Abstract
Cholesterol is an important risk factor of atherosclerosis, due to its active uptake by monocytes/macrophages. Monocyte recruitment from flowing blood to atherosclerotic foci is the key first step in the development of atherosclerosis. Cholesterol content alters cell membrane stiffness, and lateral lipid and protein diffusion. We hypothesized that cholesterol content will modulate the recruitment of monocytes to inflamed endothelial surface by altering the dynamics of adhesion receptors. We depleted or enriched the cellular cholesterol levels using methyl-β-cyclodextran in freshly isolated human monocytes. We investigated the effect of these changes on the mechanics of monocyte rolling on E-selectin surfaces at 1 dyn/cm2 in microchannels. Using imaging flow cytometry and atomic force microscopy, we characterized the distribution of lipid rafts and the E-selectin counterreceptor CD44 on the monocyte surface. We observed that lower levels of cholesterol resulted in the uniform, CD44-mediated rolling of monocytes on the E-selectin-coated surfaces. We also observed that cells depleted of cholesterol had higher membrane fluidity, and more uniform distribution of CD44 counterreceptor, which resulted in smooth motion of the cells compared to cells enriched with cholesterol. This work demonstrates that cholesterol can modulate monocyte adhesion by regulating the receptor mobility, and our results provide insights into the biophysical regulation of inflammation for the better understanding of diseases like atherosclerosis and hypercholesterolemia.
Introduction
Monocytes are key in infection and inflammation, and are also important in maintaining homeostasis (1, 2). They also play a critical role in the pathogenesis of atherosclerosis and other inflammatory diseases, and cholesterol has been shown to greatly influence this process. In most cells, plasma membrane contains the majority of the total cellular cholesterol content (3, 4, 5). Plasma membrane is segregated into lipid rafts, which are dynamic, heterogeneous regions, rich in gangliosides, cholesterol, and glycosphingolipids, and play an important role in compartmentalizing cellular processes (6, 7, 8, 9). Lipid rafts regulate a variety of cellular processes such as protein trafficking and signal transduction (10). Cholesterol is vital in maintaining the integrity of the lipid rafts (11).
The effect of cholesterol on monocyte biomechanics remains understudied despite the well-established link between cholesterol and monocytes/macrophages in atherosclerosis and other diseases. In atherogenesis, one of the first steps is the interaction of monocytes with the inflamed endothelium. The effect of cholesterol content has been studied in a variety of other cell types such as neutrophils (5, 12), fibroblasts (13), and erythrocytes (14), among others. We hypothesized that the cellular cholesterol content influences the recruitment of monocytes to endothelial surface by altering the mechanics of cellular interactions. To test this hypothesis, we altered the baseline cholesterol levels by depleting and enriching the cellular cholesterol content, and assessed the dynamics of interaction of monocytes on E-selectin-coated surfaces under flow conditions. We also correlated the observed changes in rolling interactions with changes in membrane receptor distribution that mediate the rolling process.
Materials and Methods
Cell culture
Human blood samples, without any donor identifiers, were obtained after their informed consent, and the buffy coat was isolated by the South Texas Blood and Tissue Bank (San Antonio, TX). Buffy coat was procured from the South Texas Blood and Tissue Bank. All the methodologies and procedures were approved by Institutional Review Board, The University of Texas at San Antonio (protocol No. 12-227), and the experiments were conducted in accordance with these appropriate guidelines. Primary human monocytes were isolated from buffy coat using the Monocyte Isolation Kit II by indirect magnetic labeling (Miltenyi Biotec, Auburn, CA). The monocytes were cultured in RPMI 1640 media (American Type Culture Collection, Manassas, VA) supplemented with 10% heat-inactivated FBS (Life Technologies, Grand Island, NY). The cell viability was measured by trypan blue exclusion assay using the Countess Automated Cell Counter (Life Technologies). Purity of isolated monocytes was determined by flow cytometry (LSR-II Bench Top Flow Cytometer; BD Biosciences, San Jose, CA) using FITC-conjugated anti-CD14 antibody and a suitable isotype control (Miltenyi Biotech), followed by analysis using Flow Jo software (FLOWJO, Ashland, OR).
Cholesterol depletion and enrichment
Cholesterol was depleted using Methyl-β-cyclodextrin (MβCD; Sigma-Aldrich, St. Louis, MO). MβCD solution was prepared in PBS, and varying concentrations of MβCD were used. The desired concentration of MβCD solution was mixed with the cell suspension, and incubated for 30 min at 37°C and 5% CO2. For cholesterol enrichment, MβCD-cholesterol complex (Sigma-Aldrich) was used with a cholesterol concentration of 40 mg/gm. The desired concentration of MβCD-cholesterol solution was mixed with the cell suspension, and incubated for 30 min at 37°C and 5% CO2.
