Abstract
A mitogen-activated protein (MAP) kinase gene, PMK1, is known to regulate appressorium formation and infectious hyphal growth in the rice blast fungus Magnaporthe grisea. In this study, we constructed a green fluorescent protein gene-PMK1 fusion (GFP-PMK1) to examine the expression and localization of PMK1 in M. grisea during infection-related morphogenesis. The GFP-PMK1 fusion encoded a functional protein that complemented the defect of the pmk1 deletion mutant in appressorium formation and plant infection. Although a weak GFP signal was detectable in vegetative hyphae, conidia, and germ tubes, the expression of GFP-Pmk1 was increased in appressoria and developing conidia. Nuclear localization of GFP-Pmk1 proteins was observed in a certain percentage of appressoria. A kinase-inactive allele and a nonphosphorylatable allele of PMK1 were constructed by site-directed mutagenesis. Expression of these mutant PMK1 alleles did not complement the pmk1 deletion mutant. These data confirm that kinase activity and activation of PMK1 by the upstream MAP kinase kinase are required for appressorium formation and plant infection in M. grisea. When overexpressed with the RP27 promoter in the wild-type strain, both the kinase-inactive and nonphosphorylatable PMK1 fusion proteins caused abnormal germ tube branching. Overexpression of these PMK1 mutant alleles may interfere with the function of native PMK1 during appressorium formation.
In eukaryotic cells, a family of serine/threonine protein kinases known as the mitogen-activated protein (MAP) kinases is involved in transducing a variety of extracellular signals and regulating growth and differentiation processes (31, 36). The MAP kinases are usually activated by MAP kinase kinases (MAPKK) that are in turn activated by MAPKK kinases. These MAPKK kinase-MAPKK-MAP kinase cascades are conserved in eukaryotes and have been extensively studied in various eukaryotic organisms (31). One of the best-studied MAP kinase cascades is the pheromone response pathway of the budding yeast Saccharomyces cerevisiae, which involves two partially redundant MAP kinases, FUS3 and KSS1 (13, 16). KSS1 and a few other elements of the pheromone response pathway also are involved in filamentous (invasive) growth in S. cerevisiae (10, 13).
Rice blast, caused by Magnaporthe grisea (Hebert) Barr (anamorph Pyricularia grisea Sacc), is one of the most severe diseases of rice throughout the world (40). Three MAP kinase genes have been identified and characterized for M. grisea (12, 42, 44). The PMK1 gene of M. grisea is homologous to S. cerevisiae FUS3/KSS1. It can rescue the mating defect of a fus3 kss1 yeast double mutant. Mutants with a deletion of PMK1 fail to form specialized infection structures known as appressoria and fail to grow invasively in rice plants (43). Studies with several plant-pathogenic fungi, including Ustilago maydis (6, 25, 27), Colletotrichum lagenarium (38), Cochliobolus heterostrophus (21), Fusarium oxysporum (11), Fusarium graminearum (18, 39), Botrytis cinerea (46), Pyrenophora teres (33), and Claviceps purpurea (26), have indicated that the PMK1 pathway may be well conserved in fungal pathogens for regulation of plant infection processes (42). For five fungal pathogens that form well-defined appressoria, M. grisea, C. lagenarium, Colletotrichum gloeosporioides, C. heterostrophus, and P. teres, the PMK1 pathway is essential for appressorium formation (33, 42). For U. maydis, two MAP kinases, ubc3 (kpp2) and kpp6, are similar to other fungal MAP kinases involved in mating and pathogenicity. The ubc3 (kpp2) disruption mutants are reduced in virulence and mating but still infect plants and form tumors (25, 27). The kpp6 deletion mutants are also reduced in virulence and are defective in the penetration of the plant cuticle (6).
