Summary
Current efforts to repair damaged or diseased mammalian retinas are inefficient and largely incapable of fully restoring vision. Conversely, the zebrafish retina is capable of spontaneous regeneration upon damage using Müller glia (MG)-derived progenitors. Understanding how zebrafish MG initiate regeneration may help develop new treatments that prompt mammalian retinas to regenerate. We show that inhibition of γ-aminobutyric acid (GABA) signaling facilitates initiation of MG proliferation. GABA levels decrease following damage, and MG are positioned to detect decreased ambient levels and undergo dedifferentiation. Using pharmacological and genetic approaches, we demonstrate that GABAA receptor inhibition stimulates regeneration in undamaged retinas while activation inhibits regeneration in damaged retinas.
Keywords: zebrafish, retina, regeneration, Müller glia, GABA
Graphical Abstract
Highlights
-
•
Inhibiting GABA or glutamate signaling causes spontaneous Müller glia proliferation
-
•
Activating GABA or glutamate signaling reduces damage-induced proliferation
-
•
Müller glia directly respond to extracellular GABA
-
•
Inhibition of GABA signaling activates regeneration response
Unlike mammals, zebrafish regenerate following retina damage from a resident adult stem cell (Müller glia). Dissecting the mechanisms that zebrafish use could lead to new therapeutic targets to treat retinal diseases. Patton and colleagues have discovered a mechanism by which decreased GABA levels are sensed by Müller glia to initiate a regenerative response.
Introduction
Degenerative retinal diseases and damage, such as retinitis pigmentosa, result in a loss of retinal cells and deterioration of vision, and can lead to blindness. A current effort to mitigate these effects involves intravitreal injections of stem cells or retinal precursors, hoping for successful integration and connection to existing neuronal circuits (Barber et al., 2013, Hanus et al., 2016, MacLaren et al., 2006, Pearson et al., 2012, Santos-Ferreira et al., 2015). Though improving, these therapies are inefficient and not yet capable of restoring vision (Barber et al., 2013, Bringmann et al., 2006, Pearson, 2014, Pearson et al., 2010). An alternative method would be to prompt the retina to endogenously regenerate and replace lost cells.
Mammalian retinas do not possess the ability to regenerate following disease or damage. Instead, damage commonly results in reactive gliosis (Bringmann et al., 2006, Pearson, 2014). Zebrafish, however, mount a robust spontaneous regeneration response upon damage (Goldman, 2014). In this way, Müller glia (MG) serve as adult stem cells in the retina capable of dedifferentiation, asymmetric division, and the production of progenitor cells that are capable of restoring all lost cell types (Bernardos et al., 2007, Fausett and Goldman, 2006, Nagashima et al., 2013, Rajaram et al., 2014a, Rajaram et al., 2014b, Ramachandran et al., 2012, Thummel et al., 2008, Vihtelic and Hyde, 2000, Wan et al., 2012, Zhao et al., 2014). Because overall retinal architecture and cell types are largely conserved between fish and mammals, understanding how zebrafish regulate retina regeneration may help develop novel treatments or therapeutic targets for retinal damage or diseases, especially treatments that target or induce regeneration from MG.
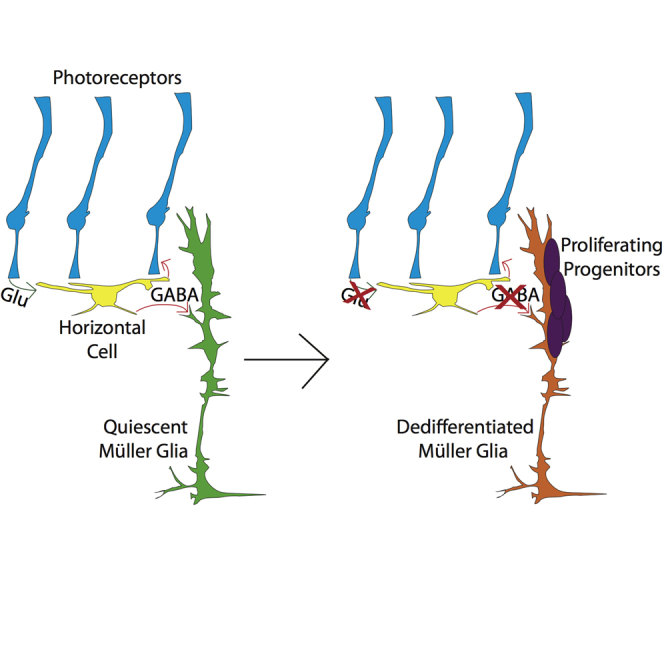
Select regions of the mammalian CNS are capable of adult neurogenesis, particularly the subgranular zone (SGZ) of the mouse hippocampus. Recently, the inhibitory neurotransmitter γ-aminobutyric acid (GABA) was shown to play an important role in regulating quiescence of radial glia-like stem cells (RGLs) in the mouse hippocampus (Song et al., 2012). Synaptic input from glutamatergic granule cells regulates activity of parvalbumin-positive (PV+) GABAergic interneurons in the SGZ. When input from granule cells is low, decreased extracellular GABA levels are detected in a non-synaptic, tonic response by GABAA receptors on RGLs, resulting in proliferation. We sought to test whether this could be an evolutionarily conserved mechanism to regulate MG-derived regeneration in the damaged zebrafish retina. In the retina, photoreceptors (PRs) release glutamate onto GABAergic horizontal cells (HCs). When PRs die they no longer stimulate HCs to release GABA. We hypothesize that MG detect decreases in ambient GABA levels and initiate regeneration in a response similar to activation of RGLs in the mouse hippocampus. We show here that disrupting GABA signaling causes spontaneous proliferation in undamaged zebrafish retinas and that increasing GABA signaling in damaged retinas suppresses regeneration.