Cholesterol content in the cells was measured using an Amplex Red Cholesterol Assay Kit (Life Technologies), as per the manufacturer’s protocol. Approximately 0.75 × 105 cells were used per well of a 96-well plate (Corning, Corning, NY). The fluorescence intensity was measured at 540 nm (excitation)/600 nm (emission) (Synergy2; Biotek, Winooski, VT). For assessment of the total cellular content, the cells were lysed by sonication. The cells were centrifuged at 300 × g for 10 min at room temperature; the pellets were resuspended in PBS, and the solution was vortexed for 60 s. The centrifugation and vortex steps were performed three times The pellet was then resuspended in PBS, sonicated for 90 s, centrifuged at 300 × g for 10 min at 4°C, resuspended in PBS, and vortexed for 45 s. The sonication with the accompanying steps was performed three times. The cell lysate was then tested for cholesterol content. For the plasma membrane cholesterol estimation, the cells were left intact. The cholesterol content was determined from a standard curve using 1–20 μg/mL cholesterol standards (the cholesterol standard provided in the kit was used as reference to obtain the standard curves; individual standard curves were obtained for each experiment). The fluorescence measurements were obtained using the automatic-gain feature, allowing the instrument software to select the best gain for a given run.
Perfusion assays on E-selectin-coated surfaces
E-selectin (R&D Systems, Minneapolis, MN) was coated on the microchannels (1 mm wide × 0.1 mm high; μ-slide V10.1; IBIDI, Munich, Germany) at a concentration of 10 μg/mL for 3 h at room temperature. After this, 1% BSA was added and incubated at room temperature for 30 min, to negate nonspecific binding. The microchannel slide was assembled on top of an inverted microscope (DMI 6000B; Leica Microsystems, Wetzlar, Germany) attached to a time-lapse digital camera (DFC-360 FX; Leica Microsystems). A monocyte suspension consisting of 0.75 × 106 cells/mL was then perfused through the microchannels using a syringe pump (Harvard Apparatus, Holliston, MA) at a constant flow rate corresponding to a wall shear stress of 1 dyn/cm2. The cell solution was perfused for 30 s for it to attain steady state. For each experimental condition, five movie clips were captured using the software LAS-AF (Leica Microsystems) at 10× magnification. Each movie was 45 s long, with a 30 s interval between each clip. Perfusion of the cells through uncoated channels treated with 1% BSA were used as controls. The images obtained from the perfusion assays were converted to TIFF stacks, and analyses were done using the software ImagePRO (Media Cybernetics, Rockville, MD). The cell movements were tracked using a semiautomated correlation tracking algorithm, and to assess the accuracy of the tracking algorithm, manual centroid tracking was used for 15 randomly chosen cells. At least 60 cells were analyzed for each video clip, and at least 300 cells were analyzed per condition per microchannel.
The following definitions are used for image analysis. “Rolling flux” refers to the number of cells actually rolling on the surface per video clip per field of view. The “cutoff velocity” was determined based on the minimum velocity of the cells that returned to the flow of the fluid stream after being stationary for at least 1 s. This was calculated to be ∼250 μm/s. The cells rolling on the surface were individually tracked using a semiautomated algorithm. The tracking algorithms yielded the distance rolled by each cell per video clip before either completely adhering or detaching from the surface. The instantaneous velocities were obtained directly from ImagePRO using the same tracking methodology. These velocities obtained were the translational velocities along the direction of fluid flow. Using these velocities, the “mean velocities” were calculated using the software MATLAB (The MathWorks, Natick, MA). A cell was considered “adhered” if it remained stationary >1 s. “Rolling duration” is the time a cell continuously spent rolling, below the cutoff velocity. The “average rolling velocity” was calculated for each “rolling duration”. The “pause time” was calculated as the duration the cells were stopped before either completely adhering on the surface or continuing the rolling motion for each rolling track. A distribution for “acceleration” was also calculated from instantaneous velocities, demonstrating how the cells have accelerated/decelerated. Pause times and acceleration were also calculated using MATLAB.
