC) of MDD and non-psychiatric control subjects.
Methods
Human mitochondrial RT2 profile PCR array plates were used to examine differentially expressed genes in dlPFC of 11 MDD and 11 control subjects. Differentially expressed genes were validated independently by qRT-PCR. Biological relevance of differentially expressed genes was analyzed by Gene Ontology (GO) and ingenuity pathways analysis (IPA).
Results
We found that 16 genes were differentially expressed in the MDD group compared with control group. Among them, 3 genes were down-regulated and 13 genes up-regulated. None of these genes were affected by confounding variables, such as age, postmortem interval, brain pH, and antidepressant toxicology. Seven differentially expressed genes were successfully validated in MDD subjects. GO and IPA analyses identified several new regulatory networks associated with mitochondrial dysfunctions in MDD.
Conclusions
Our findings suggest abnormal mitochondrial systems in the brain of MDD subjects which could be involved in the etiopathogenesis of this disorder.
Keywords: Major depression, dorsolateral prefrontal cortex, mitochondrial genes, expression, gene network
Introduction
Major depressive disorder (MDD) is a common psychiatry disorder, the lifetime prevalence of which is 15%~20%. Due to considerable morbidity and mortality, MDD is considered to be the second leading causes of disability (Ferrari et al. 2013). Despite that medication and psychotherapeutic treatments are available for clinical depression, it is urgent and critical to uncover its underlying pathogenic mechanism and find more effective drugs (Bondy 2002).
It is hypothesized that mitochondrial dysfunction in specific brain regions may be related to the cause or effect of MDD (Tobe 2013). Mitochondria are vital cellular organelles, which generate adenosine triphosphate (ATP) via mitochondrial respiratory chain (oxidative phosphorylation) in eukaryotic cells. ATP acts as the universal energy used for a wide range of cellular process (Perier and Vila 2012). For neurons, neuronal energy needs are primarily supplied by mitochondrial oxidative phosphorylation, as it is too sufficient to obtain energy through cytosolic glycolysis. That is why neuron related diseases are highly vulnerable to mitochondrial dysfunction (Ivanov et al. 2014). Besides acting as a power station, mitochondrial dynamics also regulate neuronal energy metabolism, Ca2+ homeostasis, and dendritic and axonal motility, which affect release of neurotransmitters and neurotropic factors (Mattson et al. 2008).
Mitochondrial dysfunction mainly takes part in the three metabolic processes: 1) reactive oxygen species (ROS), 2) calcium signaling, and 3) apoptosis. In ROS, mitochondria are one of the most important sources of reactive oxygen species in the mammalian cells. Moderate ROS can regulate synaptic plasticity and learning and memory. On the other hand, excessive ROS can damage mitochondrial structures and oxidative injury, which can produce free radicals, which in turn cause mitochondrial abnormalities (Sun and Chen 1998). Calcium ions act as messengers to activate intra-mitochondrial metabolic processes (McCORMACK et al. 1990). The level of calcium ions regulates the activation of mitochondrial permeability transition as well (Wong et al. 2012). Oxidative stress associated with mitochondrial dysfunction can induce cytochrome C which activates caspase dependent apoptotic pathway (Lin et al. 2012). Recently, it has been shown that apoptosis is associated with changes in mitochondrial mass consistent with mitochondrial fission (Chistiakov et al. 2014). Taken together, mitochondrial dysfunction involved in the number of vital cell physiological processes through ATP production, Ca2+, and oxidative stress.
Several lines of evidence demonstrate that mitochondrial dysfunction underlie pathophysiology of MDD. For example, chronic stress-induced depression in rodents leads to inhibition of mitochondrial respiratory chain in the cortex and cerebellum (Rezin et al. 2008). Neuroimaging studies with PET (positron emission tomography) or SPECT (single-photon emission computed tomography) have found that MDD patients show the symptoms of reduced blood flow, energy, and glucose metabolism, notably in the prefrontal cortex and basal ganglia (Beauregard 2014). When mitochondrial functions were studied in muscle biopsy, it was found that both the production of ATP and activity of respiratory chain enzymes are decreased in MDD patients (Wibom et al. 2002). Chang et al. (2015) compared the alterations of mtDNA copy number, SNPs, and oxidative damage of mtDNA in leukocytes of MDD patients and found that mtDNA copy number in MDD patients was significantly lower than the control group, however, no common 4977-bp deletion in mtDNA was detected. On the other hand, Gardner et al. (2003) reported that deletions in the mtDNA, a 9.2 kbp product encompassing nt4336–13532, were more frequent in the MDD patients compared with control subjects. They also showed a significant decrease of mitochondrial ATP production rates and mitochondrial enzyme ratios in the MDD muscles compared to controls. Two other groups investigated mitochondrial respiration via high-resolution respirometry in the intact and permeabilized platelets of MDD patients and found significant decrease of respiration efficiency in intact platelets in this population (Hroudová et al. 2013; Karabatsiakis et al. 2014). On the other hand, respiratory rates were unchanged in permeabilized platelets of MDD patients. Karabatsiakis et al. (2014) also confirmed that mitochondrial respiration of PBMCs is significantly decreased in MDD patients. Importantly, decline of respiratory activity is negatively correlated with the severity of depressive symptoms, such as loss of energy, difficulties concentrating and fatigue etc. (Karabatsiakis et al. 2014).
Recently, genetic studies have confirmed that mitochondrial genes contribute to risk of MDD. For example, using low-coverage whole-genome sequencing of 5,303 MDD and 5,337 controls, Cai et al. (2015) identified two genes to be associated with MDD: one is SIRT1 and the other one is LHPP. SIRT1 is involved in mitochondrial functions. These investigators also reported that MDD was associated with increased amounts of mtDNA (Cai et al. 2015). Another study examined the association between mtDNA sequence variations and heteroplasmy levels in the dorsolateral prefrontal cortex from a cohort of schizophrenia, bipolar, and MDD subjects and age-matched controls. Three mitochondrial variants (C10652T, T14668C and A15043G) showed a significant allelic association with MDD (Rollins et al. 2009). Altogether, these evidences clearly suggest that mitochondria dysfunction may participate in the pathophysiology of MDD.
