Abstract
Matrix metalloproteinases (MMPs) are induced from host tissues in response to Borrelia burgdorferi. Upregulation of MMPs may play a role in the dissemination of the organism through extracellular matrix tissues, but it can also result in destructive pathology. Although mice are a well-accepted model for Lyme arthritis, there are significant differences compared to human disease. We sought to determine whether MMP expression could account for some of these differences. MMP expression patterns following B. burgdorferi infection were analyzed in primary human chondrocytes, synovial fluid samples from patients with Lyme arthritis, and cartilage tissue from Lyme arthritis-susceptible and -resistant mice by using a gene array, real-time PCR, an enzyme-linked immunosorbent assay, and immunohistochemistry. B. burgdorferi infection significantly induced transcription of MMP-1, -3, -13, and -19 from primary human chondrocyte cells. Transcription of MMP-10 and tissue inhibitor of metalloprotease 1 was increased with B. burgdorferi infection, but protein expression was only minimally increased. The synovial fluid levels of MMPs from patients with high and low spirochete burdens were consistent with results seen in the in vitro studies. B. burgdorferi-susceptible C3H/HeN mice infected with B. burgdorferi showed induction of MMP-3 and MMP-19 but no other MMP or tissue inhibitor of metalloprotease. As determined by immunohistochemistry, MMP-3 expression was increased only in chondrocytes near the articular surface. The levels of MMPs were significantly lower in the more Lyme arthritis-resistant BALB/c and C57BL/6 mice. Differences between human and murine Lyme arthritis may be related to the lack of induction of collagenases, such MMP-1 and MMP-13, in mouse joints.
Borrelia burgdorferi is the causative agent of Lyme disease (39). It has been previously shown that B. burgdorferi does not produce exported proteases capable of digesting extracellular matrix proteins (17, 25). Instead, it appears to utilize proteases from its mammalian hosts to degrade extracellular matrix proteins, which allows it to disseminate. Previous studies have shown that B. burgdorferi binds host plasminogen and its activator urokinase and is able to induce production of matrix metalloproteases (MMPs), which are activated by bound plasmin (19, 22).
Human Lyme arthritis is an oligoarticular arthritis that develops late in the course of infection (39). Left untreated, B. burgdorferi infection can eventually lead to the development of an erosive arthritis with histopathologic similarities to rheumatoid arthritis (40). Studies in patients with rheumatoid arthritis have shown that an imbalance in the production of MMPs is likely to be responsible for degradation of the articular cartilage in such patients (15, 20). Among the MMPs which have been suggested to participate in cartilage degradation in patients with rheumatoid arthritis are collagenases (MMP-1, -8, and -13), stromelysins (MMP-3, -10, and -11), and gelatinases (MMP-2 and -9) (38).
Lin et al. have shown that the levels of MMP-1 and MMP-3 are elevated in the synovial fluid of patients with Lyme arthritis (27). The levels of MMP-1 and -3 are also elevated in in vitro models of Lyme arthritis, and MMP inhibition blocks cartilage degradation caused by infection with B. burgdorferi in these models (22, 27).
A full description of MMPs expressed in Lyme arthritis has not previously been reported. We were interested in whether the specific pattern of MMPs expressed in response to B. burgdorferi infection may affect the phenotypic expression of disease and explain differences between Lyme arthritis and rheumatoid arthritis and between human Lyme arthritis and animal models of the disease. Mice provide a well-accepted model of Lyme arthritis. However, interestingly, only certain strains of mice develop significant arthritis in response to B. burgdorferi infection, while other strains develop only mild or no arthritis (6, 7, 10, 41, 48). In this study, we report our findings for B. burgdorferi-induced MMP expression from chondrocytes grown in vitro, from human synovial fluid, and in cartilage obtained from arthritis-susceptible and -resistant strains of mice.
MATERIALS AND METHODS
Mice and B. burgdorferi infection.
C3H/HeN, C57BL/6, and BALB/c mice were purchased from Charles River Laboratories (Wilmington, Mass.) and Taconic (Germantown, N.Y.). The procedures used were reviewed and approved by the Tufts University Institutional Animal Care and Use Committee. Five-day-old mice were infected intradermally by needle inoculation with 1 × 104 B. burgdorferi strain N40 cells per mouse and were sacrificed 2 weeks postinfection. Cartilage was microdissected from the ankle joints by using a stereomicroscope, and total RNA was isolated by using Trizol (Invitrogen, Carlsbad, Calif.) according to the manufacturer's protocol. Successful infection of individual mice was confirmed by culturing ear samples in Barbour-Stoenner-Kelly medium and monitoring the growth of B. burgdorferi by dark-field microscopy.
Patients.
Synovial fluid was obtained from 18 patients with untreated Lyme arthritis and 15 patients with persistent Lyme arthritis after antibiotic therapy. The samples used have been previously described (27, 28). The conduct of this study was reviewed and approved by the Tufts-New England Medical Center Investigational Review Board. All patients were infected in the northeastern United States. All patients had oligoarticular arthritis involving one or both knees. All patients in both groups met the Centers for Disease Control clinical criteria for the diagnosis of Lyme disease. They had mono- or oligoarticular arthritis affecting at least one knee accompanied by a positive immunoglobulin G Western blot test for Lyme disease interpreted according to the CDC/ASTPHLD criteria (2). All 18 patients with untreated Lyme arthritis had a positive PCR test for B. burgdorferi DNA in synovial fluid, performed as described by Nocton et al. (32). All 15 synovial fluids from patients with persistent Lyme arthritis tested negative for B. burgdorferi DNA by PCR. The mean durations of symptoms at the time of sample collection were 2.2 years for the untreated group (range, 6 months to 6 years) and 1.7 years for the posttreatment group (range, 3 months to 6 years). All patients in the posttreatment group received at least 4 weeks of treatment with an antibiotic active against B. burgdorferi (the median number of courses of antibiotic was two). Samples were obtained after patients had completed their courses of antibiotics. All specimens were divided into aliquots and stored at −70°C until use.
cDNA microarray.