Lipid rafts, and receptor expression and distribution
Changes in membrane lipid raft distribution and expression due to changes in cholesterol were assessed using multispectral imaging flow cytometry (ImagestreamX Mark II; Amnis, Seattle, WA). Ganglioside GM1 was used as a marker for lipid rafts, and was fluorescently tagged with cholera toxin subunit B (Vybrant Lipid Raft Labeling Kit; Life Technologies). A final cell concentration of 106/100 μL was used. Single cell image analysis and expression levels were determined using the software IDEAS (Amnis) on images acquired at magnifications of 40× and 60×. CD44, CD15, and CD162 were fluorescently tagged with suitable antibodies (CD44-PE, CD15-PE Cy7, and CD162-BV602; BD Biosciences) and multispectral imaging flow cytometry (Amnis) was performed. Images were taken at 40× and 60×. The analysis software IDEAS was used to determine the expression levels and associated distribution changes. We determined the expression levels of the receptors by assessing the mean fluorescence intensity values. “H-variance SD” and “Bright Detail Similarity” demonstrate the distribution of the receptors and the colocalization of the receptors with membrane lipid raft marker (GM1), respectively (see details in the Supporting Material).
Membrane fluidity
Membrane fluidity was assessed using pyrenedecanoic acid (PDA; Membrane Fluidity Kit; Markergene Technologies, Eugene, OR). Approximately 105 cells were used for each experimental condition. The cells were centrifuged at 300 × g for 10 min, and the supernatant was discarded. A quantity of 20 μM solution of the probe was used with 0.1% pluronic F127 (Markergene Technologies). The cells were resuspended in 300 μL perfusion buffer (Markergene Technologies) containing the probe. The cells were incubated at room temperature for 20 min in the dark. The cells were then washed three times with 500 μL perfusion buffer (Markergene Technologies), and put in a 96-well plate (Corning) at a final volume of 200 μL. Fluorescence intensity measurements were made at 360 nm(excitation)/420 nm(emission for monomer) and 360 nm (excitation)/485 nm (emission for excimer), respectively (Synergy2; Biotek). The fluorescence measurements were obtained using the automatic-gain feature, allowing the instrument to select the best gain for the experiments.
Analysis of nanomechanical properties of membranes using atomic force microscopy
Monocytes immobilized with 0.1% linear Polyethylenimine (PEI; Sigma-Aldrich) were immersed in an appropriate culture medium and scanned in 55 mm plastic Petri dishes. Cells were scanned with a Nanoscope Catalyst (Bruker, Billerica, MA) atomic force microscope (AFM) mounted on an Eclipse Ti Inverted Epifluorescent Microscope (Nikon, Melville, NY) using the PeakForce Quantitative Nanomechanical Mapping mode (Bruker). Before and after AFM imaging, a light microscopy image was recorded for each cell. Electronic resolution of 10 × 10 μm images was kept at 256 × 256 pixels (x, number of points per line by y, number of lines). SCANASYST-AIR (Bruker) probes were used for imaging. The spring constant of the nominal value 0.02 N/m was determined for each probe with the thermal tuning. Because monocytes are extremely soft, we applied a peak force setpoint of 100 pN with feedback gain not higher than 0.3. Peak force amplitude set at 1500 nm and slow scan rates up to 0.2 Hz helped to avoid artifacts arising from dense indentation of a cell surface. Cell indentation was limited to <200 nm to assure cell membrane integrity. Cell boundaries were identified based on a cell shape and nanotopography collected in height and peak force error channels, respectively. In most cases, we imaged only a part of cell surface because of cell fragility, and scanning was stopped at any sign of cell death. We completed imaging of a single dish in 20–30 min collecting data for 3–5 cells. Cell elasticity and adhesion were captured in two additional separate channels. To limit the influence of tip-induced deformation on adhesion force, the depth of tip indentation was constrained to <200 nm (15). Nanomechanical parameters were calculated with the software Nanoscope Analysis v1.4 (Bruker) using the retrace images assuming high heterogeneity of cell surface. The Sneddon model (details in the Supporting Material) that approximates the mechanics of conical tip interactions with an object was employed to calculate nanomechanical properties of cells. A mode value of elasticity and adhesion for each cell was extracted from corresponding distribution histograms and applied in all the downstream statistical calculations.
Statistics
All the experiments were performed in triplicate, and each experiment was repeated at least three times, under independent conditions. The results are represented as mean ± SE from one representative experiment. Statistical differences between treatments were evaluated using either one-way ANOVA with Tukey’s post hoc test or Students’ t-test (GraphPad Prism, La Jolla, CA), and significance was reported at α = 0.05.
Results and Discussion
The high on- and off-rates, affinity, and avidity of selectins to their counterreceptors are crucial for the successful recruitment of leukocytes from flowing blood to inflamed endothelium. In this work, we have shown that this delicately balanced kinetics in the recruitment of monocytes to E-selectin surfaces under flow is significantly influenced by cellular cholesterol levels due to redistribution of surface receptors.
