Abstract
Cholecystokinin (CCK) was discovered in 1928 in jejunal extracts as a gallbladder contraction factor. It was later shown to be member of a peptide family, which are all ligands for the CCK1 and CCK2 receptors. CCK peptides are known to be synthetized in small intestinal endocrine I-cells and cerebral neurons. But in addition, CCK is expressed in several endocrine glands (pituitary cells, thyroid C-cells, pancreatic islets, the adrenals, and the testes); in peripheral nerves; in cortical and medullary kidney cells; in cardial myocytes; and in cells of the immune system. CCK peptides stimulate pancreatic enzyme secretion and growth, gallbladder contraction, and gut motility, satiety and inhibit acid secretion from the stomach. Moreover, they are major neurotransmitters in the brain and the periphery. CCK peptides also stimulate calcitonin, insulin, and glucagon secretion, and they may act as natriuretic peptides in the kidneys. CCK peptides are derived from proCCK with a C-terminal bioactive YMGWMDFamide sequence, in which the Y-residue is partly O-sulfated. The plasma forms are CCK-58, -33, -22, and -8, whereas the small CCK-8 and -5 are potent neurotransmitters. Over the last decades, CCK expression has also been encountered in tumors (neuroendocrine tumors, cerebral astrocytomas, gliomas, acoustic neuromas, and specific pediatric tumors). Recently, a metastastic islet cell tumor was found to cause a specific CCKoma syndrome, suggesting that circulating CCK may be a useful tumor marker.
Keywords: cholecystokinin, gastrointestinal hormones, neuropeptides, neuroendocrine tumors, tumor markers
Introduction
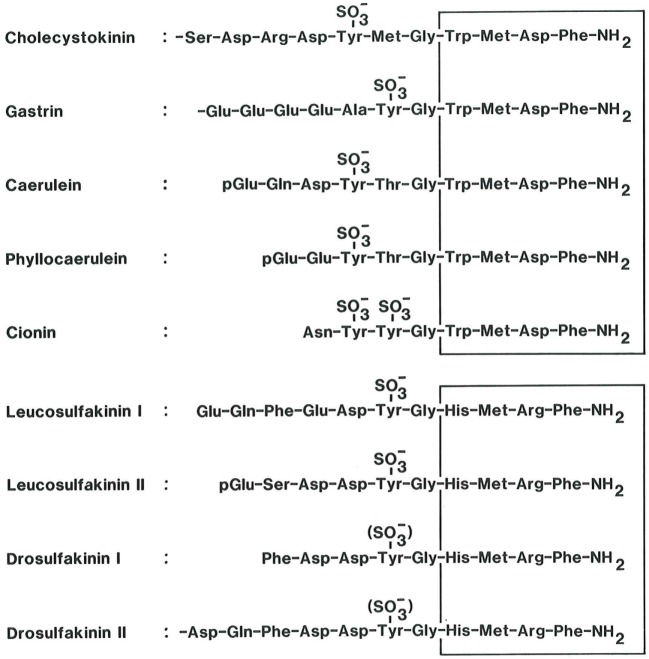
Cholecystokinin (CCK) is member of a family of regulatory peptides with a remarkably well preserved C-terminal sequence (1–3). The family also includes frog skin peptides (caerulein and phyllocaerulein) and the protochordean neuropeptide cionin, but in mammals, CCK and gastrin are the only family members (Figure 1).
Figure 1.
The homologous bioactive sequences of peptide systems belonging to the cholecystokinin (CCK) family (upper panel). CCK and the antral hormone, gastrin, are the only mammalian members of the family. Caerulein and phyllocaerulein are identified from frogskin extracts. Cionin is a neuropeptide isolated from the central ganglion of the protochord, ciona intestinalis. Note the unique disulfated sequence, which might suggest that cionin may resemble a common ancestor of CCK and gastrin. The core of the bioactive sequences, the common C-terminal tetrapeptide amide, is boxed. The lower panel shows the bioactive sequences of the insect peptides, the sulfakinins, which display some homology with vertebrate and protochordian members of the CCK family (4, 5). Also their C-terminal tetrapeptide amide sequence is boxed.
After the discovery in 1928 (6), CCK became part of the classical troika of gut hormones together with secretin and gastrin. The last decades, however, have shown that CCK, in addition to its local acute functions in digestion (gallbladder emptying and pancreatic enzyme secretion), is also a growth factor, a neurotransmitter in the brain and peripheral neurons [for reviews, see Ref. (7–9)], and besides, it may be a spermatozoan fertility factor, a natriuretic kidney peptide, an anti-inflammatory cytokine in the immune system, and a cardiac marker of heart failure. The long history has made the CCK literature comprehensive and at some points also confusing because impure CCK preparations with little attention paid to species differences and to physiological levels were used initially. In addition, most assays for measurement of CCK in plasma and elsewhere lacked specificity and sensitivity (10–12).
The biochemical concept of CCK as a single hormonal peptide from the small intestine has also changed considerably. Now CCK is known to be synthetized and released in multiple molecular forms. And the CCK gene is expressed at peptide level in a cell-specific manner in neurons, endocrine cells, and epithelial cells outside the gastrointestinal tract (Table 1). All known biological effects of CCK peptides reside in the conserved C-terminal heptapeptide sequence (Figure 1). Modification of this sequence grossly reduces or abolishes receptor binding and biological effects (13–15). The N-terminal extensions of the common C-terminus increase the biological potency and the specificity for receptor binding. Of particular importance is the tyrosyl residue in position seven [as counted from the C-terminus (Figure 1)]. The tyrosyl residue is rarely completely sulfated (16–20). The CCK2 receptor binds sulfated and unsulfated ligands equally well, whereas the CCK1 receptor is exclusive and requires Y-sulfation of the ligand.
Table 1.
The widespread expression of cholecystokinin (CCK) peptides in normal adult mammalian tissue.
| Tissue | Tissue contenta (pmol/g) | Precursor percentagec |
|---|---|---|
| Intestinal tract | ||
| Duodenal mucosa | 200 | 5 |
| Jejunal mucosa | 150 | 20 |
| Ileal mucosa | 20 | 50 |
| Colonic mucosa | 5 | 50 |
| Central nervous system | ||
| Cerebral cortex | 400 | 2 |
| Hippocampus | 350 | 2 |
| Hypothalamus | 200 | 2 |
| Cerebellum | 2 | 90 |
| Spinal cord | 40 | 10 |
| Peripheral nervous system | ||
| Vagal nerve | 25 | 5 |
| Sciatic nerve | 15 | 5 |
| Nerveplexes in colonic wall | 5 | 20 |
| Extraintestinal endocrine glands | ||
| Adenohypophysis | 25 | 100 |
| Neurohypophysis | 20 | 10 |
| Thyroid gland | 2 | 20 |
| Adrenal medulla | 1 | 50 |
| Urogenital tract | ||
| Renal cortexb | +++ | − |
| Renal medullab | +++ | − |
| Testicles | 5 | 80 |
| Spermatozoas | 1 | 50 |
| Cardiovascular system: | ||
| Atrial myocytes | 10 | 95 |
| Ventricular myocytes | 2 | 95 |
| Mononuclear immune cellsb | ++ | − |
aOrders of magnitude based on measurement of tissue extracts from different mammalian species.
bExpression determined only by immunocytochemistry.