Total RNA was extracted from tissues or cells by using Trizol (Invitrogen) according to the manufacturer's instructions. Total RNAs were reverse transcribed to incorporate biotin-16-dUTP (Roche, Indianapolis, Ind.) in order to prepare labeled cDNA probes. Prehybridization and hybridization were carried out according to the manufacturer's recommendations by using a human and mouse GE array kit (Superarray Bioscience, Frederick, Md.), followed by chemiluminescent detection to detect bound probe on specific cDNAs and to determine the intensity. Images were scanned from the X-ray film, processed, and converted to numerical values by using the ScanAlyze software. The data were analyzed by using GEArray Analyzer (Superarray Bioscience). The human gene array contained oligonucleotides to detect MMP-1, -2, -3, -7, -8, -9, -10, -11, -12, -13, -14, -15, -16, -17, -19, -20, -24, and -26, as well as tissue inhibitor of matrix metalloprotease 1 (TIMP-1), -2, -3, and -4. The mouse gene array contained oligonucleotides to detect all of the same MMPs except MMP-1 (which is not found in mice) and MMP-26 and additional oligonucleotides to detect MMP-23 and mouse collagenase-like A (McolA) and McolB.
Quantitative reverse transcriptase PCR.
Total RNA was purified by using Trizol (Invitrogen) according to the manufacturer' instructions. First-strand synthesis of cDNA from total RNA was performed by using Improm II reverse transcriptase (Promega, Madison, Wis.) according to the manufacturer' instructions. Control reactions performed in the absence of reverse transcriptase were used to control for contamination by genomic DNA. cDNA samples contaminated by genomic DNA were discarded, and the original RNA was treated with DNase before the reverse transcriptase reaction was repeated. Quantitation of cDNA from specific mRNA transcripts was accomplished by real-time quantitative reverse transcriptase PCR (RT-PCR) (ABI7700; Applied Biosystems, Foster City, Calif.) by using SYBR Green technology (Quantitect Sybr Green PCR kit; QIAGEN) as previously described (13, 46). The primers used are listed in Table 1.
TABLE 1.
Primer sequences for real-time RT-PCR
| Primer | Directiona | Sequence | Product size (bp) |
|---|---|---|---|
| Human | |||
| MMP-1 | F | 5′-CTGAAGGTGATGAAGCAGCC-3′ | 427 |
| R | 5′-AGTCCAAGAGAATGGCCGAG-3′ | ||
| MMP-3 | F | 5′-TGTAGAAGGCACAATATGGGCAC-3′ | 263 |
| R | 5′-CAGTCACTTGTCTGTTGCACACG-3′ | ||
| MMP-10 | F | 5′-CACTCTACAACTCATTCACAGAGCT-3′ | 408 |
| R | 5′-CTTGGATAACCTGCTTGTACCTCAT-3′ | ||
| MMP-13 | F | 5′-GCATCTGGAGTAACCGTATTG-3′ | 329 |
| R | 5′-GTGGTGTGGGAAGTATCATCA-3′ | ||
| MMP-19 | F | 5′-CAGGCTCTCTATGGCAAGAA-3′ | 502 |
| R | 5′-GAGCTGCATCCAGGTTAGGT-3′ | ||
| TIMP-1 | F | 5′-CTGGCTTCTGGCATCCTGTTGTT-3′ | 247 |
| F | 5′-ATGGCGGGGGTGTAGACGAA-3′ | ||
| β-ACTIN | F | 5′-CCACACCTTCTACAATGAGCTGCG-3′ | 204 |
| R | 5′-CGGAGTCCATCACGATCCA-3′ | ||
| Mouse | |||
| MMP-3 | F | 5′-ATGAAAATGAAGGGTCTTCCGG-3′ | 108 |
| R | 5′-GCAGAAGCTCCATACCAGCA-3′ | ||
| MMP-13 | F | 5′-ATGCATTCAGCTATCCTGGCCA-3′ | 222 |
| R | 5′-AAGATTGCATTTCTCGGAGCCTG-3′ | ||
| MMP-14 | F | 5′-GGCCCAAGGCAGCAACTTCA-3′ | 246 |
| R | 5′-CCTCCGAACATTGGCCTTCATC-3′ | ||
| MMP-19 | F | 5′-GCAGCTGTGGCTGGCATTCT-3′ | 139 |
| R | 5′-GCCTGAAGTCATCAGCTCCTT-3′ | ||
| TIMP-1 | F | 5′-ATGATGGCCCCCTTTGCATCTC-3′ | 112 |
| R | 5′-TGCAGAAGGCTGTCTGTGGG-3′ | ||
| TIMP-4 | F | 5′-GTGCTGAGGCTGCTGGCTTT-3′ | 101 |
| R | 5′-CACTAGAGCCGAGTGGCAGA-3′ | ||
| NIDOGEN | F | 5′-CCAGCCACAGAATACCATCC-3′ | 153 |
| R | 5′-GGACATACTCTGCTGCCATC-3′ |
F, forward; R, reverse.
Primary cultures of human chondrocytes and infection with B. burgdorferi.
Primary human chondrocytes (HCs) from a healthy donor were purchased from Cell Applications (San Diego, Calif.) and were maintained in chondrocyte growth medium (Cell Applications) containing 10% fetal calf serum (FCS) at 37°C with 5% CO2. One day prior to infection, HCs were washed, and the culture medium was replaced with chondrocyte medium without FCS. A low-passage (passage 4 to 7) clonal isolate of B. burgdorferi sensu stricto, strain N40, was cultured in Barbour-Stoenner-Kelly medium (5, 23). Spirochetes were washed three times and resuspended in chondrocyte medium without FCS. Cell cultures at 70 to 85% confluence were infected with B. burgdorferi at a ratio of cells to spirochetes of 1:10 for various times. Cells were washed and harvested in cold phosphate-buffered saline, and the cell pellets were stored at −70°C until use.
ELISAs.
