Abstract
LL-37 is a human cationic host defense peptide that is an essential component of innate immunity. In addition to its modest antimicrobial activity, LL-37 affects the gene expression and behavior of effector cells involved in the innate immune response, although its mode of interaction with eukaryotic cells remains unclear. The interaction of LL-37 with epithelial cells was characterized in tissue culture by using biotinylated LL-37 and confocal microscopy. It was demonstrated that LL-37 was actively taken up into A549 epithelial cells and eventually localized to the perinuclear region. Specific inhibitors were used to demonstrate that the uptake process was not mediated by actin but required elements normally involved in endocytosis and that trafficking to the perinuclear region was dependent on microtubules. By using nonlinear regression analysis, it was revealed that A549 epithelial cells have two receptors for LL-37B, with high and low affinity for LL-37, respectively. These results indicate the mode of interaction of LL-37 with epithelial cells and further our understanding of its role in modulating the innate immune response.
Cationic host defense peptides are key components of innate immunity that have both direct, broad-spectrum antimicrobial activity and an ability to stimulate immunity against bacteria, fungi, parasites, and viruses (14). In evolutionary terms, the peptide immune system is ancient, with a diverse repertoire of molecules, but has been maintained in virtually all advanced eukaryotes from insects to mammals. In mammals, selected gene-encoded peptides have been loosely conserved and play an important role as a first line of immune defense. Peptides appear to be a major player in local innate immunity, especially at mucosal and epithelial surfaces, providing an early line of defense against infection (2).
Peptides of the cathelicidin family are synthesized as prepropeptides and are characterized by the conserved amino-terminal sequence of the peptide pro-piece and the variable carboxy terminus (37, 38). The pro-sequence is termed “cathelin,” because this domain inhibits the activity of the first member of the cathelicidin family, cathepsin L (cathepsin L inhibitor). Molecules with a cathelin-like propeptide sequence have been isolated from multiple species including humans, monkeys, horses, cows, sheep, pigs, rabbits, and mice (35). The cathelin pro-sequence has been proposed to be involved in protecting the peptide from proteolysis during synthesis and trafficking of the peptide and/or mediating trafficking to the appropriate cellular compartment (36). The human cathelicidin hCAP18 was first cloned from cDNA isolated from human bone marrow (1). LL-37 is a proteolytically processed form of hCAP18 that is released upon stimulation of cells and is cleaved extracellularly by proteinase-3 (26). LL-37 is not only a major protein in the large granules of human neutrophils (24) but is also produced by epithelial cells, including those in the squamous epithelium (12) and lung (4), and by the epidermis and is up-regulated in response to inflammatory stimuli (11). It can be found at unstimulated mucosal surfaces at concentrations of around 2 μg/ml, and at concentrations exceeding 50 μg/ml in inflamed epithelium (4). In addition, plasma has been reported to contain hCAP18 bound to lipoproteins at a concentration of 1.2 μg/ml (25). Thus, LL-37 is an important component of both the phagocyte and epithelial defense systems in humans and has a number of activities related to its role in the immune response.
It has been demonstrated that LL-37 stimulates the expression of a wide variety of genes involved in the innate immune response, including those encoding chemokines (i.e., interleukin-8 [IL-8] and monocyte chemoattractant protein 1 [MCP-1]), differentiation factors, and anti-inflammatory cytokines (i.e., IL-10) (23). LL-37 has also been reported to be directly chemotactic for human neutrophils, monocytes, and T cells through formyl peptide receptor like-1 (FPRL-1, a Gi protein-coupled receptor) (34) and is also chemotactic for human mast cells using two different receptors, a high-affinity (dissociation constant [Kd], 2.3 μM) receptor that is not a Gi protein-coupled receptor and a low-affinity, Gi protein-coupled receptor (Kd, 112 μM), but neither of these are the FPRL-1 receptor. LL-37 has also been found to induce IL-8 production through phosphorylation and activation of the mitogen-activated protein kinases ERK-1 and -2 and p38 in human peripheral blood-derived monocytes and human bronchial epithelial cell lines, but not in B or T lymphocytes, independently of the FPRL-1 receptor (6, 30). Indeed, it was found that LL-37-induced cell signaling in a human epithelial cell line was mediated through the epidermal growth factor receptor (30). Thus, LL-37 clearly has the ability to utilize several different receptors. LL-37 also has a variety of other functions in immunity, including promotion of mast cell histamine release (19), stimulation of wound healing (15), angiogenesis (16), and modulation of dendritic cell differentiation (9).
LL-37 has been described as an antimicrobial peptide, but its activity against most bacteria is quite modest (32, 36) and may be confined to body compartments where the concentration is relatively high or the ionic strength and divalent cation concentrations are reasonably low. Nevertheless, despite its modest antibacterial activity (13), exogenously added LL-37 protects mice against Staphylococcus aureus infections (M. G. Scott and R. E. W. Hancock, unpublished data), whereas transgenic overexpression of LL-37 in mouse airways results in decreased bacterial load and mortality following challenge with either Pseudomonas aeruginosa or Escherichia coli (3). This may reflect the ability of LL-37 to boost mechanisms of innate immunity. In addition, the induction of anti-inflammatory gene products (23) has an in vivo corollary in the ability of LL-37 to demonstrate potent protection against bacterial endotoxin (lipopolysaccharide) in animal models (3, 23).
Although the effects of LL-37 on eukaryotic cells have been studied extensively, the mechanism of how LL-37 interacts with eukaryotic cells is not well understood. The aim of this study was to characterize the interaction of LL-37 with a lung epithelial cell line. By using confocal microscopy with biotinylated LL-37, in conjunction with specific inhibitors, we were able to shed light on the mechanism of uptake and localization of LL-37. Our results show that this is an active process and that LL-37 becomes localized to the perinuclear region of lung epithelia. Binding assays also reveal that there are high- and low-affinity receptors; the low-affinity receptor appears to be FPRL-1.
MATERIALS AND METHODS
Peptide synthesis.
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) and LL-37C (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTESC) were synthesized at the Nucleic Acid/Protein Synthesis Unit at the University of British Columbia (UBC) by N-(9-fluorenyl)methoxy carbonyl (Fmoc) chemistry using an Applied Biosystems model 431 peptide synthesizer.
Biotinylation of LL-37.
