Abstract
The intentional use of Bacillus anthracis, the etiological agent of anthrax, as a bioterrorist weapon in late 2001 made our society acutely aware of the importance of developing, testing, and stockpiling adequate countermeasures against biological attacks. Biodefense vaccines are an important component of our arsenal to be used during a biological attack. However, most of the agents considered significant threats either have been eradicated or rarely infect humans alive today. As such, vaccine efficacy cannot be determined in human clinical trials but must be extrapolated from experimental animal models. This article reviews the efficacy and immunogenicity of human anthrax vaccines in well-defined animal models and the progress toward developing a rugged immunologic correlate of protection. The ongoing evaluation of human anthrax vaccines will be dependent on animal efficacy data in the absence of human efficacy data for licensure by the U.S. Food and Drug Administration.
INTRODUCTION
Bacillus anthracis, the etiological agent of anthrax, is at the top of the list of bacteria, viruses, and toxins that may be used as a biological weapon against the United States of America. B. anthracis spores can be easily produced, can be aerosolized as a dry powder with a particle size of approximately 4 μm, and can remain viable in the environment for extended periods (41, 67).
B. anthracis is a gram-positive, nonhemolytic, spore-forming, facultative anaerobic bacterium. It causes three forms of anthrax, i.e., inhalational, cutaneous, and gastrointestinal, depending on the route of inoculation (19, 67). The prevailing model of infection proposes that spores introduced into the body by abrasion, inhalation, or ingestion are phagocytosed by macrophages and subsequently carried to regional lymph nodes. In this model, B. anthracis spores germinate while inside macrophages and become vegetative cells, which are then released from the macrophage and multiply in the lymphatic system (37). Multiplying vegetative cells enter the bloodstream and may reach levels of >108 CFU/ml (19).
Virulent B. anthracis harbors two plasmids, designated pX01 and pX02 (63). The pX01 plasmid contains a 44.8-kb pathogenicity island that carries the three toxin genes, cya, lef, and pagA (65). The pX02 plasmid carries three capsule genes, capA, capB, and capC, and a gene associated with depolymerization of the capsule, dep (50). The pX01 gene pagA encodes an 83-kDa protein known as protective antigen (PA). Vegetative B. anthracis cells produce two binary exotoxins. Lethal toxin (LT) is formed by the binding of the lef gene product, lethal factor (LF), to receptor-bound PA (53). By analogy, edema toxin (ET) is formed by the binding of the cya gene product, edema factor (EF), to receptor-bound PA (53). LF is a zinc metalloprotease that inactivates mitogen-activated protein kinase kinases (MAPKK) (21). The cleavage of MAPKKs prevents activation of p38 mitogen-activated protein kinase (MAPK), which subsequently prevents the induction of certain NF-κB target genes including genes necessary to prevent apoptosis of activated macrophages (66). NF-κB activation is also important for the up-regulation of cytokine genes involved in early innate immune responses (29). ET is an adenylate cyclase that increases intracellular cyclic AMP (cAMP) levels in susceptible cells. ET alters water homeostasis and is responsible for the edema that frequently occurs in patients with B. anthracis infection (19, 79, 82). By increasing cAMP concentrations in neutrophils, ET inhibits phagocytosis and blocks particulate as well as phorbol myristate acetate-induced respiratory burst responses (64). ET also differentially regulates macrophage responsiveness to lipopolysaccharide-induced production of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) (40). Based on these compelling in vitro and in vivo data, it has been proposed that B. anthracis LT and ET contribute to the ability of the bacteria to evade host innate immune responses by deregulating proinflammatory cytokines, inducing apoptosis in activated macrophages, inhibiting phagocytosis, and suppressing the respiratory burst in polymorphonuclear cells (36, 40, 48, 64).
Inhalation Anthrax
Inhalation anthrax was first described during the last half of the 19th century (52). This mysterious disease became a significant problem among British wool sorters and German ragpickers following the introduction of mohair from Turkey and alpaca from Peru to Europe in the 1830s (13). Inhalation anthrax has a clinical incubation period of 1 to 6 days, during which nonspecific symptoms of malaise, fatigue, myalgia, and fever develop. These symptoms usually persist for 2 or 3 days, and in some cases there is a short period of clinical improvement. This is followed by the sudden onset of increasing respiratory distress with dyspnea, stridor, cyanosis, increased chest pain, and sweating. Respiratory distress is typically followed by the rapid onset of shock and death within 24 to 36 h. Mortality rates of 50 to 100% have historically been reported despite appropriate treatment (13, 19, 25, 47). Only 18 cases of inhalation anthrax were reported in the United State between 1900 and 1978 (13), with the combined number of human cutaneous, gastrointestinal, and inhalation anthrax cases dropping from 20-50 cases per year in the 1950s to only 5 cases between 1980 and 1996 (14). This decrease is due to several factors including the use of human cell-free anthrax vaccines among high-risk individuals, decreased utilization of imported animal products, improved industrial hygiene, and a decrease in the incidence of anthrax in the domestic animal population (14). Owing to the infectiousness of B. anthracis spores acquired the respiratory route and the high mortality of inhalation anthrax, the primary concern with anthrax in the postindustrial era is its use as a biological weapon (25, 41). A minimum of 17 nations and some autonomous terrorist groups are thought to have or have had offensive biological weapons programs (41). As such, inhalation anthrax is considered a substantial threat despite the extremely low incidence in the general population (16).
Human Anthrax Vaccines and Correlates of Protection
Ongoing efficacy testing of the currently licensed human cell-free anthrax vaccine (AVA-Biothrax; BioPort, Lansing, Mich.) and next-generation anthrax vaccines will rely on the identification of rugged correlates of protection for humans extrapolated from animal models. The U.S. Food and Drug Administration (FDA) amended its drug and biological product regulations in 2002 in order to allow appropriate efficacy studies of animals to provide substantial evidence of effectiveness of new drug and biological products used to reduce or prevent the toxicity of chemical, biological, radiological, or nuclear substances (24a). This new rule will apply only when adequate, well-controlled clinical studies of humans cannot be ethically conducted and field efficacy studies are not feasible, as is the case with inhalation anthrax (24a). Table 1 lists the four criteria that must be met before a new drug or biological product can be licensed based on animal efficacy studies. There are several reasons why the nonhuman primate and rabbit models of inhalation anthrax are considered ideal for evaluating vaccine efficacy and determining a correlate of protection for human beings. (i) The enhancement of survival in vaccinated non-human primates and rabbits challenged with B. anthracis spores is clearly related to the prevention of mortality in exposed humans. (ii) More than one species that is expected to react with a response predictive for human beings is used. (iii) There are considerable data on the molecular basis of the toxicity of B. anthracis LT and ET. However, the roles that humoral and cell-mediated immune responses play in protecting vaccinated hosts remain to be determined. In addition, comparative information on the relationship between the nature and level of protective immune responses in vaccinated surrogate animal models and in vaccinated humans is lacking. Furthermore, additional characterization of the pathophysiological mechanism for the toxicity of B. anthracis LT and ET in vivo is also required. Therefore, the success of vaccine efficacy studies conducted with rhesus macaques and rabbits will partially depend on our ability to measure humoral and cell-mediated immune responses in these two models, which can be correlated with protection against inhalation anthrax and are predictive of efficacy in humans.
TABLE 1.
FDA criteria for animal efficacy studies of human biologics and drugsa
| Criterion | Description |
|---|---|
| 1 | There is a reasonably well understood pathophysiological mechanism for the toxicity of the chemical, biological, radiological, or nuclear substance and its amelioration or prevention by the product. |
| 2 | The effect is demonstrated in more than one animal species expected to react with a response predictive for humans, unless the effect is demonstrated in a single animal species that represents a sufficiently well-characterized animal model (meaning that the model has been adequately evaluated for its responsiveness) for predicting the response in humans. |
| 3 | The animal study end point is clearly related to the desired benefit in humans, which is generally the enhancement of survival or prevention of major morbidity. |
| 4 | The data or information on the pharmacokinetics and pharmacodynamics of the product or other relevant data or information in animals and humans is sufficiently well understood to allow selection of an effective dose in humans, and it is therefore reasonable to expect the effectiveness of the product in animals to be a reliable indicator of its effectiveness in humans. |
Data from reference 24a.