Alteration of plasma membrane cholesterol content
To modify the membrane cholesterol content, we depleted cholesterol using MβCD and enriched it using MβCD-cholesterol complex, and the levels of cellular cholesterol were estimated. Cholesterol was depleted by treating with 5 and 10 mM of MβCD and we observed a 0.6 and 0.4-fold decrease posttreatment, respectively (Fig. 1). MβCD-cholesterol complex was used to enrich the cellular cholesterol. Similarly, we observed increase of 1.6-, 2.8-, and 3.5-fold from untreated, for treatment with 0.25, 0.5, and 1 mM reagent, respectively (Fig. 1). After cholesterol depletion and enrichment, healthy cells were separated by centrifugation and were estimated to be >90% viable, similar to untreated control. Plasma membrane cholesterol accounted for ∼88% of the total cellular content in untreated monocytes; and ∼84 and ∼89% of the total cellular cholesterol content was present on the plasma membrane for cholesterol-depleted and -enriched cells, respectively (Table S1). The cholesterol levels were comparable to previous reports on monocytes/macrophages, although the exact values may depend upon the source and treatment of monocytes, and cholesterol assay techniques (16, 17, 18).
Figure 1.
Modification of baseline cellular cholesterol levels. (∗) Statistical significance compared to untreated cells. p < 0.05.
Changes in the cellular cholesterol content alters the rolling dynamics of monocytes
Upon successfully altering the cellular cholesterol content, we proceeded to study its effects on the dynamics of monocyte interactions with endothelial mimetics. We performed all our experiments using 10 mM MβCD for cholesterol depletion and 0.5 mM MβCD-cholesterol complex for cholesterol enrichment. To study any possible difference in the rolling behavior, we perfused treated and untreated cells through microfluidic channels (1 × 0.1 mm) coated with E-selectin, because E-selectin is the foremost adhesion molecule mediating monocyte-endothelial interaction (19). The cells were perfused at a venous shear stress of 1 dyn/cm2.
Upon treatment with MβCD/MβCD-cholesterol, we observed a significant change in the number of cells interacting with E-selectin-coated surface. There was a 30% increase and a 50% decrease in the number of rolling cells in the case of cholesterol-depleted and -enriched cells, respectively, as compared to untreated cells (Fig. 2 A). On following the distance traveled by individual cells by rolling before detachment from the surface, cholesterol enrichment resulted in the maximum distance traversed per track, with an approximately fourfold increase compared to untreated cells. Cholesterol depletion resulted in a twofold decrease in the distance traveled by the monocytes (Fig. 2 B).
Figure 2.
Monocyte rolling on E-selectin-coated surface. (A) Rolling flux of cells rolling on E-selectin-coated surfaces. (B) Distance traveled by rolling cells before either detaching from the surface or completely getting adhered to the surface. (C) Representative traces of instantaneous rolling velocities, sampled every 0.09 s. (D) Mean velocity of rolling cells while in contact with the surface. (E) Pause time is the time spent by a cell on a surface before moving significantly. (F) Distribution of instantaneous acceleration of rolling cells. (∗ and ϕ) Statistical significance compared to depleted and untreated cells, respectively. p < 0.05.
Next, we examined the changes in rolling dynamics due to cholesterol levels in further detail. Using a semiautomated cell tracking algorithm, we calculated translational velocities of individual cells rolling in the field of view, such as a representative trace shown in Fig. 2 C, extracted from time-lapse video microscopy of rolling cells (Movie S1). In our assays, rolling monocytes were distinguished based on their translational velocity being significantly below the hydrodynamic velocity. The mean rolling velocity of the untreated cells, including movements and pauses, was calculated to be ∼5 μm/s. Cholesterol depletion decreased the mean velocity by 45%, whereas enrichment increased it by 90% (Fig. 2 D). We observed that cholesterol depletion decreased the pause time, defined as the time spent without significant motion, by 37% and that enrichment increased it ∼15%, while simultaneously altering the maximum velocities (Fig. 2 E). This means that cholesterol-enriched cells stopped for longer duration during the course of rolling. However, they also rolled faster between pauses (Fig. 2 D), which possibly led to a more erratic rolling (Fig. 2 F) and longer rolling distances (Fig. 2 B).
