Abstract
Beta2-toxin, encoded by cpb2, is implicated in the pathogenesis of Clostridium perfringens enteritis. However, cpb2 genes from nonporcine C. perfringens isolates were not always expressed, at least in vitro. Nucleotide sequencing identified atypical cpb2 genes with 70.2 to 70.7% DNA identity to previously identified (consensus) cpb2. Atypical beta2-toxin displayed 62.3% identity and 80.4% similarity to consensus beta2-toxin. No porcine type C isolates (n = 16) and only 3.3% of porcine type A isolates (n = 60) carried atypical cpb2 genes. However, 88.5% of nonporcine isolates carried atypical cpb2 (n = 78), but beta2-toxin was not expressed. Almost half of the nonporcine consensus cpb2 genes (44.4%) carried a frameshift mutation (n = 9), resulting in an absence of beta2-toxin expression. These findings strengthen the role of beta2-toxin in the pathogenesis of enteritis in neonatal pigs. However, the identification of apparently nonexpressed, atypical cpb2 genes raises the question of whether this protein plays the same role in enteritis in other animal species.
In addition to gas gangrene and food poisoning in humans, Clostridium perfringens is a cause of economically important disease in livestock, such as cattle and swine (reviewed in reference 11). C. perfringens is classified into five types (A to E) on the basis of differential production of the four toxins, alpha-toxin, beta-toxin, epsilon-toxin, and iota-toxin. While the roles of beta-, iota-, and epsilon-toxin in the pathogenesis of enteritis are well documented (11), the roles of other toxins, such as alpha-toxin and beta2-toxin, in disease pathogenesis are still equivocal.
cpb2 encodes beta2-toxin, which was toxic to cultured epithelial cells and lethal to mice when it was administered intravenously (4). cpb2-positive C. perfringens strains are associated with the occurrence of enteric disease in domestic animals, notably pigs (2, 3, 6, 13), horses (1, 5), and dogs (12). There was an especially strong correlation between the prevalence of cpb2 in isolates from piglets with enteritis and the absence of cpb2 in isolates from healthy piglets (2, 3). However, apart from this epidemiologic association, there is little experimental evidence to support the role of beta2-toxin in pathogenesis. Immunohistochemistry identified beta2-toxin in 35 to 48% of small and large intestines of horses with typhlocolitis caused by C. perfringens (1). Furthermore, Manteca et al. demonstrated that a cpb2-positive isolate of C. perfringens produced more pronounced necrotic intestinal lesions than an isolate lacking cpb2. However, this study did not use isogenic strains, and factors other than beta2-toxin may have contributed to the severity of the lesions (8).
Recently, we determined that beta2-toxin may be differentially expressed in C. perfringens strains isolated from different host species. There was a strong correlation between beta2-toxin phenotype and genotype only in type A and C isolates obtained from diseased pigs (2). For isolates of nonporcine origin, the correlation of phenotype and genotype was only 50% (2). This study aims to determine the basis for the absence of beta2-toxin expression.
The majority of the C. perfringens isolates used in this study were received through the Clostridial Enteric Disease Unit (CEDU), University of Arizona, and were from clinical cases where C. perfringens disease was suspected. All isolates were typed by a multiplex PCR assay which amplifies cpa, cpb, cpb2, cpe, etx, and ibp genes (3).