ANIMAL MODELS OF INHALATION ANTHRAX
B. anthracis Challenge Isolates
The selection of B. anthracis isolates used in animal models of anthrax vaccine efficacy is dependent on several factors including the virulence of the isolate, the animal species, and the type of anthrax vaccine (cell-free versus modified live spore vaccine). Nonhuman primates, guinea pigs, and rabbits are susceptible to B. anthracis infection when exposed to aerosolized spores by inhalation. The rhesus macaque has a long history as a model of human inhalation anthrax, dating back to the 1950s (7, 38, 86). Guinea pigs and rabbits have also been used to test the efficacy and potency of various cell-free anthrax vaccine preparations (3, 78, 86). The initial studies performed in the United States, designed to determine the efficacy of the first human cell-free anthrax vaccines in rhesus macaques, utilized the Vollum isolate of B. anthracis to challenge the animals (86). Early studies of inhalation anthrax in macaques performed in the United Kingdom utilized the M.36 B. anthracis isolate, which was derived from the Vollum isolate by repeated passage in monkeys (7, 20, 38, 56). Auerbach and Wright were the first to report that vaccination with cell-free anthrax vaccine produced variable protection in guinea pigs challenged with various B. anthracis isolates except for the Vollum isolate (3). Further analysis of the comparative efficacy of both modified live spore and cell-free anthrax vaccines in guinea pigs revealed that a modified live spore vaccine could protect guinea pigs against multiple isolates of B. anthracis while the cell-free anthrax vaccine provided good protection only against the B. anthracis Vollum isolate and Vollum derivatives (56). Table 2 lists survival data for guinea pigs vaccinated with the cell-free anthrax vaccine or the modified live spore vaccine and challenged with six virulent B. anthracis isolates. Fellows et al. also examined the efficacy of the cell-free anthrax vaccine in guinea pigs challenged with 100 50% lethal doses (LD50) (Ames isolate equivalents) of 8 human B. anthracis isolates, 21 animal B. anthracis isolates, and 4 environmental B. anthracis isolates inoculated intramuscularly (22). As expected, survival rates varied by B. anthracis isolate from 6 to 100%, indicating that protection induced by the cell-free anthrax vaccine was limited in guinea pigs to only a few B. anthracis isolates. Because of these studies, the B. anthracis Ames isolate was referred to as a vaccine-resistant isolate and was selected as the challenge isolate for most of the vaccine efficacy studies performed in the United States and the United Kingdom after 1986. Furthermore, the guinea pig is not suitable as an animal model for testing the efficacy of cell-free anthrax vaccines due to the limited protection afforded by these vaccines in this species. However, guinea pigs continue to play a role in determining the virulence of B. anthracis isolate in vivo and in testing the potency of newly manufactured lots of human cell-free anthrax vaccines prior to efficacy studies with larger and higher-order species such as rabbits or nonhuman primates. We, along with other researchers, have observed that the virulence of particular isolates can vary over time and between laboratories (unpublished data). Coker et al. have hypothesized that virulence among pX01- and pX02-containing isolates of B. anthracis may be due to the clonality of the bacteria and the plasmid copy number (15). Complete characterization and standardization of the challenge isolates used for vaccine efficacy studies may be required to interpret the results.
TABLE 2.
Survival of guinea pigs after immunization with the human cell-free anthrax vaccine or Sterne live-spore vaccinea
| Challenge isolate | Vaccine efficacy (% survival)
|
|||
|---|---|---|---|---|
| Saline | Cell-free vaccine | Saline | Sterne spore vaccine | |
| Vollum | 0 | 100 | ||
| Vollum 1B | 13 | 100 | 40 | 100 |
| Amesb | 17 | 0 | 0 | 100 |
| 17T5c | 0 | 17 | 0 | 88 |
| NHd | 0 | 33 | 0 | 100 |
| VHe | 0 | 50 | 17 | 100 |
Data from reference 56.
Ames isolated from a cow; United States 1980.
17T5 isolated from a Kudu; South Africa 1957.
NH isolated from a human case; New Hampshire 1957.
VH isolated from a human case; South Africa 1952.
The 50% Lethal Dose
The LD50 of a toxin or biological agent is defined as an estimate of the dose at which death occurs in 50% of the population to which the agent was administered. The LD50 of B. anthracis varies both by animal species and by the route of administration within a species. In addition, it often varies between strains or isolates of B. anthracis and may change over time because of passage in the laboratory.
While many statistical methods may be used to estimate the LD50, two of the more common statistical methods are dose-response models, such as probit analysis and the Spearman-Karber Method (23, 35). Published LD50s are estimates of the “true” value for a given animal model, particular B. anthracis isolate, and route of administration. Briefly, in the probit dose-response model, an underlying assumption is that each member of the population has a threshold tolerance for the agent being tested and that, if exposed to that level or greater, the individual responds or dies. A normal distribution is used to model the population tolerances to a specified dose. Dose-response models may be used to estimate other percentiles of the dose-response curve, as well as the LD50. The Spearman-Karber method is a nonparametric statistical method that provides a point estimate of the LD50 with confidence intervals, which makes no assumptions about the distribution of the population survival probabilities. Confidence intervals are typically reported side by side with an established LD50, with the 95% confidence interval being the most common. The 95% confidence interval is interpreted as the probability (0.95) that the interval straddles the population LD50 (i.e., an interval in which we are reasonably confident that the “true” value will fall somewhere within those limits) (24). A more detailed explanation of these two statistical methods for determining LD50 values can be found in references 23 and 35.
The reported LD50 of B. anthracis spores in rabbits and nonhuman primates are presented in Table 3. The inhalation LD50s reported for the Ames and Vollum isolates of B. anthracis in rhesus macaques and cynomolgus monkeys are comparable (5.0 × 104 to 6.2 × 104), with the exception of the data reported by Glassman (31). The specific B. anthracis isolate was not reported and may be partially responsible for the low LD50 (4.1 × 103) reported by Glassman for the cynomolgus monkey. The LD50 for the chimpanzee was determined from the information reported by Albrink and Goodlow, using the Spearman-Karber method (2). The 95% confidence interval could not be determined due to the small number of animals used on the study. LD50s for other routes of administration or different isolates of B. anthracis in nonhuman primates are not available. The single reported inhalation LD50 for the Ames isolate of B. anthracis in rabbits is approximately twofold higher than the reported inhalation LD50 in nonhuman primates; this may not be significant, given that the upper limit of the 95% confidence interval for the B. anthracis Ames isolate in cynomolgus monkeys overlaps with the rabbit LD50 (88). Limitations on these published LD50s include the following the data (i) were generated several decades ago in some cases, (ii) the age of the animals varied between studies, (iii) various spore preparations and production methods were used, (iv) the health status of the animals was unknown or poorly documented, (v) some of the nonhuman primates were used in previous non-anthrax-related studies, and (vi) there was no standardized method for the generation, quantification, and particle sizing of the aerosol.
TABLE 3.
Published LD50s for rabbits and nonhuman primates
| Animal |
B. anthracis challenge
|
LD50 (CFU) | 95% confidence interval | Method | Reference | |
|---|---|---|---|---|---|---|
| Isolate | Route | |||||
| Rabbit | Ames | Subcutaneous | 1.6 × 103 | NRa | Probit | 88 |
| Rabbit | Ames | Inhalation | 1.1 × 105 | NR | Probit | 88 |
| Rhesus macaque | Ames | Inhalation | 5.5 × 104 | NR | Probit | 46 |
| Rhesus macaque | Vollum | Inhalation | 5.0 × 104 | NR | Probit | 27 |
| Cynomolgus | Ames | Inhalation | 6.2 × 104 | (3.5-11.0) × 104 | Probit | 83 |
| Cynomolgus | NR | Inhalation | 4.1 × 103 | (2.0-8.6) × 103 | SKc | 31 |
| Chimpanzee | Vollum | Inhalation | 3.7 × 105 | NDb | NR | 2 |
NR, not reported.
ND, could not be calculated due to the small sample size.
SK, Spearman-Karber method.
Pathology
The characteristics of infection with B. anthracis in humans, as seen by gross and microscopic observations, have been reproduced in multiple animal models. The most thorough documentation of the pathology in human anthrax cases revolves around the Sverdlovsk epidemic of 1979, in which a large quantity of B. anthracis spores was released from a military installation in the former Soviet Union (62). In addition, several sporadic cases of inhalation anthrax in humans have been documented over the years (6, 10, 18, 39). The comparison of the documented pathologic changes in human infection with those due to experimentally induced anthrax in laboratory animals can provide useful information concerning the suitability of a species for a model.
The gross and microscopic observations of human inhalation anthrax focus primarily on mediastinal, hemic-lymphatic, and pulmonary changes. Changes in the brain associated with hemorrhagic meningitis have also been a focus of pathologic study. Gross mediastinal lesions in human beings consist primarily of edema and hemorrhage, with similar changes within the parenchyma of mediastinal lymph nodes. This finding is considered typical; however, its presence can be variable (1, 34). Histologically, these findings appear as a fibrin-rich fluid exudation within the connective tissue of the mediastinum as well as “low-pressure” hemorrhage (34). Mediastinal lymph nodes often exhibit hemorrhage and necrosis characterized by lymphocytolysis. The presence of gram-positive bacilli has been demonstrated in these lesions by conventional staining and immunohistologic techniques. Vasculitis within the mediastinal lymph nodes is characterized by fibrinoid necrosis and infiltration by neutrophils and histiocytes.