DNA microarray analyses have been conducted to detect alteration of mitochondria-related gene expression in postmortem brain of schizophrenia and bipolar subjects (Iwamoto et al. 2005). A small fraction of genes encoding respiratory chain components was found to be downregulated in these subjects. However, there have been no studies examining the pattern of mitochondria gene expression in the MDD postmortem brain samples. In the present study, we performed quantitative analysis for expression of mitochondria-related genes in dorsolateral prefrontal cortex (dlPFC) to reveal difference between MDD and control subjects. In parallel, we conducted bioinformatics analysis to investigate corresponding pathway(s) associated with mitochondrial functions.
Material and Methods
Tissue preparation
The present studies were performed in dlPFC (Brodmann’s area 9) obtained from the right hemisphere of the brain from 11 MDD subjects and 11 non-psychiatric control subjects (referred as control subjects). Brain samples were obtained from the Brain Collection Program of the Maryland Psychiatric Research Center, Baltimore, MD. Family members/informants signed written informed consents. The prefrontal cortex was defined as the gray matter from the most anterior 1-cm coronal slice of the cortex and was further dissected according to the Brodmann atlas. All the tissues were screened for evidence of neuropathology by experienced neuropathologists. The presence of Alzheimer’s disease, infarcts, demyelinating diseases, or atrophy (or clinical history of these disorders) disqualified subjects from the study. pH of the brain was measured as discussed earlier (Dwivedi et al. 2009).
Psychological autopsy
Psychological autopsies were performed using the Diagnostic Evaluation After Death (DEAD) (Salzman et al. 1983) and the Structured Clinical Interview for the DSM-IV (SCID) (Spitzer RL 1995) as described earlier (Dwivedi et al. 2009). Briefly, one or two family members, after giving verbal informed consent, underwent an interview. Two psychiatrists independently reviewed the write-up from this interview, as well as the SCID that was completed from it, as part of their diagnostic assessment of the case. Diagnoses were made from the data obtained in this interview, medical records from the case, and records obtained from the Medical Examiner’s office. The two diagnoses were compared and discrepancies were resolved by means of a consensus conference. Control subjects were verified as free from mental illnesses using these consensus diagnostic procedures. Toxicology screening for alcohol, a comprehensive battery for illicit drug use, and screening for antidepressant or psychoactive drugs taken prior to death were performed in blood/urine of each subject. None of the subjects showed positive toxicology for alcohol or substance abuse. In each case, screening for the presence of HIV was done in blood samples, and all HIV-positive cases were excluded. This study was approved by the Institutional Review Board of the University of Alabama at Birmingham. Demographic and clinical characteristics of normal controls and MDD subjects are provided in Table 1.
Table 1.
Demographic and clinical characteristics of control and MDD subjects
| Case # | Age (yrs) | Race | Gender | PMI (hr) | Brain pH | Cause of Death | Drug Toxicology | Alcohol abuse | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | White | Male | 24 | 6.8 | ASCVD | None | None | Control |
| 2 | 35 | White | Male | 24 | 7.1 | Accidental chest injury | None | None | Control |
| 3 | 45 | White | Male | 22 | 6.8 | ASCVD | None | None | Control |
| 4 | 45 | White | Female | 12 | 6.8 | Pneumonia | OTC cold medicine | None | Control |
| 5 | 24 | White | Female | 23 | 6.7 | Embolism | None | None | Control |
| 6 | 47 | White | Male | 10 | 6.9 | ASCVD | None | None | Control |
| 7 | 37 | White | Female | 19 | 6.9 | Hemopericardium | None | None | Control |
| 8 | 31 | White | Male | 16 | 6.7 | Vehicle injury | None | None | Control |
| 9 | 28 | White | Male | 13 | 7.1 | Electrocution | None | None | Control |
| 10 | 28 | White | Male | 13 | 6.8 | Cardiac arrhythmia, natural | None | None | Control |
| 11 | 77 | White | Male | 16 | 6.9 | Drowning | None | None | Control |
|
Mean SD |
39.4 14.6 |
11 White 0 Black |
8 Males 3 Females |
17.45 5.18 |
6.86 0.14 |
||||
| 12 | 65 | White | Male | 14 | 7.1 | ASCVD | None | None | MDD |
| 13 | 55 | Black | Female | 8 | 6.9 | ASCVD | Fluoxetine, acetone | None | MDD |
| 14 | 24 | White | Male | 11 | 6.8 | Vehicle injury | Sertraline | None | MDD |
| 15 | 39 | White | Male | 36 | 6.7 | Fatty liver | None | None | MDD |
| 16 | 46 | Black | Male | 20 | 6.6 | Seizure | Fluoxetine | None | MDD |
| 17 | 59 | White | Male | 20 | 7.2 | ASCVD | Atropine, methadone | None | MDD |
| 18 | 29 | White | Female | 22 | 6.8 | Cardiomegaly | Fluoxetine, propoxyphene | None | MDD |
| 19 | 49 | White | Male | 23 | 6.8 | Undetermined | None | None | MDD |
| 20 | 58 | White | Male | 24 | 6.5 | ASCVD | None | None | MDD |
| 21 | 59 | White | Male | 9 | 6.9 | ASCVD | Lidocaine | None | MDD |
| 22 | 49 | White | Male | 24 | 6.9 | ASCVD | None | None | MDD |
|
| |||||||||
|
Mean SD |
48.4 13.0 |
10 White 1 Black |
9 Males 2 Females |
19.2 8.2 |
6.8 0.2 |
||||
ASCVD = Atherosclerosis cardiovascular disease; Controls = non-psychiatric controls; MDD = major depressive disorder; PMI = postmortem interval
RNA isolation
Total RNA was isolated using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to manufacturer’s directions and treated with DNAse 1. The RNA yield was determined by absorbance at 260 nm and the purity of RNA was determined by measuring the optical density with an absorbance ratio of 260/280 using NanoDrop®ND-1000 (NanoDrop Technologies, DE, USA). Only RNA with >1.8 purity was used. The quality of RNA was assessed using Agilent Bioanalyzer 2100 as described earlier (Dwivedi et al. 2009) and only samples with an RNA integrity number >7 were used.