PCR product, amplified with primers CPB2F and CPB2R (Table 1) from a bovine type E isolate, C. perfringens NCIB 10784, was sequenced and found to diverge significantly from cpb2 from a porcine type C isolate (GenBank accession number L77965). Due to the sequence divergence, primers designed to the sequence surrounding the previously described cpb2 gene (GenBank accession number L77965), which we will refer to as the consensus cpb2 gene, did not amplify this gene region from strain NCIB 10784. Therefore, primers upstream and downstream of the atypical cpb2 gene were designed from preliminary nucleotide sequence from a bovine type E isolate, C. perfringens 853, provided by The Institute for Genomic Research, Rockville, Md. The nucleotide sequence of the entire NCIB 10784 cpb2 gene was determined from PCR products amplified with primers EEBF2 and CPB2R and primers CPB2F and EEB2R (Table 1). The cpb2 gene from strain NCIB 10784 displayed only 70.7% DNA identity with the consensus cpb2 gene. Furthermore, deletion of a base at position 178 in the atypical cpb2 gene resulted in a frameshift such that a protein of only 73 amino acids could be produced, explaining the absence of beta2-toxin expression in this isolate.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′→3′)a | Positions on cpb2 (bp) |
|---|---|---|
| CPB2ATYPFb | ATTATGTTTAGGAATACAGTTA | 51-72 |
| CPB2CONFc | CAATTGGGGGAGTTTATCCACAA | 488-510 |
| CPB2SEQFc | GTTAAAAATTTGATATAATTGAATTG | 52-28 upstream |
| CPB2F | AGATTTTAAATATGATCCTAACC | 225-247 |
| CPB2R | CAATACCCTTCACCAAATACTC | 770-791 |
| EEB2Fb | AACATAATAAATCCTATAACCC | 256-235 upstream |
| EEB2Rb | ATAAATATAATTCTCTAAAACC | 166-187 downstream |
| HISBETA2Rc | GTCACTTCAGAATTCTTTCTATGCAC | 791-18 downstream |
| HISATYPB2Fb | CCTAATACAGTGGATCCAAATGAAGTG | 73-99 |
| HISATYPB2Rb | TATAAATAAATAGAATTCTCTAAAACC | 166-192 downstream |
Restriction endonuclease sites introduced for cloning (underlined) are indicated.
This primer does not bind to the consensus cpb2 gene region. The position on the atypical cpb2 gene region is shown.
This primer does not bind to the atypical cpb2 gene region. The position on the consensus cpb2 gene region is shown.
A triplex PCR was developed using consensus and atypical cpb2-specific forward primers (CPB2CONF and CPB2ATYPF, respectively) with a common reverse primer, CPB2R (Table 1). PCR was performed by using Taq DNA polymerase (Promega) and the supplied reaction buffer under the following conditions: (i) a 5-min hot start at 94°C; (ii) 35 cycles, with 1 cycle consisting of 1 min at 94°C, 1 min at 50°C, and 1 min/kb at 72°C; and (iii) a final extension step of 72°C for 5 min. PCR amplification of consensus and atypical cpb2 genes produced 304- and 741-bp products, respectively (data not shown). This PCR assay was used to screen 154 cpb2-positive isolates, including all five C. perfringens types, from a variety of host sources. No porcine type C isolates (n = 16) and only 3.3% of porcine type A isolates (n = 60) carried the atypical cpb2 gene; only 2.6% of all porcine isolates carried the atypical cpb2 gene (Table 2). However, the predominant allele carried by nonporcine isolates was the atypical cpb2 gene, which was present in 88.5% of these isolates (n = 78) (Table 2). All type B, nonporcine type C, type D, and type E isolates carried the atypical cpb2 gene, regardless of host origin (Table 2). Most nonporcine type A isolates also carried the atypical cpb2 gene (66.6 to 100%) (Table 2). A total of 55 cpb2-negative isolates (determined by a multiplex genotyping PCR [3]) were also examined. As expected, amplicons were not detected in these cpb2-negative isolates using the triplex PCR assay (data not shown).
TABLE 2.