MMP-10, MMP-13, and TIMP-1 were detected in synovial fluid and in human chondrocyte culture extracts in culture medium by using enzyme-linked immunosorbent assay (ELISA) kits obtained from R & D Systems (Minneapolis, Minn.) and used according to the manufacturer's instructions.
Immunohistochemistry.
To examine MMP-3 expression, slides were immunostained with anti-mouse MMP-3 monoclonal antibody (R & D Systems). Briefly, tissues were fixed in Bowen's fixative for 24 h and then placed in 70% ethanol. The hind legs were skinned, split in half longitudinally in the center, processed for decalcification, and finally embedded in paraffin. The slides were processed and immunostained by the Histology Core Facility at New England Medical Center as previously described (30).
Statistical analysis.
Experiments were repeated three to five times as indicated below. The statistical significance between groups was analyzed by using the nonparametric Mann-Whitney U test. Differences were considered statistically significant when the P value was equal to or less than 0.05.
RESULTS
Pattern of MMP induction following B. burgdorferi infection in human chondrocytes.
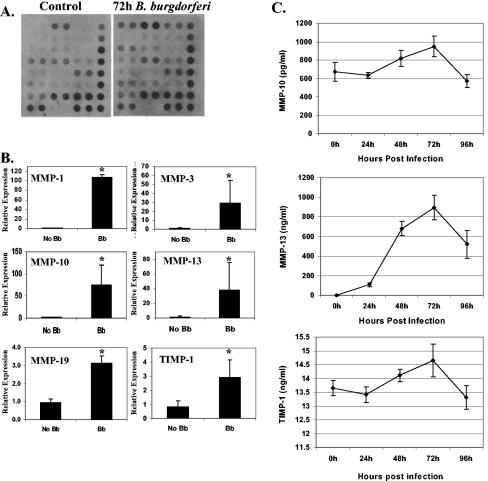
Although the roles of specific MMPs (MMP-1, -3, -8, and -9) in Lyme arthritis have been examined, the full range of MMPs that are induced in response to infection with B. burgdorferi has not previously been reported. In order to establish the pattern of MMP expression in HC following B. burgdorferi infection, primary chondrocytes were incubated with B. burgdorferi or sham incubated for 72 h. After preparation of total RNA and conversion to cDNA, changes in gene transcription were measured by using a gene array (Fig. 1A), which contained oligonucleotides to detect 18 MMPs and four TIMPs. Data for genes whose levels were found to be elevated in the array were subsequently confirmed by using real-time RT-PCR (Fig. 1B). The findings of real-time RT-PCR correlated well with the findings obtained with the gene array. Consistent with previously reported studies, the levels of both MMP-1 and MMP-3 were significantly elevated (P < 0.05) in the presence of B. burgdorferi. The levels of MMP-10 (elevated 48-fold; P ≤ 0.05) and MMP-13 (elevated 28-fold; P ≤ 0.05) and, to a lesser degree, the levels of MMP-19 (elevated 3.3. fold; P ≤ 0.05) and TIMP-1 (elevated 2.9-fold; P ≤ 0.05) were also significantly elevated in the presence of B. burgdorferi.
FIG. 1.
Pattern of MMP expression in human chondrocytes following B. burgdorferi infection. Primary human chondrocytes were infected with B. burgdorferi (Bb) (107 organisms) or sham infected for 72 h. The MMP expression pattern was examined by gene array analysis (A) and was confirmed by real-time RT-PCR (B) and ELISA (C). The experiments were repeated three times. The error bars indicate standard deviations. An asterisk indicates that the P value is ≤0.05.
It has been shown previously that the production of MMP-1 and MMP-3, as measured by ELISA, closely paralleled the increases in gene transcription with B. burgdorferi incubation (13). To determine whether increases in the levels of gene transcripts also resulted in increases in protein expression for the remaining MMPs, we performed ELISAs for MMP-10, MMP-13, and TIMP-1 (Fig. 1C). Commercially prepared ELISAs for MMP-19 are not currently available. The data for induction of MMP-13 paralleled the RT-PCR data, with an increase from undetectable to a concentration of more than 800 ng/ml, and the level peaked at 72 h. Only small increases in the amounts of MMP-10 and TIMP-1 (<30 and < 8%, respectively, at the peak) were seen, and these amounts decreased to the baseline levels by 96 h. These values did not correlate well with the 48-fold and ∼3-fold induction of MMP-10 and TIMP-1 determined by RT-PCR, suggesting that there was posttranscriptional control for expression of these proteins.
Cell type specificity of MMP induction by B. burgdorferi.
To determine the specificity of MMP induction by B. burgdorferi for HC, we also performed experiments using other primary cell cultures. We examined primary human dermal fibroblasts, which may contact B. burgdorferi during the initial entry into the body, and primary human pulmonary artery smooth muscle cells, which are found in an area where no pathology has been reported in Lyme disease. Neither of these cell lines showed induction of any MMP as determined by gene array analysis, nor was any of the MMPs induced from chondrocytes as determined by real-time RT-PCR (data not shown).
Expression of MMPs from synovial fluids of patients with treated and untreated Lyme arthritis.