LL-37C was biotinylated at the C-terminal cysteine side chain with N-α-(3-maleimidylpropionyl) biocytin (Molecular Probes, Eugene, Oreg.). LL-37C (2 mmol) and N-α-(3-maleimidylpropionyl) biocytin (23 mmol) were dissolved separately (430 and 1 mM, respectively) in 50 mM Tris buffer (pH 7) and mixed. The mixture was reacted by shaking for 2 h at room temperature. Excess N-α-(3-maleimidylpropionyl) biocytin was quenched by using 2-mercaptoethanol (Bio-Rad, Montreal, Quebec, Canada). Biotinylated LL-37 (LL-37B) was purified by using a reversed-phase fast-performance liquid chromatography column (Resource 15RPC; 3 ml; Pharmacia, Piscataway, N.J.) with a water-acetonitrile gradient containing 0.1% trifluoroacetic acid. The yield of LL37B after purification was 66% as determined by amino acid analysis. Peptide purity was confirmed by high-performance liquid chromatography and matrix-assisted laser desorption ionization—time-of-flight (MALDI-TOF) mass spectroscopy.
Liposome preparation.
A chloroform solution of either 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) (both from Avanti Polar Lipids Inc., Alabaster, Ala.), or an equimolar mixture of both lipids was dried under a stream of N2. Residual solvent was removed by desiccation under a vacuum for 2 h. The resulting lipid film was rehydrated in 10 mM phosphate buffer (pH 7.0). The suspension was put through five freeze-thaw cycles to produce multilamellar liposomes, followed by extrusion through 0.1-μm-pore-size double-stacked Poretics filters (AMD Manufacturing Inc., Mississauga, Ontario, Canada) by using an extruder device (Lipex Biomembranes, Vancouver, British Columbia, Canada).
CD spectrometry.
Circular dichroism (CD) spectra were obtained by using a J-810 spectropolarimeter (Japan Spectroscopic Company, Tokyo, Japan). Each spectrum (190 to 250 nm) was the average of 10 scans using a quartz cell with a 1-mm path length at room temperature. The scanning speed was 50 nm/min, with a step size of 0.1 nm, a response time of 2 s, and a 1-nm bandwidth. Peptide concentrations utilized were 4.0 μM for LL-37 and 4.3 μM for LL-37B in 10 mM Tris buffer (pH 7.4). The concentration of lipid was 1 mM (POPC-POPG at a molar ratio of 1:1). Spectra were baseline corrected by subtracting a blank spectrum of a sample containing all components except the peptide. Ellipticities were converted to mean residue molar ellipticities (θ), expressed in degrees times square centimeters per decimole.
Cytokine production in A549 epithelial cells.
The human epithelial cell line A549 was obtained from the American Type Culture Collection (Manassas, Va.). The A549 epithelial cell line was maintained in complete Dulbecco's modified Eagle medium (DMEM; GIBCO Laboratories, Grand Island, N.Y.), which consists of DMEM supplemented with 10% heat-inactivated fetal bovine serum (Medicorp, Montreal, Quebec, Canada). A549 cells were seeded in 24-well plates at a density of 105 cells per well in complete DMEM and were incubated overnight at 37°C under 5% CO2. The medium was removed from cells grown overnight and was replaced with fresh complete DMEM. Cationic peptides were added, and cells were incubated for 24 h at 37°C under 5% CO2. The supernatant was removed and quantified for IL-8 by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's directions (Biosource, Montreal, Quebec, Canada).
Binding assays.
A549 cells were placed in 96-well plates in complete DMEM, at a concentration of 104 cells/well, and incubated overnight at 37°C under 5% CO2. The medium was then removed from the cells, and to block uptake, the cells were treated with brefeldin A (5 μg/ml in complete DMEM) or equilibrated at 4°C for 1 h prior to the addition of LL-37B. For competition studies, the FPRL-1 agonist peptide WKYMVM (W-peptide; a generous gift from Claes Dahlgren, Phagocyte Research Laboratories, Department of Rheumatology and Inflammation Research, University of Goteborg, Goteborg, Sweden) was added at the same time as LL-37B. Cells were incubated with LL-37B (and W-peptide for the competition studies) for 15 min at 37°C. Following incubation, the cells were gently washed with phosphate-buffered saline (PBS) and incubated with streptavidin (1/200 dilution; R&D Systems, Minneapolis, Minn.) at 23°C for 60 min. Cells were washed extensively with PBS and were then incubated with 3,3′,5,5′-tetramethylbenzidine liquid substrate (Sigma) at room temperature for 30 min and protected from light. The plates were read with a Bio-Tek plate reader at an absorbance wavelength of 450 nm (correcting for background by subtracting the absorbance at 570 nm). The maximum specific binding (Bmax) and Kd were obtained by using nonlinear regression with GraphPad Prism (version 4.0; GraphPad.com, San Diego, Calif.).
RNA isolation.
A549 cells were placed in 150-mm-diameter tissue culture dishes at 106 cells/dish in complete DMEM and incubated overnight at 37°C under 5% CO2. DMEM was removed from cells grown overnight, fresh medium was added, and cells were then incubated with peptides for 4 h. After stimulation, cells were washed with PBS. Total RNA was isolated according to the manufacturer's directions by using RNAqueous (Ambion, Austin, Tex.). RNase inhibitor (Ambion) was added to the RNA sample to prevent RNA degradation. To remove contaminating genomic DNA, the RNA sample was incubated with DNase I (Ambion) for 30 min at 37°C. DNase I was subsequently inactivated by using DNase inactivation reagent (Ambion). RNA quality and quantity were assessed by using a Bioanalyzer (Agilent, Palo Alto, Calif.).
Microarrays.
Fourteen-thousand-human-oligonucleotide expression arrays, which were printed on glass slides by using the 70-mer library PRHU04 from QIAGEN (Venlo, The Netherlands; see www.operon.com for details of the library), were obtained from the Genome BC Array Facility (Vancouver Hospital, Vancouver, British Columbia, Canada). cDNA probes were prepared by reverse transcription from cellular RNA, labeled with either biotin or fluorescein from 2 μg of total RNA by using a QIAGEN LabelStar array kit labeling module according to the manufacturer's instructions, and cleaned up by using the cleanup module of the same kit. Microarray slides were subsequently hybridized by using the resonance light scattering (RLS) array detection system (Invitrogen/Genicon, San Diego, Calif.) according to the manufacturer's instructions. The array hybridization image was captured by using a GSD-501 RLS detection and imaging instrument (Invitrogen/Genicon). Hybridization signals were then quantified by using ArrayVision (version 8.0; Imaging Research Inc., St. Catherine's, Ontario, Canada) and analyzed by GeneSpring (Silicon Genetics, Redwood City, Calif.). The data were normalized by using the 50th-percentile parameter of GeneSpring. All results were derived from three independent experiments, each involving two technical replicates obtained through dye swapping. All experimental information and procedures were entered into a MIAME (minimum information about a microarray experiment)-compliant database and are available online at www.cmdr.ubc.ca/arraydata/.