Prevalence of atypical and consensus cpb2 genes in cpb2-positive C. perfringens types isolated from various animal species
| Isolate source | No. of isolates containing atypical cpb2 genes/no. of isolates tested by type:
|
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Avian | 13/14 | ||||
| Bovine | 7/10 | 2/2 | 12/12 | ||
| Canine | 5/7 | ||||
| Caprine | 2/2 | ||||
| Cervine | 2/2 | ||||
| Equine | 9/10 | ||||
| Feline | 2/2 | ||||
| Human | 8/8 | ||||
| Ovine | 4/6 | 2/2 | |||
| Porcine | 2/60 | 0/16 | |||
| Unknown | 1/1 | ||||
| Total no. | 52/119 | 1/1 | 2/18 | 4/4 | 12/12 |
A total of 23 C. perfringens isolates carrying the atypical cpb2 gene were selected for nucleotide sequence analysis: 10 type A isolates (2 avian isolates, 6 bovine isolates, 1 canine isolate, and 1 equine isolate), 1 type B isolate (unknown origin), 2 type C isolates (bovine), 4 type D isolates (2 caprine isolates and 2 ovine isolates), and 6 type E (bovine) isolates (including NCIB 10784). Nucleotide sequence data from these isolates were obtained as for the cpb2 gene from C. perfringens strain NCIB 10784, and the data were compared with data for a consensus cpb2 gene (GenBank accession number L77965).
Compared individually to the consensus cpb2 gene, the atypical cpb2 genes displayed 70.2 to 70.7% DNA identity. When the atypical cpb2 genes were compared as a group, all the atypical genes displayed 93.0% DNA identity. Two bovine type A isolates carried identical 13-bp deletions at positions 73 to 85, and one avian type A isolate carried a mutation at position 606 resulting in premature termination of translation. The remaining type A, B, and C atypical cpb2 genes displayed 99.0% DNA identity with each other and could potentially produce full-length atypical beta2-toxin protein. Five of the six type E cpb2 genes, including the one from strain NCIB 10784, were identical and carried the frameshift mutation at position 178. In the other type E isolate, 853, the sequence was identical to that of nonporcine type A and C isolates. The type D genes were identical and most similar to type E cpb2, although the cpb2 genes from type D isolates did not carry the frameshift mutations.
Nucleotide sequence upstream of the consensus cpb2 gene or the atypical cpb2 genes was similar, with 79.4% DNA identity over the 97 bp immediately upstream of cpb2. However, the consensus and atypical cpb2 sequences were significantly more divergent 5′ of this point. Primer extension analysis had identified a σ70-type promoter (TTTTAA-N17-TATAAT), which is present 35 bp upstream of the start of the consensus cpb2 gene in C. perfringens strain 13 (9), and the identical sequence is present in the consensus cpb2 gene from C. perfringens strain CWC245 (GenBank accession number L777965). Similar, but not identical, sequences are present upstream of atypical cpb2 genes from type A, type B, nonporcine type C and type D (TTTAAA-N17-TA[T/A]AAT), and type E (TTAAAA-N17-TATAAT) isolates.
When compared with sequences in GenBank, atypical cpb2 displayed similarity with only consensus cpb2 at either the nucleotide or amino acid level (data not shown). Most of the nucleotide changes occurring between atypical cpb2 genes from type A, B, and C isolates conferred either no change or conservative substitutions. Figure 1 shows an amino acid alignment between a consensus beta2-toxin protein, and representatives of types A, B, and C, type D and type E atypical beta2-toxin sequences. Atypical beta2-toxin proteins were 96.2 to 98.9% identical and 97.7 to 99.2% similar to each other, whereas atypical beta2-toxin proteins displayed only 62.3% identity and 80.4% similarity to the previously described beta2-toxin protein (4).
FIG. 1.
Amino acid alignment of C. perfringens beta2-toxin proteins expressed from consensus (GenBank accession number L77695) or atypical cpb2 genes. The type A sequence shown is the sequence of C. perfringens JGS4147 (bovine type A) and is representative of atypical type A, B, and C beta2-toxin sequences. The type D and E sequences are the sequences from C. perfringens JGS4152 (ovine type D) and NCIB 10784, respectively. The position of the frameshift mutation is indicated by the dash at position 59 in the type E sequence, and the remaining amino acid sequence shown is that which would occur in the absence of the mutation. Two or more identical amino acids in the sequences are shown on a black background, while conservative substitutions are shown on a gray or shaded background. The predicted signal sequence cleavage sites are indicated by the arrow. Amino acid numbers for each protein are indicated to the right of the sequences.