In addition to these changes in mean velocities, instantaneous velocities describe a remarkable consequence of cholesterol depletion or enrichment (Fig. 2 C). These changes are best exemplified by analyzing the acceleration between peaks and valleys in instantaneous velocities (Fig. 2 F). Rolling motion is due to the formation/dissociation of receptor–ligand bonds, and is a complex process dependent on the kinetics of bonding, cell deformability, and extension of microvilli tethers. As a result, the instantaneous rolling motion is characterized by rapid, microsecond changes in the direction of flow, which appear as peaks and valleys in the instantaneous velocity profile due to sudden acceleration or deceleration of the cell, respectively. The highly dispersed distribution of acceleration values for cholesterol-enriched cells indicated erratic rolling, whereas much tighter distributions corresponding to cholesterol-depleted cells indicated uniform rolling behavior (Fig. 2 F). Taken together, lower cholesterol levels resulted in a slower and more uniform rolling. Of note, we recently showed that monocytes infected with the intracellular pathogen Chlamydia pneumoniae decreased membrane cholesterol levels with a concomitant decrease in pause times and steadier rolling (17). The remarkable similarity in the patterns of rolling dynamics in both cases suggests that the MβCD-induced cholesterol change is a physiologically relevant model to investigate the consequence of infection and inflammation.
Cholesterol content regulates the plasma membrane fluidity and lipid raft distribution
To understand the observed differences in the dynamics of monocyte rolling, we investigated the plausible changes in membrane properties due to cholesterol treatment. We observed that the majority of cellular cholesterol is in the plasma membrane, which is corroborated by previous reports (3, 4). The first membrane property we assessed was cell membrane fluidity, which is the reciprocal of membrane viscosity (20). We tested the changes in membrane fluidity using fluorescence intensity studies with PDA. Upon interaction, the monomers of PDA form excimers that cause a shift in the emission spectrum. The ratio between the excimers and the monomers is an estimation of membrane fluidity. We observed that cholesterol depletion in human monocytes due to MβCD treatment resulted in an increased membrane fluidity as compared to the untreated control, whereas cholesterol enrichment significantly decreased the membrane fluidity (Fig. 3). A similar phenomenon is observed in C. pneumoniae-infected monocytes, where infection leads to a reduction in the cholesterol content and subsequent increase in the plasma membrane fluidity (17). Cholesterol depletion also has a similar effect on the human monocytic cell line THP-1 cells (21).
Figure 3.
Fluidity of cell membrane estimated using the fluorescent probe PDA. (∗ and ϕ) Statistical significance compared to depleted and untreated cells, respectively. p < 0.05.
Changes in membrane fluidity and cholesterol content prompted us to investigate the possible changes in the membrane lipid rafts as a result of cholesterol treatment. Membrane lipid rafts are subdomains of the plasma membrane containing high concentrations of sterols, gangliosides, and sphingolipids, and often carry receptors responsible for several cell functions (6, 7, 8, 9). GM1 gangliosides were used as markers for membrane lipid rafts (22, 23). We observed that cholesterol depletion resulted in disruption of membrane lipid rafts, and enrichment leads to higher concentration at certain focal points (Fig. 4). We also examined the distribution levels of membrane lipid rafts. H-variance SD is a measure of how distributed or localized a signal is; the lesser the H-variance SD value, the more uniformly distributed the probe is. We found that cholesterol depletion resulted in a more uniform distribution of membrane lipid rafts as compared to the untreated control, with enrichment making the lipid raft distribution more nonuniform (Fig. 4). The coalescence of lipid rafts into larger entities also occurs during cell activation (24, 25), and are shown to be effective in the capture of platelets (26) or cancer cells (27) from flow in hemostasis and metastasis, respectively.
Figure 4.
Membrane lipid raft (MLR) properties. (A) Expression levels of mean fluorescence intensity (MFI) and distribution (H variance) of plasma membrane lipid rafts. (B) Fluorescence visualization of plasma membrane lipid rafts. (∗ and ϕ) Statistical significance compared to depleted and untreated cells, respectively. p < 0.05.
Changes in membrane fluidity and lipid raft clustering were further substantiated by AFM imaging. Fig. 5 A shows the Young’s modulus maps on monocytes, primarily demonstrating their organization on the cell surface. The ranges of Young’s modulus values for monocytes obtained in our experiments are comparable with published values (28, 29). Cholesterol-enriched cells exhibited very distinct regions of low and high Young’s modulus on the plasma membrane (Fig. 5 A). The image has been magnified to show a part of the plasma membrane and is representative of the whole cell (height maps outlining the entire cell imaged are shown in Fig. S1). We estimated the Young’s modulus values using the Sneddon indentation model, which describes forces exercised on a soft flat surface by a rigid cone-shaped indenter producing an axisymmetric punch (30). The higher Young’s modulus was conceivably due to the highly clustered nature of the membrane lipid rafts. There was also a significant increase in the nonspecific adhesion force experienced by the AFM tip while scanning the cell surface with increase in the cholesterol content due to the higher resistance offered to the AFM tip during the raster scan (Fig. 5 B). The applied AFM imaging with nonmodified silica nitride probes allows for assessment of total nonspecific interactions between a cell surface and an AFM tip. At each point during a raster scanning of a cell, the QNM mode records the maximum detachment force from a retraction phase of the tip-cell interaction cycle, producing an adhesion map. These results indicate that higher degree of receptor clustering and reduced membrane fluidity due to cholesterol enrichment increases the nonspecific adhesion force, which may also contribute to the decreased rolling flux (Fig. 2 A). In contrast, cholesterol depletion led to more interspersed areas of low and high elasticity apparently arising from the disruption of the normal clustering of lipid rafts, and is expectedly followed by the significant decrease of membrane adhesion.