The following is a short review about the biology of CCK with emphasis on the recently recognized widespread expression (Table 1) and besides an update on the classic gastrointestinal effects of CCK peptides.
Biogenesis
As described earlier (9), “the exomal unit of the CCK gene is seven kilobases interrupted by two introns (23). The first of the three exons is small and non-coding. Several conserved regulatory elements have been identified in first 100 bp of the promoter, including an E-box element, a combined cAMP response element (CRE)/12-O-tetradeconoylphorbol-13-acetate response element (TRE), and a GC-rich region (24, 25). Whereas the function of the E-box and the GC-rich region is not fully clarified (26, 27), the combined CRE/TRE sequence plays an important role in the regulation of CCK transcription. The CRE/TRE binds the transcription factor CREB, which is activated by phosphorylation by several signaling pathways, including cAMP, fibroblast growth factor, pituitary adenylate cyclase-activating polypeptide, calcium, hydrolyzates, and peptones to ultimately induce CCK transcription (28–32). Only one CCK mRNA molecule has been found, and the CCK peptides are thus fragments of the same proCCK protein. The mRNA has 750 bases, of which 345 are protein coding (33, 34). The concentrations of CCK mRNA in cerebrocortical tissue are similar to that of the duodenal mucosa (34), and in the brain, there is a rapid synthesis of CCK peptides (35).
The primary translational product, preproCCK, has 115 amino acid residues. The first part is the signal peptide. The second part with considerable species variation is a spacer peptide. The bioactive CCK peptides are derived from the subsequent 58 amino acid residues (16, 18, 36–38), and the species variation is small in this sequence. The processing of proCCK is cell-specific: endocrine cells contain a mixture of the medium-sized CCK-58, -33, -22, and -8, whereas neurons mainly release CCK-8 and to some extent CCK-5 (16, 39). The endoproteolysis of proCCK occurs mainly at monobasic sites. Y-77 is mostly O-sulfated (16–20, 40), which is decisive for CCK1 receptor binding.
In the small intestine, CCK peptides are synthesized in endocrine I-cells (41), whose apical membrane is in contact with the intestinal lumen and whose basal region contains secretory granules with CCK peptides. CCK is also synthesized in pituitary corticotrophs and melanotrophs, in thyroid C-cells (17), and in adrenal medullary cells (42, 43). In the pituitary cells, CCK constitutes a small fraction of the hormones. Tumors originating from pituitary corticotrophs, however, produce larger amounts of CCK (44).”
It is the brain that expresses most CCK (16, 39, 42). Moreover, cerebral CCK neurons are more abundant than neurons of other neuropeptides (42, 45, 46). While most peptidergic neurons occur in subcortical regions, CCK is expressed in the highest concentrations in neocortical neurons (39, 42, 47). The perikarya of the cortical CCK nerves are distributed in layers II–VI, with the highest frequency in layers II and III (42, 48). CCK in mesencephalic dopamine neurons projecting to the limbic area of the forebrain (45) has aroused clinical interest because these neurons are supposed to be involved in schizophrenia.
Outside the brain, the colon contains numerous CCK neurons, whereas jejunum and ileum are less innervated (42). Colonic CCK fibers occur in the circular muscle layer, which they penetrate to form a plexus in the submucosa (42). In accordance with these locations, CCK peptides excite colonic smooth muscles and release acetylcholine from neurons in both plexus myentericus and submucosa (49). Ganglionic cell somas in pancreatic islets are also surrounded by CCK nerves (50). Moreover, CCK nerve terminals also surround pancreatic islets (51). Finally, afferent vagal nerve fibers also contain CCK (52, 53).
Endocrine and Neuronal Release
Also mentioned before (9), “CCK in circulation originates mainly from intestinal endocrine cells. The release to blood was not possible to examine until specific assays were developed (10–12, 54). The assays have confirmed that protein- and fat-rich food is the most important stimulus (11, 54). Of the constituents, protein and l-amino acids as well as digested fat cause significant CCK release (54, 55). Carbohydrates only release small amounts of CCK (54), but hydrochloric acid also stimulates release (55).
The release from neurons has been examined directly in brain slices and synaptosomes (56, 57). Potassium-induced depolarization caused a calcium-dependent release of CCK-8. Similarly, depolarization releases CCK peptides from the hypothalamic dopamine neurons that innervate the intermediate lobe of the pituitary (58).
By analogy with other neuropeptides, it is possible that overflow from peripheral CCK neurons may contribute slightly to CCK in plasma.By comparison with identified CCK peptides, it has been possible to deduce the molecular pattern of CCK in plasma. The picture has varied (12) due to species differences and because the molecular pattern along the gut varies (59, 60). Furthermore, the distribution may vary during stimulation. In man, CCK-33 predominates in plasma, but CCK-58, -22, and -8 are also present (11, 61).
In the basal state, the concentration of CCK in plasma is around 1 pmol/l, but often less. The concentration increases within 20 min to 3–5 pmol/l during meal stimulation, and then declines gradually only to reach a second peak after 1.5–2 hours. In comparison with most other pancreatic and gastrointestinal hormones (62), the concentrations of CCK in plasma are low. When food-induced CCK in plasma is mimicked by infusion of exogenous CCK, the same degree of gallbladder contraction and release of enzymes as seen during meals occurs (54, 62–64). Therefore, the low circulating concentrations of CCK are sufficient to account for the gallbladder contraction and pancreatic enzyme secretion during meals.
Because the cholecystokinetic and pancreozymic potency of CCK-33 and CCK-8 on a molar base are identical (65), it may seem less important what I-cells release during digestion.” On the other hand, CCK-58, -33, and -22 are cleared from blood at a significantly slower rate than CCK-8.
Receptors
The cellular effects of CCK peptides are mediated via two receptors (66, 67). The “alimentary” CCK-A or CCK1 receptor (66) mediates gallbladder contraction, relaxation of the sphincter of Oddi, pancreatic growth and enzyme secretion, delay of gastric emptying, and inhibition of gastric acid secretion via fundic somatostatin (68). CCK1 receptors have been found also in the anterior pituitary, the myenteric plexus, and areas of the midbrain (69, 70). The CCK1 receptor binds with high affinity CCK peptides that are amidated and sulfated, whereas the affinity for non-sulfated CCK peptides and gastrins is negligible.
