Abstract
Substrate stiffness is crucial for diverse cell functions, but the mechanisms conferring cells with mechanosensitivity are still elusive. By tailoring substrate stiffness with 10-fold difference, we showed that L-type voltage-gated Ca2+ channel current density was greater in chick ventricular myocytes cultured on the stiff substrate than on the soft substrate. Blockage of the BK channel increased the Ca2+ current density on the soft substrate and consequently eliminated substrate stiffness regulation of the Ca2+ channel. The expression of the BK channel, including the STREX-containing α-subunit that forms stretch-activated BK channel in myocytes and the BK channel function in myocytes (and also in HEK293 cells heterologously expressing STREX-containing α- and β1-subunits) was reduced in cells cultured on the stiff substrate. Furthermore, in HEK293 cells coexpressing the cardiac CaV1.2 channel and STREX-containing BK channel, the Ca2+ current density was greater in cells on the stiff substrate, which was not observed in cells expressing the CaV1.2 channel alone or coexpressing with the STREX-deleted BK channel. These results provide strong evidence to show that the stretch-activated BK channel plays a key role in functional regulation of cardiac voltage-gated Ca2+ channel by substrate stiffness, revealing, to our knowledge, a novel mechanosensing mechanism in ventricular myocytes.
Introduction
The elasticity or stiffness of extracellular matrix (ECM) often undergoes remarkable changes as a result of physiological and pathological alterations. Matrix stiffness has been increasingly recognized to play a crucial role in a variety of cell functions. For example, the stiffness of cardiac ECM can regulate diverse cell functions such as myocardial cell maturation, morphology, sarcomere organization, electromechanical coupling, and gene expression (1, 2, 3). Cardiac fibrosis is one of the most important pathophysiological processes involved in cardiac remodeling (4, 5). The elasticity of healthy mature muscles is in the range of 10–150 kPa (6). However, excessive accumulation of collagen components during fibrosis can increase by severalfold the stiffness of cardiac ECM (6, 7), which stiffens the myocardium, decreases compliance, and causes dysfunction of systole and diastole, leading to heart failure and arrhythmia (1, 8). Therefore, a clear understanding of the stiffness sensing mechanism is fundamental for gaining insights into cardiac remodeling and disease. However, how ventricular myocytes sense stiffness is still not well defined. Accumulating evidence supports that cells can detect the mechanical properties of extracellular surroundings by applying traction forces by actomyosin (myosin II) motors via focal adhesions (9, 10) and examining the mechanical response (11, 12). It is well known that Ca2+ is a ubiquitous intracellular signaling molecule (13). Intracellular Ca2+ is vital in the reorganization of the actin cytoskeleton through modulation of actin-associated protein activities (13). Increasing intracellular Ca2+ concentration ([Ca2+]i) can enhance stress fiber contractility through phosphorylation of myosin light chain kinase or activation of proteases (13, 14). We thus hypothesize that intracellular Ca2+ dynamics play an important role in underpinning the mechanosensitivity of cardiac myocytes.
The large-conductance Ca2+-sensitive K+ (BK) channel is widely expressed (15), functioning to oppose smooth muscle contraction by hyperpolarizing plasma membrane and reducing voltage-gated Ca2+ channel activation and thereby extracellular Ca2+ influx (16). The BK channel is expressed at a very low level in cardiac myocytes, leading to the notion that there is no direct link between the BK channel and cardiac function (17). However, the mouse BK channel gene was cloned from cardiac tissues (18) and the functional expression of the BK channel was documented in embryonic chick cardiac myocytes (19). The BK channel in embryonic chick ventricular myocytes is unique in that, in addition to activation by membrane potential and intracellular Ca2+, the BK channel can be activated by membrane stretch due to the presence of stress-axis regulated exon (STREX) domain in the C-terminus (20, 21, 22). L-type voltage-gated Ca2+ channels in cardiac myocytes, particularly the CaV1.2 channel, serve as the key Ca2+ influx pathway to increase the [Ca2+]i (23) that triggers Ca2+ release from sarcoplasmic reticulum and cardiac muscle contraction (24). In this study, we examined the expression of the stretch-activated BK channel and its role in mediating the regulation of L-type Ca2+ channel function in chick ventricular myocytes by substrate stiffness.
Polydimethylsiloxane (PDMS) is a widely used elastomeric material to study various cell responses, such as cell spreading and cytoskeletal morphologies in fibroblasts (25), stretch-activated action potential in dorsal root ganglion neurons under mechanical stimulation (26), and neuronal network formation (27). In this study, we use matrix-coated PDMS to study the effects of substrate stiffness on the BK channel and voltage-gated Ca2+ channel. To investigate the mechanism sensing substrate stiffness in ventricular myocytes, the mechanical properties of PDMS substrates were designed to be close to those of cardiac tissues under physiological and fibrosis conditions. Our results provide evidence to show, to our knowledge, a novel mechanism by which ventricular myocytes senses and respond to substrate stiffness.
Materials and Methods
Preparation of PDMS substrates with different stiffness
PDMS substrates were prepared by mixing Sylgard 184 (Corning, Corning, NY) in two different mass ratios of curing agent to base (1:10 and 1:50), as described in our previous study (27). In brief, the mixtures were cast into a 35-mm petri dish and cured at 60°C for 3 h. This was followed by a sterilization step, carried out by exposing them to ultraviolet radiation for 2 h. By using the spherical indentation method (28), the Young’s moduli were measured as 457 ± 39 kPa and 46 ± 11 kPa at the 1:10 and 1:50 ratios, respectively; these are referred to as “stiff” and “soft” substrates in this study.
Cell culture
White leghorn chick embryos were obtained from China Agricultural University, Beijing, China. Animal materials used for this study were obtained according to the protocols reviewed and approved by the Institutional Ethical Review Committees of both Tsinghua University and China Agricultural University. All the animal experiments were performed in line with the National Institutes of Health guidelines (NIH, Bethesda, MD; Guide for the Care and Use of Laboratory Animals).
Ventricles, dissected from 10- to 12-days-old chick embryos under sterile conditions, were washed three times with phosphate-buffered saline to avoid red blood cells and, then, cut into small pieces and exposed to Ca2+/Mg2+-free Hanks balanced salt solution (HBSS; Sigma-Aldrich, St. Louis, MO) containing 2 mg/mL collagenase (Sigma-Aldrich) for 10–15 min until they were dissociated. The cell suspension was centrifuged at 1000 rpm for 5 min. After the supernatant was discarded, the cell pellets were resuspended and cultured in high-glucose DMEM culture medium (Corning) supplemented with 1% penicillin/streptomycin and 10% heat-inactivated horse serum (HS). HS-DMEM was selected to inhibit proliferation of low-ratio cardiac fibroblasts coexisting in ventricular myocytes. The cell suspension was plated onto a 35-mm petri dish. After 2 h incubation, nonadherent cells (ventricular myocytes) were collected and transferred to dishes with 10% HS-DMEM. Ventricular myocytes were maintained in an atmosphere of 95% air and 5% CO2 at 37°C. HEK293 cells were cultured in DMEM medium with 10% fetal bovine serum with 1% penicillin/streptomycin, and grown in 95% air and 5% CO2 at 37°C.
Cell seeding and sample collection
Sterilized PDMS substrates were coated with 5 pg/mL poly-D-lysine (MW 220,000: Sigma-Aldrich) at 37°C for 40 min. Isolated ventricular myocytes were seeded onto poly-D-lysine-coated PDMS substrates at a concentration of 1–5 × 105 cells per 35-mm petri dish. Ventricular myocytes were allowed 24 h to seed and equilibrate. For mRNA and protein expression assays, after 24 h equilibration in 10% HS culture media, cells were cultured in 2% HS-DMEM for 12 h for starvation and then in 1% fresh HS-DMEM. Cell lysates in 1 mL were collected at 6 h after replacement with 1% fresh HS-DMEM for semiquantitative RT-PCR, and cell lysates in 300 μL for western blotting. For blebbistatin treatment, blebbistatin (Sigma-Aldrich) was added in culture medium with a final concentration of 5 μM for up to 24 h before cells were used for experiments.