Gross splenic pathology in cases of human infection is variable and can be insignificant. Histologically, the spleens have exhibited lymphocytolysis within the periarteriolar lymphoid sheaths and the lymphoid follicles. Variable severity between the lymphoid follicles (B-cell rich) and the periarteriolar lymphoid sheaths has been documented (1). Moderate neutrophil infiltration has been observed, as well as the presence of extracellular and intracellular bacilli. Vasculitis appears to be a minimal characteristic in the spleen.
The literature describes a great deal of variation in the pulmonary lesions associated with inhalation anthrax. Some of this variability appears to be associated with preexisting pathology in the pulmonary parenchyma. Clinically, anthrax patients with preexisting illnesses, such as chronic obstructive pulmonary disease and pulmonary fibrosis, have been described (6). Acute bronchopneumonia was described histologically in a significant percentage of the Sverdlovsk cases (62). However, the association of pneumonia with infection by B. anthracis in antibiotic-treated and untreated humans is poorly characterized, since primary infection of the lung parenchyma is usually absent. The gross findings in the lungs are commonly unspectacular, being limited to hemorrhage, edema, and atelectasis, with no apparent change in consistency and weight. Histologically, mild fibrinous exudate, hemorrhage, and alveolar histiocytosis are predominant, along with some characteristics of interstitial pneumonia, characterized by interstitial fibrin deposition. Bacilli have been identified in alveolar air spaces, with a majority being found within the alveolar exudate. It has been suggested that this accumulation of bacilli is hematogenous and is due to rupture from the interstitium rather than aerogenous deposition (28, 34). Vasculitis is typically fibrinoid but minimal.
Grossly, multifocal to coalescing areas of hemorrhage often form the extent of the neural lesions. Histologically, minimal to mild fibrin exudation, acute low-pressure hemorrhage, and infiltration by neutrophils, histiocytes, and small numbers of lymphocytes and plasma cells is documented. Mild diffuse neuronal necrosis has been observed (34). Bacillary infiltration appears to be limited to the intravascular and Virchow-Robins space.
Lesions in other organs besides the lymphoid tissues, lungs, and brain have been described, but many appear to be secondary to shock and agonal changes. The presence of bacilli in the sinusoids and glomerular capillaries appears to the most significant finding, further demonstrating the presence of septicemia.
The significance of the vasculitis found in multiple organ systems is uncertain. The histopathologic characteristics appear to be uniform among organs, indicating a generalized condition. Fibrinoid vascular necrosis is often associated with severe endothelial damage but is not a specific finding in generalized bacterial septicemia. In the Sverdlovsk outbreak, vasculitis was a variable finding depending on the organ system; however, in certain tissues, such as the lungs, vasculitis was a significant finding. The etiology of this vasculitis is not apparent. It is possible that it is induced directly by anthrax toxins or is secondary to cytokine release.
Inhalation anthrax has been studied in a variety of animal models including guinea pigs, rabbits, rhesus macaques, cynomolgus macaques, and chimpanzees. It is important to note that many of the gross and microscopic lesions found in humans with inhalation anthrax are similar to those found in experimental animal models of inhalation anthrax, suggesting a shared pathogenesis. However, variations do exist among animal models.
The experimental pathology of anthrax in primates is the most thoroughly documented. The gross findings of primate inhalation anthrax reflect the documented human lesions. Mediastinal enlargement due to different degrees of edema and hemorrhage is consistent in chimpanzees, rhesus macaques, and cynomolgus monkeys; however, in the last of these, this lesion was found in less than 40% of the experimental group (83). The lesions in hemic-lymphatic organs consist primarily of grossly observable hemorrhagic lymphadenitis. Histologically, these lesions appear as lymphocytolysis and hemorrhage with intralesional bacilli, similar to the lymphadenopathy in humans. However, splenic pathology was different between human beings and nonhuman primates, since all of the nonhuman primates studied demonstrated splenomegaly. In addition, the severity of the neutrophilic inflammation and the fibrin exudation appears to be more severe in the spleens of nonhuman primates. These lesions are in addition to the lymphoid follicular necrosis, which takes place in both nonhuman primates and humans. The pulmonary findings in primates are remarkably similar to those in humans. It is interesting that preexisting disease in these experimental animals appears to compound the anthrax-associated lesions. The presence of Pneumonyssus simicola in the lungs of rhesus macaques, as reported by Gleiser et al., may enhance the phagocytosis of B. anthracis spores by alveolar macrophages or allow the bacilli easier access to the systemic circulation (32). This mirrors our earlier statement regarding preexisting injury in lungs of humans and susceptibility to pulmonary B. anthracis infection. However, other studies of rhesus macaques have shown a lack of tropism to preexisting lesions, suggesting that specific lung lesions may not increase the susceptibility to infection. The meningeal and cerebral lesions in rhesus and cynomolgus macaques, when present, appear similar to those described in humans. The clinical signs associated with inhalation anthrax in rhesus macaques, cynomolgus monkeys, and chimpanzees have been described as nonspecific and include lethargy, anorexia, and depression (2, 27, 28, 83). The proportion of nonhuman primates with inhalation anthrax that develop overt clinical signs associated with meningitis is considerably smaller than would be expected, considering that meningitis is often a frequent histologic finding in these animals (28, 34, 83). In some reports, mild perivascular suppurative encephalitis was a microscopic feature in addition to the characteristic fibrin exudation and hemorrhage. In all organs with lesions, bacilli were seen in touch impressions, with routine hematoxylin and eosin staining, or by immunohistochemistry. Other lesions of interest seen in nonhuman primates but not in humans have been noted. Multifocal myocardial necrosis has been seen infrequently in cynomolgus monkeys (83). Gastrointestinal lesions have ranged from mild to moderate hemorrhage and occasional coagulation necrosis in cynomolgus monkeys to transmural acute colitis with necrotizing vasculitis. Models of inhalation anthrax for pure pathologic studies with nonprimate laboratory animals are infrequently documented in the literature. A model utilizing rabbits has been described recently (22, 55, 68, 88). Studies of guinea pigs and dogs were described approximately 50 years ago. Although some of the lesions observed in rabbits were similar to those found in primates, the lesions in rabbits appear to be less severe (88). These lesions include mediastinal hemorrhage, splenomegaly, and typical pulmonary changes. Encephalitis is minimal to nonexistent. This discrepancy may be attributed to the rapid progression of disease in this species. Presumably, the inflammation and necrosis do not have time to develop in rabbits, in contrast to primates.
ANIMAL MODELS OF ANTHRAX VACCINE EFFICACY
Rhesus Macaques
Wright et al., first demonstrated that a cell-free vaccine containing alum-precipitated B. anthracis PA (87), given in two doses with 2-week interval, protected between 75 and 100% of macaques challenged with 0.8 to 60.0 LD50 of B. anthracis Vollum isolate spores by inhalation at 16 to 34 weeks postimmunization (Table 4) (86). Similar protection was achieved in vaccinated animals challenged with 5 × 104 to 10 × 104 B. anthracis Vollum isolate spores injected intradermally at 2 to 58 weeks postimmunization. No significant local reactions to the vaccine were observed in the macaques, and no assessment of a humoral immune response was reported. Two years later, Belton et al. reported similar survival rates (86 to 100%) in rhesus macaques immunized with two doses of alum-precipitated PA with a 2-week interval and challenged with 10 to 15 LD50 of B. anthracis M.36 isolate spores by inhalation at 1 to 104 weeks postimmunization (7, 78). Antibody status was evaluated by a crude in vivo rabbit intradermal toxin neutralization assay (8, 76). Immunized macaques showed low in vivo toxin neutralizing antibody (TNA) levels at 12 and 18 months postimmunization; however, in vivo TNA were not detected at 2 years. Interestingly, this group reported in 1956 that rhesus macaques with undetectable TNA were still protected against inhalational exposure to B. anthracis spores (7). Henderson et al. provided convincing evidence that inhaled B. anthracis spores remain in the lung alveoli of rhesus macaques for long periods postexposure and are capable of initiating infection on phagocytosis and transport to lymphatic tissue by alveolar macrophages (38). This study also clearly showed that treatment with penicillin delayed the onset of anthrax but was ineffective at preventing disease unless accompanied by vaccination. The administration of hyperimmune horse serum also delayed the onset of disease by 20 to 25 days following aerosol challenge and protected only 45% of macaques receiving antibodies by passive transfer. A 100% survival was achieved only by vaccination prior to exposure or by treatment with antibiotics and vaccination postexposure. These results provided the first insights into the importance of an acquired immune response for complete protection against inhalation anthrax. Human cell-free anthrax vaccines containing alum-precipitated PA were further evaluated in human clinical trials designed to test safety and efficacy (12). The U.S. human cell-free anthrax vaccine (AVA) was licensed by the Michigan Department of Public Health (MDPH, Lansing, Mich.) based on the encouraging efficacy studies carried out with rhesus macaques and successful human clinical trials (12).
TABLE 4.