The CCK-B or CCK2 receptor (the “brain” receptor) is the predominant CCK receptor in the brain (67, 71). It is less specific than the CCK1 receptor and binds also non-sulfated CCK, gastrins, and C-terminal fragments such as CCK-5. It has been shown that the gastrin receptor cloned from the stomach (67) and CCK2 receptors are identical (71, 72). The gastrin/CCK2 receptor is expressed also in substantial amounts in pancreatic islet cells in man (73).
Gastrointestinal Effects
The defining functions of CCKs in digestion have been detailed regularly [for instance, see Ref. (6, 7)].
Gallbladder and Pancreas
“CCK peptides stimulate hepatic secretion mainly as bicarbonate from hepatic ductular cells (74) and act on gallbladder muscles with a potency correlated to the low plasma concentrations of sulfated CCK. From the liver and gallbladder, bile is released into the duodenum via CCK-mediated rhythmic contraction and relaxation of muscles in the common bile duct and the sphincter of Oddi. CCK regulates the secretion of pancreatic enzymes so potently that it seems sufficient to account for all enzyme secretion (63–65). CCK is also capable of releasing several small intestinal enzymes such as alkaline phosphatase (75), disaccharidase (76), and enterokinase (77). In addition, CCK stimulates the biosynthesis of pancreatic amylase, chymotrypsinogen, and trypsinogen (78–80).
While the interest in the effect of CCK on the exocrine pancreas was for many years restricted to enzyme secretion, it is now well established that CCK also stimulates fluid and bicarbonate secretion. The effect on bicarbonate secretion is in itself weak, but because CCK potentiates the secretin-induced bicarbonate secretion in the same way as secretin potentiates the CCK-induced enzyme release (81), the effect of CCK peptides on bicarbonate and fluid secretion is potent. There are species differences, so it is now assumed that CCK in man stimulates pancreatic enzyme secretion through a cholinergic pathway that is less significant in rodents (82–84).
There are also species differences regarding the endocrine pancreas. CCK peptides release insulin and glucagon more potently in man and pig than in dog and rat (51, 85–87). The difference is partly due to neurons in pancreatic islets that release CCK-8 and CCK-5 in man and pig (51), whereas rat and dog islets have no such innervation (50, 51). Moreover, islet cells in man and pig also express the CCK2 receptor abundantly (73), whereas rat islet cells express mainly the CCK1 receptor (88).
Already in 1967, Rothman and Wells (80) noted that CCK increased pancreatic weight and enzyme synthesis. Also the output of bicarbonate and protein from the hypertrophic pancreas was increased (89). Although secretin in itself is without trophic effects, the combination of secretin and CCK showed trophic effect on ductular cells with increased secretin-induced bicarbonate output (89).”
Gut Motility
Cholecystokinin contributes to control intestinal motility. The distal part of the gut is as mentioned abundantly innervated with CCK neurons (42, 90). It is therefore likely that an increase of intestinal motor activity by exogenous CCK (91) reflects neuronal control of intestinal muscles by CCK peptide transmission. Neuronal CCK acts both indirectly via acetylcholine release from postganglionic parasympathetic nerves and directly on muscle cells (49). The observation that CCK peptides stimulate intestinal blood flow is in harmony with the occurrence of CCK nerve terminals around blood vessels in the basal lamina propria and the submucosa (42).
Satiety
“In 1973, Gibbs et al. discovered that exogenous CCK inhibits food intake (92). The effect mimicked the satiety induced by food and was not seen with other gut peptides known then. The effect could be demonstrated in several mammals. Vagotomy studies indicate that peripheral CCK induces satiety via CCK1 receptors relaying the effect into afferent vagal fibers (93). The satiety signal then reaches the hypothalamus from the vagus via the nucleus tractus solitarius and area postrema.
Gastric Acid Secretion
The effect of CCK on gastric acid secretion has been uncertain. On one hand, it has been suggested that intestinal CCK was an acid inhibitor (an enterogastrone). On the other hand, the results of CCK infusions have been inconsistent. The gastrin/CCK double “knockout” mice have now shed further light on the problem showing that circulating CCK stimulates somatostatin release from fundic D-cells via CCK1 receptors, which then inhibits acid secretion from parietal cells (68).”
Novel Sites of Expression
The major sites of CCK expression are as mentioned endocrine cells in the gut, the brain, and in peripheral nerves. But the last decades have uncovered additional sites and cell types that also express the CCK gene at peptide level (Table 1). In some of these sites, proCCK is not processed to the known α-amidated peptides. Their functions are therefore still unknown. But since CCK receptors also have such widespread expression (66, 67, 70–73, 94, 95), there is both room and need for delineation of the roles of CCK released from the “new” sites.
Extraintestinal Endocrine Cells
Pituitary corticotrophs and melanotrophs express significant amounts of proCCK fragments, but the posttranslational processing results in only trace amounts of conventional α-amidated CCK peptides (43, 96). Also, thyroid C-cells produce CCK, but mainly as non-sulfated but amidated CCK-8 (17). Since C-cells are well equipped with CCK2 receptors (97), thyroid CCK-8 is probably an autocrine stimulator of growth of normal and not least malignant C-cells. Adrenal medullary cells produce small amounts of CCK, although amidated and with a low degree of sulfation (98). The significance of adrenal CCK is unknown.
Male Germ Cells
Spermatogenic cells express transiently the CCK gene in most mammals (99, 100). Less than 25% of the amidated CCK is sulfated. Interestingly, the CCK peptides in mature spermatozoes are concentrated in the acrosomal granule, which opens the possibility that CCK may play a role in fertilization due to the acrosomal reaction (100). The acrosomal expression is species-specific, as human spermatozoes in addition to CCK also express its homolog, gastrin (101).
Kidney Cells
In rodent kidneys (rat, mice, and guinea pigs), CCK has recently been shown by immunohistochemistry to be expressed both in the renal cortex and in the medulla. The cortical expression occurs in distal tubular cells and glomeruli, and the medullar CCK expression is confined to collecting ducts (102, 103). The discovery of renal CCK expression may have been stimulated by earlier findings of significant CCK1 and CCK2 receptor expression also in human kidney tissue (104, 105). It has led to suggestions of local regulatory functions of natriuresis and inflammation in the kidneys. Remarkably, the expression in diabetic mice and rat kidneys is grossly increased. This increase has been suggested to protect the diabetic kidneys somewhat against inflammatory actions of macrophages (103).
Immune Cells
Cholecystokinin immunoreactivity has consistently been found to be expressed in human and rat mononuclear cells in blood (106, 107). Moreover, CCK-8 (sulfated as well as non-sulfated) has been reported to exert a wide specter of stimulation and inhibition on lymphocytes, macrophages, and cytokine release, with ensuing anti-inflammatory effects (108–111). The field is complex due to the many players; but the clinical impact of CCK in inflammatory diseases and endotoxin shock may be significant.