Transfection
The plasmids encoding GFP, the full-length BK channel STREX-containing the α-subunit (GenBank: AB072618), and the STREX-deleted α-subunit or β1-subunit were kindly gifted from Dr. Z. Qi (Xiamen University, China). The plasmid for L-type Ca2+ channel CaV1.2 (GenBank: NC 000072.6) was kindly gifted Dr. X.-d. Liu (Tsinghua University, China). HEK293 cells were cotransfected with plasmids encoding GFP and the STREX-containing BK channel or the STREX-deleted BK channel and L-type Ca2+ channel, respectively, using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). In brief, for cells with 80% confluence in each 35-mm petri dish, 4 μg plasmid for the BK channel α-subunit and 4 μg for the CaV1.2 channel, together with 1 μg plasmid for GFP, were used. In cotransfection with the BK channel β1-subunit, an excessive amount of β1-subunit over the α-subunit (a molar ratio of 10:1 mol/mol) was used, and in cotransfection of L-type Ca2+ channel with the BK channel α-subunit and β1-subunit, the expected stoichiometry between them was 1:1. Cells 24 h after transfection were trypsinized and seeded on poly-D-lysine-coated PDMS and incubated for 24–48 h before use. The BK channels were expressed at low density to record the whole-cell currents without saturating the patch-clamp amplifier.
Semiquantitative reverse transcription polymerase chain reaction
Total RNA samples were isolated from cultured myocytes using a RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), dissolved in RNase-free water, and then quantified by measuring the absorbance at 260 nm with a spectrophotometer. The RNA samples were further treated with DNase I (Montreal Biotech, Dorval, Quebec, Canada). For each sample, 0.5 μg RNA samples were used to prepare cDNAs using a cDNA synthesis kit (Montreal Biotech) in a final volume of 20 μL.
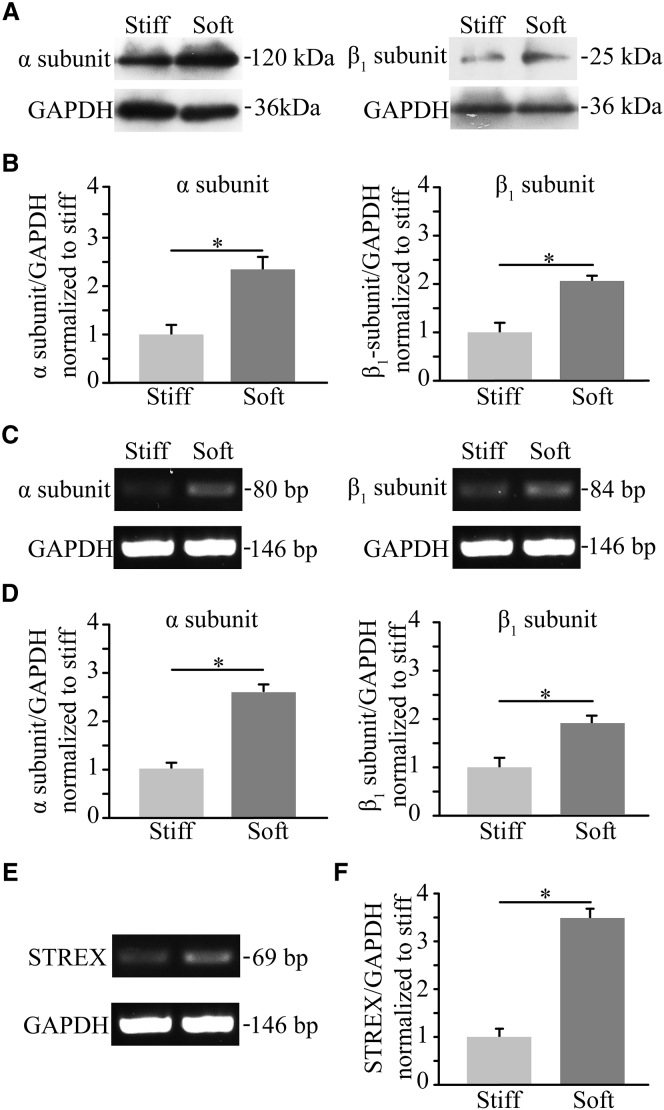
To evaluate the BK channel expression in cultured myocytes, semiquantitative PCR was performed with a PCR kit (Montreal Biotech) using a thermocycler (BioRad, Hercules, CA), and the results were normalized to the glyceraldehyde-3-phosphate dehydrogenase gene. PCR primers were designed using the software Primer 5.0 (Premier Biosoft, http://www.premierbiosoft.com/primerdesign/) and the specificity was examined using the program BLAST (NCBI, https://www.ncbi.nlm.nih.gov/). PCR reactions were performed in a total volume of 25 μL containing 1 μL cDNA, following the manufacturer’s recommendations (Montreal Biotech). The forward and reverse primer sequences used are: 5′-CCTGAGAAGGGAGTGGGAGACC-3′ and 5′-ATGTTGAGTGACGCCAAGATGC-3′ for the BK channel α-subunit with or without STREX; 5′-AGCCGAGCATGTTGTTTTGAT-3′ and 5′-ACGCACACGGCCTGACA-3′ for the STREX-containing α-subunit; 5′-TGTGCTGTCATCACCTACT-3′ and 5′-CATGGCAATAATGAGGAG-3′ for the BK channel β1-subunit. PCR consisted of 30 amplification cycles, each with denaturation at 94°C for 30 s, annealing at 58–64°C for 30 s, and elongation at 72°C for 30 s. PCR products were resolved by electrophoresis in 2% agarose gels in Tris-borate/EDTA buffer and visualized by staining with ethidium bromide. The quantification of semiquantitative PCR was performed by using the software Quantity One 4.6.3 (BioRad). The mRNA expression of the BK channel subunits was expressed with respect to that of glyceraldehyde-3-phosphate dehydrogenase in the parallel experiments, and was further normalized to that in cells on the soft substrate, as shown in Fig. 3, D–F.
Figure 3.
Substrate stiffness regulates protein and mRNA expression of the BK channel α- and β1-subunits. (A) Representative western blots showing BK channel α- and β1-subunit proteins in ventricular myocytes cultured on the soft and stiff substrates. (B) Summary of the mean protein expression from four independent experiments, normalized to that in cells on the soft and stiff substrate. (C) RT-PCR analysis of the mRNA expression of α- and β1-subunits in cells on the soft and stiff substrates. (D) Summary of the mean mRNA expression from four independent experiments, normalized to that in cells on the soft and stiff substrates. (E) RT-PCR analysis of the BK STREX-containing α-subunit in cells on the soft and stiff substrates. (F) Summary of the mRNA expression for the BK STREX-containing α-subunit from four independent experiments, normalized to that in cells on the soft and stiff substrates. ∗p < 0.05.
Western blotting
Western blotting was conducted at room temperature. Protein samples were prepared by mixing one part of cell lysate sample with one part of Laemmli Sample Buffer (BioRad) and boiled at 100°C for 3 min. Proteins were separated by electrophoresis in 10% sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidenedifluoride membrane at 200 mA for 2–3 h. After being blocked with 5% nonfat milk suspended in TBST for 2 h, the blots were incubated with the primary antibodies recognizing the BK channel α- and β1-subunits both at a dilution of 1:1000 or the primary antibody for CaV1.2 at 1:500 for 2 h, followed by incubation with HRP-conjugated secondary antibodies at 1:5000 (Santa Cruz Biotechnology, Dallas, TX) for 2 h. Proteins were visualized via chemiluminescence using a Luminol Reagent Kit (Santa Cruz Biotechnology) and signals were captured using X-AR films (Kodak, Rochester, NY).