Results
Inhibition of GABA or Glutamate Receptors Causes Spontaneous Proliferation in Undamaged Retinas
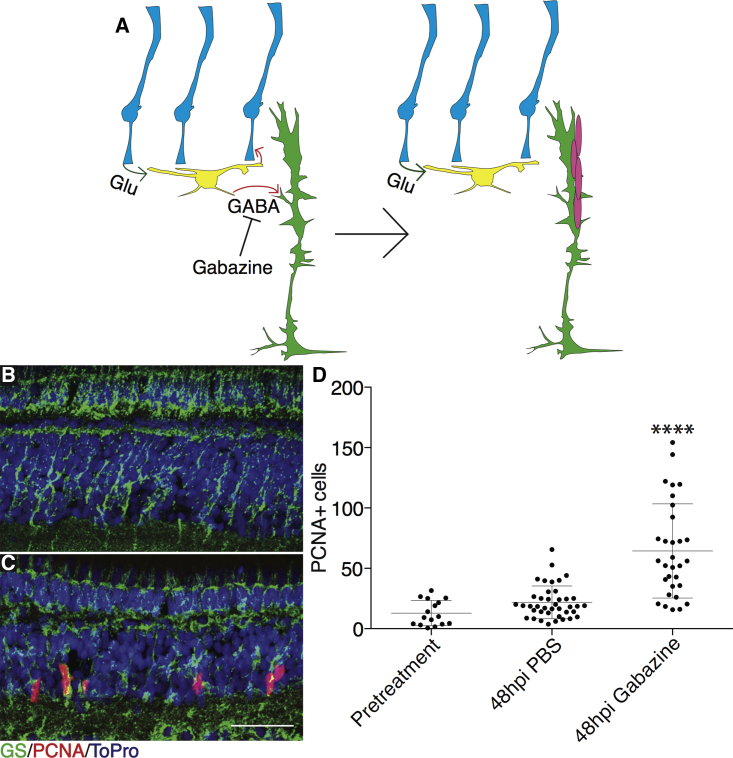
In the mouse hippocampus, dentate granule cells release synaptic GABA on PV+ interneurons, and GABA spillover is detected by neural stem cells to regulate quiescence versus activation (Chell and Frisen, 2012, Song et al., 2012). To test whether a similar neuronal network might be involved in controlling MG quiescence, we tested the effects of pharmacological inhibition of GABA signaling. GABAA antagonists were injected into undamaged retinas to determine whether inhibition of GABA signaling would cause spontaneous proliferation (Figure 1). Initially, picrotoxin or bicuculline were injected into eyes and increased MG proliferation was observed, but because these inhibitors are not completely GABAA receptor specific we switched to gabazine, an allosteric inhibitor of the GABAA receptor (Ueno et al., 1997). As early as 48 hr post injection (hpi) the number of proliferating cells, as detected by the presence of proliferating cell nuclear antigen (PCNA), was significantly greater than both uninjected and PBS-injected control eyes (Figures 1B–1D and S1A–S1J). Proliferating cells were detected in the inner nuclear layer (INL), co-labeled with glutamine synthetase (GS), which marks MG. This indicates that inhibition of GABA signaling induced the formation of MG-derived proliferating cells. Further, proliferating cells were often found in clusters, indicating a robust regenerative response with multiple divisions of MG-derived pro genitor cells. Importantly, the increase in proliferation was not simply the result of an increase in apoptosis, since TUNEL labeling showed no increase in cell death following gabazine injection (Figure S1K). Also, the effects of gabazine injection were dose dependent, arguing in favor of a specific effect via the GABAA receptor (Figure S2).
Figure 1.
Gabazine Injections Cause Time-Dependent Spontaneous Proliferation in Undamaged Retinas
(A) Model illustrating effects of gabazine injection on MG proliferation.
(B and C) Wild-type eyes were injected with PBS (B) or 12.5 nmol gabazine (C) into one eye. Fish recovered for 48 hr after gabazine injections before proliferation was measured. Representative images are small portions of entire retina. Scale bar, 100 μm.
(D) Proliferating cells were counted across whole sections by PCNA staining for pretreatment (n = 16 eyes analyzed), PBS injection (n = 40), and gabazine injection (n = 31). hpi, hours post injection. One-way ANOVA was used; error bars denote SD; ∗∗∗∗p < 0.0001.
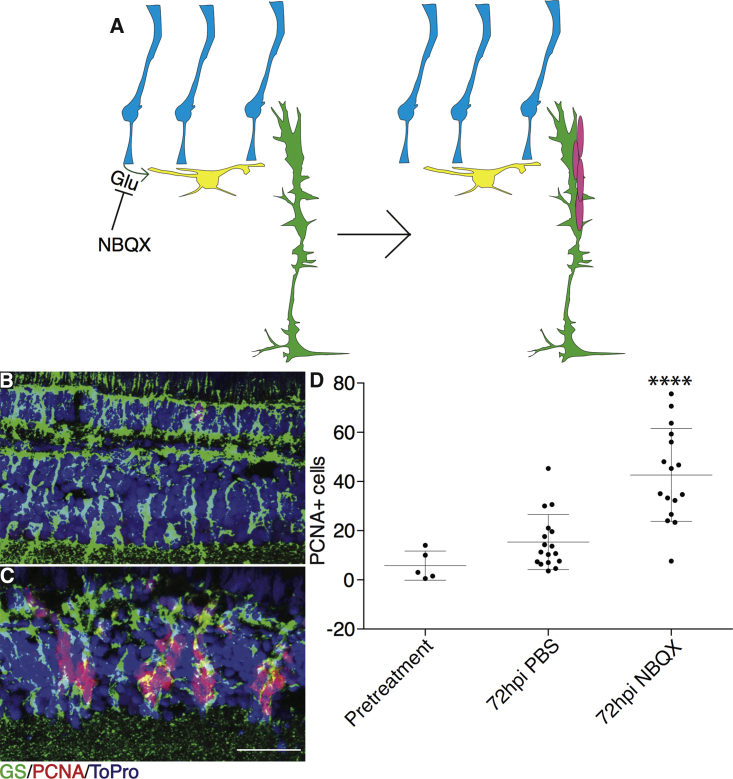
The model of neural stem cell activation in the mouse hippocampus hypothesizes that upstream neuronal activity keeps GABA levels high, maintaining quiescence. In the retina, the model predicts that inhibiting glutamate signaling upstream of GABA signaling should produce effects similar to GABA inhibition (Figure 2). To test this, we injected the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist nitro-2,3-dioxobenzoquinoxaline sulfonamide (NBQX) into undamaged eyes and measured the proliferation by PCNA staining. Proliferation was significantly greater at 48 hpi, reaching a maximum at 72 hpi (Figures 2B–2D and S3A–S3J). As above, clusters of proliferating cells in the INL that co-labeled with GS were observed at 72 hpi, indicating a robust regenerative response, and the effects were again dose dependent and not due to increased apoptosis (Figures S3K and S4).
Figure 2.
NBQX Injections Cause Time-Dependent Spontaneous Proliferation in Undamaged Retinas
(A) Model illustrating effects of NBQX injections on MG proliferation.
(B and C) Wild-type eyes were injected with PBS (B) or 25 nmol NBQX (C) into one eye. Fish recovered for 72 hr after NBQX injections (B and C) before proliferation was measured. Representative images are small portions of entire retina. Scale bar, 100 μm.
(D) Proliferating cells were counted across whole sections by PCNA staining for pretreatment (n = 5 eyes analyzed), PBS injection (n = 19), and NBQX injection (n = 16). hpi, hours post injection. One-way ANOVA was used; error bars denote SD; ∗∗∗∗p < 0.0001.