Cardiac Myocytes
Fetal mice express high levels of CCK mRNA in cardiac myocytes (112). Accordingly, adult cardiomyocytes in mice, rats, and pigs contain substantial amounts of proCCK protein (113). The processing, however, of cardiac proCCK is unique, as the result is a long triple-sulfated and N-terminally truncated fragment 25–94 with only trace amounts of the conventionally amidated and sulfated CCK peptides (113). The tissue concentration of the long proCCK fragment is higher in atrial than ventricular myocytes. The long proCCK fragment is released to plasma and may find use as a marker of the risk of mortality in heart failure patients (113).
Tumor Expression
Cholecystokinin is expressed at highly variable amounts in different neuroendocrine tumors, especially corticotrophic pituitary tumors (44), medullary thyroid carcinomas (17), phaeochromocytomas (98), and pancreatic islet cell tumors of which some may cause a specific CCKoma syndrome (114–117). CCK is also expressed in Ewing’s Sarcomas, where proCCK measurements may be used to monitor the treatment (118). Cerebral gliomas, astrocytomas, and acoustic neuromas also express CCK (119–121). The present knowledge about tumor expression of CCK was recently summarized in a review that also discussed measurements of CCK and proCCK in plasma as tumor markers (122).
Conclusion
Since the identification of CCK half a century ago as a single peptide with a sequence of 33 amino acid residues (CCK-33), the CCK story has been loaded with major revelations: first, it was shown that the C-terminus of CCK was similar to that of gastrin, and that CCK and gastrin peptides share the same receptor, the CCK2 receptor. Then, it was demonstrated that bioactive CCK occurs in multiple molecular forms—from CCK-58 to CCK-5 with and without tyrosyl O-sulfations. At variable intervals, it has since been shown that CCK peptides are expressed all over the body: in central and peripheral neurons, in intestinal and extraintestinal endocrine cells, in germ cells, kidney epithelial cells, cardiac myocytes, and immune cells. Moreover, the proCCK maturation appears to be cell specific, and tumors expressing CCK release correspondingly varying multifaceted patterns of CCK peptides. Thus, today CCK should be seen as an almost ubiquitous system of intercellular messenger peptides. The complex biology is probably characteristic for many regulatory peptides, for which the CCK system may serve as a source of inspiration for further research.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The skillful and patient secretarial assistance of Connie Bundgaard (cand.phil.) is gratefully acknowledged.
References
- 1.Larsson LI, Rehfeld JF. Evidence for a common evolutionary origin of gastrin and cholecystokinin. Nature (1977) 269:335–8. 10.1038/269335a0 [DOI] [PubMed] [Google Scholar]
- 2.Johnsen AH. Phylogeny of the cholecystokinin/gastrin family. Front Neuroendocrinol (1998) 19:73–99. 10.1006/frne.1997.0163 [DOI] [PubMed] [Google Scholar]
- 3.Johnsen AH, Rehfeld JF. Cionin: a disulfotyrosyl hybrid of cholecystokinin and gastrin from the neural ganglion of the protochordate ciona intestinalis. J Biol Chem (1990) 265:3054–8. [PubMed] [Google Scholar]
- 4.Nachman RJ, Holman GM, Haddon WF, Ling N. Leucosulfakinin, a sulfated insect neuropeptide with homology to gastrin and cholecystokinin. Science (1986) 234:71–3. 10.1126/science.3749893 [DOI] [PubMed] [Google Scholar]
- 5.Nichols R, Schneuwly SA, Dixon JE. Identification and characterization of a Drosophila homologue to the vertebrate neuropeptide cholecystokinin. J Biol Chem (1988) 263:12167–70. [PubMed] [Google Scholar]
- 6.Ivy AC, Oldberg E. A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol (1928) 86:559–613. [Google Scholar]
- 7.Jorpes JE, Mutt V. Secretin, cholecystokinin and pancreozymin. In: Jorpes JE, Mutt V, editors. Handbook of Experimental Pharmacology. (Vol. 34), New York: Springer Verlag; (1973). p. 1–179. [Google Scholar]
- 8.Rehfeld JF. Cholecystokinin. Best Pract Res Clin Endocrinol Metab (2004) 18:569–86. 10.1016/j.beem.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 9.Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem (2007) 7:1154–65. 10.2174/156802607780960483 [DOI] [PubMed] [Google Scholar]
- 10.Rehfeld JF. How to measure cholecystokinin in plasma? Gastroenterology (1984) 87:434–8. [PubMed] [Google Scholar]
- 11.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem (1998) 44:991–1001. [PubMed] [Google Scholar]
- 12.Rehfeld JF. How to measure cholecystokinin in tissue, plasma and cerebrospinal fluid. Regul Pept (1998) 78:31–9. 10.1016/S0167-0115(98)00133-5 [DOI] [PubMed] [Google Scholar]
- 13.Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem (1968) 6:156–62. 10.1111/j.1432-1033.1968.tb00433.x [DOI] [PubMed] [Google Scholar]
- 14.Mutt V, Jorpes JE. Hormonal polypeptides of the upper intestine. Biochem J (1971) 125:57–8. 10.1042/bj1250057P [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morley JS, Tracy HJ, Gregory RA. Structure-function relationships in the active C-terminal tetrapeptide sequence of gastrin. Nature (1965) 207:1356–9. 10.1038/2071356a0 [DOI] [PubMed] [Google Scholar]
- 16.Rehfeld JF, Hansen HF. Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J Biol Chem (1986) 261:5832–40. [PubMed] [Google Scholar]
- 17.Rehfeld JF, Johnsen AH, Ødum L, Bardram L, Schifter S, Scopsi L. Non-sulphated cholecystokinin in human medullary thyroid carcinomas. J Endocrinol (1990) 124:501–6. 10.1677/joe.0.1240501 [DOI] [PubMed] [Google Scholar]
- 18.Bonetto V, Jörnvall H, Andersson M, Renlund S, Mutt V, Sillard R. Isolation and characterization of sulfated and nonsulfated forms of cholecystokinin-58 and their action on gallbladder contraction. Eur J Biochem (1999) 264:336–40. 10.1046/j.1432-1327.1999.00599.x [DOI] [PubMed] [Google Scholar]