Electrophysiology
Single-channel inside-out and whole-cell patch clamp recordings were performed, using a MultiClamp 700B amplifier and pClamp 10 software (Molecular Devices, Sunnyvale, CA) as previously described in Zhao et al. (21) and Zhao and Sokabe (22), to measure voltage-gated Ca2+ channel currents and BK channel currents from myocytes cultured in vitro for 3 days or GFP-positive HEK293 cells for 24–48 h on the soft and stiff substrates. In single-channel recording, the pipette solution contained the following components: 145 mM K-gluconate, 1 mM EGTA, 10 mM HEPES, and 5 mM glucose at pH 7.4, with NaOH at 310 mOsm. The bath solution contained the same components except for various Ca2+ concentrations. The single channel open probability (Po) in a patch with multiple channels was calculated by using the software pClamp (Molecular Devices) based on the equation: Po = 1 − Pc1/N, where Pc is the probability when all of the channels are in the closed state and N is the number of channels in the patch.
Whole-cell current recordings were made, using recording pipettes with resistance of 3.0–4.0 MΩ in the extracellular solutions. The cell capacitance and series resistance (<20 MΩ) were monitored throughout the recording. Signals were filtered at 1 kHz and digitized at 10 kHz. The leak currents were corrected using the online P/6 trace subtraction. The series resistance was compensated by 80%.
The BK channel currents were recorded, using Hanks’ balanced salts solution (HBSS) containing: 1.3 mM CaCl2, 0.8 mM MgSO4, 5.4 mM KCl, 0.4 mM KH2PO4, 136.9 mM NaCl, 0.3 mM Na2HPO4, 10 mM D-glucose, and 4.2 mM NaHCO3 at pH 7.4 with NaOH at 310 mOsm. The pipette solution contained: 1 mM CaCl2, 145 mM KCl, 10 mM EGTA, and 10 mM HEPES at pH 7.3 with KOH at 290 mOsm. Extracellular solutions were applied at 0.5 mL/min. Currents were evoked by applying test pulses from −80 to +100 mV with 400-ms duration and an increment of 10 mV at 10-s intervals. The membrane potential was held at −80 mV. Whole-cell Ca2+ channel currents were recorded from cultured myocytes and HEK293 cells, mainly using Ba2+-containing bath solutions: 10 mM BaCl2, 140 mM tetraethylammonium-Cl, 10 mM HEPES, and 20 mM glucose at pH 7.3 with tetraethylammonium-OH at 310 mOsm. Recordings were also made, using Ca2+-containing Tyrode solutions: 130 mM NaCl, 2 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM HEPES, and 30 mM glucose at pH 7.3 with NaOH at 310 mOsm. The pipette solution contained: 110 mM CsCl, 10 mM EGTA, 4 mM MgATP, 0.3 mM Na-GTP, 25 mM HEPES, 10 mM Tris-phosphocreatine, and 20 U/mL creatine phosphokinase at pH 7.3 with CsOH at 290 mOsm. Extracellular solutions were applied at 0.5 mL/min. The membrane potential was held at −50 mV. Currents were evoked by applying test pulses from −80 to +60 mV with 40-ms duration and an increment of 10 mV at 10-s intervals. Voltage-dependent channel activation was studied using the tail currents. Amplitudes of the tail currents were normalized to the largest tail current and data were fitted by the Boltzmann function. To study voltage-dependent channel inactivation, patched cells were subjected to 3-s prepulse from −100 to +50 mV with 10-mV increment, followed by a 20-ms repolarization to −80 mV and then a 40-ms test pulse to +10 mV to elicit Ca2+ channel currents through the channels that remained available.
Data analysis
The data are presented as mean ± SE, where appropriate. The single channel open probability (Po)-Ca2+ concentration relationship curve was fitted with the Hill equation: Po = [Ca2+]in/(Kdn + [Ca2+]i), where n is the Hill coefficient and Kd is the dissociation constant. The Po-voltage relationship curve was fitted with the Boltzmann equation: Po = 1/[1 + exp(V1/2 − V)/k], where V1/2 and k are the half-maximal channel activation voltage and slope, respectively. The activation and inactivation curves were fitted to the Boltzmann equation: I/Imax = 1/[1 + exp(V − V1/2)/k], where the Imax, V1/2, and k values represent the maximal current, half-maximal current activation or inactivation voltage, and slope, respectively. Statistical analysis was performed using Student’s t-test, with p < 0.05 to be considered significant.
Results
Stiff substrate upregulates L-type Ca2+ channel currents in ventricular myocytes
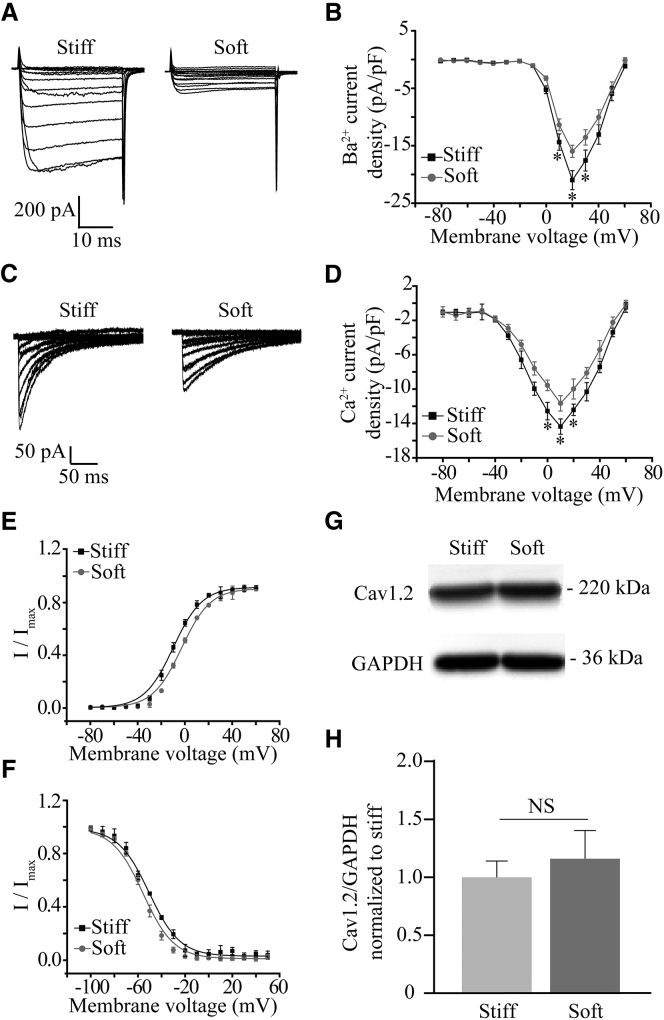
Myocytes express L-type voltage-gated CaV1.2 Ca2+ channels that play an important role in mediating Ca2+ influx and excitation-contraction coupling (23, 24). Thus, we set out to examine the effect of substrate stiffness on L-type Ca2+ channel currents in cultured chick ventricular myocytes, using Ba2+ as the charge carrier to avoid complications from Ca2+-dependent effects such as channel inactivation (29). Myocytes cultured on both stiff and soft substrates responded to depolarization pulses with inward Ca2+ channel currents (Fig. 1 A). The inward currents began to appear at ∼−30 mV, reached the maximum at +20 mV, and reversed at ∼+60 mV (Fig. 1 B). The peak current density was significantly higher in myocytes on the stiff substrate compared with that on the soft substrate (Fig. 1 B). Similar functional regulation by substrate stiffness was observed when the Ca2+ channel currents were recorded using Ca2+ as the charge carrier, under which conditions the Ca2+ channel exhibited considerable channel inactivation (Fig. 1, C and D). These results consistently indicate that substrate stiffness regulates L-type Ca2+ channel in myocytes.
Figure 1.
Stiff substrate enhances L-type Ca2+ channel currents in ventricular myocytes. (A) Representative whole-cell recordings of Ba2+ currents from ventricular myocytes cultured on the soft and stiff substrates. (B) Mean I-V relationship from 10 cells in three preparations for each case. (C) Representative whole-cell recordings of Ca2+ currents from cells cultured on the soft and stiff substrates. (D) Mean I-V relationships from nine cells in three preparations for each case. (E) Voltage dependence of channel activation determined with the tail currents from 10 cells. (F) Voltage dependence of channel inactivation from 12 cells in three preparations cultured on the soft and stiff substrates. (G) Representative western blots showing the protein expression of Ca2+ channel α-subunits in cells on the soft and stiff substrates. (H) Summary of the mean data from four independent experiments. ∗p < 0.05, comparison the current density at the same voltage. NS, no significant difference.