Figure 5.
Mechanical properties of monocytes assessed with AFM. (A) Map of Young’s modulus calculated using the Sneddon model (representing a part of imaged monocyte plasma membrane). (B) Nonspecific adhesion force between the plasma membrane and the AFM tip. (∗ and ϕ) Statistical significance compared to depleted and untreated cells, respectively. p < 0.05.
Elasticity is a complex result of membrane properties, cytoskeletal architecture, and distribution and size of organelles. We captured monocytes on PEI-covered culture dishes immediately after their treatment and instantly performed AFM imaging; this led us to believe that most changes in mechanical properties of the cells are due to alterations in the properties of the cell membrane, and the reorganization of cytoskeleton may not have a substantial contribution to the overall assessment of cell elasticity. However, exact demarcation of the individual contributions requires further investigation.
Receptor expression levels and their distribution are changed with altered cholesterol loads
Activated endothelial cells express adhesion molecules that serve to recruit monocytes to the atherosclerotic foci (31). The initial steps of this process involve tethering and rolling, which are chiefly mediated by the endothelial receptor, E-selectin, which binds to the counterreceptors CD44, CD15, and CD162 on the monocyte surface. We investigated the changes in CD44, CD15, and CD162, which are the three most commonly implicated receptors for E-selectin-mediated rolling (32). In freshly isolated monocytes, in comparison to CD44 expression levels, CD15 and CD162 were minimally expressed (Fig. S2). CD44 is ubiquitously expressed in multiple cell types, and has a profound impact on atherosclerotic lesion formation (33). Among its other functions, CD44 is implicated in the slow rolling and arrest of leukocytes (32).
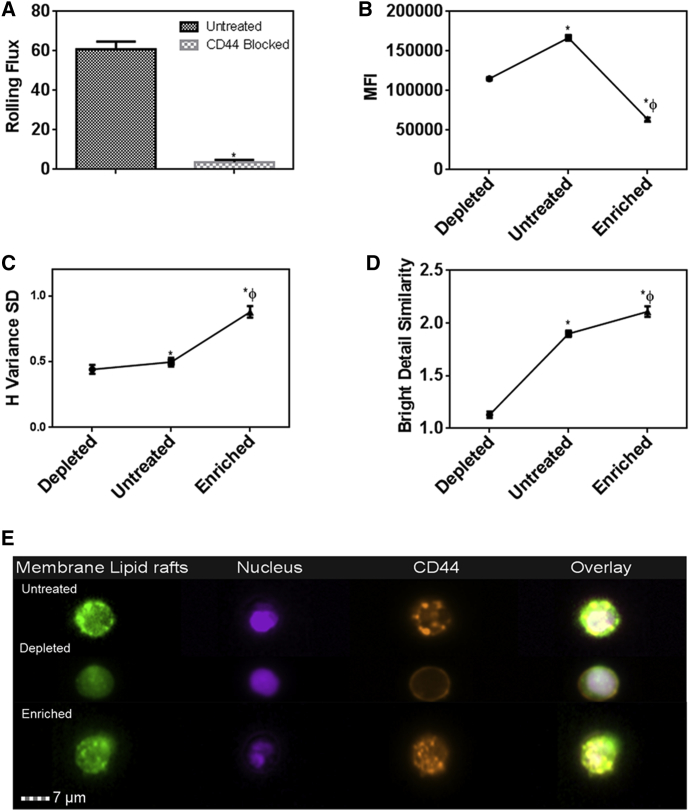
To further confirm the importance of CD44 on rolling interactions, we performed perfusion experiments on E-selectin-coated microchannels after blocking the CD44 receptor on the monocytes. CD44 blocking decreased the number of rolling cells by 95% (Fig. 6 A; Movie S2). Interestingly, both MβCD and MβCD-cholesterol treatment lowered the CD44 levels, with cholesterol enrichment having the maximum effect (Fig. 6 B). To investigate the distribution of CD44 on membrane rafts, we analyzed the colocalization of CD44 with GM1 using bright field similarity, as previous studies have shown that only raft-associated receptors colocalize with GM1 and can be visualized with CTB labeling (34, 35). As for the CD44 distribution on the surface, however, changes in H-variance SD suggest that cholesterol-depleted cells had a more uniform distribution of CD44 in comparison to the untreated control. Cholesterol enrichment resulted in a nonuniform distribution of maximum clustering (Fig. 6 C). By superimposing the fluorescence signals from the probes for lipid rafts and for CD44, we observed that these differences in CD44 distribution levels are due to the recruitment or dislodging of CD44 from lipid rafts: cholesterol enrichment also resulted in maximum recruitment of the CD44 in the lipid rafts, and cholesterol depletion resulted in them being dislodged from the lipid rafts (Fig. 6 D). Visual inspection of the images obtained from the imaging flow cytometer corroborates the clustering of the receptor in the lipid rafts on the cell surface (Fig. 6 E).