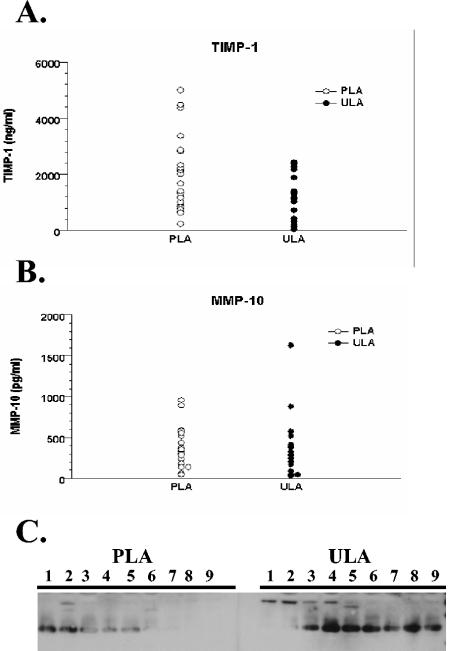
We examined synovial fluid from 19 patients with untreated Lyme arthritis and 27 patients with persistent Lyme arthritis after antibiotic therapy for the presence of MMP-10, MMP-13, MMP-19, and TIMP-1, which were found to be induced from chondrocytes incubated with B. burgdorferi. Because it is not possible to obtain normal synovial fluid, which is present in only minute amounts in healthy joints, we compared the amounts of different MMPs in patients with untreated and treated Lyme arthritis. Patients with untreated Lyme arthritis had higher burdens of B. burgdorferi, as detected by PCR for B. burgdorferi DNA. Synovial fluid samples from antibiotic-treated Lyme arthritis patients with persistent arthritis were all PCR negative for B. burgdorferi DNA. These samples had previously been used to study expression of MMP-1, MMP-3, MMP-8, and MMP-9 in Lyme arthritis (27). We used commercial ELISA kits for detecting MMP-10, MMP-13, and TIMP-1. Consistent with the ELISA data from the human chondrocyte studies, no differences were seen in the levels of MMP-10 or TIMP-1 in the synovial fluids of patients with untreated or persistent arthritis (Fig. 2). Due to specimen availability, ELISAs for MMP-13 could be performed only at dilutions higher than the recommended dilutions, and the levels were undetectable for all patients. The MMP-19 levels were compared by Western blotting. MMP-19 is secreted as a 58-kDa zymogen. The enzyme is rapidly activated to a 55-kDa form, to a 45- to 47-kDa form, and then finally to the active 28-kDa form. By Western blotting, we did not detect either the 58-kDa zymogen or the 55-kDa form in any patient. We did detect a strong band corresponding to the active 28-kDa form in 78% of the untreated Lyme arthritis patients; in only 22% of the patients with persistent Lyme arthritis was active MMP-19 detectable at the level seen in untreated patients, and another 33% of the patients had very weakly detectable enzyme. A strong 45- to 47-kDa band was detected for 56% of the untreated Lyme arthritis patients, but this band was not detected for any of the patients in the persistent Lyme arthritis group. Thus, a substantially higher proportion of patients with untreated Lyme arthritis than with persistent Lyme arthritis had evidence of either the active 28-kDa form or the intermediate 45- to 47-kDa form of MMP-19.
FIG. 2.
MMP expression in the synovial fluid of patients with untreated Lyme arthritis (ULA) and treated Lyme arthritis (PLA). Synovial fluid from 27 patients with treated Lyme arthritis and 19 patients with untreated Lyme arthritis were examined for expression of TIMP-1 (A) and MMP-10 (B) by ELISA. MMP-19 expression was determined by Western blotting. Equal volumes of samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotted onto Immobilon membranes, and probed with anti-human MMP-19 antibody. (C) Representative samples electrophoresed on a single blot. The 28- and 45- to 47-kDa bands are shown.
Induction of MMPs in murine cartilage.
Mice are a commonly utilized animal model for Lyme arthritis. However, there are notable differences between mouse Lyme arthritis and human Lyme arthritis. Although all mice can be infected with B. burgdorferi, only certain inbred strains of mice develop significant arthritis. Furthermore, even strains of mice that develop arthritis typically resolve their arthritis after 8 to 12 weeks, and there are few long-term sequelae. In order to determine whether differences in MMP expression may play a role in the development of arthritis in the murine model of Lyme disease, we examined the joints of a highly arthritis-susceptible strain of mice, C3H/HeN, for MMP expression. Because the amount of cartilage decreases with age, mice were infected at a very young age (<1 week) to maximize the amount of cartilage present at the time of sacrifice and to allow separation of cartilage tissue from bone and surrounding synovial tissue. Mice were infected with B. burgdorferi or sham infected by needle injection. By 2 weeks, the B. burgdorferi-infected mice were clearly distinguishable from the sham-infected mice by grossly visible swelling of the ankle joints. As the young mice were still growing, it was not possible to accurately determine changes in ankle diameter related to infection. After 2 weeks, the mice were sacrificed. One ankle joint was used for histological examination, and the other was utilized for RNA extraction. The histology of joints in C3H/HeN mice infected with B. burgdorferi has been described well previously. The results of the histological examination of joints from our B. burgdorferi-infected mice were consistent with prior reports (7). All infected mice showed moderate to severe synovitis with thickening of the synovial lining, inflammatory infiltration, and pockets of neo-bone formation. No cartilage degradation was seen in any of the infected mice.
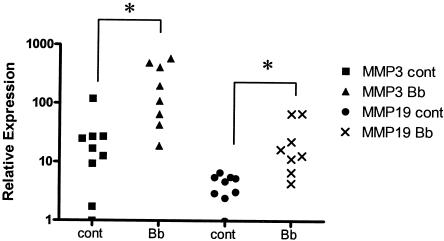
For RNA extraction, the joints were microdissected to separate cartilage from synovial tissue. Total RNA was extracted from the tissue, and the levels of gene transcription were measured by using a commercial gene array containing oligonucleotides for 22 mouse MMPs and TIMPs. All genes which showed a threefold or greater difference in expression as determined by the gene array were confirmed by real-time RT-PCR. No increase in expression of any MMP or TIMP was seen with synovial tissue fractions of our samples. In contrast, in cartilage tissue the level of MMP-3 was found to be elevated ninefold in B. burgdorferi-infected mice compared with uninfected mice. The level of MMP-19 was also found to be significantly elevated in infected mouse cartilage (6.3-fold; P = 0.00156) (Fig. 3). The level of no other MMP was found to be significantly elevated (data not shown). Based on prior studies of human synovial fluid, which showed an increase in the MMP-1 level in the synovial fluid of humans infected with B. burgdorferi, we also checked for expression of the McolA and McolB genes. Although it has been suggested previously that the mouse genome does not encode an orthologue of human MMP-1, McolA does exhibit 58.5% identity with human MMP-1 and is capable of degrading type I and II fibrillar collagens (4). A second potential mouse orthologue of human MMP-1, McolB, which like McolA is encoded in the A1-A2 region of chromosome 9, was not shown to be enzymatically active. Transcription of neither McolA nor McolB was elevated in the cartilage of mice infected with B. burgdorferi.