To ensure that antibodies raised against consensus beta2-toxin would recognize the atypical protein, a strain expressing recombinant atypical beta2-toxin was constructed. The atypical cpb2 gene, lacking the coding region for the signal sequence, was amplified as a 917-bp product from C. perfringens strain JGS4142 (bovine type A) by PCR with a 5′ primer containing a BamHI site (primer HISATYPB2F) and a 3′ primer containing an EcoRI site (primer HISATYPB2R) (Table 1). The PCR product was digested with BamHI-EcoRI and cloned into pTrcHis B (Invitrogen), generating pJGS659. pJGS659 encoded His-atypical beta2-toxin, a 268-amino-acid protein comprising 235 amino acids of the mature atypical beta2-toxin with an N-terminal extension of 33 amino acids encoded by pTrcHis B. DNA sequencing of the insert portion of pJGS659 indicated that no mutations had been introduced during PCR.
HIS-atypical beta2-toxin was purified from Escherichia coli DH5α(pJGS659) to >95% homogeneity using TALON resin (2). In Western blots, monoclonal antibody (MAb) 9E10B, raised against purified HIS-beta2-toxin (2), reacted with preparations of purified HIS-atypical beta2-toxin (data not shown).
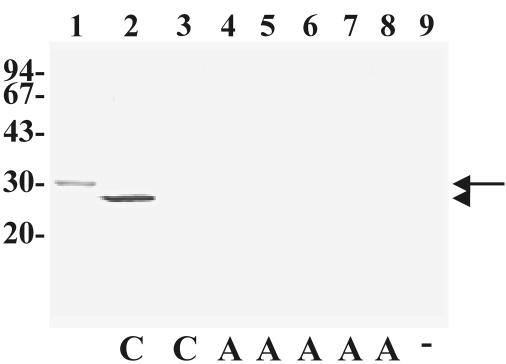
C. perfringens isolates were grown in anaerobic brain heart infusion broth (Difco) supplemented with 0.5% yeast extract and 0.05% cysteine at 37°C for 48 h. The culture supernatant fluid (CSF) was harvested by centrifugation and passage through a 0.45-μm-pore-size filter. The CSF was concentrated 40-fold by ultrafiltration through a 10,000-molecular-weight cutoff filter (Amicon), and beta2-toxin was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with MAb 9E10B. A positive-control lane loaded with purified HIS-atypical beta2-toxin was always included, and a representative blot is shown in Fig. 2. Thirty-three isolates carrying atypical cpb2 genes were selected from all five C. perfringens types and a variety of host sources and tested for beta2-toxin expression by Western blotting. As expected, beta2-toxin expression was not detected in any of the type A and E isolates that carry frameshift mutations (Table 3 and Fig. 2). However, beta2-toxin was also not detected in the 25 isolates that carried full-length atypical cpb2 genes. Beta2-toxin expression was not detected in control isolates of C. perfringens that did not carry cpb2 (n = 3) (Fig. 2).
FIG. 2.
Expression of beta2-toxin or atypical beta2-toxins in concentrated CSF from C. perfringens isolates. One microgram of HIS-atypical beta2-toxin (lane 1) or concentrated CSF from C. perfringens strains (lanes 2 to 9) was subjected to electrophoresis on SDS-10% polyacrylamide gels. CSF from C. perfringens strain 690D (porcine type A) (lane 2), strain 13 (lane 3), JGS4142 (lane 4), JGS1604 (canine type A) (lane 5), JGS1984 (unknown type origin B) (lane 6), JGS1880 (lane 7), JGS4152 (lane 8), and cpb2-negative, porcine type A (negative control) (lane 9) were used. The separated proteins were transferred to nitrocellulose by Western blotting and immunostained with a 1/20 dilution of MAb 9E10B. The positions of molecular mass standards (in kilodaltons) are shown to the left of the gel. The presence of consensus (C) or atypical (A) cpb2 genes or the absence of cpb2 (−) in that strain is indicated below the gel. The positions of HIS-atypical beta2-toxins (arrow) and beta2-toxins (arrowhead) are indicated to the right of the gel.