Summary of survival of rhesus macaques vaccinated with various B. anthracis PA-containing preparations and challenged with various B. anthracis isolates
| Vaccine, no. of doses (amt) |
Bacillus anthracis challenge
|
Reference | |||
|---|---|---|---|---|---|
| Time (wks.) | Survivala | Isolateb | Dose (LD50) | ||
| PA + Al3+, 2 (1.50 ml) | 2 | 3/3 (100) | Vollum (C) | (5-10) × 104 spores | 86 |
| 16 | 2/3 (67) | ||||
| 30 | 3/3 (100) | ||||
| 58 | 3/3 (100) | ||||
| PA + Al3+, 2 (1.00 ml) | 2 | 3/4 (75) | Vollum (A) | 0.8-1.6 | 86 |
| 5 | 4/4 (100) | Vollum (A) | 18.0-60 | ||
| PA + Al3+, 2 (1.25 ml) | 1 | 10/10 (100) | M.36 (A) | 10.0-15.0 | 7 |
| 52 | 10/10 (100) | ||||
| 104 | 6/7 (86) | ||||
| MDPH 2 (0.50 ml) | 8 | 10/10 (100) | Ames (A) | 255-760 | 45 |
| 38 | 3/8 (100) | Ames (A) | 161-247 | ||
| 100 | 7/8 (88) | Ames (A) | 239-535 | ||
| MDPH 2 (0.50 ml) | 12 | 10/10 (100) | Ames (A) | 837-961 | 70 |
| rPA + Alhydrogel,e 2 (50 μg of rPA) | 12 | 9/10 (90) | Ames (A) | 837-961 | |
| MDPH 1 (0.50 ml) | 6 | 10/10 (100) | Ames (A) | 74.4 | 46 |
| rPA + Alhydrogel,e 1 (50 μg of rPA) | 6 | 10/10 (100) | Ames (A) | 116.9 | |
| rPA + QS-21,e 1 (50 μg of rPA) | 6 | 9/9 (100) | Ames (A) | 78.3 | |
| rPA + MPL in SLT,e 1 (50 μg of rPA) | 6 | 9/10 (90) | Ames (A) | 96.0 | |
| AVA Lot FAV038,f 2 (0.50 ml) | 10 | 10/10 (100) | Namibia (A) | 398d | 22 |
| 8/10 (80) | Turkey (A) | 1,004d | |||
Number that survived/number challenged (percent survival).
C, cutaneous challenge; A, aerosol challenge.
The cutaneous LD50 for B. anthracis Vollum isolate in rhesus macaques is unknown.
LD50 expressed as Ames equivalent (Ames aerosol LD50 is approximately 5.5 × 104 CFU in rhesus macaques) (22).
Adjuvants: Alhydrogel (3% aluminum hydroxide gel), QS-21 (saponin), MPL (monophosphoryl lipid A) in SLT (2% squalene, 0.24% lecithin, 0.08% Tween 80).
AVA, anthrax vaccine adsorbed (BioPort Corp., Lansing, Mich.).
For the next 30 years, the MDPH-licensed AVA was used to immunize laboratory workers and other individuals at risk for developing anthrax due to occupational exposure, and very little experimental work relying on rhesus macaques was performed. Secretary of Defense William S. Cohen in 1997 directed the military to vaccinate approximately 2.4 million service members with the MDPH AVA vaccine in response to the emerging threat of B. anthracis used as a biological weapon. The renewed interest in anthrax vaccines stimulated new research activities with rhesus macaques as animal models in the early to middle 1990s (Table 4). Ivins et al. expanded on earlier studies to evaluate the short-term, medium-term, and long-term efficacy of AVA in rhesus macaques (45). Macaques vaccinated 2 weeks apart developed anti-PA immunoglobulin G IgG enzyme-linked immunosorbent assay (ELISA) titers as expected. A 100% survival was seen at 8 and 38 weeks postvaccination in macaques challenged with 161 to 760 LD50 of B. anthracis Ames isolate spores by inhalation. Seven of eight macaques survived an aerosol challenge with 239 to 535 LD50 of B. anthracis Ames isolate at 2 years postvaccination. The weakness of this study is that anti-PA ELISA titers were determined only at 2, 8, and 99 weeks postvaccination, which precludes evaluating the peak antibody titer and decay rate of anti-PA antibodies prior to challenge. TNA titers were also not reported. The study did provide additional evidence that AVA prevented inhalation anthrax in macaques at 38 and 102 weeks postvaccination. Ivins et al. went on to compare the efficacy of recombinant PA (rPA) combined with various adjuvants to the standard AVA (46). rPA adsorbed to a suspension of aluminum hydroxide protected 10 of 10 macaques challenged by inhalation with an average of 74.4 LD50 of B. anthracis Ames isolate spores at 6 weeks postvaccination. Similarly, rPA combined with monophosphoryl lipid A in squalene, lecithin, and Tween 80 (SLT) or combined with saponin protected 9 of 10 and 9 of 9 macaques challenged by inhalation with an average of 96.0 and 78.3 LD50 of B. anthracis Ames isolate spores, respectively. This study was the first to report on the development of both humoral and cell-mediated immune responses in macaques vaccinated with B. anthracis PA. Anti-PA IgM ELISA titers peaked at 2 weeks postvaccination, and anti-PA IgG ELISA titers peaked at 5 week postvaccination. Anti-IgM titers declined to prevaccination levels by 6 weeks postvaccination, and anti-PA IgG titers also began to decline at 6 weeks postvaccination. Lymphocyte proliferation in response to PA used as a recall antigen was measured, and the mean stimulation index was significantly increased at 5 weeks postvaccination compared to preimmunization levels. B. anthracis TNA titers were also determined in the vaccinated macaques. Animals vaccinated with AVA, rPA combined with aluminum hydroxide, or rPA combined with saponin developed neutralizing antibodies at 5 weeks postvaccination; however, animals vaccinated with rPA combined with monophosphoryl lipid A in SLT failed to develop neutralizing antibodies. Vaccine preparations containing the adjuvant monophosphoryl lipid A in SLT were less immunogenic than AVA or rPA combined with aluminum hydroxide or saponin but protected 90% of vaccinated macaques. Toxin neutralizing antibody titers did not correlate well with protection against inhalation anthrax, as shown by the 90% survival in the monophosphoryl lipid A in SLT group, which did not develop neutralizing antibodies. Ivins et al. did assert that protective immunity in rhesus macaques is most probably complex and may not be readily reduced to a single correlate or surrogate marker or immunity (46).
The ability of a vaccine to protect against diverse isolates of B. anthracis is also critical to the evaluation of efficacy. Fellows et al. challenged AVA-vaccinated macaques with B. anthracis Namibia isolate or B. anthracis Turkey isolate (22). The macaques received two doses of AVA 4 weeks apart and were challenged 10 weeks postvaccination. AVA protected 10 of 10 animals challenged with the Namibia isolate and 8 of 10 animals challenged with the Turkey isolate. The variability in protection against different B. anthracis isolates seen in guinea pigs (Table 2) was not observed in the macaques, indicating broader protection in AVA-vaccinated nonhuman primates (22).
Human cell-free anthrax vaccines have demonstrated excellent protection against inhalation anthrax in rhesus macaques, with an overall survival rate of 97% across multiple vaccine preparations, dosages, challenge isolates, challenge levels, and challenge times (7, 22, 45, 46, 70, 86). Unlike the guinea pig, cell-free anthrax vaccines protect 95% of rhesus macaques exposed to aerosolized “vaccine-resistant” B. anthracis Ames spores (22, 45, 46, 56, 70). Taken together, these studies demonstrate that AVA- and rPA-based vaccines provide broad, high-level protection in rhesus macaques. However, there is a significant lack of data on the development of humoral and cell-mediated immunity following vaccination of nonhuman primates. These data are necessary to define a durable correlate or surrogate marker of protection that can be used to predict the efficacy of anthrax vaccines in humans under current FDA guidelines.
Rabbits
Belton and Strange developed the conditions and methods required for large-scale (1,000 liters of culture medium per week) production of PA from B. anthracis cultures (9). Mass-produced alum-precipitated PA protected rabbits challenged intradermally with 250 LD50 of the M.36 isolate of B. anthracis in a PA dose-dependent manner (9). Rabbits receiving five doses of the vaccine preparation were partially protected against an intradermal challenge with B. anthracis Vollum isolate spores at 5, 8, 14, and 16 weeks postvaccination (86). However, rabbits challenged at 23 weeks postvaccination were not protected. The vaccination schedule used by Wright et al. differs significantly from schedules most commonly used by investigators today (86). The administration of only two doses 14 days apart may have increased the duration of immunity compared to that induced by five doses administered every other day. The authors suggested that effective immunity could be induced with fewer vaccine doses but chose to adhere to the five-dose schedule for consistency and for comparison with previous observations. Auerbach and Wright investigated antigenic heterogeneity among virulent B. anthracis isolates used to challenge rabbits vaccinated with alum-precipitated PA (3). The vaccine preparation protected 100% of the rabbits challenged intradermally with 26 of 33 B. anthracis isolates cultured from human, bovine, caprine, canine, and porcine cases from various parts of the world. Two antibiotic-resistant B. anthracis isolates were also used to challenge the rabbits. The vaccine preparation protected 100% of rabbits challenged with a streptomycin-resistant B. anthracis isolate (SR 8) and a streptomycin-, aureomycin-, and terramycin-resistant B. anthracis isolate (STAR) (3). The study clearly demonstrated that alum-precipitated PA provided broad protection against a large number of B. anthracis isolates and two antibiotic-resistant isolates in rabbits challenged intradermally.