FIG. 3.
Pattern of MMP expression in B. burgdorferi-infected C3H/HeN mice. Five-day-old C3H/HeN mice were infected with B. burgdorferi (104 cells) and sacrificed 2 weeks postinfection. Ankle joint cartilage was microdissected, and total RNA was harvested. cDNA from total RNA was analyzed for MMP expression by using a gene array (data not shown) and then real-time RT-PCR. Expression of MMP-3 and expression of MMP-19 were significantly induced in infected mice (n = 8) compared to sham-infected controls (n = 9). cont, control; Bb, B. burgdorferi. *, P < 0.01.
Localization of MMP-3 expression in the joints of B. burgdorferi-infected mice.
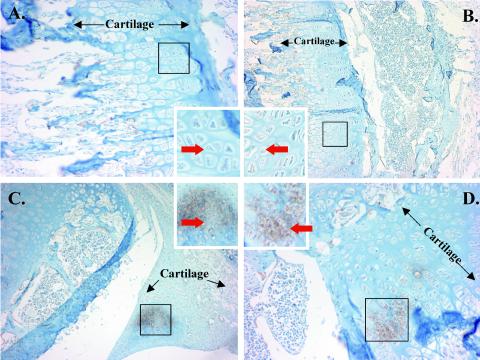
We also confirmed the expression of MMP-3 in B. burgdorferi-infected mouse joints by immunohistochemistry (Fig. 4). There was patchy staining for MMP-3 in infected animals around chondrocyte cells located near the surface of the articular cartilage. No staining for MMP-3 was seen around tendons, ligaments, or bone or in the inflamed synovium. The patterns of MMP-3 staining in infected and uninfected mice were clearly distinguishable, and no areas of increased deposition of stain were seen in uninfected mouse joints.
FIG. 4.
Localization of MMP-3 expression in vivo in murine joints. Eight C3H/HeN mice were infected with B. burgdorferi N40 or sham infected by needle inoculation. The mice were sacrificed after 2 weeks. Expression of MMP-3 was determined by immunohistochemical staining by using a monoclonal antibody to MMP-3. The brown areas enclosed in boxes in B. burgdorferi-infected mice (C and D) show deposition of stain, indicating the presence of MMP-3. Similar areas in uninfected mice (A and B) are also enclosed in boxes to show the absence of staining for MMP-3. The red arrows indicate examples of chondrocytes. The black arrows delimit areas that include resting and proliferating cartilage. The insets are magnified views of the areas in the boxes. Representative slides from two infected mice and two uninfected mice are shown.
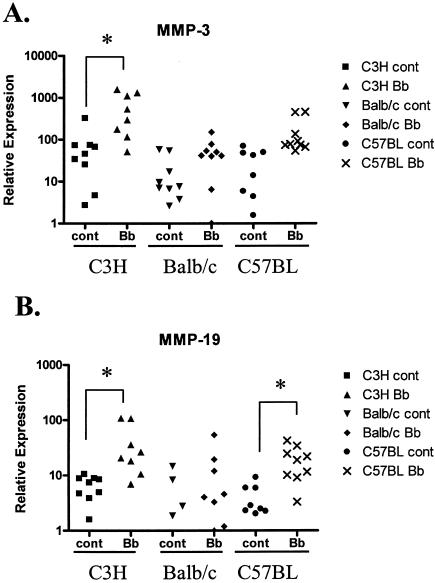
Comparison of MMP expression following B. burgdorferi infection in different strains of mice.
We were also interested in examining whether differences in the severity of arthritis seen in different mouse strains could be related to the level of MMP induction. We compared MMP expression in three very well characterized strains of mice, C3H/HeN, BALB/c, and C57BL/6. The severity of arthritis with B. burgdorferi infection was greatest in the C3H/HeN mice. Both BALB/c and C57BL/6 mice were more resistant to the development of arthritis and developed only mild to moderate amounts of arthritis.
Again, all mice were infected with B. burgdorferi by needle injection at an age of <1 week. The infections were again allowed to progress for 2 weeks before animals were sacrificed and tissues were harvested. None of the B. burgdorferi-infected BALB/c mice showed any grossly observable swelling of any joint when they were compared to sham-infected mice. Cartilage from joints from infected BALB/c mice did not show increases in the level of any MMP or TIMP, including MMP-3 and MMP-19, compared with controls as determined by both gene array analysis (data not shown) and real-time RT-PCR (Fig. 5). In contrast, in C57BL/6 mice there was noticeable swelling of the rear ankle joints, although the swelling was less than that in C3H/HeN mice. The level of MMP-3 in the joints of infected C57BL/6 mice trended toward an increase compared with controls, although the difference was not significant (P = 0.059). However, the increases in MMP-3 transcription in the joints of infected C3H/HeN were significantly greater than those in C57BL/6 mice (P = 0.0014). The level of MMP-19 was increased in B. burgdorferi-infected C57BL/6 mice (sixfold increase; P = 0.000165), which is similar to what was seen in C3H/HeN mice (Fig. 5). The levels of MMP-19 were not significantly different in C3H/HeN and C57BL/6 mice. Again, the levels of no other MMPs or TIMPs were found to be elevated in the joints of infected C57BL/6 mice.
FIG. 5.
Comparison of MMP expression following B. burgdorferi infection in different strains of mice. C3H/HeN, BALB/c, and C57BL/6 mice were infected with B. burgdorferi strain N40 or sham infected and sacrificed 2 weeks postinfection. Ankle joint cartilage was microdissected, and total RNA was harvested. cDNA generated from total RNA was analyzed for MMP expression by real-time RT-PCR for MMP-3 (A) and MMP-19 (B). cont, control; Bb, B. burgdorferi. *, P < 0.01.