- 19.Reeve JR, Jr, Liddle RA, McVey DC, Vigna SR, Solomon TE, Keire DA. Identification of nonsulfated cholecystokinin-58 in canine intestinal extracts and its biological properties. Am J Physiol Gastrointest Liver Physiol (2004) 287:G326–33. 10.1152/ajpgi.00520.2003 [DOI] [PubMed] [Google Scholar]
- 20.Agersnap M, Rehfeld JF. Nonsulfated cholecystokinins in the small intestine of pigs and rats. Peptides (2015) 71:121–7. 10.1016/j.peptides.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 21.Bardram L, Rehfeld JF. Processing-independent radioimmunoanalysis: a general analytical principle applied to progastrin and its products. Anal Biochem (1988) 175:537–43. 10.1016/0003-2697(88)90580-5 [DOI] [PubMed] [Google Scholar]
- 22.Paloheimo LI, Rehfeld JF. A processing-independent assay for human procholecystokinin and its products. Clin Chim Acta (1994) 229:49–65. 10.1016/0009-8981(94)90228-3 [DOI] [PubMed] [Google Scholar]
- 23.Deschenes RJ, Haun RS, Funckes CL, Dixon JE. A gene encoding rat cholecystokinin. Isolation, nucleotide sequence, and promoter activity. J Biol Chem (1985) 260:1280–6. [PubMed] [Google Scholar]
- 24.Nielsen FC, Pedersen K, Hansen TV, Rourke IJ, Rehfeld JF. Transcriptional regulation of the human cholecystokinin gene: composite action of upstream stimulatory factor, Sp1, and members of the CREB/ATF-AP-1 family of transcription factors. DNA Cell Biol (1996) 15:53–63. 10.1089/dna.1996.15.53 [DOI] [PubMed] [Google Scholar]
- 25.Hansen TV. Cholecystokinin gene transcription: promoter elements, transcription factors and signaling pathways. Peptides (2001) 22:1201–11. 10.1016/S0196-9781(01)00443-0 [DOI] [PubMed] [Google Scholar]
- 26.Rourke IJ, Hansen TV, Nerlov C, Rehfeld JF, Nielsen FC. Negative cooperativity between juxtaposed E-box and cAMP/TPA responsive elements in the cholecystokinin gene promoter. FEBS Lett (1999) 448:15–8. 10.1016/S0014-5793(99)00320-8 [DOI] [PubMed] [Google Scholar]
- 27.Hansen TV, Rehfeld JF, Nielsen FC. Function of the C-36 to T polymorphism in the human cholecystokinin gene promoter. Mol Psychiatry (2000) 5:443–7. 10.1038/sj.mp.4000705 [DOI] [PubMed] [Google Scholar]
- 28.Hansen TV, Rehfeld JF, Nielsen FC. Mitogen-activated protein kinase and protein kinase A signaling pathways stimulate cholecystokinin transcription via activation of cyclic adenosine 3’, 5’-monophosphate response element-binding protein. Mol Endocrinol (1999) 13:466–75. 10.1210/mend.13.3.0257 [DOI] [PubMed] [Google Scholar]
- 29.Deavall DG, Raychowdhury R, Dockray GJ, Dimaline R. Control of CCK gene transcription by PACAP in STC-1 cells. Am J Physiol Gastrointest Liver Physiol (2000) 279:G605–12. [DOI] [PubMed] [Google Scholar]
- 30.Bernard C, Sutter A, Vinson C, Ratineau C, Chayvialle J, Cordier-Bussat M. Peptones stimulate intestinal cholecystokinin gene transcription via cyclic adenosine monophosphate response element-binding factors. Endocrinology (2001) 142:721–9. 10.1210/endo.142.2.7924 [DOI] [PubMed] [Google Scholar]
- 31.Gevrey JC, Cordier-Bussat M, Nemoz-Gaillard E, Chayvialle JA, Abello J. Co-requirement of cyclic AMP- and calcium-dependent protein kinases for transcriptional activation of cholecystokinin gene by protein hydrolysates. J Biol Chem (2002) 277:22407–13. 10.1074/jbc.M201624200 [DOI] [PubMed] [Google Scholar]
- 32.Hansen TV, Rehfeld JF, Nielsen FC. KCl and forskolin synergistically up-regulate cholecystokinin gene expression via coordinate activation of CREB and the co-activator CBP. J Neurochem (2004) 89:15–23. 10.1046/j.1471-4159.2003.02252.x [DOI] [PubMed] [Google Scholar]
- 33.Deschenes RJ, Lorenz L, Haun RS, Roos BR, Collier KJ, Dixon JE. Cloning and sequence analysis of cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A (1984) 81:726–30. 10.1073/pnas.81.3.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubler U, Chua AO, Hoffman BJ, Collier KJ, Eng J. Cloned cDNA to cholecystokinin mRNA predicts an identical preprocholecystokinin in pig brain and gut. Proc Natl Acad Sci U S A (1984) 81:4307–10. 10.1073/pnas.81.14.4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goltermann N, Rehfeld JF, Roigaard-Petersen H. In vivo biosynthesis of cholecystokinin in rat cerebral cortex. J Biol Chem (1980) 255:6181–5. [PubMed] [Google Scholar]
- 36.Jorpes JE, Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand (1966) 66:196–202. 10.1111/j.1748-1716.1966.tb03185.x [DOI] [PubMed] [Google Scholar]
- 37.Dockray GJ, Gregory RA, Huchinson JB, Harris JI, Runswick MJ. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature (1978) 274:711–3. 10.1038/274711a0 [DOI] [PubMed] [Google Scholar]
- 38.Reeve JR, Jr, Eysselein V, Walsh JH, Ben-Avram CM, Shively JE. New molecular forms of cholecystokinin. Microsequence analysis of forms previously characterized by chromatographic methods. J Biol Chem (1986) 261:16392–7. [PubMed] [Google Scholar]
- 39.Rehfeld JF. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem (1978) 253:4022–30. [PubMed] [Google Scholar]
- 40.Agersnap M, Zhang MD, Harkany T, Hökfelt T, Rehfeld JF. Nonsulfated cholecystokinins in cerebral neurons. Neuropeptides (2016) 60:37–44. 10.1016/j.npep.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 41.Buffa R, Solcia E, Go VL. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology (1976) 70:528–30. [PubMed] [Google Scholar]
- 42.Larsson LI, Rehfeld JF. Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res (1979) 165:201–18. 10.1016/0006-8993(79)90554-7 [DOI] [PubMed] [Google Scholar]
- 43.Rehfeld JF. Procholecystokinin processing in the normal human anterior pituitary. Proc Natl Acad Sci U S A (1987) 84:3019–23. 10.1073/pnas.84.9.3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehfeld JF, Lindholm J, Andersen BN, Bardram L, Cantor P, Fenger M, et al. Pituitary tumors containing cholecystokinin. N Engl J Med (1987) 316:1244–7. 10.1056/NEJM198705143162004 [DOI] [PubMed] [Google Scholar]
