Main Text
In this issue of the Biophysical Journal, a group from the Fabry laboratory in Erlangen, Germany, presents a method for determining the mechanical properties of cells in a high throughput platform, and a statistical approach by which to avoid bias associated with different stress and strain histories over the cell population (1). These are important issues, insofar as it is now well established that the rheological properties of cells are heterogeneous and often significantly nonlinear, which in turn poses serious difficulties in achieving high throughput and consistency of parameter estimation over differing protocols (2). Lange et al. (1) have made a large step forward here. Our thoughts in this commentary focus on the new and notable nature of the technology.
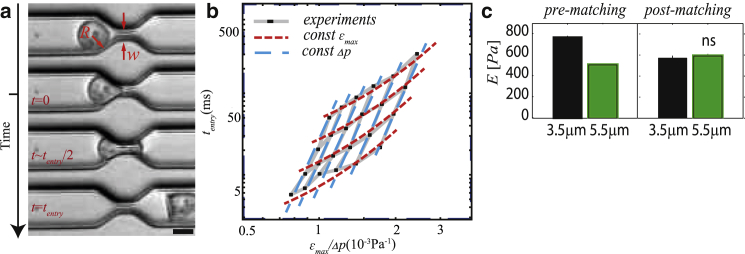
The essence of their rheological measurements reflects an old and venerable technique of pipette aspiration (3, 4, 5), wherein cells are subjected to a pressure difference (stress) driving them into or out of a lumen smaller than their intrinsic diameter (strain), and noting the temporal evolution of the resulting strain as the cell enters or exits the pipette. In their manifestation of this idea, Lange et al. (1) have a series of eight parallel microfluidic channels, each with a microconstriction, with a large number of cells in an upstream reservoir (see Fig. 1 a). Strain, εmax, is straightforward: it is basically the fractional change in the undeformed cell diameter to an effective luminal diameter. Stress (Δp) is inferred from the cell velocities just before the constriction encounter, using an empirical relationship to net fluid flow, and the resulting Poiseuille drop in the constriction (6). One of the clever techniques here is to invoke Kirchhoff’s law in parallel circuits to account for the effect of parallel neighbors encountering the obstructions, thus changing the pressure drops throughout (6). The characteristic time for deformation, tentry, is taken as the time from the constriction encounter to the cell’s full deformation into (and exit from) the constriction. These are put together with the now reasonably well-established power law description of cellular rheology (2). In particular, the strain is related to stress and a characteristic deformation time through a power law, often written as εmax ∼ , where E is an effective elastic modulus and β is the power-law exponent.
Figure 1.
(a) A cell of radius R transverses a microfluidic channel and a constriction of narrower width w. The pressure difference is Δp. At t = 0 the cell enters a constriction and leaves it at t = tentry. Scale bar, 10 μm. (b) The experimental data are binned for cells flowing under comparable Δp and εmax. This binning has a gridlike structure. (c) The width of the channel is varied from 3.5 to 5.5 μm. With raw data, it appears that E is protocol-dependent whereas it is protocol-independent for post histogram matching. This is also true for β (not shown here). All figures were taken or adapted from Lange et al. (1). To see this figure in color, go online.
In experiments on K562 leukemia cells, a striking pattern emerged within the very wide dispersed cloud of tentry versus εmax/Δp data points. In particular, due to the variation in undeformed cell size (R in Fig. 1 a), each cell is subjected to a different maximal strain as it deforms into the constriction; similarly, each cell is exposed to different stresses secondary both to its own size as well as the pressure coupling to its neighbor’s encounters. Binning the data over these different histories, as shown in Fig. 1 b, appears to largely resolve the high degree of raw variability. That this should reveal stress or strain stiffening is not surprising, but it does lead to an important insight. Utilizing raw averages of all data necessarily weights those bins with larger number of cells and conversely, and this leads to bias in E and β insofar as there is true stress or strain stiffening. Lange et al. (1) propose a clever but elementary (and assumption free) bootstrapping approach—repeated random sampling of bins such that equal numbers of data points are sampled within each bin, a procedure called “histogram matching”. That this has the potential to remove bin number bias was checked by comparing data obtained with narrow and wide channels on the same K562 cell line. The modulus without and with histogram matching is shown in Fig. 1 c. Cells appear stiffer when measured with a narrow channel using just raw data, but with histogram matching, that difference disappears, presumably revealing a more robust measurement of the true cell properties.