TABLE 3.
Correlation of the presence of the cpb2 allele with beta2-toxin expression in C. perfringens types isolated from various animal species
| Isolate source | Type | No. of isolates positive for beta2-toxin expression/no. of isolates tested
|
|
|---|---|---|---|
| Atypical cpb2 | Consensus cpb2 | ||
| Avian | A | 0/4 | 1/1 |
| Bovine | A | 0/6 | 2/3 |
| C | 0/2 | ||
| E | 0/12 | ||
| Canine | A | 0/1 | 0/2 |
| Caprine | D | 0/2 | |
| Equine | A | 0/2 | 1/1 |
| Ovine | A | 1/2 | |
| D | 0/2 | ||
| Porcine | A | 0/1 | 1/1 |
| C | 2/3 | ||
| Unknown | B | 0/1 | |
| Total no. | 0/33 | 8/13 | |
Consensus cpb2 genes were not common in nonporcine isolates, with only 11.5% of nonporcine isolates carrying the consensus cpb2 gene (n = 78) (Table 2). Interestingly, only five of the nine nonporcine isolates carrying consensus cpb2 genes expressed beta2-toxin protein (Table 3 and Fig. 2). The nucleotide sequences of these nine consensus cpb2 genes were determined from PCR products amplified with primers CPB2SEQF and CPB2R and primers CPB2F and HISBETA2R (Table 1). Identical frameshift mutations resulting from the insertion of an additional A within a poly(A) tract from positions 4 to 10 were identified in the cpb2 genes from type A isolates of bovine, canine, and ovine origin, including strain 13 (7). This frameshift mutation could result in translation of a truncated protein of 4.5 kDa, explaining the absence of beta2-toxin expression in these strains. Interestingly, the published sequence of cpb2 from strain 13 does not contain this frameshift mutation (10), but this mutation was identified in the cpb2 gene from strain 13 obtained from a different source (Bruce A. McClane, personal communication). The other five cpb2 genes from nonporcine hosts did not contain the frameshift mutation, and beta2-toxin expression was detected in each of these isolates (Table 3).
With one exception, strain JGS1475, all strains of porcine origin carrying cpb2 expressed beta2-toxin (n = 4) (Table 3 and Fig. 2). In addition, we previously demonstrated that 96.9% of consensus cpb2-carrying porcine isolates expressed beta2-toxin protein (n = 32) (2). The nucleotide sequence of cpb2 from strain JGS1475 was determined, but it was identical to the cpb2 sequence from strain CWC245 (GenBank accession number L777965) (data not shown), so it is not known why this isolate does not express beta2-toxin. Regardless, it is clear that in general, consensus genes from porcine isolates are expressed, yet atypical genes from nonporcine C. perfringens isolates are not expressed. Furthermore, consensus genes from nonporcine C. perfringens isolates are expressed at different levels.
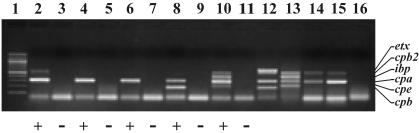
Given that the sequences upstream of consensus and atypical cpb2 genes were similar, but not identical, the defect in expression of atypical genes could occur at the transcriptional level. Reverse transcription-PCR (RT-PCR) was used to identify cpb2 transcripts in C. perfringens isolates carrying atypical genes. Tri Reagent (Medical Research Center) was used to extract total RNA from mid-log-phase cultures, as transcript from a consensus cpb2 gene is abundant at this phase of the cell cycle (9). RNA was reverse transcribed into total cDNA using the Advantage RT-for-PCR kit (Clontech) and was used as a template in the multiplex PCR (3) to identify the presence of any cpb2 transcript. cpa transcript was detected in all C. perfringens isolates tested and served as an internal positive control. Transcripts for the cpb, cpe, etx, and ibp genes, encoding other toxins, were also detected in the multiplex PCR assay, depending on the C. perfringens type. Reaction mixtures with no Moloney murine leukemia virus (MMLV) reverse transcriptase added were used as negative controls to determine whether contaminating bacterial DNA was present. RNA was prepared from C. perfringens strains JGS1880 (bovine type C), JGS1906 (bovine type A), JGS4142, NCIB 10784, and 13 and subjected to RT-PCR.