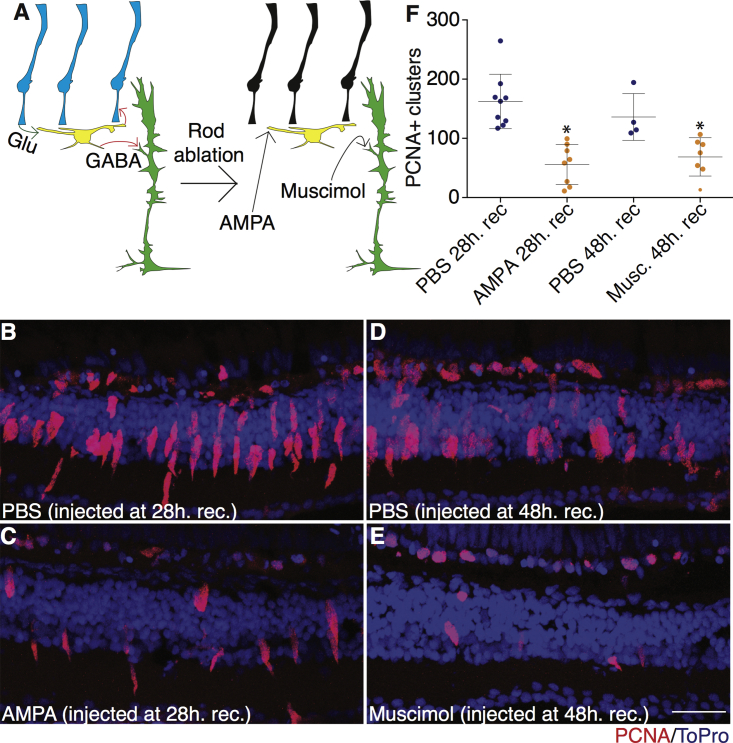
Activation of GABA or Glutamate Receptors Suppresses Regeneration in Damaged Retinas
The previous experiments showed an increase in proliferation without damage. A converse set of experiments was devised to determine whether activating GABAA receptors or AMPA receptors would suppress regeneration after damage (Figure 3A). To test this, we ablated rod PRs using Tg(zop:nfsb-EGFP)nt19 fish treated with metronidazole (MTZ). Eyes were injected with muscimol, a GABAA receptor agonist, at 48 hr after MTZ treatment, or AMPA, a glutamate receptor agonist, at 28 hr after MTZ treatment. All retinas were collected at 52 hr after MTZ treatment, when MG proliferation begins (Figure S5). Although the number of proliferating MG in a region of the retina may vary, the timing of MG proliferation was relatively uniform across the retina. Both injections showed a significant decrease in proliferating cells compared with PBS injections, as measured by PCNA expression (Figures 3B–3F). These results are consistent with the overall model that neural networks control ambient GABA levels that can be sensed by MG to either maintain quiescence or initiate a regenerative response.
Figure 3.
AMPA and Muscimol Injections Suppress Regeneration in Damaged Retinas
(A) Model illustrating effects of muscimol and AMPA injections on MG proliferation.
(B–E) Tg(zop:nfsb-EGFP)nt19 fish were treated with 10 mM metronidazole for 24 hr, then allowed to recover. Fish were then anesthetized and injected with either AMPA at 28-hr recovery (C) or muscimol at 48-hr recovery (E). PBS controls were also anesthetized and injected at 28-hr (B) or 48-hr recovery (D). All injected eyes were removed at 52-hr recovery. Proliferation was assessed by PCNA staining. Representative images are small portions of the entire retina. Scale bar, 100 μm.
(F) Clusters of proliferating cells were measured across entire sections for PBS at 28 hr recovery (n = 9 eyes analyzed), AMPA at 28 hr recovery (n = 8), PBS at 48 hr recovery (n = 4), and muscimol at 48 hr recovery (n = 7). Two-tailed Student’s t test was used; error bars denote SD; ∗p < 0.05.
MG Are Poised to Detect Changes in GABA Levels
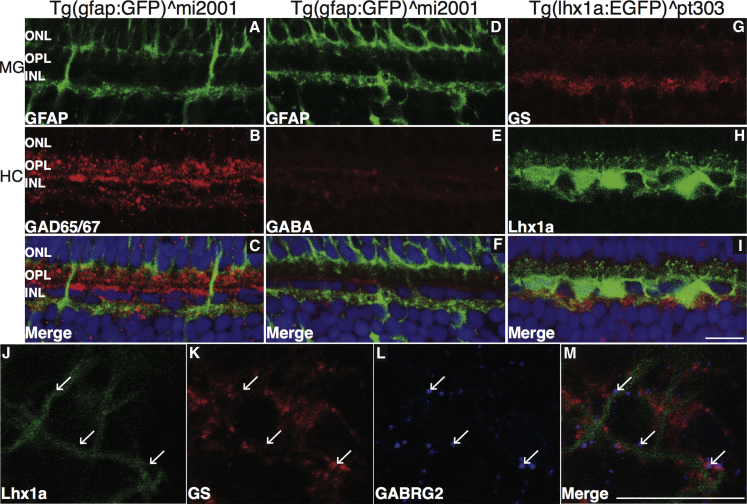
If the model that MG quiescence is controlled by GABA levels is correct, several predictions arise. First, for MG to detect GABA released from HCs, their processes should be in close proximity. In mice, the transcription factor Lhx1 is specific to HCs (Liu et al., 2000, Poche et al., 2007). We tested whether this was true in zebrafish and found that in Tg(lhx1a:EGFP)pt303 reporter fish, GFP is indeed restricted to HCs in the retina (Figures 4H and 4I). To test whether MG and HC processes are closely associated, we immunostained cross-sections of retinas from two lines of zebrafish that express GFP in either MG (Tg(gfap:GFP)mi2001) or HCs (Tg(lhx1a:EGFP)pt303) with antibodies against GABA, glutamic acid decarboxylase 65/67 (GAD65/67), or GS. We found that HC and MG processes co-localize in the INL (Figures 4A–4I). Although overall monitoring of GABA levels in the retina is likely due to contacts between MG and multiple cells across the three main layers of the retina, this result is intriguing in that there appears to be a specialized connection between MG and HCs in the fish retina. To more closely determine the nature of this interaction, we stained flat-mounted Tg(lhx1a:EGFP)pt303 retinas with GS, which showed that MG processes wrap around HC processes in the INL (Figures S6A–S6E).
Figure 4.
Close Association of MG and HC Processes in the INL
(A–I) Tg(gfap:GFP)mi2001 and Tg(lhx1a:EGFP)pt303 retina sections were stained for GAD65/67 (A–C), GABA (D–F), or GS (G–I). Scale bar, 100 μm.
(J–M) Co-localization of MG and HC markers was observed in the INL. Tg(lhx1a:EGFP)pt303 retinas were removed, stained for GS and GABRG2, and the area of co-localization imaged in flat mount. Arrows indicate GABRG2 puncta. Scale bar, 100 μm.
See also Figure S6.
Second, when we immunostained for the presence of GABAA receptors we detected the γ2 subunit of the GABAA receptor (GABRG2) on MG processes that flank HC processes (Figures 4J–4M). This was validated by detection of mRNAs encoding GABRG2 by RT-PCR of RNA isolated from sorted MG, consistent with previous work (Ramachandran et al., 2012, and data not shown). In the mouse, GABRG2 has been shown to be essential for neurogenesis in the SGZ (Song et al., 2012). Identifying GABRG2 expression and localization in MG suggests that a similar role could be played by fish MG.
A third prediction is that following injury when a regenerative response is initiated, retinal GABA levels should decrease. To determine this, we monitored GABA levels in whole retina by high-performance liquid chromatography (HPLC) after rod ablation in Tg(zop:nfsb-EGFP)nt19 fish treated with MTZ. We observed a significant decrease in whole-retina GABA levels at 52 hpi (Figure S6F).