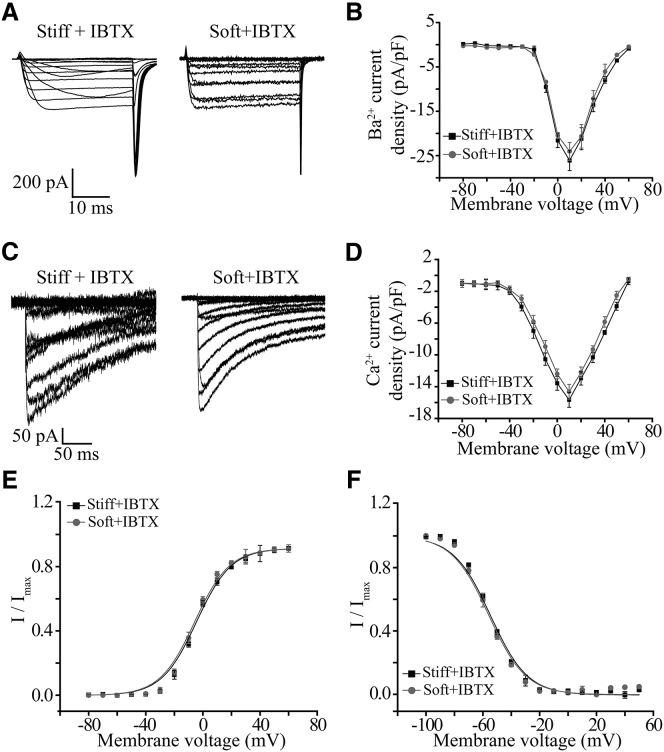
As aforementioned, chick ventricular myocytes express the BK channel, particularly the stretch-activated BK channel. We hypothesize that under physiological condition, BK channel activation causes membrane hyperpolarization that may reduce voltage-gated Ca2+ channel activation and Ca2+ influx. Therefore, we next examined the role of the BK channel in mediating functional regulation of the L-type Ca2+ channel by substrate stiffness. Treatment with IBTX, a BK channel blocker, abolished the difference in inward Ca2+ current density in myocytes on the soft and stiff substrates without altering the I-V relationship curve when the Ca2+ channel currents were carried by Ba2+ (Fig. 2, A and B) or Ca2+ (Fig. 2, C and D). These results provide initial evidence to support that the BK channel is crucial in the regulation of the L-type Ca2+ channel function by substrate stiffness.
Figure 2.
BK channel is critical in substrate stiffness regulation of L-type Ca2+ channel in ventricular myocytes. (A) Representative whole-cell recordings of Ba2+ currents from cells cultured on the soft and stiff substrates after treatment with 100 nM IBTX for 10 min. (B) Mean I-V relationships from 12 cells in three preparations for each case. (C) Representative whole-cell recordings of Ca2+ currents from cells cultured on the soft and stiff substrates after treatment with 100 nM IBTX for 10 min. (D) Mean I-V relationships from 10 cells in three preparations for each case. (E) Voltage dependence of L-type channel activation determined with tail currents from 12 cells cultured on the soft and stiff substrates after treatment with 100 nM IBTX for 10 min. (F) Voltage dependence of L-type channel inactivation from 12 cells in three preparations on the soft and stiff substrates after treatment with 100 nM IBTX for 10 min.
We also evaluated whether substrate stiffness affected voltage-dependent activation and inactivation of L-type Ca2+ channel in myocytes. The half-maximal activation voltage (V1/2) was −3.6 ± 0.3 mV in cells on the soft substrate, which was significantly more positive than −10.1 ± 0.9 mV on the stiff substrates (p < 0.05), but there was no distinct change in the slope (k) (4.8 ± 0.3 and 5.1 ± 0.3 on the soft and stiff substrates, respectively; Fig. 1 E). As shown in Fig. 1 F, the stiff substrate caused a positive shift in the voltage-dependent channel inactivation curve. The half-inactivation voltage (V1/2) was shifted from −55.5 ± 3.2 mV on the soft substrate to −44.3 ± 2.2 mV on the stiff one (p < 0.05), while the slope (k) was not significantly different (9.1 ± 0.4 and 8.9 ± 0.8 on the soft and stiff substrates, respectively). These results indicate that substrate stiffness changes L-type Ca2+ channel currents by modulating the channel activation and (particularly) the inactivation. Blockage of the BK channel with IBTX reversed these substrate stiffness-induced effects on voltage-dependent channel activation and inactivation (Fig. 2, E and F). As mentioned above, CaV1.2 represents the cardiac L-type voltage-gated Ca2+ channel (23, 24). Western blotting was performed to analyze the CaV1.2 protein expression in myocytes on the soft and stiff substrates. There was no significant difference in the protein expression (Fig. 1, G and H). Taken together, these results suggest that substrate stiffness regulates the cardiac L-type Ca2+ channel function without effecting its protein expression, and that the BK channel is critical in substrate stiffness regulation of L-type Ca2+ channel in myocytes.
Stiff substrate downregulates the expression of the BK channel α- and β1-subunits
To further explore the role of the BK channel in substrate stiffness regulation of the cardiac L-type Ca2+ channel, western blotting was carried out to examine the BK channel expression. The results show a duplet with 120 and 25 kDa, corresponding to the anticipated size for the BK channel α- and β1-subunits, respectively, as described in Shi et al. (30). The protein expression levels for both α- and β1-subunits in myocytes on the soft substrate were remarkably greater than those on the stiff substrate (Fig. 3, A and B). To investigate whether such substrate stiffness-dependent changes in the protein expression resulted from their mRNA expression, semiquantitative RT-PCR assays were performed. The mRNA expression levels for both BK channel α- and β1-subunits were elevated in myocytes on the soft substrate compared to those on the stiff substrate (Fig. 3, C and D). Previous studies showed that that several alternative splicing isoforms of the BK channel α-subunit are expressed in chick cardiac myocytes, including the STREX-containing α-subunit that forms the stretch-activated BK channel (20, 21, 22). Hence, semiquantitative RT-PCR assays were performed to further evaluate expression of the STREX-containing α-subunit in myocytes and the effect of substrate stiffness on its expression. The mRNA transcript for the STREX-containing α-subunit was detected in myocytes on the soft and stiff substrates and, furthermore, the expression level was significantly higher in myocytes on the soft substrate (Fig. 3, E and F). These results suggest that substrate stiffness regulates the expression of BK channel STREX-containing α- and β1-subunits.
Stiff substrate inhibits the BK channel function by reducing channel open probability and sensitivity to Ca2+ and voltage
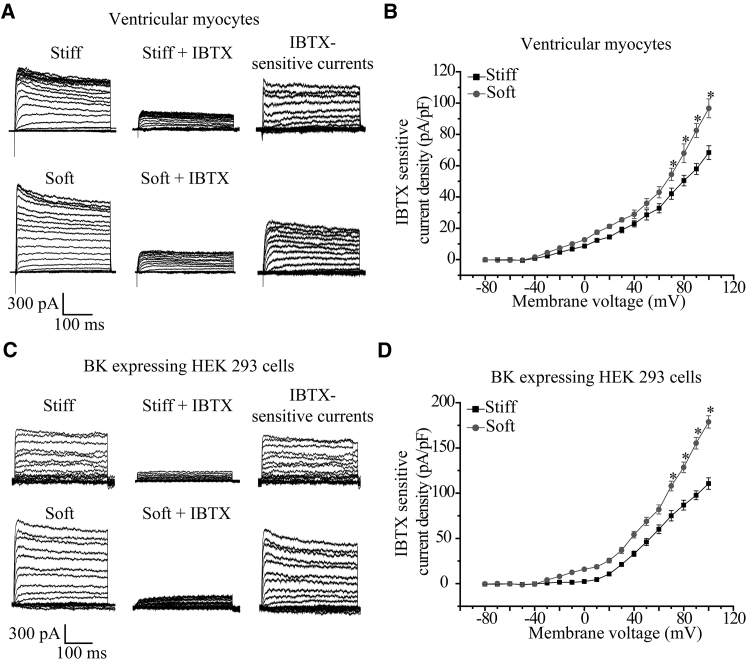
To characterize the effects of substrate stiffness on the BK channel function, whole-cell recordings were made to measure the BK channel currents in myocytes. There were robust outward K+ currents, which were strongly suppressed by treatment with 100 nM IBTX, indicating that the outward currents were mainly carried by the BK channel (Fig. 4, A and B). The BK channel current density in myocytes was significantly greater in cells on the soft substrate; for example, the current density at +100 mV was 126 ± 3.6 pA/pF relative to 86.5 ± 3.2 pA/pF on the stiff substrate (p < 0.05) (Fig. 4, A and B).