LDH assay for cytotoxicity.
A549 cells were maintained and passaged as described above. The epithelial cells were seeded in 24-well plates at a density of 105 cells/well in complete DMEM and incubated at 37°C under 5% CO2 overnight. The medium was removed from the cells and replaced with fresh complete medium. Cells were treated with LL-37 at a range of concentrations (5 to 50 μg/ml) for 24 h. Two wells of cells were treated with 1% Triton X-100 as a positive control for maximum lactate dehydrogenase (LDH) release. After 24 h of incubation at 37°C under 5% CO2, the supernatants were removed and centrifuged at 4,500 × g for 5 min to remove contaminating cells, and the level of LDH was measured in duplicate by using a cytotoxicity detection kit (Roche, Mannheim, Germany) in a 96-well plate.
Antibodies.
Murine monoclonal anti-golgin 97 (α-golgin 97), used at a 1/200 dilution, was obtained from Molecular Probes; rabbit monoclonal α-calnexin, used at a 1/1,000 dilution, was obtained from Sigma; murine monoclonal α-LAMP-2, used at a 1/200 dilution, was obtained from the Developmental Studies Hybridoma Bank (Iowa City, Iowa); murine monoclonal α-nucleolin, used at a 1/200 dilution, was obtained from Molecular Probes; anti-LL-37 clone 3D11, used at a 1/200 dilution, was a generous gift from Pieter S. Hiemstra and G. Sandra Tjabringa, Department of Pulmonology, Leiden University Medical Center, Leiden, The Netherlands; α-cathepsin D, used at a 1/200 dilution, was obtained from Scripps Laboratories (San Diego, Calif.); and Alexa-conjugated secondary antibodies, used at a 1/200 dilution, were obtained from Molecular Probes.
Immunofluorescence and inhibition studies.
A549 cells were seeded onto 12-mm-diameter coverslips at a density of 105 cells per coverslip in complete DMEM (see above) and incubated overnight at 37°C under 5% CO2. The following day, the medium was removed from cells grown overnight and replaced with fresh complete DMEM. For experiments at low temperatures, cells were maintained for 15 min on ice before addition of peptide and were kept on ice throughout the experiment. Biocytin was used as a control for all conditions. To induce microtubule depolymerization, cells were treated with 2 μg of nocodazole/ml for 30 min prior to addition of LL-37B. Actin polymerization was blocked by using 2 μg of cytochalasin D (Sigma)/ml for 30 min prior to LL-37B addition. To inhibit Gi-coupled receptors, cells were treated with 100 ng of pertussis toxin (List Biological Laboratories Inc., Campbell, Calif.)/ml for 2 h prior to LL-37B addition. The W-peptide was added at 50 and 500 nM with LL-37B to compete for FPRL-1 receptor binding. Endocytosis was inhibited by treating A549 cells with 5 μg of brefeldin A (Sigma)/ml for 1 h prior to LL-37B addition. Biocytin (Sigma) was used as a negative control for all conditions.
LL-37B was incubated with cells for 30 min to 4 h at 4 and 37°C under 5% CO2. Following peptide incubation, coverslips were washed with PBS and fixed with 4% paraformaldehyde. The coverslips were washed extensively after fixing, and the cells were permeabilized by using 0.1% Triton X-100 in PBS and probed with streptavidin (Molecular Probes). Cells were washed extensively with PBS and then probed with biotin-Oregon green (Molecular Probes) to detect LL-37B and Alexa-conjugated phalloidin (Molecular Probes) to detect actin. LL-37 localization was confirmed by using unlabeled LL-37, which was detected by using α-LL-37 clone 3D11 (1/200 dilution) and an Alexa-conjugated secondary antibody (Molecular Probes). Coverslips were mounted in VectaShield with 4′,6′-diamidino-2-phenylindole (DAPI) to stain for host cell DNA (Vector Laboratories, Burlingame, Calif.). Coverslips were viewed by using a Bio-Rad Radiance confocal microscope.
Colocalization studies with endocytic markers.
A549 cells were prepared as described above for immunofluorescence. Following fixation and extensive washing with PBS, cells were permeabilized with 0.2% saponin in PBS and then probed with antibodies specific for components of the endocytic transport mechanism along with streptavidin (Molecular Probes). Coverslips were washed extensively with 0.2% saponin in PBS and probed with Alexa-conjugated antibodies and biotin-Oregon green (Molecular Probes). Coverslips were mounted in VectaShield with DAPI to stain for host cell DNA (Vector Laboratories) and were viewed by using a Bio-Rad Radiance confocal microscope.
Statistical analysis.
Results are generally expressed as means ± standard errors from three independent experiments. The paired Student t test was used to test for significance.
RESULTS
Biotinylated LL-37 and LL-37 have similar structural and biological properties.