- 45.Hökfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for co-existence of dopamine and CCK in meso-limbic neurons. Nature (1980) 285:476–8. 10.1038/285476a0 [DOI] [PubMed] [Google Scholar]
- 46.Crawley JN. Comparative distribution of cholecystokinin and other neuropeptides. Why is this peptide different from all other peptides? Ann N Y Acad Sci (1985) 448:1–8. 10.1111/j.1749-6632.1985.tb29900.x [DOI] [PubMed] [Google Scholar]
- 47.Fallon JH, Seroogy KB. The distribution and some connections of cholecystokinin neurons in the rat brain. Ann N Y Acad Sci (1985) 448:121–32. 10.1111/j.1749-6632.1985.tb29912.x [DOI] [PubMed] [Google Scholar]
- 48.Hendry SH, Jones EG, Beinfeld MC. Cholecystokinin-immunoreactive neurons in rat and monkey cerebral cortex make symmetric synapses and have intimate associations with blood vessels. Proc Natl Acad Sci U S A (1983) 80:2400–4. 10.1073/pnas.80.8.2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vizi SE, Bertaccini G, Impiciattore M, Knoll J. Evidence that acetylcholine released by gastrin and related peptides contributes to their effect on gastrointestinal motility. Gastroenterology (1973) 64:268–77. [PubMed] [Google Scholar]
- 50.Larsson LI, Rehfeld JF. Peptidergic and adrenergic innervation of pancreatic ganglia. Scand J Gastroenterol (1979) 14:433–7. [PubMed] [Google Scholar]
- 51.Rehfeld JF, Larsson LI, Goltermann N, Schwartz TW, Holst JJ, Jensen SL, et al. Neural regulation of pancreatic hormone secretion by the C-terminal tetrapeptide of CCK. Nature (1980) 284:33–8. 10.1038/284033a0 [DOI] [PubMed] [Google Scholar]
- 52.Dockray GJ, Gregory RA, Tracy HJ, Zhou WY. Transport of cholecystokinin-octapeptide-like immunoreactivity towards the gut in afferent vagal fibres in cat and dog. J Physiol (1981) 314:501–11. 10.1113/jphysiol.1981.sp013721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehfeld JF, Lundberg J. Cholecystokinin in feline vagal and sciatic nerves: concentration, molecular forms and transport velocity. Brain Res (1983) 275:341–7. 10.1016/0006-8993(83)90995-2 [DOI] [PubMed] [Google Scholar]
- 54.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest (1985) 75:1144–52. 10.1172/JCI111809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Himeno S, Tarui S, Kanayama S, Kuroshima T, Shinomura Y, Hayashi C, et al. Plasma cholecystokinin responses after ingestion of liquid meal and intraduodenal infusion of fat, amino acids, or hydrochloric acid in man: analysis with region specific radioimmunoassays. Am J Gastroenterol (1983) 78:703–7. [PubMed] [Google Scholar]
- 56.Dodd PR, Edwardson JA, Dockray GJ. The depolarisation-induced release of cholecystokinin octapeptide from rat synaptosomes and brain slices. Regul Pept (1980) 1:17–29. 10.1016/0167-0115(80)90003-8 [DOI] [Google Scholar]
- 57.Emson PC, Lee CM, Rehfeld JF. Cholecystokinin octapeptide: vesicular localization and calcium dependent release from rat brain in vitro. Life Sci (1980) 26:2157–63. 10.1016/0024-3205(80)90603-7 [DOI] [PubMed] [Google Scholar]
- 58.Rehfeld JF, Hansen HF, Larsson LI, Stengaard-Pedersen K, Thorn NA. Gastrin and cholecystokinin in pituitary neurons. Proc Natl Acad Sci U S A (1984) 81:1902–5. 10.1073/pnas.81.6.1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maton PN, Selden AC, Chadwick VS. Differential distribution of molecular forms of cholecystokinin in human and porcine small intestinal mucosa. Regul Pept (1984) 8:9–19. 10.1016/0167-0115(84)90024-7 [DOI] [PubMed] [Google Scholar]
- 60.Rehfeld JF, Holst JJ, Jensen SL. The molecular nature of vascularly released cholecystokinin from the isolated perfused porcine duodenum. Regul Pept (1982) 3:15–28. 10.1016/0167-0115(82)90003-9 [DOI] [PubMed] [Google Scholar]
- 61.Rehfeld JF, Sun G, Christensen T, Hillingsø JG. The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab (2001) 86:251–8. 10.1210/jcem.86.1.7148 [DOI] [PubMed] [Google Scholar]
- 62.Hornnes PJ, Kühl C, Holst JJ, Lauritzen KB, Rehfeld JF, Schwartz TW. Simultaneous recording of the gastro-entero-pancreatic hormonal peptide response to food in man. Metabolism (1980) 29:777–9. 10.1016/0026-0495(80)90203-6 [DOI] [PubMed] [Google Scholar]
- 63.Anagnostides AA, Chadwick VS, Selden AC, Barr J, Maton PN. Human pancreatic and biliary responses to physiological concentrations of cholecystokinin octapeptide. Clin Sci (Lond) (1985) 69:259–63. 10.1042/cs0690259 [DOI] [PubMed] [Google Scholar]
- 64.Kerstens PJ, Lamers CB, Jansen JB, de Jong AJ, Hessels M, Hafkenscheid JC. Physiological plasma concentrations of cholecystokinin stimulate pancreatic enzyme secretion and gallbladder contraction in man. Life Sci (1985) 36:565–9. 10.1016/0024-3205(85)90638-1 [DOI] [PubMed] [Google Scholar]
- 65.Solomon TE, Yamada T, Elashoff J, Wood J, Beglinger C. Bioactivity of cholecystokinin analogues: CCK-8 is not more potent than CCK-33. Am J Physiol (1984) 247:G105–11. [DOI] [PubMed] [Google Scholar]
- 66.Wank SA, Harkins R, Jensen RT, Shapira H, de Weerth A, Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc Natl Acad Sci U S A (1992) 89:3125–9. 10.1073/pnas.89.7.3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, et al. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci U S A (1992) 89:3605–9. 10.1073/pnas.89.8.3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen D, Zhao CM, Håkanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L. Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology (2004) 126:476–87. 10.1053/j.gastro.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 69.You ZB, Herrera-Marschitz M, Pettersson E, Nylander I, Goiny M, Shou HZ, et al. Modulation of neurotransmitter release by cholecystokinin in the neostriatum and substantia nigra of the rat: regional and receptor specificity. Neuroscience (1996) 74:793–804. 10.1016/0306-4522(96)00149-2 [DOI] [PubMed] [Google Scholar]