Increased awareness that B. anthracis, used as a biological weapon, posed a significant threat to the United States and the desire to immunize military personnel stimulated the development of the rabbit model for human inhalation during the 1990s (16, 25, 26, 41, 42). The rabbit model has several attractive features compared to the well-developed rhesus macaque model. (i) Rabbits are considered lower-order species and may partially replace or reduce the number of higher-order species, such as nonhuman primates, required for vaccine efficacy studies. (ii) The FDA will probably require that efficacy be demonstrated in two animal models expected to react with a response predictive for humans (Table 1). (iii) Rabbits are easier to house and safer to handle than nonhuman primates. Pitt and colleagues compared the efficacy of rPA and AVA against inhalation anthrax in guinea pigs, rabbits, and rhesus macaques and described the pathology of cutaneous and inhalation anthrax in the rabbit model (69, 88). They reported that AVA is highly efficacious against inhalation anthrax in rabbits (Table 5). Pitt et al. vaccinated 128 rabbits with two doses of various dilutions of two lots of AVA and challenged the animals with 42 to 184 LD50 of the Ames isolate of B. anthracis at 10 weeks postvaccination (68). Animals receiving the undiluted vaccine or the 1:4, 1:16, or 1:64 dilutions were protected, whereas there was only 1 survivor from 30 animals receiving a 1:256 dilution of the vaccine. Anti-PA IgG antibody titers were determined by ELISA at 6 and 10 weeks postvaccination. TNA titers were determined at 6 weeks. Mean anti-PA IgG antibody titers were higher at 6 weeks than at 10 weeks. The mean anti-PA IgG antibody and TNA titers decreased for all groups as the vaccine was progressively diluted. The authors compared the association between anti-PA IgG antibody titers and TNA titers with survival by using logistic regression analysis. They concluded that the TNA and anti-PA IgG antibody titers at 6 weeks as well as the anti-PA antibody titer at 10 weeks were significant predictors of survival at the 10-week challenge. Little et al. reported that two doses of rPA adsorbed to Al2O3 (Alhydrogel; Biosector, Frederikssund, Denmark) protected rabbits from inhaled B. anthracis Ames isolate spores out to 10 weeks postvaccination (55). A graded dose response was observed in animals that received one or two doses of the rPA vaccine preparation. The anti-PA IgG titer and the TNA titer were significant predictors of survival at weeks 10 and 8 post-vaccination, respectively, in rabbits receiving two doses of the vaccine. Thus, anti-PA and TNA titers correlate with survival in rabbits vaccinated with either AVA or rPA vaccines. These studies demonstrate that anti-PA antibody and TNA titers can predict survival in rabbits challenged at 10 weeks. However, the authors did not determine if anti-PA or TNA titers are predictive of survival in rabbits challenged up to 1 year after vaccination. Human anthrax vaccines should provide protection against a broad range of B. anthracis isolates. Fellows et al. vaccinated rabbits with two doses of undiluted AVA at 0 and 4 weeks (22). The rabbits were challenged at 10 weeks with 300 to 2,700 LD50 (B. anthracis Ames isolate equivalent) of six B. anthracis isolates exhibiting the greatest virulence in vaccinated guinea pigs. The AVA vaccine protected 90 to 100% of the rabbits challenged with the K5926/India, K7978/Namibia, K4539/France, K8091/Norway, K9729/Turkey, and K1938/Indonesia B. anthracis isolates. Strong anti-PA antibody titers were detected by ELISA 1 week prior to challenge. These studies clearly demonstrate that the human cell-free anthrax vaccine provides short-term high-level protection against inhalation anthrax in rabbits challenged with a panel of diverse B. anthracis isolates. Anti-PA and TNA titers correlate with survival in rabbits at 10 weeks postimmunization. However, the level of protection and the correlation of survival with anti-PA and TNA titers should to be assessed at greater intervals between vaccination and challenge, such as 26 or 52 weeks. In addition, the role of vaccine-induced cell-mediated immunity was not addressed in these studies.
TABLE 5.
Summary of survival of rabbits vaccinated with various B. anthracis PA-containing preparations and challenged with various B. anthracis isolates
| Vaccine preparation, doses (amt)d |
Bacillus anthracis challenge
|
Reference | |||
|---|---|---|---|---|---|
| Time (wk) | Survivala | Isolate | Dose (LD50) | ||
| AVA lot FAV008 | 6 | Ames (A)b | 82-184 | 70 | |
| 2 (Undil.) | 8/8 (100) | ||||
| 2 (1/4 dil.) | 10/10 (100) | ||||
| 2 (1/16 dil.) | 9/10 (90) | ||||
| 2 (1/64 dil.) | 9/10 (90) | ||||
| 2 (1/256 dil.) | 1/10 (10) | ||||
| AVA Lot FAV032 | 6 | Ames (A) | 42-126 | 70 | |
| 2 (Undil.) | 10/10 (100) | ||||
| 2 (1/4 dil.) | 10/10 (100) | ||||
| 2 (1/16 dil.) | 15/20 (75) | ||||
| 2 (1/64 dil.) | 14/20 (70) | ||||
| 2 (1/256 dil.) | 0/20 (0) | ||||
| AVA Lot FAV032 | 6 | 22 | |||
| 2 (0.5 ml) | 7/10 (70) | K7978/Namibia (A) | 1,305c | ||
| 9/9 (100) | K5926/India (A) | 1,448c | |||
| 9/10 (90) | K8091/Norway (A) | 360c | |||
| 10/10 (100) | K4539/France (A) | 1,191c | |||
| 10/10 (100) | K9729/Turkey (A) | 790c | |||
| 10/10 (100) | K1938/Indonesia (A) | 2,743c | |||
| rPA-Alhydrogel | 4 | Ames (A) | 59-847 | 55 | |
| 1 (100 μg) | 28/30 (93) | ||||
| 1 (24 μg) | 42/64 (66) | ||||
| 1 (5 μg) | 16/37 (43) | ||||
| 1 (1 μg) | 4/22 (18) | ||||
| 1 (0.2 μg) | 1/10 (10) | ||||
| 1 (0.08 μg) | 0/10 (0) | ||||
| rPA-Alhydrogel | 10 | Ames (A) | 10.5-528 | 55 | |
| 2 (10 μg) | 12/12 (100) | ||||
| 2 (1 μg) | 10/12 (83) | ||||
| 2 (0.2 μg) | 4/12 (33) | ||||
| 2 (0.08 μg) | 0/8 (0) | ||||
Number that survived/number challenged (percent survival).
A, aerosol challenge.
LD50 expressed as Ames equivalent (Ames Inhalation LD50 is approximately 1.1 × 105 CFU in rabbits) (70).
Undil., undiluted; dil., dilution.