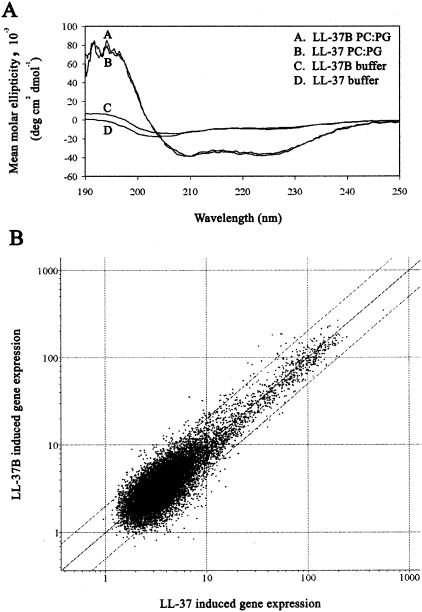
CD spectroscopy was used to compare the secondary structures of LL-37 and LL-37B in aqueous solution and in the presence of large unilamellar POPC-POPG (1:1) vesicles. The CD spectra are shown in Fig. 1A. Both LL-37 and LL-37B were largely unstructured in free solution, as indicated by the minima near 205 nm. Upon addition of lipid vesicles, both peptides adopted an α-helical structure, as indicated by the two minima at 210 and 222 nm and the maxima at 195 nm. The similarity of the secondary structures of LL-37 and LL-37B both in the absence and in the presence of a membrane environment indicated that the structure and membrane-binding properties of LL-37 were not affected by biotinylation. LL-37 and its biotinylated counterpart were also tested in certain biological assays to confirm their functional similarity. A549 cells were exposed to either LL-37 or LL-37B at 10 μg/ml for 4 h, and RNA was isolated, converted to labeled cDNA, and used to probe microarrays for patterns of gene expression essentially as described previously (23). Of the 14,000 genes for which expression levels were tested, only 14 genes had expression levels that differed more than fourfold between the two peptides (Fig. 1B). Because this was a control experiment demonstrating that the patterns of gene expression were very similar for the two peptides, and LL-37-induced gene expression has been reported previously (23), this data set has been made available only through the Internet at www.cmdr.ubc.ca/arraydata/. LL-37 has previously been demonstrated to induce production of IL-8, a potent neutrophil chemokine, in lung epithelial cells (23, 33). IL-8 production in A549 cells in response to treatment by LL-37 or LL-37B at a range of concentrations was studied by ELISA. Production of IL-8 increased with increasing LL-37 and LL-37B stimulation at concentrations between 5 and 50 μg/ml: levels of IL-8 were 53 ± 8.9, 95 ± 12.3, 123 ± 9.1, and 295 ± 14.2 μg/ml at LL-37 or LL-37B concentrations of 5, 10, 25, and 50 μg/ml, respectively. No significant differences were observed between the two peptides (P > 0.05) (data not shown).
FIG. 1.
Structural and biological properties of LL-37 and LL-37B. (A) CD spectra of LL-37 and LL-37B. LL-37 (4 μM) and LL-37B (4.3 μM) were incubated in 10 mM Tris-HCl buffer (pH 7.4) in the presence and absence of 1 mM POPC-POPG (1:1) liposomes. PC, POPC; PG, POPG. (B) A549 cells were incubated with LL-37 or LL-37B for 4 h, the RNA was harvested, and total RNA was used to make labeled cDNA probes (biotin or fluorescein), which were hybridized onto microarrays spotted with 14,000 human genes in duplicate and labeled as described in Materials and Methods. RLS images were quantified, the densities were normalized, and the average intensities for LL-37 were plotted against those of LL-37B on a log scale. The plot represents averages of three biological replicates and two technical replicates for each biological replicate.
It has been well documented that LL-37 can be cytotoxic to certain eukaryotic cell lines (20), although we recently published the finding that LL-37 is not cytotoxic to human primary monocytes at the concentrations used in this study (6). LDH release experiments were done to ensure that the level of cytotoxicity exhibited by LL-37 toward A549 cells was known. There were no significant differences (P > 0.05) in LDH release between LL-37 and LL-37B. The level of LDH release in response to 10 μg of LL-37 or LL-37B/ml was less than 3%, not statistically significantly different from that of control cells not treated with peptide. At the highest concentrations studied here (50 μg/ml), we observed 35 to 44% LDH release from the A549 epithelial cells.
Uptake of LL-37 into cells is an active process resulting in localization to the perinuclear region.
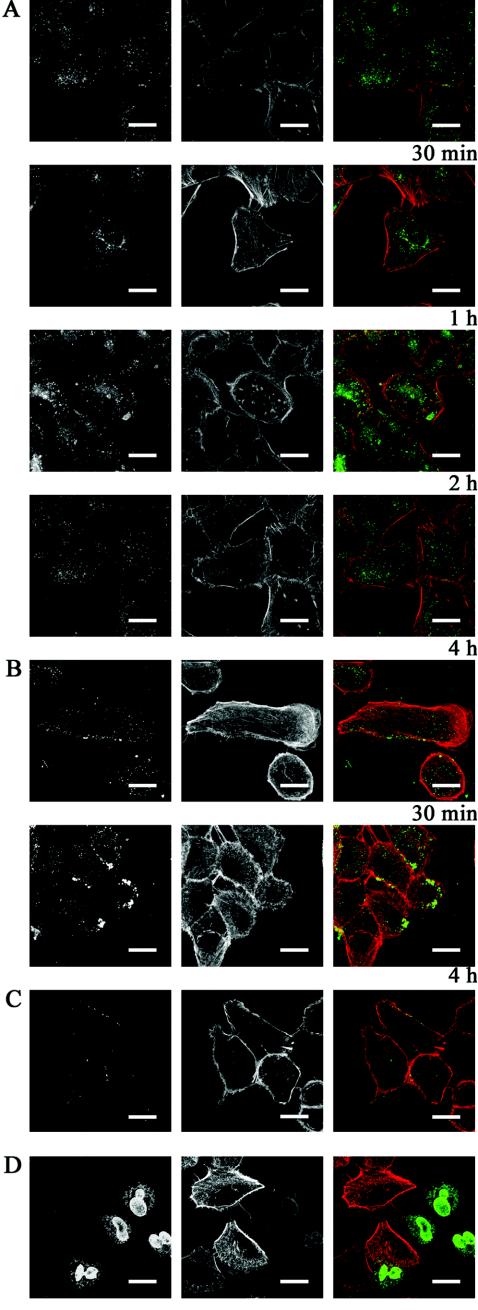
The human lung epithelial cell line A549 was incubated with 10 μg of LL-37B/ml at 37°C. LL-37B was taken up into the cells and at 30 min was already detected in small circular structures reminiscent of vesicles throughout the cytosol (Fig. 2A). By 1 h, most of these vesicles had congregated around the perinuclear region. At 4 h, the majority of the peptide was localized around the perinuclear region, with some peptide accumulating around the nuclear membrane (Fig. 2A). Similar observations were made by using unlabeled LL-37, visualized with specific antibody (Fig. 2B), although in this case, because labeling was direct, the signal strength was somewhat lower. To determine whether LL-37 was taken up into cells through an active or a passive mechanism, A549 cells were incubated with 10 μg of LL-37B/ml at 4°C. Under these conditions, LL-37B bound to and colocalized with actin at the edge of the cell after 4 h (Fig. 2C), indicating that uptake into cells was temperature dependent, as would be expected if uptake was dependent on receptors.