PCR array
RT reaction was performed with 400 ng of total RNA using an RT2 First-strand Kit (SABiosciences, Qiagen Inc., Valencia, CA). The quality of RNA was confirmed by using an RT2 RNA QC PCR Array Kit (SABiosciences, Qiagen Inc., Valencia, CA, USA). Two hundred nanograms of random-primed cDNAs were processed for quantitative real-time reverse-transcriptase PCR (qRT-PCR) of 84 genes involved in biogenesis and function of mitochondria and housekeeping genes by using an RT2Profiler™ PCR Array Kit for human mitochondria (PAHS-087A, SABiosciences, Qiagen Inc., Valencia, CA, USA). An integrated web-based software package for the PCR Array System was used to calculate ΔΔCt-based fold-change from the raw threshold cycle data.
Real-time PCR validation
Seven genes which showed significant change were selected to verify the PCR array results. Quantitative real-time PCR expression analysis was carried out using MX3005P sequence detection system (Agilent Technologies, Santa Clara, CA, USA) in dlPFC of each depressed (n = 11) and control (n = 11) subjects in which PCR array was determined. Reaction mixture (20 μl) was prepared, which included 10 μl of EvaGreen 2X qPCR MasterMix-ROX (Applied Biology Materials, Richmond, BC, Canada), 0.2 mM of each oligo primer and 1 μl of diluted cDNA. Gene transcripts were quantified and normalized using GeNorm algorithm. Following reference genes were used: B2M, HPRT1, RPL13A, GAPDH, ACTB. The primer sequences for these genes are mentioned in Supplementary Table 1. All biological samples were tested in triplicate, and SD values of the means were calculated using standard statistical methods. The 2-ΔΔCT method was used for quantification of the transcript expression (Schmittgen and Livak 2008). Specific annealing of the oligonucleotides was controlled by dissociation kinetics performed at the end of each PCR run. The efficiency of each primer pair was measured on a PCR product by serial dilution. Quantitative real-time PCR primer sequences are provided in Supplementary Table 1.
Statistical and bioinformatics analyses
All data were analyzed using Statistical Package for the Social Sciences (SPSS), version 23 (IBM, USA). The data are reported as the mean ± SD. Similar statistical procedure was followed to examine the difference in expression of genes that were validated. The differences in age, sex, pH of the brain and postmortem interval (PMI), between MDD subjects and controls were analyzed using the independent-sample “t” test. An α level ≤0.05 was considered statistically significant. The relationships between gene expression and PMI, age, and pH of the brain were determined by Pearson product-moment correlation analysis. The confounding variables were used as covariates during data analysis. The effects of gender on various measures were determined by an independent sample “t” test comparing males and females. Similarly, an independent sample “t” test was used to compare the depressed subjects who showed presence of antidepressant at the time of death with the depressed subjects who did not. To exploit the Ct obtained from PCR-Array, gene expression heatmaps were generated using the heatmap function of the gplots R package (v.2.16.0). PCR array results were analyzed using the ΔΔCt method (PCR Array Data Analysis Webportal: http://www.sabiosciences.com/pcrarraydataanalysis.php (SABiosciences, Qiagen, Valencia, CA, USA). The fold change was normalized to the arithmetic mean of five reference genes (RPL13A, GAPDH, ACTB, B2M, and HPRT1). Mitochondria related gene expression with differences greater than 1.3-fold (p≤0.05) was considered as differential expressed genes. Gene Ontology (GO) term enrichments were performed TopGO R package (v.2.18) with the classic Fisher method. The differentially expressed genes and fold change were uploaded into data analysis software (Ingenuity System Inc., Redwood City, CA, USA). To view the molecular connections and regulatory network, canonical pathway analysis were conducted with IPA software. In the analysis of validation in MDD, the housekeeping gene GAPDH was selected as the reference and the relative expressions value were expressed by ΔCt = Ct target gene-Ct GAPDH, which means that the higher value of ΔCt of the mitochondrial genes, the lower its transcription level.
Results
Subject demography
The demographic characteristics of MDD subjects and normal control subjects are provided in Table 1. There were 8 males and 3 females in the control group, and 9 males and 2 females in the MDD group. The age range was 24–77 years; the PMI was in the range of 8–36 hr. There were no significant differences in age (t = 1.5, df = 20, p = 0.15) or PMI (t = 0.59, df = 20, p = 0.56) between MDD subjects and control subjects. The mean brain pH values of MDD subjects and controls were 6.8±0.13 and 6.8±0.2, respectively, which were not different between these groups (t = 0.37, df = 20, p = 0.71).
Transcriptional profiling of mitochondrial genes
The transcriptional profiling of 84 mitochondrial genes was obtained by the human mitochondrial RT2 profiler PCR array and was used to produce a heatmap scheme (Figure 1a) and scatter plot (Figure 1b). The figure describes the difference between MDD and control groups. On the basis of Ct value of each gene in the heatmap figure, high Ct value represented the low expression.
Figure 1.