DISCUSSION
MMPs have been shown to play a role in cartilage degradation and breakdown in various types of arthritis (1, 26, 33, 34, 43, 44). In this study, we sought to develop a comprehensive picture of MMP induction by B. burgdorferi. Based on previous reports showing that there was good correlation of in vitro chondrocyte models with human samples, we applied gene array technology to an in vitro chondrocyte model of Lyme arthritis in order to focus our studies. Our results show that compared with other causes of arthritis (1, 26, 33, 34, 43, 44), B. burgdorferi appears to induce only a very limited number of MMPs (MMP-1, -3, -13, and -19). MMP-10 and TIMP-1 transcripts were induced by incubation with B. burgdorferi, but there were only slight increases in actual protein expression. The lack of TIMP induction is important because in pathological situations, increases in MMP levels are typically out of proportion to increases in TIMP levels (3, 31, 49); this appears to be the case with exposure to B. burgdorferi, where the levels of MMP-1 and MMP-3 transcripts are elevated 30- to 107-fold, whereas TIMP-1 expression is increased only 2.9-fold.
Other cell types have also been reported to produce MMPs in response to incubation with B. burgdorferi. Human peripheral blood monocytes, neutrophils, and human keratinocyte cells incubated with B. burgdorferi have been shown to produce pro-MMP-9, which has been postulated to play a role in the dissemination of the organism through skin and blood vessels (19). In our studies performed with primary human fibroblasts and human pulmonary artery cells, we did not find induction of any MMPs following B. burgdorferi incubation. This suggests that the induction of MMPs by B. burgdorferi is cell specific. Fibroblasts and pulmonary artery cells contain the machinery to produce at least some MMPs (MMP-1, -2, and -19 and TIMP-1, etc.) (18, 21); the lack of induction in the presence of B. burgdorferi suggests either that these cells may lack a specific receptor for recognizing B. burgdorferi or that other critical elements in B. burgdorferi-related induction pathways of MMPs are absent. Cell-specific induction of MMPs may help to explain why pathological inflammation and degradation are seen in certain tissues but not in others despite the fact that B. burgdorferi is typically widely disseminated and can be recovered from multiple sites.
We used the results of our in vitro studies to guide our studies of synovial fluid from patients with Lyme arthritis. We compared the levels of MMP-10, -13, and -19 and TIMP-1 in synovial fluids from patients with untreated Lyme arthritis and patients with persistent Lyme arthritis. In patients with untreated Lyme arthritis, the mechanism of arthritis is clearly due to the presence of the organism. The cause of persistent Lyme arthritis is less clear. Four basic hypotheses have been proposed: persistent infection, retained spirochetal antigens, infection-induced autoimmunity resulting from T-cell epitope autoimmunity, and nonspecific bystander activation (41). Regardless of the mechanism, the most important point for our studies is that these patients clearly have lower (if any) bacterial burdens in the joint. The levels of MMP-1 and MMP-3 have previously been shown to be elevated in untreated patient synovial fluids, whereas the levels of MMP-8 and MMP-9 were elevated in patients with persistent disease (27). Consistent with the ELISA data in our time course studies of expression, no differences in MMP-10 or TIMP-1 were seen for the different groups of patients. The level of MMP-19 was found to be increased in the synovial fluid of patients with untreated Lyme arthritis, as would be predicted by our in vitro studies.
We next sought to use our data to better understand the similarities and differences between human and murine Lyme arthritis. Mice are a well-established model for Lyme arthritis. Differences in the development of Lyme arthritis in different strains of mice have been extensively studied at the level of the host immune response to B. burgdorferi (6-9, 12, 36, 37, 47, 48). There appear to be at least two separate mechanisms controlling the development of arthritis in the different strains of mice. In BALB/c mice, the severity of arthritis appears to be related to spirochete burden. C57BL/6 mice appear to have a mechanism for resistance to Lyme arthritis, which is independent of spirochete burden. Immunocompetent mice with both BALB/c and C57BL/6 backgrounds typically develop milder arthritis than C3H/HeN mice (12, 14, 24, 35-37, 45, 47, 50).
The expression of MMPs and other end effector molecules in cartilage degradation in murine Lyme arthritis has not been reported previously. We found that MMP-3 expression closely paralleled the severity of arthritis in the three strains of mice. Similar to humans with Lyme arthritis, C3H/HeN mice showed clear increases in the MMP-3 levels in the joints. C57BL/6 mice showed lower, nonsignificant increases in the levels of MMP-3, and BALB/c mice, which did not develop clinical arthritis, showed no increases in the levels of MMP-3. Also, C3H/HeN and C57BL/6 mice showed significant increases in the levels of MMP-19, while BALB/c mice did not. Both MMP-3 and MMP-19 are active in cleaving multiple components of the extracellular matrix (16, 42). In addition, MMP-3 can activate other MMPs and is thought to play a critical role in the development of human rheumatoid arthritis. The patchy localization of MMP-3 induction and the restriction to chondrocytes near the articular surface of cartilage are consistent with the hypothesis that direct contact with the organism is responsible for MMP-3 induction. The lack of MMP-3 induction from tissue other than chondrocytes in our immunohistochemical studies confirmed our RT-PCR studies with mouse joint tissue and previously reported in vitro studies (27) which showed that only chondrocytes in the joint respond to B. burgdorferi infection with MMP-3 induction.