MG Directly Detect Changes in GABA Levels
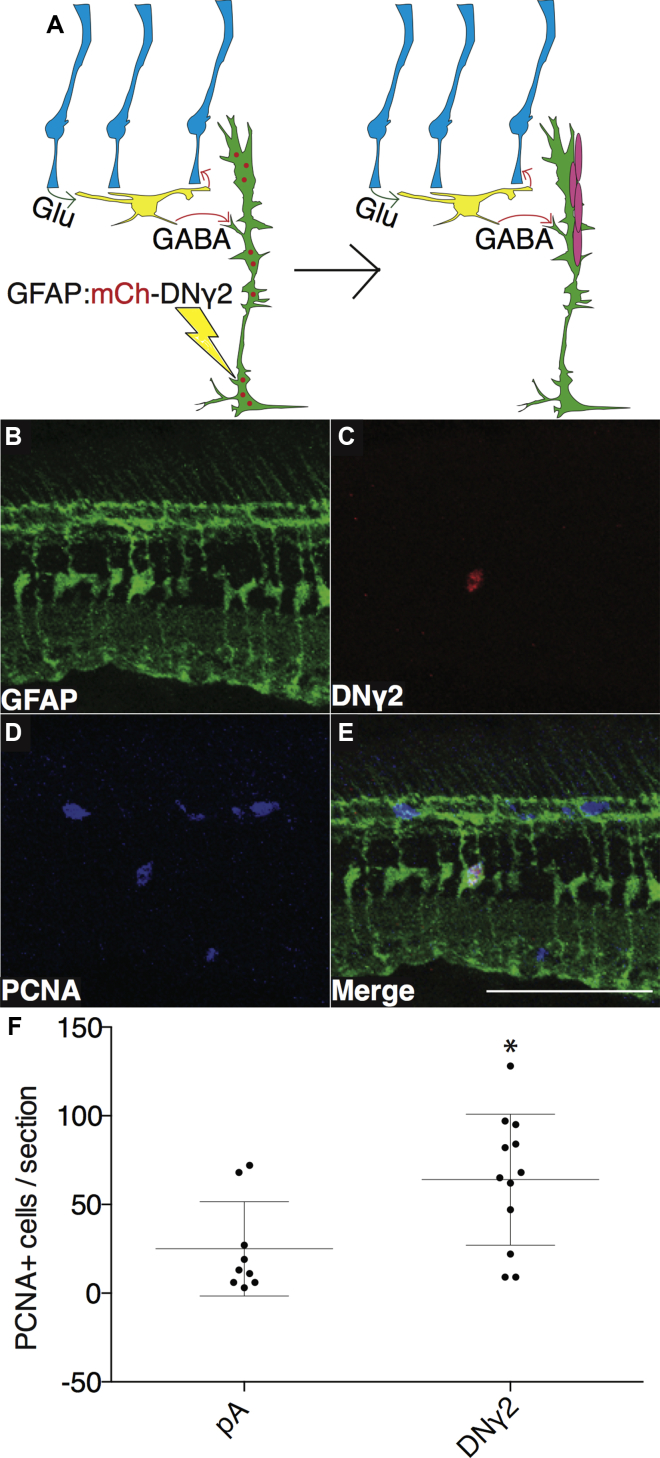
Together, the aforementioned data support the idea that MG are poised to detect decreased GABA levels following damage, but it remained uncertain whether the effects were mediated directly by MG. The different agonists and antagonists could be acting indirectly to cause MG proliferation, since many other cells contain GABAA or glutamate receptors and many cells also release GABA or glutamate. As a first step to address this, we used a genetic approach by creating a construct expressing a dominant-negative version of the zebrafish γ2 subunit of the GABAA receptor (DNγ2), following an identical human mutation that underlies an inherited form of epilepsy (Harkin et al., 2002, Kang et al., 2009). The zebrafish glial fibrillary acidic protein (GFAP) promoter was used to drive expression of an mCherry-tagged version of the DNγ2 isoform in MG. Injection and electroporation of this construct into Tg(gfap:GFP)mi2001 fish was performed followed by analysis of proliferating cells that co-localize with mCherry, GFP, and PCNA. Successful electroporation and expression of the construct is expected to be a rare event, especially because the DNγ2 mutation is caused by a premature stop codon which will result in nonsense-mediated decay, making visualization of mCherry potentially difficult. Nevertheless, detecting even rare cells exhibiting co-localization of the three markers would strongly argue in favor of a direct response by MG. As shown in Figure 5, we observed a significant increase in the total number of proliferating cells, marked by PCNA and co-localizing with GFP and mCherry (Figures 5 and S7). Some of the proliferating cells observed in Figures 5 and S7 are in the outer nuclear layer and are likely rod precursors, while those in the GCL are likely microglia. Proliferation of these cells is probably due to limited damage that occurs during electroporation, but the increased proliferation in MG with DNγ2 is most consistent with inhibition of GABA signaling.
Figure 5.
Expression of DNγ2 in MG of Undamaged Retina Causes Increased Proliferation
(A) Model illustrating effects of electroporation of DNγ2 into MG on proliferation.
(B–E) A GFAP:mCh-DNγ2 construct was electroporated into one retina of undamaged Tg(gfap:GFP)mi2001 fish. GFP expression (B), mCherry expression (C), and staining for PCNA (D) all co-labeled in the same cell (E). Scale bar, 100 μm.
(F) Total number of PCNA-expressing cells was measured for pA electroporation (n = 9 eyes analyzed) and DNγ2 (n = 12). Two-tailed Student’s t test was used; error bars denote SD; ∗p < 0.05.
See also Figure S7.
Inhibition of GABA Signaling Induces a Bona Fide Regenerative Response
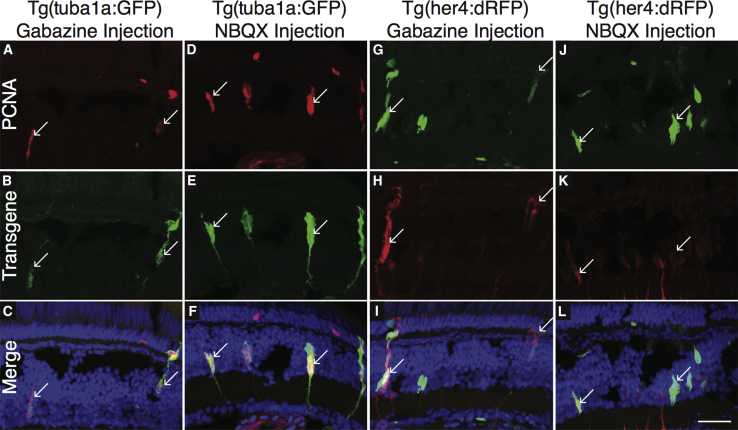
Besides caveats as to whether inhibition of GABA signaling is mediated directly by MG, another potential caveat is whether the observed increase in proliferation is part of a bona fide regenerative response. To test whether proliferation resulting from gabazine or NBQX injections accurately replicates regeneration, we analyzed markers of regeneration following injections. Drugs were injected into Tg(tuba1a:GFP) and Tg(her4:dRFP) fish, marking activation of α-tubulin 1a and Notch signaling, respectively. Both genes have been shown to be upregulated during regeneration (Fausett and Goldman, 2006, Hayes et al., 2007, Ramachandran et al., 2012, Wan et al., 2012). After injection, both were found to be upregulated and associated with PCNA-expressing cells (Figure 6), suggesting that drug-induced proliferation is accurately replicating what is observed during damage-induced retinal regeneration.