- 70.Honda T, Wada E, Battey JF, Wank SA. Differential gene expression of CCK(A) and CCK(B) receptors in the rat brain. Mol Cell Neurosci (1993) 4:143–54. 10.1006/mcne.1993.1018 [DOI] [PubMed] [Google Scholar]
- 71.Pisegna JR, de Weerth A, Huppi K, Wank SA. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Commun (1992) 189:296–303. 10.1016/0006-291X(92)91557-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee YM, Beinborn M, McBride EW, Lu M, Kolakowski LF, Jr, Kopin AS. The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J Biol Chem (1993) 268:8164–9. [PubMed] [Google Scholar]
- 73.Saillan-Barreau C, Dufresne M, Clerc P, Sanchez D, Corominola H, Moriscot C, et al. Evidence for a functional role of the cholecystokinin-B/gastrin receptor in human fetal and adult pancreas. Diabetes (1999) 48:2015–21. 10.2337/diabetes.48.10.2015 [DOI] [PubMed] [Google Scholar]
- 74.Shaw RA, Jones RS. The choleretic action of cholecystokinin and cholecystokinin octapeptide in dogs. Surgery (1978) 84:622–5. [PubMed] [Google Scholar]
- 75.Dyck WP, Martin GA, Ratliff CR. Influence of secretin and cholecystokinin on intestinal alkaline phosphatase secretion. Gastroenterology (1973) 64:599–602. [PubMed] [Google Scholar]
- 76.Dyck WP, Bonnet D, Lasater J, Stinson C, Hall FF. Hormonal stimulation of intestinal disaccharidase release in the dog. Gastroenterology (1974) 66:533–8. [PubMed] [Google Scholar]
- 77.Götze H, Götze J, Adelson JW. Studies on intestinal enzyme secretion; the action of cholecystokinin-pancreozymin, pentagastrin and bile. Res Exp Med (Berl) (1978) 173:17–25. 10.1007/BF01851370 [DOI] [PubMed] [Google Scholar]
- 78.Bragado MJ, Tashiro M, Williams JA. Regulation of the initiation of pancreatic digestive enzyme protein synthesis by cholecystokinin in rat pancreas in vivo. Gastroenterology (2000) 119:1731–9. 10.1053/gast.2000.20242 [DOI] [PubMed] [Google Scholar]
- 79.Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol (2001) 63:77–97. 10.1146/annurev.physiol.63.1.77 [DOI] [PubMed] [Google Scholar]
- 80.Rothman SS, Wells H. Enhancement of pancreatic enzyme synthesis by pancreozymin. Am J Physiol (1967) 213:215–8. [DOI] [PubMed] [Google Scholar]
- 81.Debas HT, Grossman MI. Pure cholecystokinin: pancreatic protein and bicarbonate response. Digestion (1973) 9:469–81. 10.1159/000197476 [DOI] [PubMed] [Google Scholar]
- 82.Soudah HC, Lu Y, Hasler WL, Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol (1992) 263:G102–7. [DOI] [PubMed] [Google Scholar]
- 83.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology (2001) 121:1380–90. 10.1053/gast.2001.29557 [DOI] [PubMed] [Google Scholar]
- 84.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology (2004) 127:957–69. 10.1053/j.gastro.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 85.Jensen SL, Rehfeld JF, Holst JJ, Nielsen OV, Fahrenkrug J, Schaffalitzky de Muckadell OB. Secretory effects of cholecystokinins on the isolated perfused porcine pancreas. Acta Physiol Scand (1981) 111:225–31. 10.1111/j.1748-1716.1981.tb06730.x [DOI] [PubMed] [Google Scholar]
- 86.Hermansen K. Effects of cholecystokinin (CCK)-4, nonsulfated CCK-8 and sulfated CCK-8 on pancreatic somatostatin, insulin, and glucagons secretion in the dog: studies in vitro. Endocrinology (1984) 114:1770–5. 10.1210/endo-114-5-1770 [DOI] [PubMed] [Google Scholar]
- 87.Otsuki M, Sakamoto C, Yuu H, Maeda M, Morita S, Ohki A, et al. Discrepancies between the doses of cholecystokinin or caerulein-stimulating exocrine and endocrine responses in perfused isolated rat pancreas. J Clin Invest (1979) 63:478–84. 10.1172/JCI109325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monstein HJ, Nylander AG, Salehi A, Chen D, Lundquist I, Håkanson R. Cholecystokinin-A and cholecystokinin-B/gastrin receptor mRNA expression in the gastrointestinal tract and pancreas of the rat and man. A polymerase chain reaction study. Scand J Gastroenterol (1996) 31:383–90. 10.3109/00365529609006415 [DOI] [PubMed] [Google Scholar]
- 89.Petersen H, Solomon T, Grossman MI. Effect of chronic pentagastrin, cholecystokinin, and secretin on pancreas of rats. Am J Physiol (1978) 234:E286–93. [DOI] [PubMed] [Google Scholar]
- 90.Schultzberg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, et al. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience (1980) 5:689–744. [DOI] [PubMed] [Google Scholar]
- 91.Gutiérrez JG, Chey WY, Dinoso VP. Actions of cholecystokinin and secretin on the motor activity of the small intestine in man. Gastroenterology (1974) 67:35–41. [PubMed] [Google Scholar]
- 92.Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature (1973) 245:323–5. 10.1038/245323a0 [DOI] [PubMed] [Google Scholar]
- 93.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science (1981) 213:1036–7. 10.1126/science.7268408 [DOI] [PubMed] [Google Scholar]
- 94.Reubi JC, Waser B, Gugger M, Friess H, Kleeff J, Kayed H, et al. Distribution of CCK1 and CCK2 receptors in normal and diseased human pancreatic tissue. Gastroenterology (2003) 125:98–106. 10.1016/S0016-5085(03)00697-8 [DOI] [PubMed] [Google Scholar]
- 95.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev (2006) 86:805–47. 10.1152/physrev.00014.2005 [DOI] [PubMed] [Google Scholar]
- 96.Rehfeld JF. Accumulation of nonamidated preprogastrin and preprocholecystokinin products in porcine pituitary corticotrophs. Evidence of post-translational control of cell differentiation. J Biol Chem (1986) 261:5841–7. [PubMed] [Google Scholar]
- 97.Reubi JC, Waser B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int J Cancer (1996) 67:644–7. [DOI] [PubMed] [Google Scholar]
- 98.Bardram L, Hilsted L, Rehfeld JF. Cholecystokinin, gastrin and their precursors in pheochromocytomas. Acta Endocrinol (1989) 120:479–84. [DOI] [PubMed] [Google Scholar]
- 99.Persson H, Ericsson A, Schalling M, Rehfeld JF, Hökfelt T. Detection of cholecystokinin in spermatogenic cells. Acta Physiol Scand (1988) 134:565–6. 10.1111/j.1748-1716.1998.tb08534.x [DOI] [PubMed] [Google Scholar]