While murine Lyme arthritis has many similarities to human Lyme arthritis, there are some notable differences. First, murine Lyme arthritis typically develops within the first 2 to 4 weeks of infection and resolves over an 8- to 12-week period after infection, although B. burgdorferi remains present long after this period in the absence of treatment (9, 11). In contrast, human Lyme arthritis generally develops later in the course of disease and can persist or recur intermittently for years in the absence of therapy (41). Certain inbred strains of mice have been reported to develop late recurrence of arthritis in the first year after infection, but the recurrence is usually much milder than the original arthritis. Additionally, humans may develop an antibiotic treatment-resistant arthritis after antibiotic therapy, which has not been reported in mice. Arthritis-susceptible mice (i.e., C3H/HeN mice) develop predominantly tenosynovitis and tendonitis and have few long-term sequelae once the arthritis has resolved. Humans also develop prominent synovitis, but they can also develop true articular arthritis with permanent cartilage degradation and erosions. It is tempting to speculate that differences in MMP expression may account for some of the differences in the course of arthritis. The level of MMP-1, an enzyme that is closely associated with the progression of rheumatoid arthritis, was found to be elevated in humans with Lyme arthritis and in in vitro models of Lyme arthritis. MMP-13, another collagenase, was also induced from human chondrocytes infected with B. burgdorferi, but the level was not increased in joints of mice infected with B. burgdorferi. While stromelysins (such as MMP-3), gelatinases, and other MMPs can degrade multiple components of the cartilage extracellular matrix (29), the components targeted by these enzymes are typically quickly regenerated. Irreparable cartilage damage occurs only with the degradation of the interstitial collagens in the cartilage matrix. Collagenases, such as MMP-1 and MMP-13, are thought to play the major role in degradation of the interstitial collagens, and the absence of these enzymes in the joints of B. burgdorferi-infected mice may explain some of the differences between murine and human Lyme arthritis.
In summary, we found that MMP induction in response to B. burgdorferi is very specific and is limited to only certain tissue types and to a very limited subset of MMPs. The expression patterns of MMPs in mouse joints and in human joints appear to be similar, with the exception of the collagenases MMP-1 and MMP-13. Future studies with genetically altered mice expressing human MMP-1 may provide further insight which confirms the role of MMP-1 in the phenotypic differences between human and murine Lyme arthritis.
Acknowledgments
We thank Annette Shepard-Barry for her help with the immunohistochemistry studies.
This work was supported by the following grants from the National Institutes of Health: grants R01AI44240, R01 AI50043, and U01AI058266 to L.T.H. and grant R01AR20358 to A.C.S.
Editor: D. L. Burns
REFERENCES
- 1.Ahrens, D., A. E. Koch, R. M. Pope, M. Stein-Picarella, and M. J. Niedbala. 1996. Expression of matrix metalloproteinase 9 (96-kd gelatinase B) in human rheumatoid arthritis. Arthritis Rheum. 39:1576-1587. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 3.Baker, A. H., D. R. Edwards, and G. Murphy. 2002. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 115:3719-3727. [DOI] [PubMed] [Google Scholar]
- 4.Balbin, M., A. Fueyo, V. Knauper, J. M. Lopez, J. Alvarez, L. M. Sanchez, V. Quesada, J. Bordallo, G. Murphy, and C. Lopez-Otin. 2001. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J. Biol. Chem. 276:10253-10262. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold, S. W. 1991. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J. Infect. Dis. 163:419-420. (Letter.) [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 8.Barthold, S. W., and M. de Souza. 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172:778-784. [DOI] [PubMed] [Google Scholar]
- 9.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 10.Barthold, S. W., K. D. Moody, G. A. Terwilliger, P. H. Duray, R. O. Jacoby, and A. C. Steere. 1988. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J. Infect. Dis. 157:842-846. [DOI] [PubMed] [Google Scholar]
- 11.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 13.Behera, A. K., C. M. Thorpe, J. M. Kidder, W. Smith, E. Hildebrand, and L. T. Hu. 2004. Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways. Infect. Immun. 72:2864-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, C. R., and S. L. Reiner. 1999. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 67:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawston, T. E., E. Mercer, M. de Silva, and B. L. Hazleman. 1984. Metalloproteinases and collagenase inhibitors in rheumatoid synovial fluid. Arthritis Rheum. 27:285-290. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborti, S., M. Mandal, S. Das, A. Mandal, and T. Chakraborti. 2003. Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 253:269-285. [DOI] [PubMed] [Google Scholar]
- 17.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasu, M. R., R. E. Barrow, M. Spies, and D. N. Herndon. 2003. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns 29:527-531. [DOI] [PubMed] [Google Scholar]
- 19.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2001. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 69:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravallese, E. M., J. M. Darling, A. L. Ladd, J. N. Katz, and L. H. Glimcher. 1991. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 34:1076-1084. [DOI] [PubMed] [Google Scholar]
- 21.Hieta, N., U. Impola, C. Lopez-Otin, U. Saarialho-Kere, and V. M. Kahari. 2003. Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J. Investig. Dermatol. 121:997-1004. (See comments.) [DOI] [PubMed] [Google Scholar]
- 22.Hu, L. T., M. A. Eskildsen, C. Masgala, A. C. Steere, E. C. Arner, M. A. Pratta, A. J. Grodzinsky, A. Loening, and G. Perides. 2001. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 44:1401-1410. [DOI] [PubMed] [Google Scholar]