Figure 6.
Injection of Gabazine or NBQX into Undamaged Eyes Causes Upregulation of Factors Associated with Regeneration
Gabazine (A–C, G–I) or NBQX (D–F, J–L) was injected into one eye of Tg(tuba1a:GFP) (A–F) or Tg(her4:dRFP) (G–L) fish. Fish were allowed to recover for 72 hr, after which retinas were removed and stained for PCNA. Both GFP and dRFP expression co-labeled with PCNA. Arrows indicate co-localization of transgene and PCNA. Scale bar, 100 μm.
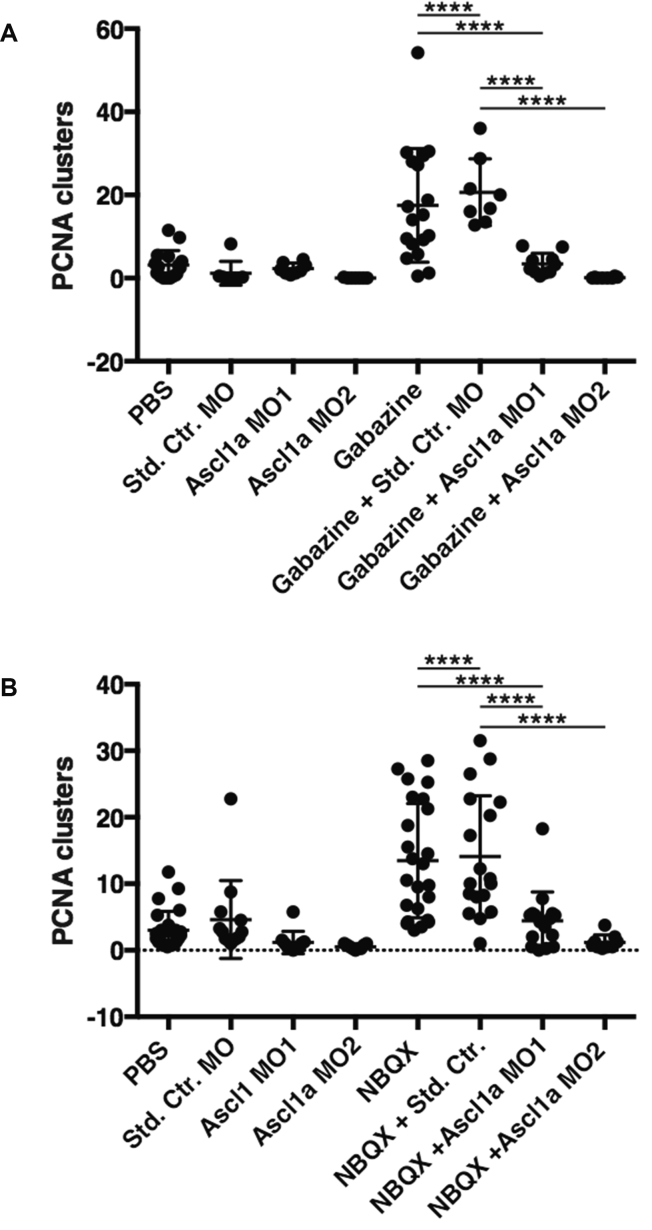
To further test whether a bona fide regenerative response was initiated, we combined gabazine treatment with knockdown of Ascl1. Ascl1 is a transcription factor that is necessary for MG dedifferentiation and retina regeneration in both fish and mice (Brzezinski et al., 2011, Fausett et al., 2008, Pollak et al., 2013, Ramachandran et al., 2010, Ramachandran et al., 2011, Ueki et al., 2015, Wohl and Reh, 2016). Dedifferentiated MG express α1-tubulin (as used in the Tg(tuba1a:GFP) fish) whose expression is due in part to binding of Ascl1 to an E box within the promoter (Fausett et al., 2008). If gabazine treatment is inducing a bona fide regenerative response via MG, the prediction is that proliferation should be inhibited by knockdown of Ascl1. As shown in Figure 7, we observed a significant decrease in the number of proliferating cells and clusters of proliferating cells when antisense morpholinos targeting Ascl1 were co-injected with gabazine or NBQX compared with gabazine or NBQX injection alone or with co-injection of a standard control morpholino. These results indicate that inhibition of GABA signaling induces a bona fide regenerative response. While the majority of these experiments used expression of PCNA as a readout of DNA replication and proliferation, the results support the idea that detection of PCNA is truly indicative of a regenerative response, since expression of α1-tubulin is observed in dedifferentiated MG and proliferating progenitor cells.
Figure 7.
Knockdown of Ascl1a Inhibits Retinal Proliferation Induced by Neurotransmitter Inhibition
(A) Wild-type eyes were injected with PBS (n = 16), 0.75 nmol of a standard control morpholino (Std. Ctr., n = 8), or one of two morpholinos targeting Ascl1a (Ascl1a MO1 [n = 8] and Ascl1a MO2 [n = 7]). Gabazine was also injected alone (n = 18) or in combination with morpholinos (n = 8, 10, and 8, respectively).
(B) Wild-type eyes were injected with PBS (n = 26), 0.75 nmol of a standard control morpholino (Std. Ctr., n = 13), or one of two morpholinos targeting Ascl1a (Ascl1a MO1 [n = 10] and Ascl1a MO2 [n = 9]). NBQX was also injected alone (n = 24) or in combination with morpholinos (n = 18, 16, and 9, respectively). Following 1–2 hr of recovery, the injected eyes were electroporated. Seventy-two hours after injection the treated eyes were removed for immunohistochemistry and scoring. Clusters of proliferating cells were counted and averaged across four whole retinal sections per eye by PCNA staining.
n Values denote the number of eyes analyzed. One-way ANOVA was used; error bars = SD; ∗∗∗∗p < 0.0001.
Discussion
Our data support a mechanism whereby decreased ambient GABA levels are sensed by MG to initiate retina regeneration. Due to their expression of GABAA receptors, MG are poised to detect this decrease. While other cells may also play a role, we hypothesize that HCs are positioned to mediate the decrease in GABA following PR death due to close association between HCs and MG in a specialized layer of the INL. In the retina, MG contact all cells and, therefore, a potential interaction between HC and MG is not surprising. However, this interaction may be unique to zebrafish and within a layer of the retina not found in mammals. This interaction, therefore, may provide the zebrafish retina with one of its unique qualities: its ability to regenerate. In the mouse brain, GABA is involved in neurogenesis in the SGZ and subventricular zone (Bordey, 2007, Liu et al., 2005, Pallotto and Deprez, 2014, Song et al., 2012). Multiple reports have also indicated a role for GABA in controlling proliferation of progenitor cells (Braun and Jessberger, 2014, Giachino et al., 2014, Liu et al., 2005, Pallotto and Deprez, 2014, Ramirez et al., 2012, Song et al., 2013, Tozuka et al., 2005). Our results suggest that this also holds for the retina.