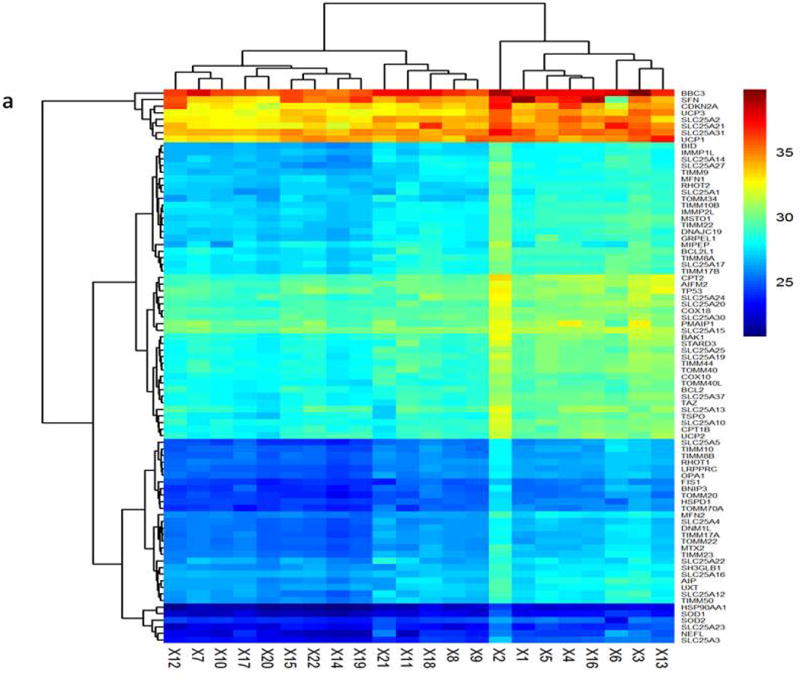
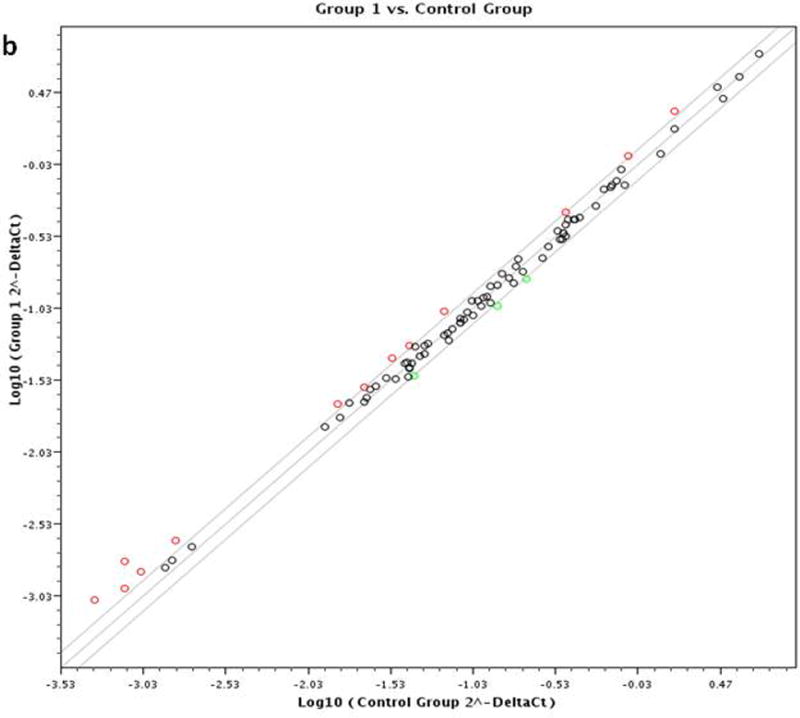
Figure 1a: Hierarchical clustering analysis of mitochondria gene expression profile in MDD and control subjects. Each row represented different sample, each column represented different genes. Red represents high Ct value with low expression. Blue represent low Ct value with high expression. Figure 1b: Scatter plot based on the fold change of each gene. Group 1 means MDD Group; Control means healthy control.
There were a total of sixteen genes (fold change ≥ 1.3) that were differentially expressed in the MDD group compared with control group. Among them, three genes were down-regulated in the MDD subjects. These 3 genes included: SLC25A23 (phosphate carrier), SCL25A12 (aspartate/glutamate carrier), and STARD3 (lipid trafficking proteins) (Table 2). There were also 13 mRNA upregulated changes in the MDD patients. These upregulated genes and related expression information are listed in the Table 3. The fold change of SFN (stratifin) was 2.24 and ranked the 1st among all the differentially expressed genes. In addition, TIMM8B, the p-value of which was 0.008453, was associated with MDD. TIMM8B encodes translocase of inner mitochondrial membrane 8 homolog B and shows moderate to high expression in human central nervous systems. The studies related to TIMM8B are rarely reported. Our study provides the first proof for the TIMM8B and SFN in MDD.
Table 2.
Down-regulated mitochondria genes expressed in dlPFC of MDD subjects
| # | Gene Symbol | UniGene ID | Functional Gene Grouping | Fold Change |
|---|---|---|---|---|
| 1 | SLC25A27 | 9481 | Solute carrier family 25, member 27 | −1.35 |
| 2 | SLC25A12 | 8604 | Solute carrier family 25 (aspartate/glutamate carrier), member 12 | −1.31 |
| 3 | STARD3 | 10948 | StAR-related lipid transfer (START) domain containing 3 | −1.29 |
Table 3.
Up-regulated mitochondria genes expressed in dlPFC of MDD subjects
| # | Gene Symbol | UniGene ID | Functional Gene Grouping | Fold Change |
|---|---|---|---|---|
| 1 | SLC25A30 | 253512 | Solute carrier family 25, member 30 | 1.29 |
| 2 | TIMM8B | 26521 | Translocase of inner mitochondrial membrane 8 homolog B (yeast) | 1.29 |
| 3 | SOD2 | 6648 | Superoxide dismutase 2, mitochondrial | 1.32 |
| 4 | BCL2 | 596 | B-cell CLL/lymphoma 2 | 1.35 |
| 5 | UCP2 | 7351 | Uncoupling protein 2 (mitochondrial, proton carrier) | 1.4 |
| 6 | SLC25A23 | 79085 | Solute carrier family 25 (mitochondrial carrier; phosphate carrier), member 23 | 1.42 |
| 7 | MIPEP | 4285 | Mitochondrial intermediate peptidase | 1.43 |
| 8 | TP53 | 7157 | Tumor protein p53 | 1.45 |
| 9 | SLC25A31 | 83447 | Solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 31 | 1.46 |
| 10 | UCP1 | 7350 | Uncoupling protein 1 (mitochondrial, proton carrier) | 1.53 |
| 11 | CDKN2A | 1029 | Cyclin-dependent kinase inhibitor 2A | 1.55 |
| 12 | BBC3 | 27113 | BCL2 binding component 3 | 1.86 |
| 13 | SFN | 2810 | Stratifin | 2.24 |
Effect of confounding variables
The effects of potential confounding variables, namely, age, gender, PMI, and brain pH were evaluated with respect to the expression of mitochondrial genes, in which we had found differences between control and MDD subjects. As shown in Supplemental Table 2, there were no significant effects of any of these variables on the expression of mitochondrial genes. There were 8 males and 3 females in the control group and 9 males and 2 females in the MDD group. Comparison of expression levels between males and females showed no effects in both control and MDD groups (Supplemental Table 3). Out of 11 MDD subjects, 7 had no antidepressant toxicology whereas 4 showed antidepressant toxicology at the time of death. Except MIPEP and BDC3 genes, the expression levels of other mitochondrial genes were similar in groups with and without antidepressant toxicology (Supplemental Table 4).