Figure 4.
Soft substrate increases the BK channel currents in ventricular myocytes and HEK293 heterologously expressing the stretch-activated BK channel. (A) Representative whole-cell recordings of outward K+ currents from cells on the soft and stiff substrates before (left panels), and 10 min after, application of 100 nM IBTX (middle panels) and IBTX-sensitive currents (right panels). (B) Mean I-V relationships of the IBTX-sensitive currents from 10 cells in three preparations for each case. (C) Representative whole-cell recordings of outward K+ channel currents from HEK293 cells heterologously expressing the BK channel STREX-containing α- and β1-subunits and cultured on the soft and stiff substrates before (left panels), and 10 min after, application of 100 nM IBTX (middle panels) and IBTX-sensitive current (right panels). (D) Mean I-V relationships of the IBTX-sensitive current in 11 cells from three preparations for each case. ∗p < 0.05, comparison to the current density at the same voltage.
Myocytes express stretch-activated BK channels as well as stretch-insensitive and other types of K+ channels. In HEK293 cells, a widely used heterologous mammalian cell expression system, there was no detectable expression of the BK channel α- and β1-subunits both at mRNA and protein levels (Fig. S1 A in the Supporting Material), and only a very small K+ current density that was largely insensitive to IBTX (Fig. S1, B and C), consistently supporting negligible endogenous expression of the BK channel. Thus, to provide direct evidence for substrate stiffness regulation of stretch-activated BK channel, the BK channel STREX-containing α- and β1-subunits cloned from chick ventricular myocytes were transiently coexpressed in HEK293 cells. Fig. 4 C shows representative IBTX-sensitive BK channel K+ currents, which are similar to those reported in Shi et al. (30). As observed for the native BK channel in myocytes, the current density of recombinant stretch-activated BK channel in HEK293 cells on the soft substrate was considerably higher than that on the stiff substrate; for example, the current density at +100 mV was 134 ± 4.6 pA/pF on the soft substrate, which was significantly greater than 92.5 ± 3.1 pA/pF on the stiff substrate (p < 0.05) (Fig. 4 D).
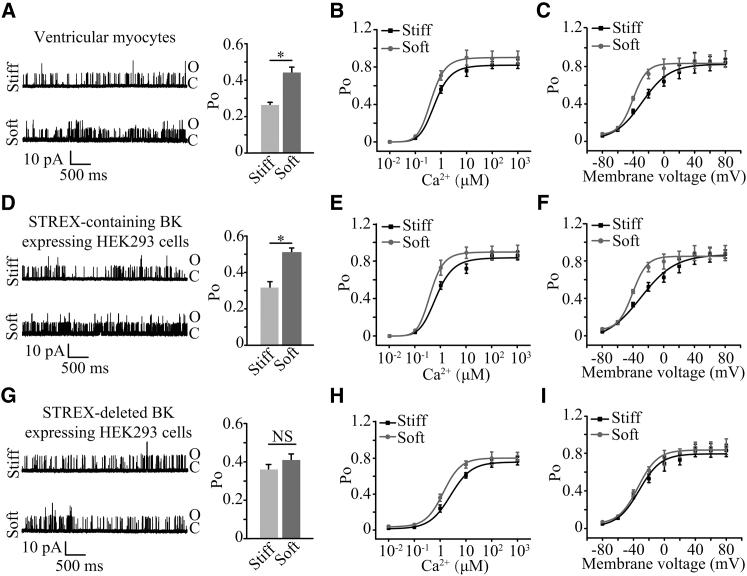
To gain further insights into substrate stiffness regulation of the BK channel, single channel recordings were made to examine the single channel conductance, Ca2+, and voltage sensitivity of the BK channel in myocytes. While single channel conductance was not altered by substrate stiffness, the channel open probability was higher on the soft substrate than that on the stiff substrate (Fig. 5 A). Previous studies reported that the channel opening probability was strongly dependent on the [Ca2+]i (21, 22). The increase in the [Ca2+]i (0.01–1000 μM) greatly enhanced the open probability of the BK channel in myocytes on both soft and stiff substrates (Fig. 5 B). The Po-Ca2+ concentration relationship in myocytes on the soft substrates was noticeably left-shifted with respect to that on the stiff substrate; Hill coefficient also significantly increased from 2.2 ± 0.07 on the stiff substrate to 2.8 ± 0.01 on the soft substrate (p < 0.05), suggesting an increase in the Ca2+ sensitivity on the soft substrate. The BK channel activation also strongly depends on voltage or membrane potential. Thus, we examined the influence of substrate stiffness on the voltage-dependence of channel activation. The Po-V relationship was left-shifted in myocytes on the soft substrate (Fig. 5 C). V1/2 was −28.4 ± 3.0 mV for the stiff substrate, which was much higher than −40.2 ± 0.7 mV for the soft substrate (p < 0.05). Similar effects by substrate stiffness on the open probability (Fig. 5 D), and the sensitivity to Ca2+ (Fig. 5 E) and voltage (Fig. 5 F), were observed in HEK293 cells heterologously expressing STREX-containing α- and β1-subunits. However, in HEK293 cells expressing the STREX-deleted BK channel, such substrate stiffness-induced change in whole-cell current density (Fig. S2, A and B) and single channel open probability (Fig. 5 G) was not observed. There were similar Po-Ca2+ and Po-voltage relationships on the soft and stiff substrates (Fig. 5, H and I).
Figure 5.
Substrate stiffness regulates the single channel open probability, Ca2+ and voltage sensitivity of the BK channel. (A) Representative inside-out recordings of single-channel currents in ventricular myocytes on soft and stiff substrates at a membrane voltage of +40 mV with 0 μM Ca2+ (left panel), and summary of Po from 10 cells in three preparations for each case (right panel). (B) The Po-Ca2+ relationships of the BK channel currents from seven cells in three preparations. The membrane potential was held at +40 mV. (C) The Po-voltage relationships of the BK channel currents from eight cells in three preparations for each case. 3 μM Ca2+ was used. (D) Representative inside-out recording of single-channel currents in HEK293 cells heterologously expressing the BK channel STREX-containing α- and β1-subunits at a membrane potential of +40 mV with 0 μM Ca2+ (left panel), and summary of Po from 11 cells for each case (right panel). (E) The Po-Ca2+ relationships of the BK channel currents in HEK293 cells heterologously expressing the BK channel STREX-containing α- and β1-subunits on the soft and stiff substrates from seven cells in three preparations for each case. The membrane potential was held at +40 mV. (F) The Po-voltage relationship curves of the BK channel currents in HEK293 cells heterologously expressing the BK STREX-containing channel α- and β1-subunits on the soft and stiff substrates from seven cells in three preparations for each case. 3 μM Ca2+ was used. (G) Representative inside-out recording of single-channel currents in HEK293 cells heterologously expressing BK channel STREX-deleted α- and β1-subunits at a membrane voltage of +40 mV with 0 μM Ca2+ (left panel), and summary of Po from 11 cells for each case (right panel). (H) The Po-Ca2+ relationships of the BK channel currents in HEK293 cells heterologously expressing the BK STREX-deleted channel α- and β1-subunits on the soft and stiff substrates from seven cells in three preparations for each case. The membrane potential was held at +40 mV. (I) The Po-voltage relationships of the BK channel currents in HEK293 cells heterologously expressing the BK channel STREX-deleted α- and β1-subunits on the soft and stiff substrates from seven cells in three preparations for each case. 3 μM Ca2+ was used. ∗p < 0.05. NS, no significant difference.