Our data suggest that PR damage is communicated via GABA, based on the timing of events in different experiments. Nevertheless, the possibility exists that MG directly sense changes in glutamate as well. Maximum proliferation for gabazine injections was observed at 48 hpi while maximum proliferation for NBQX injections was observed at 72 hpi. This suggests that GABA affects MG more proximally than glutamate. Furthermore, muscimol injection into damaged retinas only produced an effect when injected at 48 hr after MTZ treatment, while AMPA injections only produced an effect when injected at 28 hr after MTZ treatment. Injecting muscimol earlier or AMPA at later times did not cause significant changes in proliferation. The overall timing best supports the idea that GABA acts directly on MG and glutamate is upstream.
Previous studies have suggested that tumor necrosis factor α (TNF-α) (Nelson et al., 2013), Notch (Conner et al., 2014), leptin, and interleukin-6 (IL-6) (Zhao et al., 2014), are involved in initiating retina regeneration in zebrafish. It is possible that these and other, as yet undiscovered, signals act synergistically to mount a full, robust regenerative response. However, even though TNF-α, Notch, leptin, and IL-6 are all relatively early markers of regeneration, it is not clear what signals induce their expression, especially because numerous gene expression changes accompany differential expression of these factors. An attractive hypothesis based on our data is that decreased GABA is the primary signal for retinal regeneration initiation and that other signals follow to act synergistically. This is supported by the fact that injection of gabazine or NBQX causes an upregulation of the Notch reporter Her4, suggesting that GABA is upstream of Notch signaling. This is also in line with earlier studies showing that Notch signaling is important for later stages of regeneration and development (Hayes et al., 2007, Karl et al., 2008, Kassen et al., 2007, Olena et al., 2015, Raymond et al., 2006). In addition, leptin mRNA was observed to increase following injury, suggesting that it is induced by some other signal. Interestingly, IL-6 mRNA was not detected in regenerating retinas, indicating that its source originates from outside the retina and may be prompted to increase only after damage occurs (Zhao et al., 2014). Inflammatory signals such as TNF-α and IL-6 may be released by endogenous immune cells (e.g., microglia [Fischer et al., 2014]) or those invading from the vasculature after damage.
Retinitis pigmentosa and age-related macular degeneration arise from dysfunction and death of PRs. We have focused on PR regeneration but questions remain as to how bipolar, amacrine, or ganglion cells regenerate. It may be that feedback mechanisms are in place whereby HC activity would be affected by the death of other cells. For example, dopaminergic amacrine cells have been found to have processes that project to the HC layer and may also affect HC activity (Herrmann et al., 2011). There are likely other mechanisms by which the retina senses bipolar, amacrine, or ganglion cell death as well. MG processes appear to surround the cell bodies of amacrine and ganglion cells, suggesting a different mechanism to sense cell death, such as juxtacrine or paracrine signaling. These mechanisms may also be involved in the sensation of PR and HC death. Identifying a method to induce spontaneous MG proliferation and the production of progenitor cells may be sufficient if the new progenitors can then follow endogenous cues to differentiate into whichever cell is needed. New therapies that activate MG in this manner could lead to robust endogenous regeneration and counteract many retinal diseases. These might include both agonists and antagonists of neurotransmitter signaling, but could also be targeted at intracellular cascades downstream of GABA signaling (Andang et al., 2008, Fernando et al., 2011, Jagasia et al., 2009, Quadrato et al., 2012, Quadrato et al., 2014, Ramirez et al., 2012).
Lastly, the question remains of why teleost retinas have maintained a robust regenerative response while mammalian retinas are largely incapable of repair. The cellular organization found in zebrafish may be a key difference. In zebrafish, the HCs form a monolayer that is separated from the rest of the INL by the network of HC and MG processes, observed in the current study. A consequence of this organization is that the HCs contain processes that project into the INL as well as into the outer plexiform layer (OPL). In mice, however, the HCs are co-mingled with other cells in the INL and only contain projections into the OPL (Matsuoka et al., 2012, Poche et al., 2007). Investigating HC development and potential interactions between HCs and MG could greatly inform about how MG respond to damage in the mammalian retina. It may be that MG are inefficient or blocked from detecting changes in GABA after damage, but perhaps alteration of signaling by pharmacological agents such as gabazine could push MG down a regenerative path.
Experimental Procedures
Zebrafish Lines and Maintenance
Animals used in this study were treated in accordance with Vanderbilt's Institutional Animal Care and Use Committee. Zebrafish lines used in this study include Tg(gfap:GFP)mi2001 (Raymond et al., 2006), which marks differentiated MG, Tg(zop:nfsb-EGFP)nt19 (Montgomery et al., 2010), which marks rods and is used for rod ablation, Tg(lhx1a:EGFP)pt303 (Swanhart et al., 2010), which marks HCs, Tg(tuba1a:GFP) (Fausett and Goldman, 2006), which marks dedifferentiated MG and progenitor cells, and Tg(her4:dRFP) (Yeo et al., 2007), which marks Notch-activated cells. All fish were maintained in a 14:10-hr light/dark (L/D) cycle at 28°C unless otherwise noted. All animals were between 3 and 7 months old.
Metronidazole-Induced Rod Damage
Rod ablation was induced similarly to previously established protocols (Montgomery et al., 2010). In brief, Tg(zop:nfsb-EGFP)nt19 transgenic zebrafish were transferred to egg water containing 10 mM MTZ for 24 hr in darkness at 28°C. Fish were then transferred to normal egg water and returned a 14:10-hr L/D cycle for recovery. The extent of regeneration was assayed at the indicated times post recovery after MTZ treatment.
HPLC Analysis of GABA
Whole retinas were dissected from Tg(zop:nfsb-EGFP)nt19 transgenic zebrafish following recovery from MTZ treatment. Protein was extracted from ten whole retinas for each time point and subjected to HPLC. The levels of each amino acid and derivations were quantified.
Drug and Morpholino Injections
Different neurotoxins were injected into the vitreous using a protocol adapted from previous studies (Rajaram et al., 2014a, Rajaram et al., 2014b, Thummel et al., 2008). The drugs included gabazine (Sigma S106), NBQX (Abcam ab210046), muscimol (Sigma M1523), and AMPA (Sigma A9111). In brief, zebrafish were anesthetized in 0.016% tricaine, an incision was made in the sclera with a sapphire knife, and a blunt-end 30-gauge needle inserted. Drugs (0.5 μL) were injected into one eye of adult zebrafish. Fish were immediately placed into a recovery tank; times indicated are hours of recovery.