FIG. 2.
Localization of LL-37 in A549 epithelial cells. A549 cells were incubated with (A) 10 μg of LL-37B/ml (2.2 μM) for 30 min, 1 h, 2 h, and 4 h at 37°C, (B) 10 μg of LL-37/ml (2.2 μM) for 30 min and 4 h at 37°C to confirm uptake and localization of LL-37, (C) 10 μg of LL-37B/ml (2.2 μM) for 4 h at 4°C, or (D) 50 μg of LL-37B/ml (11 μM) for 4 h. Immunohistochemical analysis was performed with the appropriate antibody to detect LL-37B or LL-37. Detection of LL-37B or LL-37 (left panels) and detection of F-actin (center panels) are shown merged in the panels on the right (green, LL-37B; red, F-actin). Bars, 20 μm.
At a higher, somewhat cytotoxic concentration of LL-37B (50 μg/ml), the peptide was localized to the nucleus and around the nuclear membrane after 4 h (Fig. 2D), although colocalization studies demonstrated that the peptide was excluded from the nucleoli (data not shown).
Uptake of LL-37 incorporates elements of endocytosis, and LL-37 trafficking is mediated by microtubules.
Three separate experiments were performed to determine whether LL-37B was entering cells through an endocytotic pathway. The effects of specific inhibitors on intracellular accumulation and localization were investigated.
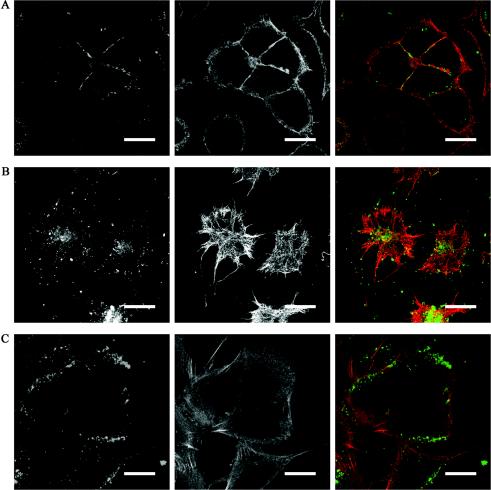
Brefeldin A is a fungal metabolite that is widely utilized as an endocytosis inhibitor but has been reported to have multiple effects, dependent on cell type and species (10). This compound targets the ADP-ribosylation factor GTPase exchange factor in the secretory pathway, leading to the blockage of protein transport from the endoplasmic reticulum to the Golgi apparatus. It also affects endocytosis and related events such as vesicular assembly and secretion and antigen presentation. Control confocal microscopy experiments were performed to confirm that, after 1 h of treatment, brefeldin A caused the characteristic disappearance of the Golgi apparatus in A549 cells (data not shown). Cells were pretreated with brefeldin A for 1 h prior to addition of 10 μg of LL-37B/ml. In brefeldin A-treated cells after 4 h, there was no intracellular accumulation of LL-37B, and the majority of the peptide was seen on the outside of the cell (Fig. 3A), consistent with the requirement for functional endocytic pathways for the uptake of LL-37.
FIG. 3.
Uptake and trafficking of LL-37 in A549 epithelial cells. A549 cells were incubated with either (A) brefeldin A, to inhibit endocytosis, (B) cytochalasin D, to disrupt actin polymerization, or (C) nocodazole, a microtubule-disrupting agent, prior to incubation with 10 μg of LL-37B/ml (2.2 μM) for 4 h. Cells were prepared for immunofluorescence as described in Materials and Methods. Right panels represent a merger of LL-37 (from left panels) (green) and actin (A, C) or microtubules (B) (from center panels) (red). Bars, 20 μm.
Conversely, A549 cells were pretreated for 30 min with cytochalasin D, which binds G-actin and prevents the polymerization of actin monomers, prior to addition of 10 μg of LL-37B/ml. There was no observable difference in the uptake and accumulation of LL-37B after 4 h (Fig. 3B), demonstrating that uptake and trafficking of LL-37 did not require the polymerization of actin. However, when cells were treated with nocodazole, which depolymerizes microtubules, no accumulation of peptides in the perinuclear region occurred (Fig. 3C). Under these conditions, after 4 h, LL-37B was retained close to the periphery of the cell, rather than migrating to a perinuclear location as was observed with control cells. These results are consistent with the conclusion that the trafficking of LL-37B from the cell periphery to the perinuclear region was mediated by microtubules. Colocalization studies were also performed with endocytic markers that included LAMP-2, golgin-97, nucleolin, calnexin, and cathepsin D at 15 and 30 min and at 1, 2, and 4 h, but no colocalization was observed (data not shown).
Uptake of LL-37 is not mediated by Gi protein-coupled receptors.
LL-37 was recently proposed to be chemotactic for macrophages, neutrophils, endothelial cells (via FPRL-1), and mast cells (by different Gi protein-coupled receptors) (18, 34), although other events, including induction of the chemokine IL-8, have been shown to be independent of the Gi protein-coupled receptor FPRL-1 in human monocytes and epithelial cell lines (16). It has been previously reported that epithelial cells express FPRL-1 (5, 17), and indeed FPRL-1 expression at the mRNA level was confirmed by reverse transcription-PCR in the A549 epithelial cell line (data not shown). To determine if the uptake of LL-37B was mediated by a Gi protein-coupled receptor, such as FPRL-1, cells were incubated with pertussis toxin (100 ng/ml) for 2 h prior to addition of 10 μg of LL-37B/ml in order to inactivate all Gi protein-coupled receptors. LL-37B uptake and localization to the perinuclear region were unaffected at 4 h (data not shown). In addition, the FPRL-1 hexapeptide agonist WKYMVM (8) was investigated as a potential competitive inhibitor. LL-37 was still able to enter and traffic to the perinuclear region of the cell in the presence of this agonist at 50 and 500 nM (data not shown), results comparable to the Kd of 160 nM for agonist binding to FPRL-1.
LL-37B is bound by two receptors, and the low-affinity receptor appears to be FPRL-1.