RNA from all C. perfringens isolates contained approximately equivalent amounts of cpa transcript (Fig. 3). As reported previously, strain 13 RNA contained cpb2-specific transcript (9). By PCR, strains JGS1880, JGS1906, and JGS4142 did not contain cpb2-specific product, indicating that very little or no transcription occurred in these isolates carrying atypical cpb2 genes (Fig. 3). The absence of atypical cpb2 gene expression in these strains probably occurs at the transcriptional level, at least in broth culture. Unexpectedly, however, RNA from strain NCIB 10784 contained cpb2-specific transcript (Fig. 3), but expression of beta2-toxin was not detected in this isolate due to a frameshift mutation in the cpb2 gene. The putative −35 promoter sequence in type E atypical cpb2 genes varies slightly from the −35 promoter sequence found in type A, B, C, and D atypical genes, and these −35 promoter sequences are slightly different from those found for consensus genes (TTAAAA, TTTAAA, and TTTTAA, respectively). The absence of transcription in the type A and C isolates examined may be a result of this divergent −35 sequence, whereas the type E cpb2 −35 sequence may still be able to promote transcription. However, these studies were performed with bacteria grown in vitro, and there may be signals involved in up-regulation of atypical cpb2 gene expression that are present only in the host, or possibly the type A and C isolates may lack any required activators of cpb2 transcription. Further experiments will be necessary to confirm these hypotheses.
FIG. 3.
Transcriptional analysis of atypical cpb2 genes. Total cDNA, prepared by reverse transcription from RNA, was subjected to multiplex PCR analysis (3), and the amplicons were visualized after electrophoresis in a 1.5% agarose gel. Lanes: 1, 100-bp ladder; 2 and 3, strain 13 cDNA; 4 and 5, JGS1906 cDNA; 6 and 7, JGS4142 cDNA; 8 and 9, JGS1880 cDNA; 10 and 11, NCIB 10784 cDNA; 12, JGS1984 DNA; 13, strain 294 DNA (bovine type E); 14, strain 13 DNA; 15, JGS4142 DNA; 16, no-template control. MMLV reverse transcriptase was added (+) or not added (−) to the reaction mixtures in lanes 2 to 11. The positions of the 655-bp etx, 567-bp cpb2, 446-bp ibp, 324-bp cpa, 233-bp cpe and 196-bp cpb gene products are indicated to the right of the gel.
Consensus cpb2 genes are found predominantly in C. perfringens strains isolated from porcine hosts. With only a few exceptions, these genes are expressed and beta2-toxin protein can be detected in culture supernatant by Western blotting. However, atypical cpb2 genes predominate in C. perfringens strains isolated from nonporcine species. These genes were either not transcribed or full-length protein was not translated in bacteria grown in broth culture, although expression may still occur in vivo. Atypical genes present in types D and E are more similar to each other than those from type A and B and nonporcine type C isolates and may indicate divergent evolution of atypical cpb2 genes in different C. perfringens types. As cpb2 genes are plasmid-borne, at least in some strains (4, 10), there is the potential for mobility and the subsequent transfer of cpb2 among strains of C. perfringens. However, the strong correlation of porcine isolates carrying consensus cpb2 genes suggests that if atypical gene transfer does occur, it is not maintained in porcine isolates. Correspondingly, consensus genes transferred into nonporcine isolates are also apparently not maintained. Interestingly, almost half of the consensus genes in nonporcine isolates carry frameshift mutations (44.4%), resulting in an absence of beta2-toxin expression.