Morpholino Injections
Standard control morpholino or ascl1a morpholinos were injected as described above. 0.5 μL of 3′-lissamine-tagged morpholinos (Gene Tools) at a concentration of 1.5 mM were injected with or without neurotoxins into one eye of adult zebrafish and electroporated (75 V/pulse, two pulses, 1-s intervals between pulses). Fish were given 72 hr following injection to allow for recovery and proliferation before eyes were removed for immunohistochemistry and scoring. Morpholinos used were ascl1a MO1 (5′-ATC TTG GCG GTG ATG TCC ATT TCG C-3′), ascl1a MO2 (5′-AAG GAG TGA GTC AAA GCA CTA AAG T-3′) (Cau and Wilson, 2003), and a standard control morpholino (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′).
Immunohistochemistry and TUNEL Labeling
Zebrafish were euthanized in 0.08% tricaine and whole eyes were removed and fixed in 9:1 ethanolic formaldehyde (PCNA staining) or 4% paraformaldehyde (all other staining) overnight. Eyes were then washed in PBS and cryoprotected in 30% sucrose for 4 hr at room temperature. Eyes were transferred to a solution containing two parts optimal cutting temperature compound (OCT) and one part 30% sucrose overnight followed by transfer to 100% OCT for 2 hr and then embedded in OCT for cryosectioning. Antibodies used were PCNA (Sigma, P8825; Abcam, ab2426), GS (Millipore, mab302), GABA (Sigma, A0310), GABAA receptor γ2 subunit (Novus Biologicals, NB300-151), GAD65 + GAD67 (Abcam, ab11070), GFP (Torrey Pines BioLabs, TP401), and mCherry (Novus Biologicals, NBP1-96752). TUNEL labeling was performed following immunohistochemistry. The in situ cell death detection kit, TMR Red (Roche Applied Sciences, 12156792910) was used to detect apoptotic cells.
Design of Dominant-Negative γ2 and Electroporation
A dominant-negative form of the γ2 subunit of the GABAAR was previously characterized in humans (Harkin et al., 2002, Kang et al., 2009). The mutation is in a conserved position in zebrafish and generates a premature codon. A plasmid containing the zebrafish mRNA sequence of γ2 until the premature stop codon was created by GeneArt. The sequence was cloned into a Tol2 backbone to create the vector GFAP:mCh(no-stop)-DNγ2. A control vector GFAP:mCh(no-stop)-pA was also created. Both constructs were electroporated into retinas following a protocol adapted from previous studies (Rajaram et al., 2014a, Rajaram et al., 2014b, Thummel et al., 2008). In brief, fish were anesthetized, the outer cornea was removed, an incision was made in the sclera with a sapphire knife, and a blunt-end 30-gauge needle was inserted into the vitreous. 0.5 μL of plasmid DNA at a concentration of 2 ng/μL was injected into the vitreous of one eye. Anesthetized fish were allowed to recover and then re-anesthetized, and injected eyes were electroporated (50 V/pulse, four pulses, 1-s intervals between pulses). Treated fish were placed in recovery tanks for the times indicated.
Statistical Analysis
Two-tailed Student’s t test was performed when comparing two means and a one-way ANOVA when comparing three or more means. The tests used are indicated in each figure, as well as the number of eyes measured. In most cases, experiments were performed using a single clutch of fish that were divided among different treatment groups. When multiple clutches were required the fish were mixed, then distributed among treatment groups to reduce bias. One to four sections from one eye were measured and the resulting values averaged to arrive at the reported values. Eyes that were damaged were not included in analysis, based on disrupted morphology and/or extremely high proliferation.
Author Contributions
M.B.R., D.D., and J.G.P. conceived, designed, and performed all experiments and wrote the paper.
Acknowledgments
We wish to thank members of the Patton laboratory, Elizabeth Beilharz, Alissa Guarnaccia, and Mariana Jimenez for help and advice. Transgenic zebrafish lines were shared by Pamela Raymond (Tg(gfap:GFP)mi2001), David Hyde (Tg(zop:nfsb-EGFP)nt19), Neil Hukriede (Tg(lhx1a:EGFP)pt303), Daniel Goldman (Tg(tuba1a:GFP)), and Ajay Chitnis (Tg(her4:dRFP)). This work was supported by grants from the NIH RO1 EY024354 and R21 EY019759 to J.G.P., a Vanderbilt Vision Research Center NEI Core Grant (P30-EY008126), and additional support from the Stevenson family and Gisela Mosig endowments to Vanderbilt University.
Published: March 9, 2017
Footnotes
Supplemental Information includes seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.02.007.
Supplemental Information
References
- Andang M., Hjerling-Leffler J., Moliner A., Lundgren T.K., Castelo-Branco G., Nanou E., Pozas E., Bryja V., Halliez S., Nishimaru H. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- Barber A.C., Hippert C., Duran Y., West E.L., Bainbridge J.W., Warre-Cornish K., Luhmann U.F., Lakowski J., Sowden J.C., Ali R.R. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA. 2013;110:354–359. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos R.L., Barthel L.K., Meyers J.R., Raymond P.A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res. Rev. 2007;53:124–134. doi: 10.1016/j.brainresrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Braun S.M., Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development. 2014;141:1983–1986. doi: 10.1242/dev.104596. [DOI] [PubMed] [Google Scholar]
- Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S.N., Osborne N.N., Reichenbach A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Brzezinski J.A.t., Kim E.J., Johnson J.E., Reh T.A. Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development. 2011;138:3519–3531. doi: 10.1242/dev.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E., Wilson S.W. Ash1a and Neurogenin1 function downstream of Floating head to regulate epiphysial neurogenesis. Development. 2003;130:2455–2466. doi: 10.1242/dev.00452. [DOI] [PubMed] [Google Scholar]
- Chell J.M., Frisen J. Noisy neurons keep neural stem cells quiet. Cell Stem Cell. 2012;11:282–284. doi: 10.1016/j.stem.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Conner C., Ackerman K.M., Lahne M., Hobgood J.S., Hyde D.R. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J. Neurosci. 2014;34:14403–14419. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett B.V., Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett B.V., Gumerson J.D., Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando R.N., Eleuteri B., Abdelhady S., Nussenzweig A., Andang M., Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.J., Zelinka C., Gallina D., Scott M.A., Todd L. Reactive microglia and macrophage facilitate the formation of Muller glia-derived retinal progenitors. Glia. 2014;62:1608–1628. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C., Barz M., Tchorz J.S., Tome M., Gassmann M., Bischofberger J., Bettler B., Taylor V. GABA suppresses neurogenesis in the adult hippocampus through GABAB receptors. Development. 2014;141:83–90. doi: 10.1242/dev.102608. [DOI] [PubMed] [Google Scholar]