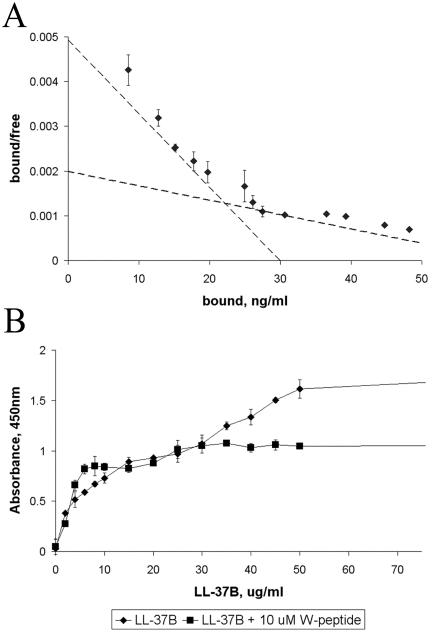
To determine whether A549 epithelial cells have a specific receptor(s) for LL-37, binding assays were performed on cells that had been treated with 5 μg of brefeldin A/ml to inhibit LL-37B uptake. Nonlinear regression analysis indicated that LL-37 is able to bind to two classes of receptors, a higher-affinity receptor with a Kd of 0.76 ± 0.21 μM (3.90 μg/ml) and a lower-affinity receptor with a Kd of 2.46 ± 0.29 μM (12.6 μg/ml) (Fig. 4A). When the binding kinetics experiment was repeated using a low temperature (4°C) instead of brefeldin A to inhibit uptake, the Kd values obtained were within 30% of each other. Competitive binding assays using a 60-fold molar excess of the FPRL-1 agonist W-peptide showed that the binding of LL-37B to the low-affinity receptor was inhibited by the presence of W-peptide, favoring the conclusion that FPRL-1 was the low-affinity receptor (Fig. 4B). The Kd values were somewhat lower (i.e., affinity was higher) than the estimated values of 2.2 and 123 μM based on radioactive analysis of mast cell receptors (12). However, W-peptide was unable to block binding to either receptor, consistent with the idea that this receptor was absent from mast cells.
FIG. 4.
Kinetics of binding of LL-37B to A549 epithelial cells. (A) Binding of LL-37B to A549 epithelial cells displayed by a Scatchard plot. A549 cells were preincubated with brefeldin A (5 μg/ml) for 1 h prior to addition of 2 to 1,000 μg of LL-37B/ml for 15 min at 37°C. Kd values were obtained by nonlinear regression analysis. (B) Competitive binding studies with the FPRL-1 agonist W-peptide. A549 cells were preincubated with brefeldin A (5 μg/ml) for 1 h prior to addition of 2 to 1,000 μg of LL-37B/ml alone (♦) or with 10 μM W-peptide (▪) for 15 min at 37°C. Streptavidin conjugated to horseradish peroxidase and tetramethylbenzidine were added sequentially to visualize binding. Each point represents the mean of three individual experiments ± standard error; each point was assayed in duplicate.
DISCUSSION
The mechanism of interaction of cationic host defense peptides with human cells has not been well characterized to date. There have been a number of studies looking at the uptake by eukaryotic cells of several peptides belonging to different structural classes (7, 10, 27-29). In this study, we examined the interaction of the human cathelicidin peptide LL-37 with the human lung epithelial cell line A549. Using LL-37B together with confocal microscopy, we were able to visualize the uptake of this peptide by epithelial cells.
It was important to demonstrate that the labeling of LL-37 with biotin did not alter the structural or biological behavior of this peptide. LL-37B and LL-37 adopted similar α-helical secondary structures upon membrane binding as assessed by CD spectrometry that were consistent with findings of published Fourier-transform infrared spectroscopy studies for LL-37 (20). Also, similar patterns of A549 cell gene expression were observed in response to LL-37 and LL-37B, with only 14 of 14,000 genes differing more than fourfold in expression levels. In addition, the two peptides stimulated similar levels of IL-8 expression and LDH release. These results indicate that LL-37B provides an excellent model for visualizing the interactions between epithelial cells and LL-37.
The images presented here demonstrate that, in the presence of fetal bovine serum, LL-37B is actively taken up into A549 cells. Over a period of 4 h, at concentrations that are achievable in vivo, the peptide first became rapidly dispersed throughout the cytoplasm in small vesicles and then trafficked to the perinuclear region. The localization of LL-37B to the perinuclear region was confirmed by using unlabeled LL-37, which was visualized with specific antibodies. The vesicular structures seen at all time points were suggestive of an endocytic process by which LL-37 was transported into the cell. Similar observations have been made with SynB3 and SynB5, synthetic linear analogues of the β-hairpin structured peptide protegrin-1 (10). By using the K562 cell line and TAMRA-labeled peptide, it was demonstrated that uptake of SynB3 and SynB5 was inhibited at low temperatures, but at 37°C these peptides became localized to the perinuclear region in a punctate pattern consistent with, but not proving, a location within endocytic vesicles (10). It was recently shown that LL-37 can bind extracellular DNA, that the LL-37-DNA complex is taken up through caveola-independent membrane raft endocytosis and cell surface proteoglycans, and that the resulting complex is found in vesicular structures at the cell membrane and throughout the cytoplasm (22). Conversely, PR-39, a proline-arginine-rich peptide, enters eukaryotic cells and localizes throughout the cytoplasm, binding to several cytoplasmic proteins (7).
Since cationic host defense peptides are reminiscent of peptides with nuclear localization signals, we had anticipated that LL-37 would migrate to the nucleus, something that was observed in these studies only when higher, somewhat toxic concentrations were used. The observation of nuclear localization can indeed be an artifact, as was, for example, demonstrated for the cationic arginine-rich Tat peptide from human immunodeficiency virus, which had been shown to localize to the nucleus via protein transduction (28). Recently it has been shown that cell fixation promotes penetration of the nucleus by Tat due to the strong cationic nature of this peptide (10, 21). At 37°C, such cell-penetrating peptides, including Tat, apparently are not localized to the nucleus, as previously proposed, but instead are distributed throughout the cytoplasm in a punctate pattern in epithelial cells (21). As observed here for LL-37, endocytosis is decreased in cells at low temperatures, and at 4°C there was no intracellular accumulation of peptide; the majority of the peptide was seen around the peripheries of cells.