Validation in the MDD samples by qRT-PCR
Seven differentially transcribed mitochondria genes were selected to validate in the MDD and control subjects (n =11 in each group). The results are shown in Figure 2. As can be seen, the expression of TIMM8B (t = 2.87, df = 20, p = 0.01) and SLC25A23 (t = 2.04, df = 20, p = 0.02), SFN (t = 2.46, df = 20, p = 0.023), SLC25A30 (t = 2.54, df = 20, p = 0.021), and UCP2 (t = 3.0, df = 20, p = 0.007), were upregulated and SLC25A12 (t = 4.03, df = 20, p = 0.001), and SLC25A27 (t = 5.59, df = 20, p <0.001), were downregulated in MDD subjects as compared with control subjects. The fold changes in in these genes were almost similar when replicated as compared with the qRT-PCR profile array data.
Figure 2.
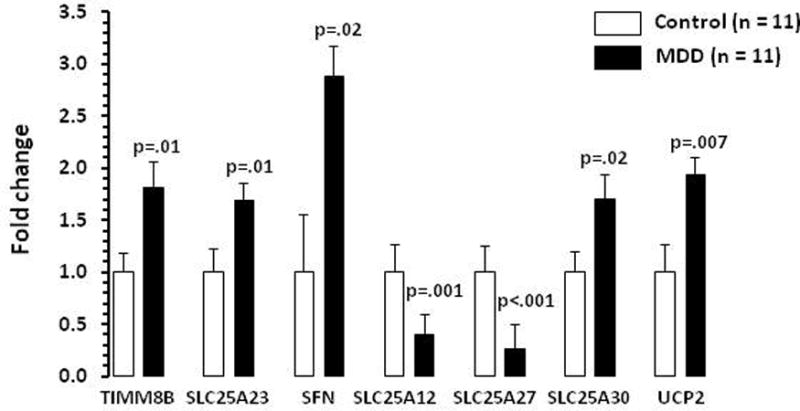
Bar diagram showing expression levels of genes that were tested for validation analysis in dlPFC of control and MDD subjects. Data are the mean ± SD. N = 11 in each group.
Gene ontology (GO) analysis
To obtain information about biological processes, we performed GO analysis with TopGO packages of R software. Gene ontology analysis was performed to identify significantly enriched biological processes, molecular functions, and cellular components. For biological processes, we found a total of 416 GO terms with p-value <0.01. These GO terms include direct or indirect functions towards mitochondria. The top 20 statistically significant GO terms associated with mitochondria biological processes are listed in Supplemental Table 5. The most significantly GO terms were related to mitochondrial transport, establishment of protein localization in mitochondrion, protein localization to mitochondrion, single-organism intracellular transport, intracellular transport, apoptotic mitochondrial changes, etc. For molecular functions, there were 29 GO terms, the p-value of which was<0.01. Supplemental Table 6 shows top 20 molecular function terms from GO enrichment analysis. Theses molecular function terms are involved in many multiple signaling and binding activities including MDM2/MDM4 family protein binding, protein phosphatase 2A binding, protein serine/threonine kinase inhibitor activity, etc. Twenty two GO terms of cellular component category reached significance level (p< 0.01) (Supplemental Table 7). Of these 20 GO terms include mitochondrion, mitochondrial part, organelle envelope, etc. GO term enrichment analysis provided a brief view of biological processes, cellular components and molecular functions associated with MDD.
Pathway analysis
We uploaded 16 differentially expressed genes into IPA analysis tool. Canonical pathway analysis was first performed to find these gene specific affected pathways. The results are shown in the Figure 3. The top canonical pathway for these gene sets includes p53 signaling, Myc-mediated apoptosis signaling, induction of apoptosis by HIV1, PEDF signaling, etc. These results indicate an association between apoptosis and mitochondria gene sets in MDD. There were 4 differentially expressed genes (BBC3, Bcl2, CDKN2A, TP53) enriched in P53 pathway (p =3.16× 10−7) and 3 genes (Bcl2, CDKN2A, TP53) in the Myc-mediated apoptosis signaling (6.44×10−6).
Figure 3.
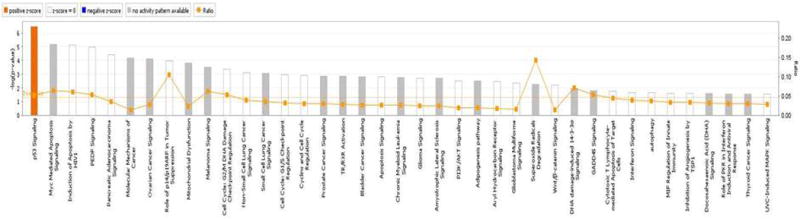
Canonical pathway analysis of genes differentially expressed in dlPFC of MDD subjects. The line represents the ratio of the number of genes represented within each pathway to the total number of genes in the pathway.