Figure 6.
Monocyte counterreceptors for E-selectin. (A) Changes in rolling flux due to treatment with CD44 blocking antibody. (B) Expression levels of CD44. (C) Distribution of CD44 on the plasma membrane. (D) Colocalization of CD44 with plasma membrane lipid rafts. (E) Fluorescence visualization of CD44. (∗ and ϕ) Statistical significance compared to depleted and untreated cells, respectively. p < 0.05.
The changes in the distribution of CD44 on the lipid rafts due to cholesterol depletion/enrichment provides a basis for the vast differences in the characteristics of rolling interaction between CD44 and E-selectin: Cholesterol depletion results in a modest decrease in CD44 expression levels but a more uniform redistribution on the plasma membrane due to higher membrane fluidity (36). This may increase the probability of interaction between the cell and the E-selectin-coated surface, leading to a higher number of cells rolling on the surface. A more uniform distribution also calls for steady rolling. On the other hand, cholesterol enrichment decreased the total CD44 levels but increased the recruitment of CD44 in the membrane lipid rafts and hence clustering of the receptors. This may explain the more erratic rolling observed in the enriched cells, which at higher speeds traveled a longer distance. These observations were consistent with our recent finding that receptor distribution due to cholesterol changes resulting from bacterial infection critically influences the rolling dynamics (17). Further, we observed that the expression levels of other E-selectin counterreceptors CD15 and CD162 were significantly lower than CD44 levels (Fig. S2), and hence may not have a significant effect on the rolling dynamics described in this work.
In summary, we have demonstrated that by altering CD44 distribution on a monocyte surface, cholesterol modulates the avidity of CD44 with an endothelial surface mimic, thereby altering the overall rolling dynamics. Rolling of leukocytes on the endothelium marks one of the initial and most important stages of their immune response. Non-steady rolling with longer pause times due to cholesterol enrichment enhances the leukocyte-endothelial interactions, increasing the probability of activation and firm arrest (37). Our work highlights the importance of biophysical regulation by cholesterol levels as another possible mechanism in addition to the well-established biochemical pathways in the pathophysiology of atherosclerosis.
Author Contributions
A.K.S. and A.K.R. designed the study, analyzed the data, and wrote the article; A.K.S. performed the experiments; S.F.D. performed blocking of CD44 molecules on monocytes; P.O. performed AFM imaging; and P.O., M.G., and T.H.-M.H. analyzed AFM data.
Acknowledgments
The authors thank Dr. Shankar J. Evani and Dr. Anand Srinivasan for technical assistance.
The authors acknowledge the assistance of the Immune Defense Core Facility at the University of Texas at San Antonio, supported by a grant from the National Institute on Minority Health and Health Disparities, National Institutes of Health (No. G12MD007591). This work was also supported by a grant from the National Institutes of Health (No. HL112629).
Editor: Philip LeDuc.
Footnotes
Amit Saha’s present address is Department of Biomedical, Chemical and Material Engineering, San José State University, San José, California.
Supporting Materials and Methods, two figures, one table, and two movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30235-7.
Supporting Material
No monocytes were seen rolling in the center of the channel where wall effects are negligible.