To summarize, the above results provide consistent evidence that substrate stiffness regulates the open probability and the sensitivity to Ca2+ and voltage of the BK channel in myocytes and such functional regulation depends on the STREX domain in the BK channel.
Substrate stiffness regulation of L-type Ca2+ channel depends on stretch-activated BK channel
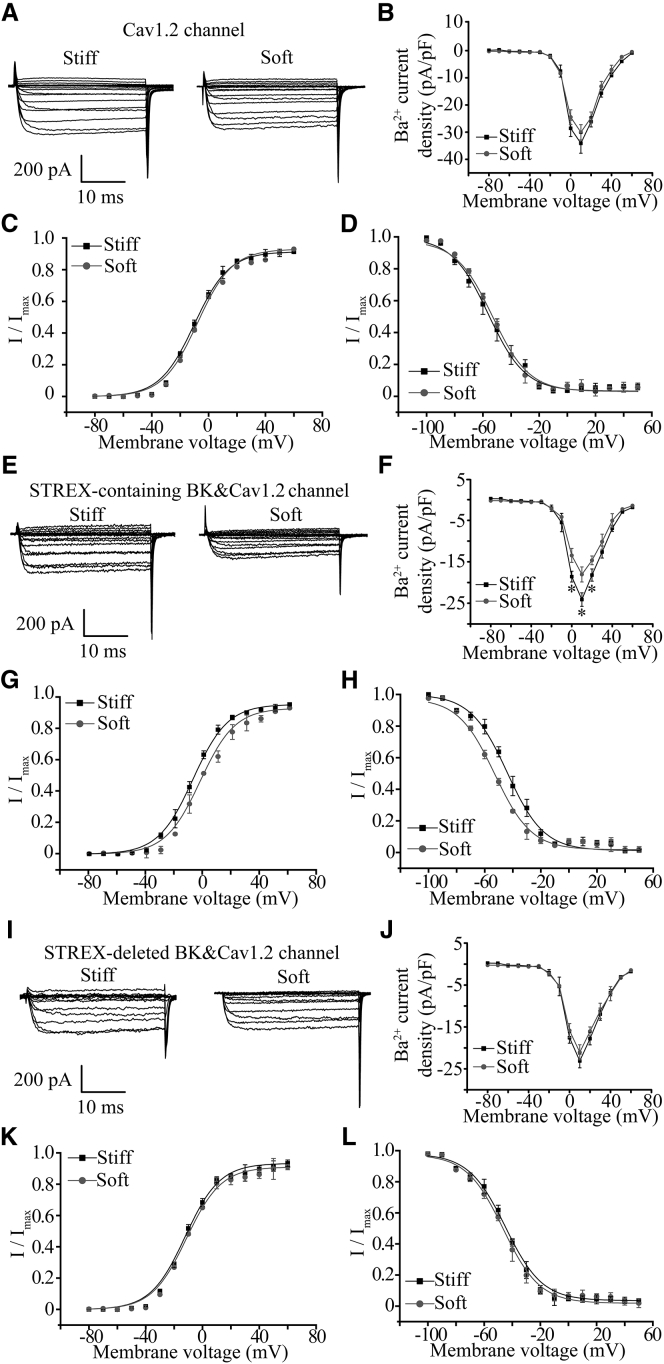
To further confirm the importance of the BK channel (particularly the STREX-containing stretch-activated channel) in substrate stiffness regulation of cardiac L-type Ca2+ channel, whole-cell Ca2+ channel currents were determined in HEK293 cells that expressed the CaV1.2 channel alone or coexpressed the STREX-containing BK or STREX-deleted BK channel, then were cultured on substrates with different stiffness. In cells only expressing the CaV1.2 channel, there was no significant difference in the Ca2+ channel current density in cells cultured on the soft and stiff substrates (Fig. 6, A and B). Substrate stiffness also had no effect on voltage-dependent channel activation or inactivation (Fig. 6, C and D). In striking contrast, in cells coexpressing the CaV1.2 Ca2+ channel and STREX-containing BK channel, the Ca2+ current density at voltages from 0 to 20 mV in cells on the stiff substrate was significantly higher than that on the soft substrate (Fig. 6, E and F). Stiff substrates also caused a negative shift in the voltage-dependent activation curve with the V1/2 value changing from −2.5 ± 0.4 mV on the soft substrate to −7.6 ± 0.8 mV on the stiff substrate (p < 0.05) without change in the slope value (4.3 ± 0.3 and 4.5 ± 0.5 on the soft and stiff substrates, respectively). Moreover, stiff substrate induced a positive shift in the voltage-dependent inactivation curve with the V1/2 changing from −53.2 ± 3.1 mV on the soft substrate to −41 ± 3.6 mV on the stiff substrate (p < 0.05) and the k value changing from 9.2 ± 0.8 on the soft substrate to 8.8 ± 1.2 on the stiff substrate (Fig. 6, G and H). However, in cells coexpressing CaV1.2 channel and STREX-deleted BK channel, the Ca2+ current density exhibited no significant difference in cells on the soft and stiff substrates (Fig. 6, I and J). The Ca2+ current density in cells coexpressing the STREX-deleted BK channel was slightly lower than, but not significantly different from, that in cells expressing the Ca2+ channel alone. Voltage-dependent channel activation and inactivation were not significantly altered by substrate stiffness (Fig. 6, K and L).
Figure 6.
Regulation of L-type CaV1.2 Ca2+ channel function by substrate stiffness requires the STREX-containing BK channel. (A) Representative whole-cell recordings of Ba2+ currents from HEK293 cells expressing the CaV1.2 channel on the soft and stiff substrates. (B) Summary of the mean I-V relationships from 10 cells in three preparations for each case. (C) Voltage dependence of the CaV1.2 channel activation determined with tail currents from 10 cells for each case. (D) Voltage dependence of the CaV1.2 channel inactivation from recordings in 10 HEK293 cells expressing the CaV1.2 channel in three preparations cultured on the soft and stiff substrates. (E) Representative whole-cell recordings of Ba2+ currents from HEK293 cells coexpressing the STREX-containing BK channel and the CaV1.2 Ca2+ channel on the soft and stiff substrates. (F) Summary of the mean I-V relationships from 12 cells in three preparations for each case. (G) Voltage dependence of the CaV1.2 channel activation determined with tail currents from 12 cells for each case. (H) Voltage dependence of the CaV1.2 channel inactivation from 11 cells in three preparations cultured on the soft and stiff substrates. (I) Representative whole-cell recordings of Ba2+ currents from HEK293 cells coexpressing the STREX-deleted BK channel and the CaV1.2 channel on the soft and stiff substrates. (J) Summary of the mean I-V relationships from 12 cells in three preparations for each case. (K) Voltage dependence of the CaV1.2 channel activation determined with tail currents from 12 cells for each case. (L) Voltage dependence of the CaV1.2 channel inactivation from 11 cells in three preparations cultured on the soft and stiff substrates. ∗p < 0.05, comparison of the current density at the same voltage.