Discussion
To our knowledge, this is the first study to explore the mitochondria gene expression in the postmortem brain of MDD subjects. In this study, we used mitochondrial PCR array profiling to find alterations in 84 mitochondria related gene expression. We found 16 specific genes differentially expressed between MDD and control subjects. In order to examine if the changes in mitochondrial genes in MDD subjects can be replicated, we independently examined expression of TIMM8B, SLC25A23, SFN, SLC25A30, UCP2, SLC25A12, and SLC25A27 in dlPFC that we found to be altered in the MDD group. We were able to replicate the findings, suggesting that these changes are consistent. We did not find any effect of confounding variables such as age, sex, postmortem interval, and pH of the brain on any of the genes that showed significant change in the MDD group. Two genes MIPEP and BBC3 were downregulated in the MDD subjects who showed positive antidepressant toxicology. Interestingly, these two genes were upregulated in the MDD group, suggesting that antidepressant toxicology had no overall effect on the expression of these two genes.
Three downregulated genes, including SLC25A27, SLC25A12, and STARD3 were significantly decreased in the dlPFC of MDD subjects. Of interest to this study was SLC25A27. The product of SLC25A27 is uncoupling proteins 4 (UCP4), which is located on the inner mitochondrial membrane and plays an important role in reducing ROS and alleviation of oxidative stress, consequently, inhibition of apoptosis, ATP inadequacy, etc. (Ho et al. 2012). It has earlier been reported that downregulation of SLC25A27 could negatively influence these processes. Abnormalities in UCP4 have been reported in schizophrenia, autism spectrum disorder, and Huntington’s disease (Anitha et al. 2012; Besson et al. 2010; Mouaffak et al. 2011; Yasuno et al. 2007). This paper provides evidence that downregulation of SLC25A27 may contribute to neuropsychiatric disorders.
We also found that the expression of 13 genes was significantly increased in dlPFC of MDD subjects. These include: SLC25A30, TIMM8B, SOD2, BCL2, UCP2, SLC25A23, MIPEP, TP53, SLC25A31, UCP1, CDKN2A, BBC3, and SFN. Among these 13 genes, 3 genes SLC25A30, SLC25A23, and SLC25A31 belong to the member of solute carrier family 25.SLC25A23 encodes the human APC2 (ATP-Mg/Pi carrier), which is a part of Ca2+sensitive mitochondrial carriers. SLC25A23 mediates the influx of Ca2+ and regulates ATP levels in neurons (Rueda et al. 2015). Le-Niculescu et al. (2009) performed gene expression profiling studies in blood samples of mood disorder patients and identified SLC25A23 as one of the candidate biomarker genes with convergent functional genomics, which can elevate low mood state (MDD). Our results in postmortem brain samples confirm this finding and suggest that upregulation of SLC25A23 may be associated with MDD.
SLC25A31,which encodes ANT4 protein (adenine nucleotide translocases), helps in supplying ADP to mitochondria and in release of ATP into cytoplasm (Dolce et al. 2005). So far, there is no published report of SLC25A31 and SLC25A30 in MDD. Our study is the first one to demonstrate such changes in this disorder. Stratifin, also called 14-3-3 sigma, is encoded by SFN gene. Genetics studies have shown that SFN is associated with schizophrenia. A reduction in mRNA and protein expression of diverse 14-3-3 isoforms have been reported in different brain areas of subjects with schizophrenia (English et al. 2009; Middleton et al. 2005; Vawter et al. 2001). Recently, (Rivero et al. 2015) performed western blot analysis of this protein in the PFC of MDD and control subjects. They found that the immunoreactivity values of 14-3-3 sigma were not alteration in MDD subjects. Although data from in vitro, animal, and human postmortem studies supported that 14-3-3 protein plays critical roles in various neurodegenerative disorders including schizophrenia and bipolar, so far, there have been no report of SFN in pathophysiology of MDD. Our study provides strong evidence of altered SFN expression in brain of MDD subjects.
Our study showed that superoxide dismutase 2 (SOD2) was significantly increased in dlPFC of MDD subjects, which successfully replicated Szuster-Ciesielska et al. (2008) findings. SOD2 expresses proteins of mitochondrial antioxidant enzymes. ROS induces upregulation of SOD2 through activation of cellular signaling pathways. Evidence shows that ROS or RNS increase and decrease antioxidant defense in depression (Maes et al. 2011). The increase of ROS/RNS and reduction of antioxidant defenses may cause damage of DNA and fatty acids (Aprioku 2013). We found SOD2 upregulation bears further testimony to oxidative stress in the MDD patients.
In this study, we performed Gene Ontology analysis on the three categories (“Molecular function”, “Biological process” and “Cellular component”) and listed the top 20 significantly enriched GO terms. For molecular function part, there are 29 GO terms, the p-value of which is below 0.01. These 29 GO terms include MDM2/MDM4 family protein binding, protein kinase inhibitor activity, transporter activity, superoxide dismutase activity, etc. The results demonstrated the altered expression genes of mitochondria takes part in several crucial physiological functions. For cellular component part, the 22 GO terms, with p-value <0.01, were identified with topGO software. The significant GO terms were mainly associated with mitochondrial membrane, envelope, cytoplasm and mitochondrial matrix, etc. For biological process, we found 416 GO terms with p-value <0.01. These GO terms included cell apoptosis, cell aging, neuron death, B cell differentiation, chromatin assembly, etc. These results suggest that the mitochondrial associated genes may participate in MDD pathophysiology via several biological processes. Further studies will be needed to confirm the involvement of these biological processes.