References
- 1.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 2.Gratchev A., Sobenin I., Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology. 2012;217:476–482. doi: 10.1016/j.imbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Lange Y., Ramos B.V. Analysis of the distribution of cholesterol in the intact cell. J. Biol. Chem. 1983;258:15130–15134. [PubMed] [Google Scholar]
- 4.Lange Y., Ye J., Steck T.L. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J. Lipid Res. 1999;40:2264–2270. [PubMed] [Google Scholar]
- 5.Oh H., Mohler E.R., 3rd, Diamond S.L. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arterioscler. Thromb. Vasc. Biol. 2009;29:1290–1297. doi: 10.1161/ATVBAHA.109.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 7.Brown D.A., London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 8.Pike L.J. Lipid rafts: bringing order to chaos. J. Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Head B.P., Patel H.H., Insel P.A. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundbaek J.A., Andersen O.S., Nielsen C. Cholesterol-induced protein sorting: an analysis of energetic feasibility. Biophys. J. 2003;84:2080–2089. doi: 10.1016/S0006-3495(03)75015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roduit C., van der Goot F.G., Kasas S. Elastic membrane heterogeneity of living cells revealed by stiff nanoscale membrane domains. Biophys. J. 2008;94:1521–1532. doi: 10.1529/biophysj.107.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierini L.M., Eddy R.J., Maxfield F.R. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 13.Kwik J., Boyle S., Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth A.M., Braunmüller S., Stone H.A. The effects of membrane cholesterol and simvastatin on red blood cell deformability and ATP release. Microvasc. Res. 2012;83:347–351. doi: 10.1016/j.mvr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Friedrichs J., Legate K.R., Benoit M. A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods. 2013;60:169–178. doi: 10.1016/j.ymeth.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran M.P., Lichtenstein A.H., Lamon-Fava S. The effect of 17β-estradiol on cholesterol content in human macrophages is influenced by the lipoprotein milieu. J. Mol. Endocrinol. 2011;47:109–117. doi: 10.1530/jme-10-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evani S.J., Ramasubramanian A.K. Biophysical regulation of Chlamydia pneumoniae-infected monocyte recruitment to atherosclerotic foci. Sci. Rep. 2016;6:19058. doi: 10.1038/srep19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aryal B., Rotllan N., Fernández-Hernando C. ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression. Nat. Commun. 2016;7:12313. doi: 10.1038/ncomms12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkel E.J., Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 20.Nipper M.E., Majd S., Haidekker M.A. Characterization of changes in the viscosity of lipid membranes with the molecular rotor FCVJ. Biochim. Biophys. Acta. 2008;1778:1148–1153. doi: 10.1016/j.bbamem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Saha A.K., Dallo S.F., Ramasubramanian A.K. Cellular cholesterol regulates monocyte deformation. J. Biomech. 2016;52:83–88. doi: 10.1016/j.jbiomech.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita A., Cheng J., Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol. Biol. Cell. 2007;18:2112–2122. doi: 10.1091/mbc.E07-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols B.J. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr. Biol. 2003;13:686–690. doi: 10.1016/s0960-9822(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy C., Nelson M.D., Bamezai A.K. Analysis of detergent-free lipid rafts isolated from CD4+ T cell line: interaction with antigen presenting cells promotes coalescing of lipid rafts. Cell Commun. Signal. 2011;9:31. doi: 10.1186/1478-811X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta N., DeFranco A.L. Lipid rafts and B cell signaling. Semin. Cell Dev. Biol. 2007;18:616–626. doi: 10.1016/j.semcdb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodin S., Tronchère H., Payrastre B. Lipid rafts are critical membrane domains in blood platelet activation processes. Biochim. Biophys. Acta. 2003;1610:247–257. doi: 10.1016/s0005-2736(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.C., Park M.J., Kim Y.N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. quiz 1404–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross S.E., Jin Y.S., Gimzewski J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 29.Leporatti S., Gerth A., Donath E. Elasticity and adhesion of resting and lipopolysaccharide-stimulated macrophages. FEBS Lett. 2006;580:450–454. doi: 10.1016/j.febslet.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Kuznetsova T.G., Starodubtseva M.N., Zhdanov R.I. Atomic force microscopy probing of cell elasticity. Micron. 2007;38:824–833. doi: 10.1016/j.micron.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Galkina E., Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo A., Peired A.J., Frenette P.S. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuff C.A., Kothapalli D., Puré E. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J. Clin. Invest. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennies C.M., Lehn M.A., Janssen E.M. Quantitating MHC class II trafficking in primary dendritic cells using imaging flow cytometry. J. Immunol. Methods. 2015;423:18–28. doi: 10.1016/j.jim.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janes P.W., Ley S.C., Magee A.I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murai T., Sato C., Kawashima H. Membrane cholesterol modulates the hyaluronan-binding ability of CD44 in T lymphocytes and controls rolling under shear flow. J. Cell Sci. 2013;126:3284–3294. doi: 10.1242/jcs.120014. [DOI] [PubMed] [Google Scholar]
- 37.Muller W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013;50:7–22. doi: 10.1177/0300985812469883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No monocytes were seen rolling in the center of the channel where wall effects are negligible.