At higher concentrations, LL-37B was taken up into the nuclei of cells, and it was observed that in these cells, staining of F-actin by phalloidin was lost, presumably reflecting the binding of LL-37 to F-actin (31). At lower concentrations, when cells were not permeabilized for immunohistochemical analysis, cells treated with LL-37 still stained for actin (Fig. 2). This indicates that LL-37 itself is able to permeabilize the eukaryotic membrane. Similarly, it has been demonstrated that Tat can permeate cell membranes without disturbing membrane integrity (27, 28). In contrast, the host defense peptide PR-39 has been shown to enter mesenchymal cells without permeabilizing the plasma membrane (7).
Specific inhibitors were utilized to explore the possibility that LL-37 was taken up by an endocytic process. Uptake of LL-37 was inhibited when endocytosis was disrupted with brefeldin A; however, disruption of actin polymerization by cytochalasin D did not block the uptake and perinuclear localization of the peptide. Thus, although actin is not required for uptake of LL-37, it seems possible that other elements of endocytosis are required. LL-37 trafficking to the perinuclear region was dependent on an intact network of microtubules, since the microtubule-disrupting agent nocodazole prevented cellular accumulation of the peptide. Cellular markers involved in endocytic transport such as LAMP-1, golgin-97, calnexin, and cathepsin D were also examined but did not colocalize with LL-37B at the time points studied. Since LL-37 is a highly charged molecule, it is possible that this alters the endosomal/lysosomal compartment and therefore prevents the association of some of the markers normally involved in endocytosis. Alternatively, LL-37 may be colocalizing with components of the endocytic pathway at time points different from those used in this study. In contrast to our LL-37 observations, it has been suggested that, once internalized, linearized protegrin-1 peptides follow a conventional endocytic pathway toward late endosomes and lysosomes (10). LL-37-DNA complexes have been shown to colocalize with a marker for lipid rafts, cholera toxin subunit B, in vesicular structures, but no colocalization was seen with caveolin, a well-established marker of caveolae at the site of endocytosis (22).
It is worth speculating on the significance of the pattern of uptake and localization observed. L-37 has been shown to induce the production of IL-8, a neutrophil-specific chemokine, in whole human blood (23) as well as in cultured epithelial cell lines (23, 33). Therefore, correlations were sought between the process of uptake and this functional end point. Using real-time reverse transcription-PCR, we were able to confirm the 2.5-fold up-regulation of IL-8 (data not shown) in response to LL-37. Using brefeldin A to inhibit the uptake and localization of LL-37, we were able to demonstrate that IL-8 levels remained unchanged relative to those for the control (P > 0.05). This is consistent with the conclusion that IL-8 expression was dependent on the localization of LL-37 to the perinuclear region within epithelial cells, although this conclusion is somewhat weakened by the known pleiotropic nature of brefeldin A.
Since LL-37 is internalized into epithelial cells, we wanted to characterize the binding of LL-37 to the surfaces of cells that would precede uptake. Binding of LL-37B to A549 epithelial cells was demonstrated to involve two receptors, and the low-affinity binding receptor appeared to be FPRL-1. These observations are consistent with other studies of LL-37 interaction with cells indicating multiple receptors on the cell surface (6, 18, 30, 34). The binding was concentration dependent and saturable. Although binding experiments are indeed often performed for 1 to 2 h at 4°C, we determined in preliminary experiments that equilibrium binding was achieved in less than 15 min; thus, all subsequent binding studies assessed binding after 15 min. Receptor densities were estimated at more than 107 binding sites/cell. However, we consider these numbers to be overestimates caused by the requirement for us to use an amplification step to assess LL-37B, as well as by quenching of fluorescence due to the presence of cells.
We were able to demonstrate that the FPRL-1 agonist WKYMVM inhibited the binding of LL-37B to the low-affinity receptor, favoring the concept that the Gi protein-coupled receptor FPRL-1 is this low-affinity receptor. Nevertheless, we have demonstrated that pertussis toxin does not affect uptake or localization, and in agreement with this finding, it has been shown previously that FPRL-1 is not a functional receptor for mast cell chemotaxis (18) or for monocyte or epithelial cell signaling (6, 30). Therefore, since uptake of LL-37B was not affected by competition with the FPRL-1 agonist, it seems likely that the high-affinity receptor is responsible for uptake. Current unresolved questions include the nature of the LL-37 high-affinity receptor and the impact of different serum components on uptake.
Acknowledgments
Financial assistance was received from the Canadian Bacterial Diseases Network and the Functional Pathogenomics of Mucosal Immunity (FPMI) program grant funded by Genome Prairie and Genome BC, with additional assistance from Inimex Pharmaceuticals Inc. Y.E.L. is the recipient of a Canadian Cystic Fibrosis Foundation studentship, R.E.W.H. holds a Canada Research chair, M.G.S. and A.R. hold NSERC Industrial postdoctoral fellowships, and D.J.D. holds a Canadian Cystic Fibrosis Foundation fellowship.
We thank Pieter S. Hiemstra and G. Sandra Tjabringa for providing the LL-37 antibody, Brett Finlay for providing the antibodies used in colocalization studies, Claes Dahlgren for providing the W-peptide, and Johan Bylund, Silvana Doria, Mary Kinloch, Lin Hu, and Brian Calvert for technical assistance. We also acknowledge the assistance of Marc Rousseau and Byron Kuo in the interpretation of the microarray data.