IMMUNE RESPONSES AND CORRELATES OF PROTECTION
Humoral Immunity
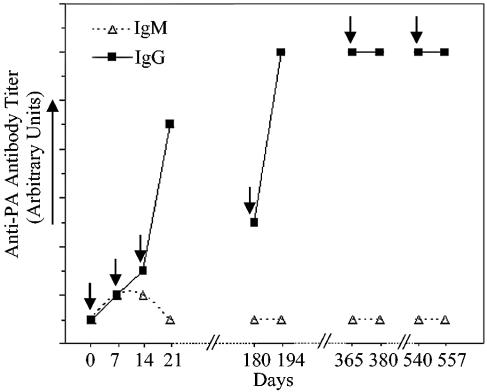
Cell-free anthrax vaccines containing PA and the adjuvant aluminum hydroxide or aluminum sulfate induce anti-PA antibodies in vaccinated humans, rhesus macaques, rabbits, and guinea pigs (7, 22, 44-46, 56, 60, 68, 71, 72, 74, 80, 81). The development of anti-PA IgM, IgG, and IgA responses is variable, depending on the species, route of administration, and adjuvant. PA-containing vaccines administered subcutaneously or intramuscularly stimulate transient low-titer anti-PA IgM responses in humans and rhesus macaques (46, 80) (Fig. 1). In contrast, guinea pigs vaccinated with the human cell-free anthrax vaccine develop high-titer anti-PA IgM responses (80). PA-containing vaccines administered subcutaneously or intramuscularly induce high-titer anti-PA IgG antibodies in humans, rabbits, rhesus macaques, and guinea pigs (4, 22, 43, 46, 60, 68, 71, 74, 80, 81). Humans, rhesus macaques, rabbits, and guinea pigs exhibit different IgG subclasses, which differ structurally and functionally. Human IgG is composed of IgG1, IgG2, IgG3, and IgG4 (33). Rhesus macaque IgG is composed of only three subclasses: IgG1, IgG2, and IgG4 (75). Rabbits and guinea pigs have only IgG1 and IgG2 subclasses (33). Humans vaccinated with PA-containing vaccines adsorbed to aluminum adjuvants developed predominantly IgG1 anti-PA antibodies (5). IgG4, IgG3, and IgG2 anti-PA antibodies were also detected but were present at lower titers and were seen less frequently than IgG1. Guinea pigs vaccinated with PA-containing vaccines adsorbed to aluminum adjuvants produced anti-PA IgG1 but not IgG2 antibodies (60). However, guinea pigs vaccinated with PA-containing vaccines prepared with Ribi (monophosphoryl lipid A, trehalose dicorynomycolate, mycobacterial cell wall skeleton, squalene, and Tween 80) produced both anti-PA IgG1 and IgG2 antibodies (60). The specific subclasses of anti-PA IgG induced in rhesus macaques and rabbits have not been reported. The significance of the predominance of anti-PA IgG1 induced by vaccination in humans is not clear but is consistent with responses observed following the administration of tetanus toxoid (51). Anti-PA IgA antibodies have been detected in mice immunized intranasally with PA-containing preparations but not in mice vaccinated intradermally or subcutaneously (11, 17). Humans, rhesus macaques, and rabbits vaccinated with cell-free anthrax vaccines intramuscularly or subcutaneously are not likely to produce significant quantities of anti-PA IgA (11, 30).
FIG. 1.
Representative Anti-PA IgM and IgG antibody titers in AVA-vaccinated humans and macaques. Transient anti-PA IgM antibodies and increasing anti-PA IgG antibody levels are observed after three doses of AVA (↓) at 0, 7, and 14 days. Anti-PA IgG titers decline until the first AVA booster at 180 days and then increase and remain high before and after the second and third AVA boosters at 12 and 18 months.
The functionality and specificity of anti-PA antibodies induced by immunization are as important as the level of antibody produced. The ability of anti-PA antibodies to neutralize B. anthracis LT in vitro has been used to evaluate the functionality of anti-PA antibodies. Polyclonal anti-PA antibodies from vaccinated rhesus macaques, rabbits, and guinea pigs can neutralize LT both in vitro and in vivo (7, 46, 60, 68, 74). Passive transfer of anti-PA antibodies or hyperimmune serum to a naive host confers protection against B. anthracis challenge (38, 49, 54, 74). Furthermore, Welkos et al. have demonstrated that anti-PA antibodies can enhance the phagocytosis of B. anthracis spores by macrophages and inhibit spore germination (85). This group has also proposed that the enhancement of spore phagocytosis mediated by anti-PA antibodies leads to increased killing of B. anthracis spores by macrophages, albeit by an undefined mechanism (84). Reed et al. utilized a competitive assay to measure antibody responses to important epitopes of PA (73). Vaccinated rabbits developed antibodies against amino acids (aa) 581 to 601 and 671 to 721 of PA. Antibodies that recognize aa 581 to 601 of PA block the binding of LF to PA, and antibodies that recognize aa 671 to 721 of PA block the binding of PA to the anthrax toxin receptor (57, 58). Maynard et al. demonstrated that antibodies with higher affinity to aa 671 to 721 of PA were enhanced for toxin neutralization in vitro and afforded increased protection to rats challenged with LT (59). Quantitative measurements of the specificity of anti-PA antibodies for aa 581 to 601 or aa 671 to 721 of PA have not been reported for vaccinated rhesus macaques or humans. Studies designed to determine the affinity of anti-PA antibodies for PA and the ability of anti-PA antibodies to enhance the phagocytosis of B. anthracis spores in vaccinated human beings and rhesus macaques are under way.
Cell-Mediated Immunity
Cell-free anthrax vaccines containing PA and the adjuvant aluminum hydroxide or aluminum sulfate induce clonal expansion of PA-specific T cells as measured by ex vivo proliferation assays with PA as a recall antigen (46, 60, 68). We have demonstrated that the frequency of PA-specific T cells increases in rhesus macaques following vaccination with AVA (Fig. 2) (A. M. Brys, K. A. Hayes, L. E. Mathes, N. A. Niemuth, R. E. Hunt, J. E. Estep, D. M. Robinson, and A. J. Phipps, Abstr. 5th Int. Conf. Anthrax, abstr. P619, 2002). Lymphocyte proliferative responses correlated with the dilution of vaccine after the first two doses of AVA but reached a plateau after the third dose, irrespective of the dilution of the vaccine administered. The responses were maintained for up to 1 year following three doses of vaccine. We also characterized T-cell responses by evaluating ex vivo cytokine production. Stimulation of lymphocytes with rPA ex vivo resulted in significant increases in IL-4 and IL-2 mRNA levels without increases in gamma interferon, IL-6, IL-1β, or TNF-α mRNA levels, indicating a predominantly Th2 profile (Fig. 3) (C. L. K. Sabourin, S. L. Casbohm, Y. W. Choi, N. A. Niemuth, R. E. Hunt, J. E. Estep, D. M. Robinson, and A. J. Phipps, Abstr. 5th Int. Conf. Antrhax, abstr. P620, 2002). The Th2-type response in conjunction with increasing anti-PA IgG titers in macaques is to be expected following immunization with a protein antigen, such as PA, adsorbed to aluminum. The importance of the frequency of PA-specific memory T cells or the level of Th2-type cytokines in long-term protection against inhalation anthrax remains to be determined.
FIG. 2.
Modulation of T-cell proliferation by AVA dilution in macaques. Peripheral blood mononuclear cells were isolated at 0, 28, 56, 210, and 365 days from macaques receiving a saline placebo, undiluted AVA, 1:5 AVA dilution, 1:10 AVA dilution, 1:20 AVA dilution, and 1:40 AVA dilution at 0, 4, and 26 weeks. The mean stimulation index (SI) and standard error of the mean of each dilution group (n = 10 animal per group) were determined and plotted against time.
FIG. 3.
Fold increases in cytokine mRNA levels from PA-stimulated PBMC at 30 weeks. Peripheral blood mononuclear cells were isolated at 30 weeks from rhesus macaques receiving three doses of AVA at 0, 4, and 26 weeks (n = 60 animals) and stimulated with PA ex vivo. Fold increases in gamma interferon (IFN-γ), IL-2, IL-4, IL-6, IL-1β, and TNF-α mRNA levels were measured by real-time reverse transcriptase PCR. Each plus sign represents saline placebo controls, and dots represent AVA-vaccinated animals.
CONCLUSION
AVA- and rPA-based anthrax vaccines provide high-level and lasting protection against aerosol challenge with a diverse panel of B. anthracis isolates in rhesus macaques. AVA also protects rabbits against aerosol challenge with multiple B. anthracis isolates in a vaccine dose-dependent manner. The duration of immunity in rabbits is unknown. AVA induces anti-PA IgG antibodies in all species examined so far. Further evaluation of the specific IgG subclasses, antibody specificity, antibody avidity, and TNA activity in vaccinated macaques and human beings will probably reveal similar patterns in humoral immunity. The predominance of T-cell Th2-type cytokine responses that we have observed in rhesus macaques has also been described for other human aluminum-adjuvanted vaccines such as tetanus toxoid. The challenge will be to determine how these complex acquired immune responses correlate with protection in animal models and if the data can be extrapolated to humans. Valuable information on the importance of humoral and cell-mediated immunity in protection can be gained by careful analysis of vaccine failures, as has been the case with human immunodeficiency virus vaccines in nonhuman primates and humans. Studies designed to establish the relationship between the type(s) and level of immune responses and the protection in rhesus macaques vaccinated with dilutions of AVA that are predicted to provide limited protection are under way. Correlates of protection can be derived only from studies in which the vaccine fails to protect, which has not been the experience thus far with anthrax vaccines in nonhuman primates. If a single immune parameter, such as the anti-PA IgG titer, can be identified as the primary surrogate marker of protection, logistic regression analysis can be applied to determine the effect of titer on survival outcome. This approach has been applied by to the rabbit model by Pitt et al. and Little et al. (55, 68). If multiple immune parameters are identified as critical surrogate markers of protection, predictive models may be built by using several approaches including logistic discriminant analysis and cluster analysis. These types of analysis and their application to medical research are reviewed by Solberg (77) and McLachlan (61). The lessons learned from these studies will be of great importance for future studies of vaccine efficacy and the establishment of rugged correlates of protection in cases where human efficacy trials are untenable, as is true for most biodefense vaccines.