Myosin-mediated traction forces are critical in substrate stiffness regulation of the BK and L-type Ca2+ channels
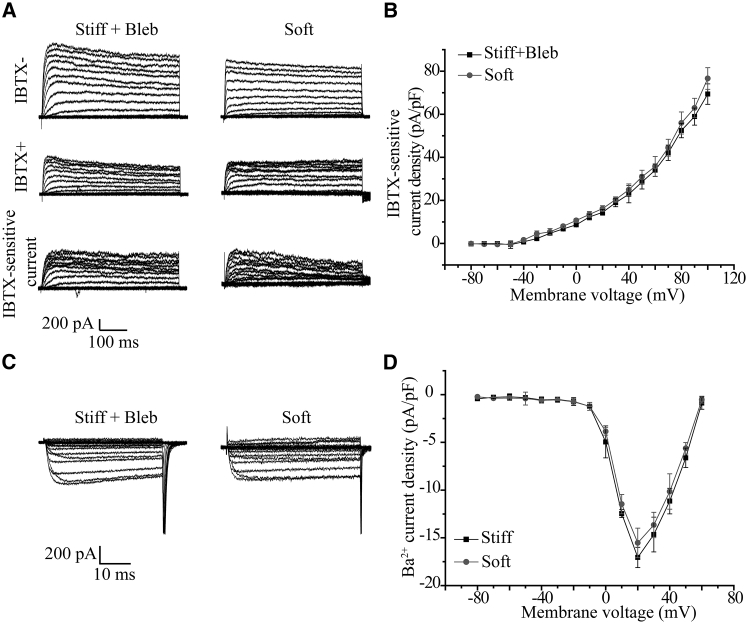
Cells can detect the mechanical properties of ECM by the mechanical response to the traction forces generated by myosin II in stress fibers (9, 10). Traction forces are greater in cells on the stiff substrate (31). Blebbistatin is known to specifically inhibit myosin and thereby the traction forces (31). Therefore, we finally examined whether myosin-mediated traction forces are critically involved in sensing the substrate stiffness by examining the effects of treatment with blebbistatin at a low dose on the BK channel and L-type Ca2+ channel in myocytes on the stiff substrate. Treatment with 5 μM blebbistatin resulted in relatively higher BK channel currents (Fig. 7, A and B) and lower Ca2+ channel currents (Fig. 7, C and D), which were similar in amplitude to those on the soft substrate (Fig. 7, B and D). These results suggest that myosin-mediated traction forces are critical in sensing substrate stiffness and regulating the BK and L-type Ca2+ channels in myocytes.
Figure 7.
Increased BK channel current and decreased L-type Ca2+ channel current in ventricular myocytes on stiff substrate after treatment with low-dose blebbistatin. (A) Representative whole-cell recordings of outward K+ currents from cells after treatment with 5 μM blebbistatin on the stiff substrate and cells on the soft substrate before (upper panels), and 10 min after, application of 100 nM IBTX (middle panels) and IBTX-sensitive currents (bottom panels). (B) Mean I-V relationships from 10 cells in three preparations for each case. (C) Representative whole-cell recordings of Ba2+ currents from cells on the stiff substrates after treatment with low-dose blebbistatin and cells on the soft substrate without blebbistatin treatment. (D) Mean I-V relationships from 11 cells in three preparations for each case.
Discussion
In this study, we have shown that substrate stiffness regulates the BK and L-type voltage-gated Ca2+ channels in ventricular myocytes and that the stretch-activated BK channel is critical in substrate stiffness regulation of the cardiac L-type Ca2+ channel. These findings reveal, to our knowledge, a novel mechanosensing mechanism in cardiac myocytes.
The L-type Ca2+ channel plays a critical role in mediating Ca2+ influx as a signal in the excitation-contraction coupling in cardiac myocytes. It has been documented that the stiffness of cardiac ECM undergoes remarkable increase during cardiac fibrosis (32). In this work, we showed that the change in substrate stiffness significantly altered the cardiac L-type Ca2+ channel function in chick ventricular myocytes (Fig. 1, A–D), suggesting that the cardiac L-type Ca2+ channel may function significantly differently under physiological and disease conditions. We further demonstrated that such substrate stiffness regulation mainly results from change in voltage-dependent channel activation and inactivation (Fig. 1, E and F) but not protein expression (Fig. 1 H). We also provided evidence to show that stiff substrate regulates the BK channel in chick ventricular myocytes. Stiff substrate downregulated mRNA and protein expression of both BK channel α- and β1-subunits (Fig. 3, A–D) and reduced the BK channel currents (Fig. 4, A–D). Further analysis of single channel recordings revealed that stiff substrate reduced the channel open probability (Fig. 5, A and D) and its sensitivity to voltage and Ca2+ (Fig. 5, B–F). The BK channel is long known to be expressed in cardiac myocytes but its role in cardiac function is less well established (17). Here, we showed that inhibition of the BK channel abolished substrate stiffness regulation of the cardiac L-type Ca2+ channel (Fig. 2, A–D), supporting the idea that the BK channel plays a critical role in substrate stiffness regulation of the L-type voltage-gated Ca2+ channel in chick cardiac myocytes.
Previous studies demonstrated that the STREX-containing α-subunit forms a stretch-activated BK channel in embryonic chick cardiac myocytes and in recombinant systems and the sensitivity of the BK channel to membrane stretch is determined by the STREX domain located in the intracellular C-terminus (21, 22). Here we have confirmed the expression of the STREX-containing α-subunit in chick ventricular myocytes and further demonstrated that its expression level was downregulated on the stiff substrate (Fig. 3, E and F). In HEK293 cells heterologously coexpressing the cardiac Cav1.2 channel and the STREX-containing BK channel, the Ca2+ channel current (Fig. 6, E and F) and voltage-dependent channel activation and inactivation (Fig. 6, G and H) were significantly altered by substrate stiffness in a similar manner to that observed in ventricular myocytes (Fig. 1, E and F). However, these effects of substrate stiffness on L-type Ca2+ channel were largely lost in HEK293 cells expressing the CaV1.2 channel alone (Fig. 6, A–D) or coexpressing with STREX-deleted BK channel (Fig. 6, I–L). These results, together with the aforementioned observation that pharmacological inhibition of the BK channel prevented substrate stiffness regulation of the cardiac L-type Ca2+ channel, provide consistent evidence to indicate that the STREX-containing BK channel plays an essential role in the process during which substrate stiffness regulates the L-type Ca2+ channel in myocytes. However, despite that this intracellularly located STREX domain is firmly established as the sensor for membrane stretch, it is unlikely to directly detect the stiffness of extracellular matrix and substrates. Instead, the STREX domain is more likely to serve as a transducer in the mechanism by which cells sense substrate stiffness. It is known that cells detect ECM by application of actomyosin-mediated traction forces (13, 31). We showed that treatment of myocytes on the stiff substrate with low-dose blebbistatin significantly increased the BK channel current (Fig. 7, A and B) and at the same time lowered the L-type Ca2+ channel current (Fig. 7, C and D), with the amplitudes of maximal K+ and Ca2+ channel currents being similar to those in cells on the soft substrate (Fig. 7, B and D). These results suggest that myosin-mediated traction forces are critically engaged in substrate stiffness sensing and regulation of the BK channel and L-type Ca2+ channel in myocytes.
While our results provide strong evidence to support a critical role of the STREX-containing BK channel in functional regulation of the cardiac L-type Ca2+ channel by substrate stiffness, it remains unclear how the BK channel regulates the Ca2+ channel. Increased BK channel activity can induce the membrane hyperpolarization that inhibits Ca2+ channel activation. This mechanism may occur under physiological conditions but it is highly unlikely in our experimental conditions where the membrane potential was clamped. It is tempting to hypothesize a physical coupling between the BK channel and L-type Ca2+ channel via which activation of the BK channel evokes conformational changes in the L-type Ca2+ channel. This hypothesis is consistent with the changes in voltage-dependent activation and inactivation. There is accumulating evidence to show that change in substrate stiffness can cause membrane protein and cytoskeleton remodeling (33, 34), and previous studies reported that the BK channel and the voltage-gated Ca2+ channel form protein complexes in neurons and other types of cells, where Ca2+ influx through the voltage-gated Ca2+ channel facilitates BK channel activation (35, 36). In cardiac myocytes, L-type Ca2+ channel serves as the main Ca2+ influx pathway to stimulate ryanodine receptor-mediated Ca2+-induced Ca2+ release to further increase the [Ca2+]i and thereby activate the Ca2+-activated K+ channels including the BK channel (23, 24). Therefore, there are two possible mechanisms by which Ca2+ induces BK channel activation in cardiac myocytes: Ca2+ release from sarcoplasmic reticulum through the ryanodine receptor, and direct Ca2+ influx mediated by the L-type Ca2+ channel. Another interesting observation was that expression of both STREX-containing α- and β1-subunits was significantly regulated by substrate stiffness (Fig. 7, B and D). It is known that the transcriptional coactivators Yap and Taz (also known as WWTR1), identified as a part of the Hippo pathway, are located in the nucleus in epithelial and mesenchymal stem cells cultured on the stiff substrates but translocate to the cytoplasm in cells on the soft substrates (37). It remains to be investigated in future studies whether such transcriptional mechanisms contribute to substrate stiffness regulation of the BK channel expression.