When we analyzed the canonical pathways, we found that p53- and Myc-mediated apoptosis signaling pathways were significantly enriched in the brain of MDD subjects. These two signaling pathways were identified around Bcl2, TP53 and CDKN2A genes. These results reveal strong possibility of apoptosis as pathophysiological process of cell death, which may significantly contribute to the understanding of the etiology of MDD. Bcl2 is a member of apoptosis family genes, which is linked with other apoptotic-regulatory factors at the mitochondria membrane and regulates the apoptosis. Bcl-2 family members are activated by various types of stresses and form pores in the mitochondrial outer membranes (Gross et al. 1999). Cytochrome C and other pro-apoptotic factors are released from the intermembrane space and triggercaspase-3 pathway (Gogvadze et al. 2006). It has been reported that BCL2 confer risk of MDD susceptibility and antidepressant treatment outcome in a Han Chinese population. A significant association between the rs2279115C allele associated with BCL2 and treatment resistant depression in males (corrected p=0.048) were found (Zhang et al. 2014). On the other hand, resistance to the development of stress-induced behavioral despair in the forced swim test is associated with elevated hippocampal anti-apoptotic Bcl-xl expression (Shishkina et al. 2010). We speculate that apoptotic pathways interact with the other pathways including oxidative and inflammatory, which may result in the development of MDD phenotype.
Besides p53- and Myc-mediated apoptosis signaling pathways, we also found PI3K-AKT and Wnt/β-catenin signaling pathways were significantly enriched in the canonical pathway analysis. PI3k-mediated signaling plays a crucial role in many physiological functions in the brain, including cell survival, synaptic plasticity, protein synthesis, and membrane trafficking. The function of PI3K is to activate the downstream target Akt, which is central to the PI3K-AKT pathway. We have earlier reported reduced catalytic activation and altered expression of specific PI3-kinase regulatory subunits in postmortem brain of depressed suicide subjects (Dwivedi et al. 2008). We have also reported that the catalytic activity and expression of the other 2 important genes in the PI3K/AKT pathway, PDK and PTEN, were altered in the prefrontal cortex and hippocampus of depressed suicide subjects (Dwivedi et al. 2010). Our study also supports the evidence that aberrant PI3K-kinase signaling may possible involved in the pathogenic mechanisms of depression.
Interestingly, canonical pathway analysis yielded Wnt signaling, which has been shown to be involved in affective disorders. For example, several genetics studies have found an association of Wnt signaling pathway genes in patients with MDD (Inkster et al. 2010). The mechanism of action of several mood stabilizing agents have been shown to be associated with Wnt signaling (Zhou et al. 2009). Dysregulated expression of several Wnt pathway-related genes has been reported in patients with bipolar disorder and suicide (Matigian et al. 2007; Ren et al. 2013). A recent study by explored the influence on GSK-3β expression, a critical component of Wnt signaling, in the rapid antidepressant action of ketamine in a mouse model of depression (Beurel 2011). The study demonstrated that ketamine can rapidly increase the phosphorylation of both GSK-3α and GSK-3β. Taken together, these studies confirmed the important role of the Wnt signaling system in depression and treatment response.
In summary, we identified 16 differentially expressed mitochondria genes by human RT2 PCR array. These differentially genes not only contain mitochondrial transporter family proteins but also include specific genes related to oxidative stress and apoptotic pathways. We also conducted GO analysis and IPA analysis and found several new regulatory networks. Overall, our study provides strong evidence that mitochondrial genes play a critical role in the MDD and indicate potential therapeutic targets and biomarkers to predict the MDD.
Supplementary Material
Acknowledgments
This research was supported by grants from National Institute of Mental Health (R01MH082802, R01MH100616 and R01MH101980) to Dr. Yogesh Dwivedi. We sincerely thank the Maryland Brain Collection for providing postmortem brain samples.
Footnotes
Statement of interest
None to declare.
References
- Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, Matsuzaki H, Miyachi T, Yamada S, Tsujii M. Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism. 2012;3:12. doi: 10.1186/2040-2392-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013;14:158–172. [PMC free article] [PubMed] [Google Scholar]
- Beauregard M. Functional neuroimaging studies of the effects of psychotherapy. Dialogues Clin Neurosci. 2014;16:75–81. doi: 10.31887/DCNS.2014.16.1/mbeauregard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M-T, Dupont P, Fridell Y-WC, Liévens J-C. Increased energy metabolism rescues glia‐induced pathology in a Drosophila model of Huntington’s disease. Hum Mol Genet. 2010;19:3372–3382. doi: 10.1093/hmg/ddq249. [DOI] [PubMed] [Google Scholar]
- Beurel E. Regulation by glycogen synthase kinase-3 of inflammation andT cells in CNS diseases. Neurological Functions of the Masterswitch Protein Kinase–GSK-3. 2011 doi: 10.3389/fnmol.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4:7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Bigdeli TB, Kretzschmar W, Li Y, Liang J, Song L, Hu J, Li Q, Jin W, Hu Z. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–591. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-C, Jou S-H, Lin T-T, Lai T-J, Liu C-S. Mitochondria DNA Change and Oxidative Damage in Clinically Stable Patients with Major Depressive Disorder. PLoS One. 2015;10:e0125855. doi: 10.1371/journal.pone.0125855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res Int. 2014;2014:238463. doi: 10.1155/2014/238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633–637. doi: 10.1016/j.febslet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Teppen T, Zhang H, Mondal A, Roberts RC, Conley RR, Pandey GN. Lower phosphoinositide 3-kinase (PI 3-kinase) activity and differential expression levels of selective catalytic and regulatory PI 3-kinase subunit isoforms in prefrontal cortex and hippocampus of suicide subjects. Neuropsychopharmacology. 2008;33:2324–2340. doi: 10.1038/sj.npp.1301641. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, Pandey GN. Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Modulation in Activation and Expression of PTEN, Akt1, and PDK1: Further Evidence Demonstrating Altered Phosphoinositide 3-kinase Signaling in Postmortem Brain of Suicide Subjects. Biological psychiatry. 2010;67:1017. doi: 10.1016/j.biopsych.