- 100.Persson H, Rehfeld JF, Ericsson A, Schalling M, Pelto-Huikko M, Hökfelt T. Transient expression of the cholecystokinin gene in male germ cells and accumulation of the peptide in the acrosomal granule: possible role of cholecystokinin in fertilization. Proc Natl Acad Sci U S A (1989) 86:6166–70. 10.1073/pnas.86.16.6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schalling M, Persson H, Pelto-Huikko M, Odum L, Ekman P, Gottlieb C, et al. Expression and localization of gastrin messenger RNA and peptide in spermatogenic cells. J Clin Invest (1990) 86:660–9. 10.1172/JCI114758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aunapuu M, Roosaar P, Järveots T, Kurrikoff K, Kõks S, Vasar E, et al. Altered renal morphology in transgenic mice with cholecystokinin overexpression. Transgenic Res (2008) 17:1079–89. 10.1007/s11248-008-9204-5 [DOI] [PubMed] [Google Scholar]
- 103.Miyamoto S, Shikata K, Miyasaka K, Okada S, Sasaki M, Kodera R, et al. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: anti-inflammatory effect of cholecystokinin. Diabetes (2012) 61:897–907. 10.2337/db11-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Weerth A, Jonas L, Schade R, Schöneberg T, Wolf G, Pace A, et al. Gastrin/cholecystokinin type B receptors in the kidney: molecular, pharmacological, functional characterization, and localization. Eur J Clin Invest (1998) 28:592–601. 10.1046/j.1365-2362.1998.00310.x [DOI] [PubMed] [Google Scholar]
- 105.von Schrenck T, de Weerth A, Bechtel S, Eschenhagen T, Weil J, Wolf G, et al. Evidence for CCK(B) receptors in the guinea-pig kidney: localization and characterization by 125I-gastrin binding studies and by RT-PCR. Naunyn Schmiedebergs Arch Pharmacol (1998) 358:287–92. 10.1007/PL00005255 [DOI] [PubMed] [Google Scholar]
- 106.Okahata H, Nishi Y, Muraki K, Sumii K, Miyachi Y, Usui T. Gastrin/cholecystokinin-like immunoreactivity in human blood cells. Life Sci (1985) 36:369–73. 10.1016/0024-3205(85)90123-7 [DOI] [PubMed] [Google Scholar]
- 107.Sacerdote P, Breda M, Barcellini W, Meroni PL, Panerai AE. Age-related changes of beta-endorphin and cholecystokinin in human and rat mononuclear cells. Peptides (1991) 12:1353–6. 10.1016/0196-9781(91)90219-F [DOI] [PubMed] [Google Scholar]
- 108.De la Fuente M, Carrasco M, Del Rio M, Hernanz A. Modulation of murine lymphocyte functions by sulfated cholecystokinin octapeptide. Neuropeptides (1998) 32:225–33. 10.1016/S0143-4179(98)90041-5 [DOI] [PubMed] [Google Scholar]
- 109.Carrasco M, Del Rio M, Hernanz A, De la Fuente M. Inhibition of human neutrophil functions by sulfated and nonsulfated cholecystokinin octapeptides. Peptides (1997) 18:415–22. 10.1016/S0196-9781(96)00338-5 [DOI] [PubMed] [Google Scholar]
- 110.Meng AH, Ling YL, Zhang XP, Zhang JL. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World J Gastroenterol (2002) 8:712–7. 10.3748/wjg.v8.i4.712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li S, Ni Z, Cong B, Gao W, Xu S, Wang C, et al. CCK-8 inhibits LPS-induced IL-1beta production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock (2007) 27:678–86. 10.1097/shk.0b013e3180ze26dd [DOI] [PubMed] [Google Scholar]
- 112.Lay JM, Gillespie PJ, Samuelson LC. Murine prenatal expression of cholecystokinin in neural crest, enteric neurons, and enteroendocrine cells. Dev Dyn (1999) 216:190–200. [DOI] [PubMed] [Google Scholar]
- 113.Goetze JP, Johnsen AH, Kistorp C, Gustafsson F, Johnbeck CB, Rehfeld JF. Cardiomyocyte expression and cell-specific processing of procholecystokinin. J Biol Chem (2015) 290:6837–43. 10.1074/jbc.M114.622670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Madsen OD, Larsson LI, Rehfeld JF, Schwartz TW, Lernmark A, Labrecque AD, et al. Cloned cell lines from a transplantable islet cell tumor are heterogeneous and express cholecystokinin in addition to islet hormones. J Cell Biol (1986) 103:2025–34. 10.1083/jcb.103.5.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madsen OD, Karlsen C, Nielsen E, Lund K, Kofod H, Welinder B, et al. The dissociation of tumor-induced weight loss from hypoglycemia in a transplantable pluripotent rat islet tumor results in the segregation of stable alpha- and beta-cell tumor phenotypes. Endocrinology (1993) 133:2022–30. 10.1210/endo.133.5.8404649 [DOI] [PubMed] [Google Scholar]
- 116.Rehfeld JF, Federspiel B, Bardram L. A neuroendocrine tumor syndrome from cholecystokinin secretion. N Engl J Med (2013) 368:1165–6. 10.1056/NEJMc1215137 [DOI] [PubMed] [Google Scholar]
- 117.Rehfeld JF, Federspiel B, Agersnap M, Knigge U, Bardram L. The uncovering and characterization of a CCKoma syndrome in enteropancreatic neuroendocrine tumor patients. Scand J Gastroenterol (2016) 51:1172–8. 10.1080/00365521.2016.1183706 [DOI] [PubMed] [Google Scholar]
- 118.Reubi JC, Koefoed P, Hansen TV, Stauffer E, Rauch D, Nielsen FC, et al. Procholecystokinin as marker of human Ewing sarcomas. Clin Cancer Res (2004) 10:5523–30. 10.1158/1078-0432.CCR-1015-03 [DOI] [PubMed] [Google Scholar]
- 119.Oikonomou E, Buchfelder M, Adams EF. Cholecystokinin (CCK) and CCK receptor expression by human gliomas: evidence for an autocrine/paracrine stimulatory loop. Neuropeptides (2008) 42:255–65. 10.1016/j.npep.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 120.Camby I, Salmon I, Danguy A, Pasteels JL, Brotchi J, Martinez J, et al. Influence of gastrin on human astrocytic tumor cell proliferation. J Natl Cancer Inst (1996) 88:594–600. 10.1093/jnci/88.9.594 [DOI] [PubMed] [Google Scholar]
- 121.Rehfeld JF, van Solinge WW, Tos M, Thomsen J. Gastrin, cholecystokinin and their precursors in acoustic neuromas. Brain Res (1990) 530:235–8. 10.1016/0006-8993(90)91288-R [DOI] [PubMed] [Google Scholar]
- 122.Rehfeld JF. Cholecystokinin expression in tumors: biogenetic and diagnostic implications. Future Oncol (2016) 12:2135–47. 10.2217/fon-2015-0053 [DOI] [PubMed] [Google Scholar]