Acknowledgments
We are grateful to Lawrence E. Mathes, Lee Silverman, and Kathleen Hayes for their critical review of the paper and helpful comments.
REFERENCES
- 1.Abramova, F. A., L. M. Grinberg, O. V. Yampolskaya, and D. H. Walker. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 90:2291-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrink, W. S. and R. J. Goodlow. 1959. Experimental inhalation anthrax in the chimpanzee. Am. J. Pathol. 35:1055-1065. [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach, S. and G. G. Wright. 1955. Studies on immunity in anthrax. VI. Immunizing activity of protective antigen against various strains of Bacillus anthracis. J. Immunol. 75:129-133. [PubMed] [Google Scholar]
- 4.Baillie, L., R. Hebdon, H. Flick-Smith, and D. Williamson. 2003. Characterisation of the immune response to the UK human anthrax vaccine. FEMS Immunol. Med. Microbiol. 36:83-86. [DOI] [PubMed] [Google Scholar]
- 5.Baillie, L. W., K. Fowler, and P. C. Turnbull. 1999. Human immune responses to the UK human anthrax vaccine. J. Appl. Microbiol. 87:306-308. [DOI] [PubMed] [Google Scholar]
- 6.Barakat, L. A., H. L. Quentzel, J. A. Jernigan, D. L. Kirschke, K. Griffith, S. M. Spear, K. Kelley, D. Barden, D. Mayo, D. S. Stephens, T. Popovic, C. Marston, S. R. Zaki, J. Guarner, W. J. Shieh, H. W. Carver, R. F. Meyer, D. L. Swerdlow, E. E. Mast, and J. L. Hadler. 2002. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA 287:863-868. [DOI] [PubMed] [Google Scholar]
- 7.Belton, F. C., H. M. Darlow, and D. W. Henderson. 1956. The use of anthrax antigen to immunise man and monkey. Lancet 271:476-479. [DOI] [PubMed] [Google Scholar]
- 8.Belton, F. C., and D. W. Henderson. 1956. A method for assaying anthrax immunising antigen and antibody. Br. J. Exp. Pathol. 37:156-160. [PMC free article] [PubMed] [Google Scholar]
- 9.Belton, F. C., and R. E. Strange. 1954. Studies on a protective antigen produced in vitro from Bacillus anthracis: medium and methods of production. Br. J. Exp. Pathol. 35:144-152. [PMC free article] [PubMed] [Google Scholar]
- 10.Borio, L., D. Frank, V. Mani, C. Chiriboga, M. Pollanen, M. Ripple, S. Ali, C. DiAngelo, J. Lee, J. Arden, J. Titus, D. Fowler, T. O'Toole, H. Masur, J. Bartlett, and T. Inglesby. 2001. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA 286:2554-2559. [DOI] [PubMed] [Google Scholar]
- 11.Boyaka, P. N., A. Tafaro, R. Fischer, S. H. Leppla, K. Fujihashi, and J. R. McGhee. 2003. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 170:5636-5643. [DOI] [PubMed] [Google Scholar]
- 12.Brachman, P. S., H. Gold, S. A. Plotkin, F. R. Fekety, M. Werrin, and N. R. Ingraham. 1962. Field evaluation of a human anthrax vaccine. Am. J. Public Health 52:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brachman, P. S. 1980. Inhalation anthrax. Ann. N. Y. Acad. Sci. 353:83-93. [DOI] [PubMed] [Google Scholar]
- 14.Brachman, P. S., and A. F. Kaufmann. 1998. Anthrax, p. 95-107. In A. S. Evans and P. S. Brachman (ed.), Bacterial infections of humans. Plenum Medical Book Co., New York, N.Y.
- 15.Coker, P. R., K. L. Smith, P. F. Fellows, G. Rybachuck, K. G. Kousoulas, and M. E. Hugh-Jones. 2003. Bacillus anthracis virulence in Guinea pigs vaccinated with anthrax vaccine adsorbed is linked to plasmid quantities and clonality. J. Clin. Microbiol. 41:1212-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole, L. A. 1996. The specter of biological weapons. Sci. Am. 275:60-65. [DOI] [PubMed] [Google Scholar]
- 17.Debin, A., R. Kravtzoff, J. V. Santiago, L. Cazales, S. Sperandio, K. Melber, Z. Janowicz, D. Betbeder, and M. Moynier. 2002. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine 20:2752-2763. [DOI] [PubMed] [Google Scholar]
- 18.Dewan, P. K., A. M. Fry, K. Laserson, B. C. Tierney, C. P. Quinn, J. A. Hayslett, L. N. Broyles, A. Shane, K. L. Winthrop, I. Walks, L. Siegel, T. Hales, V. A. Semenova, S. Romero-Steiner, C. Elie, R. Khabbaz, A. S. Khan, R. A. Hajjeh, and A. Schuchat. 2002. Inhalational anthrax outbreak among postal workers, Washington, D.C., 2001. Emerg. Infect. Dis. 8:1066-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 20.Druett, H. A., D. W. Henderson, L. Packman, and S. Peacock. 1953. Studies on respiratory infection. I. The influence of particle size on respiratory infection with anthrax spores. J. Hyg. 51:359-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 22.Fellows, P. F., M. K. Linscott, B. E. Ivins, M. L. Pitt, C. A. Rossi, P. H. Gibbs, and A. M. Friedlander. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19:3241-3247. [DOI] [PubMed] [Google Scholar]
- 23.Finney, D. J. 1971. Probit analysis. Cambridge University Press, Cambridge, United Kingdom.
- 24.Fisher, L. D., and G. van Belle. 1993. Biostatistics—a methodology for the health sciences. John Wiley & Sons, Inc., New York, N.Y.
- 24a.Food and Drug Administration. 2002. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. 21 CFR 314 and 601. U.S. Food and Drug Administration, Department of Health and Human Services, Rockville, Md. [PubMed]
- 25.Friedlander, A. M. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335-349. [PubMed] [Google Scholar]
- 26.Friedlander, A. M., P. R. Pittman, and G. W. Parker. 1999. Anthrax vaccine: evidence for safety and efficacy against inhalational anthrax. JAMA 282:2104-2106. [DOI] [PubMed] [Google Scholar]
- 27.Friedlander, A. M., S. L. Welkos, M. L. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, and P. Mikesell. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1243. [DOI] [PubMed] [Google Scholar]
- 28.Fritz, D. L., N. K. Jaax, W. B. Lawrence, K. J. Davis, M. L. Pitt, J. W. Ezzell, and A. M. Friedlander. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Investig. 73:691-702. [PubMed] [Google Scholar]
- 29.Gao, J. J., V. Diesl, T. Wittmann, D. C. Morrison, J. L. Ryan, S. N. Vogel, and M. T. Follettie. 2002. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J. Leukoc. Biol. 72:1234-1245. [PubMed] [Google Scholar]
- 30.Gaur, R., P. K. Gupta, A. C. Banerjea, and Y. Singh. 2002. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine 20:2836-2839. [DOI] [PubMed] [Google Scholar]
- 31.Glassman, H. N. 1966. Industrial inhalation anthrax, discussion. Bacteriol. Rev. 30:657-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gleiser, C. A., C. C. Berdjis, H. A. Hartman, and W. S. Gochenour. 1963. Pathology of experimental respiratory anthrax in Macaca mulatta. Br. J. Exp. Pathol. 44:416-426. [PMC free article] [PubMed] [Google Scholar]
- 33.Golub, E. S. 1987. Heterogeneity of Immunoglobulins, p. 54-72. In E. S. Golub (ed.), Immunology: a synthesis. Sinauer Associates, Inc., Sunderland, Mass.