Previous studies reported that Ba2+ can block (38, 39) or activate the BK channel via the Ca2+-bowl site (40) or be ineffective in activating the BK channel (41). This study used Ba2+ as the charge carrier to measure the L-type Ca2+ channel currents to rule out Ca2+-dependent mechanisms such as channel inactivation (Fig. 1 C). Here, we showed that substrate stiffness regulation of L-type voltage Ca2+ channels was observed regardless of Ca2+ or Ba2+ as the charge carrier (Fig. 1, A–D). Furthermore, IBTX was effective in inhibiting the BK channel currents (Fig. 2, B and D). The L-type Ca2+ channel current carried by Ba2+ was significantly regulated by substrate stiffness in HEK293 cells coexpressing the STREX-containing BK channel and the CaV1.2 channel (Fig. 6 F). All these results suggest that Ba2+ at the concentration used in this study was ineffective in inhibiting the cardiac BK channel endogenously expressed in myocytes and heterologously expressed in HEK293 cells.
In conclusion, this study manifests a key role for the STREX-containing BK channel in substrate stiffness regulation of L-type Ca2+ channel function in cardiac myocytes. This finding helps us to understand the influence of mechanical properties of ECM or biomaterials on cardiac function and remodeling under physiological and pathophysiological conditions, but also provides useful information for tissue engineering approaches to repair and regenerate cardiac tissues.
Author Contributions
X.F., H.Z., L.J., and B. Li conceived the research, designed the experiments, and prepared and revised the article; H.Z., Y.Y., X.W., S.L., B. Liu, and J.D. performed the experiments and analyzed the data; and all authors participated in discussion and commented on the article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (under grant Nos. 11272184, 11432008, and 11672161) and the Department of Education, Henan Provincial Government.
Editor: Miriam Goodman.
Footnotes
Hucheng Zhao, Yang Yu, and Xiaoan Wu contributed equally to this article.
Supporting Materials and Methods and two figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30229-1.
Contributor Information
Bo Li, Email: libome@mail.tsinghua.edu.cn.
Linhua Jiang, Email: l.h.jiang@leeds.ac.uk.
Xiqiao Feng, Email: fengxq@mail.tsinghua.edu.cn.
Supporting Material
References
- 1.Weber K.T., Sun Y., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 2.Galie P.A., Westfall M.V., Stegemann J.P. Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovasc. Pathol. 2011;20:325–333. doi: 10.1016/j.carpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forte G., Pagliari S., Aoyagi T. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng. Part A. 2012;18:1837–1848. doi: 10.1089/ten.TEA.2011.0707. [DOI] [PubMed] [Google Scholar]
- 4.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 5.Heymans S., Luttun A., Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat. Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 6.Levental I., Georges P.C., Janmey P.A. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 7.Engler A.J., Griffin M.A., Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers E.L., Pugh M.E., Doyle T.P. Percutaneous vascular stent implantation as treatment for central vascular obstruction due to fibrosing mediastinitis. Circulation. 2011;123:1391–1399. doi: 10.1161/CIRCULATIONAHA.110.949180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajaj P., Tang X., Bashir R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J. Biomed. Mater. Res. A. 2010;95:1261–1269. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- 10.Pelham R.J., Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo C.M., Buxton D.B., Wang Y.L. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiger B., Spatz J.P., Bershadsky A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T., Sokabe M. Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Curr. Opin. Cell Biol. 2010;22:669–676. doi: 10.1016/j.ceb.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Ridley A.J., Schwartz M.A., Horwitz A.R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Pleyte K., Rusch N.J. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- 16.Brenner R., Peréz G.J., Aldrich R.W. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 17.Sato T., Saito T., Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 18.Ko J.H., Ibrahim M.A., Han J. Cloning of large-conductance Ca2+-activated K+ channel α-subunits in mouse cardiomyocytes. Biochem. Biophys. Res. Commun. 2009;389:74–79. doi: 10.1016/j.bbrc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 19.Kawakubo T., Naruse K., Sokabe M. Characterization of a newly found stretch-activated KCa,ATP channel in cultured chick ventricular myocytes. Am. J. Physiol. 1999;276:H1827–H1838. doi: 10.1152/ajpheart.1999.276.6.H1827. [DOI] [PubMed] [Google Scholar]
- 20.Naruse K., Tang Q.Y., Sokabe M. Stress-Axis Regulated Exon (STREX) in the C terminus of BK(Ca) channels is responsible for the stretch sensitivity. Biochem. Biophys. Res. Commun. 2009;385:634–639. doi: 10.1016/j.bbrc.2009.05.105. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H.C., Agula H., Li L.M. Membrane stretch and cytoplasmic Ca2+ independently modulate stretch-activated BK channel activity. J. Biomech. 2010;43:3015–3019. doi: 10.1016/j.jbiomech.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H., Sokabe M. Tuning the mechanosensitivity of a BK channel by changing the linker length. Cell Res. 2008;18:871–878. doi: 10.1038/cr.2008.88. [DOI] [PubMed] [Google Scholar]
- 23.Benitah J.P., Alvarez J.L., Gómez A.M. L-type Ca2+ current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Takamatsu H., Nagao T., Adachi-Akahane S. L-type Ca2+ channels serve as a sensor of the SR Ca2+ for tuning the efficacy of Ca2+-induced Ca2+ release in rat ventricular myocytes. J. Physiol. 2003;552:415–424. doi: 10.1113/jphysiol.2003.050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo J.H., Sakai K., Yui N. Adsorption state of fibronectin on poly(dimethylsiloxane) surfaces with varied stiffness can dominate adhesion density of fibroblasts. Acta Biomater. 2013;9:5493–5501. doi: 10.1016/j.actbio.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y.W., Cheng C.M., Chen C.C. Understanding sensory nerve mechanotransduction through localized elastomeric matrix control. PLoS One. 2009;4:e4293. doi: 10.1371/journal.pone.0004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q.Y., Zhang Y.Y., Zhao H.C. Stiff substrates enhance cultured neuronal network activity. Sci. Rep. 2014;4:6215. doi: 10.1038/srep06215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M.G., Cao Y.P., Feng X.Q. Spherical indentation method for determining the constitutive parameters of hyperelastic soft materials. Biomech. Model. Mechanobiol. 2014;13:1–11. doi: 10.1007/s10237-013-0481-4. [DOI] [PubMed] [Google Scholar]
- 29.Tao J., Liu P., Cao Y.Q. Effects of familial hemiplegic migraine type 1 mutation T666M on voltage-gated calcium channel activities in trigeminal ganglion neurons. J. Neurophysiol. 2012;107:1666–1680. doi: 10.1152/jn.00551.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L., Zhang H., Zhang L. Chronic exercise normalizes changes in Cav 1.2 and KCa 1.1 channels in mesenteric arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 2015;172:1846–1858. doi: 10.1111/bph.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 32.Engler A.J., Carag-Krieger C., Discher D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacot J.G., McCulloch A.D., Omens J.H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih Y.R.V., Tseng K.F., Lee O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 35.Cox D.H. Modeling a Ca2+ channel/BKCa channel complex at the single-complex level. Biophys. J. 2014;107:2797–2814. doi: 10.1016/j.bpj.2014.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkefeld H., Fakler B. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J. Neurosci. 2008;28:8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupont S., Morsut L., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 38.Neyton J., Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel. J. Gen. Physiol. 1988;92:569–586. doi: 10.1085/jgp.92.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neyton J., Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J. Gen. Physiol. 1988;92:549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y., Zeng X.H., Lingle C.J. Barium ions selectively activate BK channels via the Ca2+-bowl site. Proc. Natl. Acad. Sci. USA. 2012;109:11413–11418. doi: 10.1073/pnas.1204444109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberhauser A., Alvarez O., Latorre R. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J. Gen. Physiol. 1988;92:67–86. doi: 10.1085/jgp.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.