- 23.Hu, L. T., G. Perides, R. Noring, and M. S. Klempner. 1995. Binding of human plasminogen to Borrelia burgdorferi. Infect. Immun. 63:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurtenbach, U., C. Museteanu, J. Gasser, U. E. Schaible, and M. M. Simon. 1995. Studies on early events of Borrelia burgdorferi-induced cytokine production in immunodeficient SCID mice by using a tissue chamber model for acute inflammation. Int. J. Exp. Pathol. 76:111-123. [PMC free article] [PubMed] [Google Scholar]
- 25.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, R. Hu, S. A. Limentani, and R. A. Rogers. 1995. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 171:1258-1265. [DOI] [PubMed] [Google Scholar]
- 26.Lark, M. W., E. K. Bayne, J. Flanagan, C. F. Harper, L. A. Hoerrner, N. I. Hutchinson, I. I. Singer, S. A. Donatelli, J. R. Weidner, H. R. Williams, R. A. Mumford, and L. S. Lohmander. 1997. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J. Clin. Investig. 100:93-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, B., J. M. Kidder, R. Noring, A. C. Steere, M. S. Klempner, and L. T. Hu. 2001. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J. Infect. Dis. 184:174-180. [DOI] [PubMed] [Google Scholar]
- 28.Lin, B., R. Noring, A. C. Steere, M. S. Klempner, and L. T. Hu. 2000. Soluble CD14 levels in the serum, synovial fluid, and cerebrospinal fluid of patients with various stages of Lyme disease. J. Infect. Dis. 181:1185-1188. [DOI] [PubMed] [Google Scholar]
- 29.Mandal, M., A. Mandal, S. Das, T. Chakraborti, and C. Sajal. 2003. Clinical implications of matrix metalloproteinases. Mol. Cell. Biochem. 252:305-329. [DOI] [PubMed] [Google Scholar]
- 30.Matsuse, H., A. K. Behera, M. Kumar, H. Rabb, R. F. Lockey, and S. S. Mohapatra. 2000. Recurrent respiratory syncytial virus infections in allergen-sensitized mice lead to persistent airway inflammation and hyperresponsiveness. J. Immunol. 164:6583-6592. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed, F. F., D. S. Smookler, and R. Khokha. 2003. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann. Rheum. Dis. 62:ii43-ii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi, K., R. Kawai, M. Hara, Y. Okada, S. Tachibana, and Y. Ogura. 1996. Increased matrix metalloproteinases as possible cause of osseoarticular tissue destruction in long-term haemodialysis and beta 2-microglobulin amyloidosis. Virchows Arch. 428:37-46. [DOI] [PubMed] [Google Scholar]
- 34.Okada, Y., H. Nagase, E. D. Harris, Jr., D. J. Taylor, N. T. Cheung, P. T. Dawes, L. C. Tetlow, M. Lees, Y. Ogata, D. E. Woolley, T. Morodomi, J. J. Enghild, K. Suzuki, A. Yasui, I. Nakanishi, G. Salvesen, D. Ahrens, A. E. Koch, R. M. Pope, M. Stein-Picarella, M. J. Niedbala, K. Ohashi, R. Kawai, M. Hara, S. Tachibana, Y. Ogura, B. L. Gruber, D. Sorbi, D. L. French, M. J. Marchese, G. J. Nuovo, R. R. Kew, L. A. Arbeit, S. Chubinskaya, K. Huch, K. Mikecz, G. Cs-Szabo, K. A. Hasty, K. E. Kuettner, A. A. Cole, U. Machein, W. Conca, O. Lindy, Y. T. Konttinen, T. Sorsa, Y. Ding, S. Santavirta, A. Ceponis, C. Lopez-Otin, M. W. Lark, E. K. Bayne, J. Flanagan, C. F. Harper, L. A. Hoerrner, N. I. Hutchinson, I. I. Singer, S. A. Donatelli, J. R. Weidner, H. R. Williams, R. A. Mumford, and L. S. Lohmander. 1987. Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J. Rheumatol. 14:41-42. [PubMed] [Google Scholar]
- 35.Pennington, P. M., C. D. Allred, C. S. West, R. Alvarez, and A. G. Barbour. 1997. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect. Immun. 65:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaible, U. E., M. D. Kramer, C. Museteanu, G. Zimmer, H. Mossmann, and M. M. Simon. 1989. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J. Exp. Med. 170:1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaible, U. E., R. Wallich, M. D. Kramer, C. Museteanu, and M. M. Simon. 1991. A mouse model for Borrelia burgdorferi infection: pathogenesis, immune response and protection. Behring Inst. Mitt. 88:59-67. [PubMed] [Google Scholar]
- 38.Shaw, T. 2000. Inhibition of matrix metalloproteinases in rheumatoid arthritis. Oxford University Press, Oxford, United Kingdom.
- 39.Steere, A. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 40.Steere, A. C., P. H. Duray, and E. C. Butcher. 1988. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 31:487-495. [DOI] [PubMed] [Google Scholar]
- 41.Steere, A. C., and L. Glickstein. 2004. Elucidation of Lyme arthritis. Nat. Rev. Immunol. 4:143-152. [DOI] [PubMed] [Google Scholar]
- 42.Stracke, J. O., M. Hutton, M. Stewart, A. M. Pendas, B. Smith, C. Lopez-Otin, G. Murphy, and V. Knauper. 2000. Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. J. Biol. Chem. 275:14809-14816. [DOI] [PubMed] [Google Scholar]
- 43.Tchetverikov, I., L. R. Lard, J. DeGroot, N. Verzijl, J. M. TeKoppele, F. C. Breedveld, T. W. Huizinga, and R. Hanemaaijer. 2003. Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Ann. Rheum. Dis. 62:1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchibori, M., Y. Nishida, I. Tabata, H. Sugiura, H. Nakashima, Y. Yamada, and N. Ishiguro. 2004. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in pigmented villonodular synovitis suggests their potential role for joint destruction. J. Rheumatol. 31:110-119. [PubMed] [Google Scholar]
- 45.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, X. G., J. M. Kidder, J. P. Scagliotti, M. S. Klempner, R. Noring, and L. T. Hu. 2004. Analysis of differences in the functional properties of the substrate binding proteins of the Borrelia burgdorferi oligopeptide permease (opp) operon. J. Bacteriol. 186:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 48.Weis, J. J., L. Yang, K. P. Seiler, and R. M. Silver. 1997. Pathological manifestations in murine Lyme disease: association with tissue invasion and spirochete persistence. Clin. Infect. Dis. 25:S18-24. [DOI] [PubMed] [Google Scholar]
- 49.Woessner, J. F., Jr. 2002. MMPs and TIMPs—an historical perspective. Mol. Biotechnol. 22:33-49. [DOI] [PubMed] [Google Scholar]
- 50.Zimmer, G., U. E. Schaible, M. D. Kramer, G. Mall, C. Museteanu, and M. M. Simon. 1990. Lyme carditis in immunodeficient mice during experimental infection of Borrelia burgdorferi. Virchows Arch. A Pathol. Anat. Histopathol. 417:129-135. [DOI] [PubMed] [Google Scholar]