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JA, Dicker P, Föcking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9:3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Johansson A, Wibom R, Nennesmo I, von Döbeln U, Hagenfeldt L, Hällström T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Ho P, Ho J, Liu H-F, So D, Tse Z, Chan K-H, Ramsden DB, Ho S-L. Mitochondrial neuronal uncoupling proteins: a target for potential disease-modification in Parkinson’s disease. Transl Neurodegener. 2012;1:3. doi: 10.1186/2047-9158-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hroudová J, Fišar Z, Kitzlerová E, Zvěřová M, Raboch J. Mitochondrial respiration in blood platelets of depressive patients. Mitochondrion. 2013;13:795–800. doi: 10.1016/j.mito.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, Matthews PM. Pathway-based approaches to imaging genetics association studies: Wnt signaling, GSK3beta substrates and major depression. Neuroimage. 2010;53:908–917. doi: 10.1016/j.neuroimage.2010.02.065. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Malkov AE, Waseem T, Mukhtarov M, Buldakova S, Gubkina O, Zilberter M, Zilberter Y. Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices. J Cereb Blood Flow Metab. 2014;34:397–407. doi: 10.1038/jcbfm.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsiakis A, Böck C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich DE, Kolassa I-T. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Translational Psychiatry. 2014;4:e397. doi: 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H, Kurian S, Yehyawi N, Dike C, Patel S, Edenberg H, Tsuang M, Salomon D, Nurnberger J, Niculescu A. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry. 2009;14:156–174. doi: 10.1038/mp.2008.11. [DOI] [PubMed] [Google Scholar]
- Lin T-K, Cheng C-H, Chen S-D, Liou C-W, Huang C-R, Chuang Y-C. Mitochondrial dysfunction and oxidative stress promote apoptotic cell death in the striatum via cytochrome c/caspase-3 signaling cascade following chronic rotenone intoxication in rats. Int J Mol Sci. 2012;13:8722–8739. doi: 10.3390/ijms13078722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Matigian N, Windus L, Smith H, Filippich C, Pantelis C, McGrath J, Mowry B, Hayward N. Expression profiling in monozygotic twins discordant for bipolar disorder reveals dysregulation of the WNT signalling pathway. Molecular psychiatry. 2007;12:815–825. doi: 10.1038/sj.mp.4001998. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCORMACK JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Peng L, Lewis DA, Levitt P, Mirnics K. Altered expression of 14-3-3 genes in the prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2005;30:974–983. doi: 10.1038/sj.npp.1300674. [DOI] [PubMed] [Google Scholar]
- Mouaffak F, Kebir O, Bellon A, Gourevitch R, Tordjman S, Viala A, Millet B, Jaafari N, Olié JP, Krebs MO. Association of an UCP4 (SLC25A27) haplotype with ultra-resistant schizophrenia. Pharmacogenomics. 2011;12:185–193. doi: 10.2217/pgs.10.179. [DOI] [PubMed] [Google Scholar]
- Perier C, Vila M. Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009332. doi: 10.1101/cshperspect.a009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Rizavi HS, Khan MA, Dwivedi Y, Pandey GN. Altered Wnt signalling in the teenage suicide brain: focus on glycogen synthase kinase-3β and β-catenin. International Journal of Neuropsychopharmacology. 2013;16:945–955. doi: 10.1017/S1461145712001010. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Cardoso MR, Gonçalves CL, Scaini G, Fraga DB, Riegel RE, Comim CM, Quevedo J, Streck EL. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem Int. 2008;53:395–400. doi: 10.1016/j.neuint.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Rivero G, Gabilondo AM, García-Sevilla JA, La Harpe R, Morentín B, Meana JJ. Up-regulated 14-3-3β and 14-3-3ζ proteins in prefrontal cortex of subjects with schizophrenia: effect of psychotropic treatment. Schizophr Res. 2015;161:446–451. doi: 10.1016/j.schres.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Rollins B, Martin MV, Sequeira PA, Moon EA, Morgan LZ, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG. Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PloS one. 2009;4:e4913. doi: 10.1371/journal.pone.0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda CB, Traba J, Amigo I, Llorente-Folch I, González-Sánchez P, Pardo B, Esteban JA, del Arco A, Satrústegui J. Mitochondrial ATP-Mg/Pi Carrier SCaMC-3/Slc25a23 Counteracts PARP-1-Dependent Fall in Mitochondrial ATP Caused by Excitotoxic Insults in Neurons. J Neurosci. 2015;35:3566–3581. doi: 10.1523/JNEUROSCI.2702-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman S, Endicott J, Clayton P, Winokur G. Diagnostic evaluation after death (DEAD) National Institute of Mental Health Neuroscience Research Branch; Rockville: 1983. [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Berezova IV, Bulygina VV, Dygalo NN. Resistance to the development of stress-induced behavioral despair in the forced swim test associated with elevated hippocampal Bcl-xl expression. Behav Brain Res. 2010;213:218–224. doi: 10.1016/j.bbr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Spitzer RLWJ, Gibbon M, First MD. Structural Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute, New York, NY: Biometrics Research; 1995. [Google Scholar]
- Sun AY, Chen Y-M. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998;5:401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- Tobe EH. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat. 2013;9:567–573. doi: 10.2147/NDT.S44282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WH, Donovan DM, Webster M, Freed WJ, Becker KG. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Res Bull. 2001;55:641–650. doi: 10.1016/s0361-9230(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Wibom R, Hagenfeldt L, von Döbeln U. Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Anal Biochem. 2002;311:139–151. doi: 10.1016/s0003-2697(02)00424-4. [DOI] [PubMed] [Google Scholar]
- Wong R, Steenbergen C, Murphy E. Methods Mol Biol. Springer; 2012. Mitochondrial permeability transition pore and calcium handling; pp. 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno K, Ando S, Misumi S, Makino S, Kulski JK, Muratake T, Kaneko N, Amagane H, Someya T, Inoko H. Synergistic association of mitochondrial uncoupling protein (UCP) genes with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144:250–253. doi: 10.1002/ajmg.b.30443. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Hong W, Wang Z, Peng D, Chen J, Yuan C, Yu S, Xu L, Fang Y. Influence of BCL2 gene in major depression susceptibility and antidepressant treatment outcome. J Affect Disord. 2014;155:288–294. doi: 10.1016/j.jad.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G, Manji HK. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34:1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