The findings presented here strengthen the possible role of beta2-toxin in the pathogenesis of enteritis in neonatal pigs. However, the presence of apparently nonexpressed, atypical cpb2 genes in C. perfringens strains isolated from nonporcine hosts raises the question of whether this protein plays the same role in enteritis in other animal species. Answering these questions will require the development of appropriate animal models of C. perfringens enteritis.
Nucleotide sequence accession number.
The atypical cpb2 sequence data were submitted to the DDBJ/EMBL/GenBank databases under accession numbers AY609161 to AY609183.
Acknowledgments
We thank Jeremy W. Coombs for excellent technical assistance. MAb 9E10B was kindly provided by Carol W. Maddox, University of Illinois at Urbana-Champaign. We also thank Ian T. Paulsen, The Institute for Genomic Research, Rockville, Md., for the C. perfringens 853 nucleotide sequence.
Sequencing of the C. perfringens 853 nucleotide sequence was part of a genome sequencing project funded by NIAID grant 1 U01 AI49921-01.
Editor: J. T. Barbieri
REFERENCES
- 1.Bacciarini, L. N., P. Boerlin, R. Straub, J. Frey, and A. Gröne. 2003. Immunohistochemical localization of the Clostridium perfringens β2-toxin in the gastrointestinal tract of horses. Vet. Pathol. 40:376-381. [DOI] [PubMed] [Google Scholar]
- 2.Bueschel, D. M., B. H. Jost, S. J. Billington, H. T. Trinh, and J. G. Songer. 2002. Prevalence of cbp2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet. Microbiol. 94:121-129. [DOI] [PubMed] [Google Scholar]
- 3.Garmory, H. S., N. Chanter, N. P. French, D. Bueschel, J. G. Songer, and R. W. Titball. 2000. Occurrence of Clostridium perfringens β2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol. Infect. 124:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibert, M., C. Jolivet-Reynaud, and M. R. Popoff. 1997. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 203:65-73. [DOI] [PubMed] [Google Scholar]
- 5.Herholz, C., R. Miserez, J. Nicolet, J. Frey, M. Popoff, M. Gibert, H. Gerber, and R. Straub. 1999. Prevalence of β2-toxigenic Clostridium perfringens in horses with intestinal disorders. J. Clin. Microbiol. 37:358-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaasen, H. L., M. J. Molkenboer, J. Bakker, R. Miserez, H. Hani, J. Frey, M. R. Popoff, and J. F. van den Bosch. 1999. Detection of the beta2 toxin gene of Clostridium perfringens in diarrhoeic piglets in The Netherlands and Switzerland. FEMS Immunol. Med. Microbiol. 24:325-332. [DOI] [PubMed] [Google Scholar]
- 7.Mahoney, D. E., and T. I. Moore. 1976. Stable L-forms of Clostridium perfringens and their growth on glass surfaces. Can. J. Microbiol. 22:953-959. [DOI] [PubMed] [Google Scholar]
- 8.Manteca, C., G. Daube, T. Jauniaux, A. Linden, V. Pirson, J. Detilleux, A. Ginter, P. Coppe, A. Kaeckenbeeck, and J. G. Mainil. 2002. A role for the Clostridium perfringens beta2 toxin in bovine enterotoxaemia? Vet. Microbiol. 86:191-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtani, K., H. I. Kawsar, K. Okumura, H. Hayashi, and T. Shimizu. 2003. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol. Lett. 222:137-141. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Songer, J. G. 1996. Clostridial enteric disease of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiede, S., R. Goethe, and G. Amtsberg. 2001. Prevalence of beta2 toxin gene of Clostridium perfringens type A from diarrhoeic dogs. Vet. Rec. 149:273-274. [DOI] [PubMed] [Google Scholar]
- 13.Waters, M., A. Savoie, H. S. Garmory, D. Bueschel, M. R. Popoff, J. G. Songer, R. W. Titball, B. A. McClane, and M. R. Sarker. 2003. Genotyping and phenotyping of beta2-toxigenic Clostridium perfringens fecal isolates associated with gastrointestinal diseases in piglets. J. Clin. Microbiol. 41:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]