Editor: V. J. DiRita
REFERENCES
- 1.Agerberth, B., H. Gunne, J. Odeberg, P. Kogner, H. G. Boman, and G. H. Gudmundsson. 1995. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 92:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals, R. 2000. Epithelial antimicrobial peptide in host defense against infection. Respir. Res. 1:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., D. J. Weiner, A. D. Moscioni, R. L. Meegalla, and J. M. Wilson. 1999. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67:6084-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnans, C., B. Mainprice, P. Chanez, J. Bousquet, and V. Urbach. 2003. Lipoxin A4 stimulates a cytosolic Ca2+increase in human bronchial epithelium. J. Biol. Chem. 278:10879-10884. [DOI] [PubMed] [Google Scholar]
- 6.Bowdish, D. M. E., D. J. Davidson, D. P. Speert, and R. E. W. Hancock. 2003. The human cationic peptide LL-37 induces activation of the extracellular signal regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 172:3758-3765. [DOI] [PubMed] [Google Scholar]
- 7.Chan, Y. R., and R. L. Gallo. 1998. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130Cas*. J. Biol. Chem. 273:28978-28985. [DOI] [PubMed] [Google Scholar]
- 8.Dahlgren, C., T. Christophe, F. Boulay, P. N. Madianos, M. J. Rabiet, and A. Karlsson. 2000. The synthetic chemoattractant Trp-Lys-Try-Met-Val-DMet activated neutrophils preferentially through the lipoxin A4 receptor. Blood 95:1810-1818. [PubMed] [Google Scholar]
- 9.Davidson, D. J., A. J. Currie, G. S. D. Reid, D. M. E. Bowdish, K. L. MacDonald, R. C. Ma, R. E. W. Hancock, and D. P. Speert. 2004. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 172:1146-1156. [DOI] [PubMed] [Google Scholar]
- 10.Drin, G., S. Cottin, E. Blanc, A. R. Rees, and J. Temsamani. 2003. Studies on the internalization mechanism of cationic cell-penetrating peptides. J. Biol. Chem. 278:31192-31201. [DOI] [PubMed] [Google Scholar]
- 11.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 12.Frohm Nilsson, M., B. Sandstedt, O. Sorensen, G. Weber, N. Borregaard, and M. Stahle-Backdahl. 1999. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 67:2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudmundsson, G. H., B. Agerberth, J. Odeberg, T. Bergman, B. Olsson, and R. Salcedo. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238:325-332. [DOI] [PubMed] [Google Scholar]
- 14.Hancock, R. E. W., and T. J. Falla. 1996. Antimicrobial peptides: broad-spectrum antibiotics from nature. Clin. Microbiol. Infect. 1:226-229. [DOI] [PubMed] [Google Scholar]
- 15.Heilborn, J. D., M. F. Nilsson, G. Kratz, G. Weber, O. Sorensen, N. Borregaard, and M. Stahle-Backdahl. 2003. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Investig. Dermatol. 120:379-389. [DOI] [PubMed] [Google Scholar]
- 16.Koczulla, R., G. von Degenfeld, C. Kupatt, F. Krotz, S. Zahler, T. Gloe, K. Issbrucker, P. Unterberger, M. Zaiou, C. Lebherz, A. Karl, P. Raake, A. Pfosser, P. Boekstegers, U. Welsch, P. S. Hiemstra, C. Vogelmeier, R. L. Gallo, M. Clauss, and R. Bals. 2003. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 111:1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le, Y., J. J. Oppenheim, and J. M. Wang. 2001. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 12:91-105. [DOI] [PubMed] [Google Scholar]
- 18.Niyonsaba, F., K. Iwabuchi, A. Someya, M. Hirata, H. Matsuda, H. Ogawa, and I. Nagaoka. 2002. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyonsaba, F., A. Someya, M. Hirata, H. Ogawa, and I. Nagaoka. 2001. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur. J. Immunol. 31:1066-1075. [DOI] [PubMed] [Google Scholar]
- 20.Oren, Z., J. C. Lerman, G. H. Gudmundsson, B. Agergerth, and Y. Shai. 1999. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 341:501-513. [PMC free article] [PubMed] [Google Scholar]
- 21.Richard, J. P., K. Melikov, E. Vives, C. Ramos, B. Verbeure, M. J. Gait, L. V. Chernomordik, and B. Lebleu. 2003. Cell penetrating peptides: a reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 278:585-590. [DOI] [PubMed] [Google Scholar]
- 22.Sandgren, S., A. Wittrup, F. Cheng, M. Jonsson, E. Eklund, S. Busch, and M. Belting. 2004. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J. Biol. Chem. 279:17951-17956. (First published 11 February 2004; doi:10.1074/jbc.M311440200.) [DOI] [PubMed] [Google Scholar]
- 23.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. W. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen, O., K. Arnligots, J. B. Cowland, D. F. Bainton, and N. Borregaard. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 25.Sorensen, O., T. Bratt, A. H. Johnsen, M. T. Madsen, and N. Borregaard. 1999. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J. Biol. Chem. 274:22445-22451. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen, O. E., P. Follin, A. H. Johnsen, J. Calafat, G. S. Tjabringa, P. S. Hiemstra, and N. Borregaard. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951-3959. [DOI] [PubMed] [Google Scholar]
- 27.Stauber, R. H., and G. N. Pavlakis. 1998. Intracellular trafficking and interaction of the HIV-1 Tat protein. Virology 252:126-136. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, T., S. Futaki, M. Niwa, S. Tanaka, K. Ueda, and Y. Sugirua. 2002. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 277:2437-2443. [DOI] [PubMed] [Google Scholar]
- 29.Takeshima, K., A. Chikushi, K.-K. Lee, S. Yonehara, and K. Matsuzaki. 2003. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J. Biol. Chem. 278:1310-1315. [DOI] [PubMed] [Google Scholar]
- 30.Tjabringa, G. S., J. Aarbiou, D. K. Ninaber, J. W. Drijfhout, O. E. Sorensen, N. Borregaard, K. F. Rabe, and P. S. Hiemstra. 2003. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 171:6690-6696. [DOI] [PubMed] [Google Scholar]
- 31.Weiner, D. J., R. Bucki, and P. A. Janmey. 2002. The antimicrobial activity of the cathelicidin LL-37 is inhibited by F-actin bundles and restored by gelsolin. Am. J. Respir. Cell Mol. Biol. 28:738-745. [DOI] [PubMed] [Google Scholar]
- 32.Yan, H., and R. E. W. Hancock. 2001. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, D., O. Chertov, and J. J. Oppenheim. 2001. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J. Leukoc. Biol. 69:691-697. [PubMed] [Google Scholar]
- 34.Yang, D., Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaiou, M., and R. L. Gallo. 2002. Cathelicidins, essential gene-encoded mammalian antibiotics. J. Mol. Med. 80:549-561. [DOI] [PubMed] [Google Scholar]
- 36.Zaiou, M., V. Nizet, and R. L. Gallo. 2003. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Investig. Dermatol. 120:810-816. [DOI] [PubMed] [Google Scholar]
- 37.Zanetti, M., R. Gennaro, and D. Romeo. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1-5. [DOI] [PubMed] [Google Scholar]
- 38.Zanetti, M., R. Gennaro, M. Scocchi, and B. Skerlavaj. 2000. Structure and biology of cathelicidins. Adv. Exp. Med. Biol. 479:203-218. [DOI] [PubMed] [Google Scholar]