- 34.Grinberg, L. M., F. A. Abramova, O. V. Yampolskaya, D. H. Walker, and J. H. Smith. 2001. Quantitative pathology of inhalational anthrax. I. Quantitative microscopic findings. Mod. Pathol. 14:482-495. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton, M. A., R. C. Russo, and R. V. Thurston. 1977. Trimmed Spearman-Karber method for estimating the median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 11:714-719. [Google Scholar]
- 36.Hanna, P. 1999. Lethal toxin actions and their consequences. J. Appl. Microbiol. 87:285-287. [DOI] [PubMed] [Google Scholar]
- 37.Hanna, P. C., and J. A. Ireland. 1999. Understanding Bacillus anthracis pathogenesis. Trends Microbiol. 7:180-182. [DOI] [PubMed] [Google Scholar]
- 38.Henderson, D. W., S. Peacock, and F. C. Belton. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. 54:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtz, T. H., J. Ackelsberg, J. L. Kool, R. Rosselli, A. Marfin, T. Matte, S. T. Beatrice, M. B. Heller, D. Hewett, L. C. Moskin, M. L. Bunning, and M. Layton. 2003. Isolated case of bioterrorism-related Inhalational anthrax, New York City, 2001. Emerg. Infect. Dis. 9:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoover, D. L., A. M. Friedlander, L. C. Rogers, I. K. Yoon, R. L. Warren, and A. S. Cross. 1994. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect. Immun. 62:4432-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inglesby, T. V., D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Hauer, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, K. Tonat, and the Working Group on Civilian Biodefense. 1999. Anthrax as a biological weapon: medical and public health management. JAMA 281:1735-1745. [DOI] [PubMed] [Google Scholar]
- 42.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon. 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 43.Ivins, B., P. Fellows, L. Pitt, J. Estep, J. Farchaus, A. Friedlander, and P. Gibbs. 1995. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine 13:1779-1784. [DOI] [PubMed] [Google Scholar]
- 44.Ivins, B. E., P. F. Fellows, and G. O. Nelson. 1994. Efficacy of a standard human anthrax vaccine against Bacillus anthracis spore challenge in guinea-pigs. Vaccine 12:872-874. [DOI] [PubMed] [Google Scholar]
- 45.Ivins, B. E., P. F. Fellows, M. L. M. Pitt, J. E. Estep, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Efficacy of a standard human anthrax vaccine against Bacillus anthracis aerosol spore challenge in rhesus macaques. Salisbury Med. Bull. 87:125-126. [Google Scholar]
- 46.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, Jr., P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 47.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, and J. L. Gerberding. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim, S. O., Q. Jing, K. Hoebe, B. Beutler, N. S. Duesbery, and J. Han. 2003. Sensitizing anthrax lethal toxin-resistant macrophages to lethal toxin-induced killing by tumor necrosis factor-alpha. J Biol. Chem. 278:7413-7421. [DOI] [PubMed] [Google Scholar]
- 49.Kobiler, D., Y. Gozes, H. Rosenberg, D. Marcus, S. Reuveny, and Z. Altboum. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koehler, T. M. 2002. Bacillus anthracis genetics and virulence gene regulation. Curr. Top. Microbiol. Immunol. 271:143-164. [DOI] [PubMed] [Google Scholar]
- 51.Kroon, F. P., M. J. van Tol, Jol-van der Zijde CM, R. van Furth, and J. T. van Dissel. 1999. Immunoglobulin G (IgG) subclass distribution and IgG1 avidity of antibodies in human immunodeficiency virus-infected individuals after revaccination with tetanus toxoid. Clin. Diagn. Lab. Immunol. 6:352-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaForce, F. M. 1978. Woolsorters' disease in England. Bull. N. Y. Acad. Med. 54:956-963. [PMC free article] [PubMed] [Google Scholar]
- 53.Leppla, S. H. 1995. Anthrax toxins, p. 543-572. In J. Moss, B. Iglewski, M. Vaughan and A. T. Tu (ed.), Bacterial toxins and virulence factors in disease. Matcel Dekker, Inc., New York, N.Y.
- 54.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 56.Little, S. F. and G. B. Knudson. 1986. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect. Immun. 52:509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 56:1807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142:707-715. [DOI] [PubMed] [Google Scholar]
- 59.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 60.McBride, B. W., A. Mogg, J. L. Telfer, M. S. Lever, J. Miller, P. C. Turnbull, and L. Baillie. 1998. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine 16:810-817. [DOI] [PubMed] [Google Scholar]
- 61.McLachlan, G. J. 1992. Cluster analysis and related techniques in medical research. Stat. Methods Med. Res. 1:27-48. [DOI] [PubMed] [Google Scholar]
- 62.Meselson, M., J. Guillemin, M. Hugh-Jones, A.Langmuir, I. Popova, A. Shelokov, and O. Yampolskaya. 1994. The Sverdlovsk anthrax outbreak of 1979. Science 266:1202-1208. [DOI] [PubMed] [Google Scholar]
- 63.Mikesell, P., B. E. Ivins, J. D. Ristroph, and T. M. Dreier. 1983. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect. Immun. 39:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Brien, J., A. Friedlander, T. Dreier, J. Ezzell, and S. Leppla. 1985. Effects of anthrax toxin components on human neutrophils. Infect. Immun. 47:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okinaka, R. T., K. Cloud, O. Hampton, A. R. Hoffmaster, K. K. Hill, P. Keim, T. M. Koehler, G. Lamke, S. Kumano, J. Mahillon, D. Manter, Y. Martinez, D. Ricke, R. Svensson, and P. J. Jackson. 1999. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 181:6509-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park, J. M., F. R. Greten, Z. W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048-2051. [DOI] [PubMed] [Google Scholar]
- 67.Pile, J. C., J. D. Malone, E. M. Eitzen, and A. M. Friedlander. 1998. Anthrax as a potential biological warfare agent. Arch. Intern. Med. 158:429-434. [DOI] [PubMed] [Google Scholar]
- 68.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 69.Pitt, M. L. M., B. Ivins, J. E. Estep, J. Farchaus, and A. Friedlander. 1996. Comparative efficacy of a recombinant protective antigen vaccine against inhalation anthrax in guinea pigs, rabbits, and rhesus monkeys. Abstr. 96th. Gen. Meet. Am. Soc. Microbiol. 1996, abstr. E-70, p. 278.
- 70.Pitt, M. L. M., B. E. Ivins, J. E. Estep, J. Farchaus, and A. M. Friedlander. 1996. Comparison of the efficacy of purified protective antigen and MDPH (anthrax vaccine adsorbed) to protect non-human primates from inhalation anthrax. Salisbury Med. Bull. 87:130. [Google Scholar]
- 71.Pittman, P. R., J. A. Mangiafico, C. A. Rossi, T. L. Cannon, P. H. Gibbs, G. W. Parker, and A. M. Friedlander. 2000. Anthrax vaccine: increasing intervals between the first two doses enhances antibody response in humans. Vaccine 19:213-216. [DOI] [PubMed] [Google Scholar]
- 72.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reed, D. S., J. Smoll, P. Gibbs, and S. F. Little. 2002. Mapping of antibody responses to the protective antigen of Bacillus anthracis by flow cytometric analysis. Cytometry 49:1-7. [DOI] [PubMed] [Google Scholar]
- 74.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shearer, M. H., R. D. Dark, J. Chodosh, and R. C. Kennedy. 1999. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin. Diagn. Lab. Immunol. 6:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith, H., J. Keppie, and J. L. Stanley. 1954. Observations on the cause of death in experimental anthrax. Lancet 267:474-476. [DOI] [PubMed] [Google Scholar]
- 77.Solberg, H. E. 1978. Discriminant analysis. Crit. Rev. Clin. Lab. Sci. 9:209-242. [DOI] [PubMed] [Google Scholar]
- 78.Strange, R. E. and F. C. Belton. 1954. Studies on a protective antigen produced in vitro from Bacillus anthracis: purification and chemistry of the antigen. Br. J. Exp. Pathol. 35:153-165. [PMC free article] [PubMed] [Google Scholar]
- 79.Tippetts, M. T., and D. L. Robertson. 1988. Molecular cloning and expression of the Bacillus anthracis edema factor toxin gene: a calmodulin-dependent adenylate cyclase. J. Bacteriol. 170:2263-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turnbull, P. C., M. G. Broster, J. A. Carman, R. J. Manchee, and J. Melling. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect. Immun. 52:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turnbull, P. C., S. H. Leppla, M. G. Broster, C. P. Quinn, and J. Melling. 1988. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med. Microbiol. Immunol. 177:293-303. [DOI] [PubMed] [Google Scholar]
- 82.Ulmer, T. S., S. Soelaiman, S. Li, C. B. Klee, W. J. Tang, and A. Bax. 2003. Calcium dependence of the interaction between calmodulin and anthrax edema factor. J. Biol. Chem. 278:29261-29266. [DOI] [PubMed] [Google Scholar]
- 83.Vasconcelos, D., R. Barnewall, M. Babin, R. Hunt, J. Estep, C. Nielsen, R. Carnes, and J. Carney. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 88:1201-1209. [DOI] [PubMed] [Google Scholar]
- 84.Welkos, S., A. Friedlander, S. Weeks, S. Little, and I. Mendelson. 2002. In-vitro characterisation of the phagocytosis and fate of anthrax spores in macrophages and the effects of anti-PA antibody. J. Med. Microbiol. 51:821-831. [DOI] [PubMed] [Google Scholar]
- 85.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]
- 86.Wright, G. G., T. W. Green, and R. G. Kanode, Jr. 1954. Studies on immunity in anthrax. V. Immunizing activity of alum-precipitated protective antigen. J. Immunol. 73:387-391. [PubMed] [Google Scholar]
- 87.Wright, G. G., M. A. Hedberg, and J. B. Slein. 1954. Studies on immunity in anthrax. III. Elaboration of protective antigen in a chemically defined, non-protein medium. J. Immunol. 72:263-269. [PubMed] [Google Scholar]
- 88.Zaucha, G. M., L. M. Pitt, J. Estep, B. E. Ivins, and A. M. Friedlander. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982-992. [PubMed] [Google Scholar]