- Goldman D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus J., Zhao F., Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br. J. Ophthalmol. 2016;100:122–127. doi: 10.1136/bjophthalmol-2015-306972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin L.A., Bowser D.N., Dibbens L.M., Singh R., Phillips F., Wallace R.H., Richards M.C., Williams D.A., Mulley J.C., Berkovic S.F. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am. J. Hum. Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S., Nelson B.R., Buckingham B., Reh T.A. Notch signaling regulates regeneration in the avian retina. Dev. Biol. 2007;312:300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R., Heflin S.J., Hammond T., Lee B., Wang J., Gainetdinov R.R., Caron M.G., Eggers E.D., Frishman L.J., McCall M.A. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–110. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R., Steib K., Englberger E., Herold S., Faus-Kessler T., Saxe M., Gage F.H., Song H., Lie D.C. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.Q., Shen W., Macdonald R.L. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J. Neurosci. 2009;29:2845–2856. doi: 10.1523/JNEUROSCI.4772-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl M.O., Hayes S., Nelson B.R., Tan K., Buckingham B., Reh T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen S.C., Ramanan V., Montgomery J.E., T Burket C., Liu C.G., Vihtelic T.S., Hyde D.R. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev. Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Liu W., Wang J.H., Xiang M. Specific expression of the LIM/homeodomain protein Lim-1 in horizontal cells during retinogenesis. Dev. Dyn. 2000;217:320–325. doi: 10.1002/(SICI)1097-0177(200003)217:3<320::AID-DVDY10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang Q., Haydar T.F., Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Matsuoka R.L., Jiang Z., Samuels I.S., Nguyen-Ba-Charvet K.T., Sun L.O., Peachey N.S., Chedotal A., Yau K.W., Kolodkin A.L. Guidance-cue control of horizontal cell morphology, lamination, and synapse formation in the mammalian outer retina. J. Neurosci. 2012;32:6859–6868. doi: 10.1523/JNEUROSCI.0267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J.E., Parsons M.J., Hyde D.R. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J. Comp. Neurol. 2010;518:800–814. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M., Barthel L.K., Raymond P.A. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–4521. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.M., Ackerman K.M., O'Hayer P., Bailey T.J., Gorsuch R.A., Hyde D.R. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J. Neurosci. 2013;33:6524–6539. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olena A.F., Rao M.B., Thatcher E.J., Wu S.Y., Patton J.G. miR-216a regulates snx5, a novel notch signaling pathway component, during zebrafish retinal development. Dev. Biol. 2015;400:72–81. doi: 10.1016/j.ydbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotto M., Deprez F. Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front Cell. Neurosci. 2014;8:166. doi: 10.3389/fncel.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A. Advances in repairing the degenerate retina by rod photoreceptor transplantation. Biotechnol. Adv. 2014;32:485–491. doi: 10.1016/j.biotechadv.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A., Barber A.C., West E.L., MacLaren R.E., Duran Y., Bainbridge J.W., Sowden J.C., Ali R.R. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transpl. 2010;19:487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A., Barber A.C., Rizzi M., Hippert C., Xue T., West E.L., Duran Y., Smith A.J., Chuang J.Z., Azam S.A. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poche R.A., Kwan K.M., Raven M.A., Furuta Y., Reese B.E., Behringer R.R. Lim1 is essential for the correct laminar positioning of retinal horizontal cells. J. Neurosci. 2007;27:14099–14107. doi: 10.1523/JNEUROSCI.4046-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J., Wilken M.S., Ueki Y., Cox K.E., Sullivan J.M., Taylor R.J., Levine E.M., Reh T.A. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013;140:2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G., Benevento M., Alber S., Jacob C., Floriddia E.M., Nguyen T., Elnaggar M.Y., Pedroarena C.M., Molkentin J.D., Di Giovanni S. Nuclear factor of activated T cells (NFATc4) is required for BDNF-dependent survival of adult-born neurons and spatial memory formation in the hippocampus. Proc. Natl. Acad. Sci. USA. 2012;109:E1499–E1508. doi: 10.1073/pnas.1202068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G., Elnaggar M.Y., Duman C., Sabino A., Forsberg K., Di Giovanni S. Modulation of GABAA receptor signaling increases neurogenesis and suppresses anxiety through NFATc4. J. Neurosci. 2014;34:8630–8645. doi: 10.1523/JNEUROSCI.0047-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram K., Harding R.L., Bailey T., Patton J.G., Hyde D.R. Dynamic miRNA expression patterns during retinal regeneration in Zebrafish: reduced dicer or miRNA expression suppresses proliferation of Muller glia-derived neuronal progenitor cells. Dev. Dyn. 2014;243:1591–1605. doi: 10.1002/dvdy.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram K., Harding R.L., Hyde D.R., Patton J.G. miR-203 regulates progenitor cell proliferation during adult zebrafish retina regeneration. Dev. Biol. 2014;392:393–403. doi: 10.1016/j.ydbio.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Fausett B.V., Goldman D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat. Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Zhao X.F., Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc. Natl. Acad. Sci. USA. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Zhao X.F., Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Muller glia-derived progenitors in the injured retina. Nat. Cell Biol. 2012;14:1013–1023. doi: 10.1038/ncb2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M., Hernandez-Montoya J., Sanchez-Serrano S.L., Ordaz B., Ferraro S., Quintero H., Pena-Ortega F., Lamas M. GABA-mediated induction of early neuronal markers expression in postnatal rat progenitor cells in culture. Neuroscience. 2012;224:210–222. doi: 10.1016/j.neuroscience.2012.08.044. [DOI] [PubMed] [Google Scholar]
- Raymond P.A., Barthel L.K., Bernardos R.L., Perkowski J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T., Postel K., Stutzki H., Kurth T., Zeck G., Ader M. Daylight vision repair by cell transplantation. Stem Cells. 2015;33:79–90. doi: 10.1002/stem.1824. [DOI] [PubMed] [Google Scholar]
- Song J., Zhong C., Bonaguidi M.A., Sun G.J., Hsu D., Gu Y., Meletis K., Huang Z.J., Ge S., Enikolopov G. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489:150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Sun J., Moss J., Wen Z., Sun G.J., Hsu D., Zhong C., Davoudi H., Christian K.M., Toni N. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 2013;16:1728–1730. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanhart L.M., Takahashi N., Jackson R.L., Gibson G.A., Watkins S.C., Dawid I.B., Hukriede N.A. Characterization of an lhx1a transgenic reporter in zebrafish. Int. J. Dev. Biol. 2010;54:731–736. doi: 10.1387/ijdb.092969ls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R., Kassen S.C., Montgomery J.E., Enright J.M., Hyde D.R. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Ueki Y., Wilken M.S., Cox K.E., Chipman L., Jorstad N., Sternhagen K., Simic M., Ullom K., Nakafuku M., Reh T.A. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA. 2015;112:13717–13722. doi: 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Bracamontes J., Zorumski C., Weiss D.S., Steinbach J.H. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic T.S., Hyde D.R. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wan J., Ramachandran R., Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev. Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl S.G., Reh T.A. miR-124-9-9∗ potentiates Ascl1-induced reprogramming of cultured Muller glia. Glia. 2016;64:743–762. doi: 10.1002/glia.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo S.Y., Kim M., Kim H.S., Huh T.L., Chitnis A.B. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev. Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Zhao X.F., Wan J., Powell C., Ramachandran R., Myers M.G., Jr., Goldman D. Leptin and IL-6 family cytokines synergize to stimulate Muller glia reprogramming and retina regeneration. Cell Rep. 2014;9:272–284. doi: 10.1016/j.celrep.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.