It should be noted that while previous works (7, 8, 9) have already achieved higher throughputs of 102–104 cells/s, each of these has advantages and disadvantages. Gossett et al. (7) has by far the highest throughput (>103 cells/s) with offline analysis, but does not extract actual material properties (which Lange et al. (1) does). Also, due to the very high rate, the measurements are limited by camera memory. Otto et al. (8) has a smaller throughput (102–103 cells/s), but the cells are analyzed in real-time, which can be done continuously until the cells run out. Byun et al. (9) is probably closest in approach to the present method, and has a similar throughput, with analysis done online. There are also differences in the timescales at which mechanics is probed. These range from microseconds in Gosset et al. (7), milliseconds in Otto et al (8), and milliseconds to seconds in both Lange et al. (1) and Byun et al. (9). Moreover, there are also differences in whether there is physical contact or not (yes in Lange et al. (1) and Byun et al. (9); no in Gosset et al. (7) and Otto et al. (8)). All (1, 7, 8, 9) can do fluorescence in parallel.
The techniques employed here have clear applications in a wide variety of cell types and interventions. To be sure, these ideas also raise as many questions as they answer (e.g., the physical origin of the strain and stress stiffening, and whether these are reflected in just the modulus or perhaps in the power-law exponent). But with these new tools in hand, new hypotheses can be entertained and tested with throughputs in the thousands of cells per hour, and with histogram matching, at least some portion of intrinsic bias can be circumvented. In addition, the approach described here is accessible to virtually any laboratory, and importantly, with no need for highly specialized technology or significant resource commitments. Finally, we note that fluorescently tagged protein expression levels can be measured in parallel with cell mechanics (such as done here with laminA labeling) on a cell-by-cell basis, in turn leading to more direct assays of the relationship between mechanics and protein expression. One hopes that the approaches outlined in Lange et al. (1) will constitute another significant step forward in our ability to probe cells’ intrinsic properties with high throughput, and with a measure of independence of the history distribution in response to variable protocols and interventions.
Editor: David Piston.
References
- 1.Lange, J. R., C. Metzner, …, B. Fabry. Unbiased high precision cell mechanical measurements with microconstrictions. Biophys. J. 112:1472–1480. [DOI] [PMC free article] [PubMed]
- 2.Kollmannsberger P., Fabry B. Linear and nonlinear rheology of living cells. Annu. Rev. Mater. Res. 2011;41:75–97. [Google Scholar]
- 3.Sung K.L., Dong C., Skalak R. Leukocyte relaxation properties. Biophys. J. 1988;54:331–336. doi: 10.1016/S0006-3495(88)82963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai M.A., Frank R.S., Waugh R.E. Passive mechanical behavior of human neutrophils: power-law fluid. Biophys. J. 1993;65:2078–2088. doi: 10.1016/S0006-3495(93)81238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 1989;56:151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange J.R., Steinwachs J., Fabry B. Microconstriction arrays for high-throughput quantitative measurements of cell mechanical properties. Biophys. J. 2015;109:26–34. doi: 10.1016/j.bpj.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gossett D.R., Tse H.T.K., Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA. 2012;109:7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto O., Rosendahl P., Guck J. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods. 2015;12:199–202. doi: 10.1038/nmeth.3281. 4, 202. [DOI] [PubMed] [Google Scholar]
- 9.Byun S., Son S., Manalis S.R. Characterizing deformability and surface friction of cancer cells. Proc. Natl. Acad. Sci. USA. 2013;110:7580–7585. doi: 10.1073/pnas.1218806110. [DOI] [PMC free article] [PubMed] [Google Scholar]