To determine the expression and localization of PMK1 in M. grisea, in this study we constructed a green fluorescent protein gene-PMK1 fusion (GFP-PMK1) that could complement the pmk1 mutant for appressorium formation and plant infection. A low level of GFP-Pmk1 expression was detectable in all cell types, but stronger GFP signals were observed in appressoria and developing conidia. Localization of the GFP-Pmk1 fusion protein in nuclei was observed during appressorium formation. Since Kss1 has a kinase-independent function of inhibiting filamentous growth in yeast (2, 3, 7, 34), we also constructed a kinase-negative allele and a nonphosphorylatable allele of PMK1 to assess whether activation and catalytic activity are required for appressorium development and pathogenesis. These PMK1 mutant alleles were also overexpressed in the wild-type strain to determine their interference with the function of native PMK1 gene.
MATERIALS AND METHODS
Strains and culture conditions.
Wild-type M. grisea strains, the pmk1 mutant nn78, and transformants generated in this study (Table 1) were cultured at 25°C on oatmeal agar plates for conidiation (43). Mycelia collected from 2-day-old 5× YEG cultures shaken at 150 rpm at room temperature were used for preparation of protoplasts and isolation of genomic DNA or protein (46). Fungal transformation and isolation of monoconidial cultures were performed as described previously (43). Hygromycin-resistant and zeocin-resistant transformants were selected on media with 250 μg of hygromycin B (Calbiochem)/ml and 200 μg of zeocin (Invitrogen)/ml, respectively. For examination of GFP expression in young conidia and conidiophores, small blocks of 4-day-old oatmeal cultures were placed over a glass slide, overlaid with a cover glass, and incubated in a moist chamber at 25°C with constant light for 24 h.
TABLE 1.
Wild-type strains and mutants of M. grisea used in this study
| Strain | Brief description | Background | Reference |
|---|---|---|---|
| Guy11 | Wild type, MAT1-2 | NAa | 20 |
| 70-15 | Wild type, MAT1-1 | NA | 8 |
| nn78 | A pmk1 deletion mutant | Guy11 | 43 |
| Xh14 | Native promoter::GFP-PMK1 | nn78 | This study |
| AEF-4 | Native promoter::GFP-PMK1AEF | nn78 | This study |
| K53R-110 | Native promoter::GFP-PMK1K53R | nn78 | This study |
| MT36-17 | Single copy of native promoter::GFP-PMK1K53R | Guy11 | This study |
| MT37-12 | Multiple copies of native promoter::GFP-PMK1AEF | Guy11 | This study |
| MK36-3 | Multiple copies of RP27 promoter::GFP-PMK1K53R | Guy11 | This study |
| MK36-2 | Single copy of RP27 promoter::GFP-PMK1K53R | Guy11 | This study |
| MK37-3 | Single copy of RP27 promoter::GFP-PMK1AEF | Guy11 | This study |
| MK37-1 | Multiple copies of RP27 promoter::GFP-PMK1AEF | Guy11 | This study |
NA, not applicable.
Assays for appressorium formation and plant infection.
Conidia were harvested from 10-day-old oatmeal agar cultures and resuspended to 5 × 104 conidia/ml in sterile distilled water. Appressorium formation was assayed on plastic microscope coverslips and the hydrophobic surface of GelBond membranes (Cambrex) as previously described (45). GFP fluorescence was examined with a Nikon E800 epifluorescence microscope. To visualize nuclear DNA and cell wall, samples were stained with 1 μg of Calcofluor/ml and 1 μg of Hoechst 33258/ml as described previously (17). Appressorial penetration and infectious hyphae formation were assayed with onion epidermal cells (45). For plant infection assays, conidia were resuspended to 105 conidia/ml in a 0.25% gelatin solution. Two-week-old seedlings of the rice cultivar CO39 were used for spray and injection infection assays (45). Plant incubation and inoculation were performed as described previously (41, 45). Lesion formation was examined 7 days after inoculation.
Molecular techniques.
Standard molecular biology procedures were followed for Western and Southern blot analyses (35). Fungal DNA was extracted by the cetyltrimethylammonium bromide (CTAB) protocol (43). For protein isolation, about 150 to 200 mg of mycelia were resuspended in 2 ml of extraction buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 50 mM NaF, 2 mM phenylmethylsulfonyl fluoride, 5 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% glycerol) and 10 μl of protease inhibitor cocktail (Sigma). Samples were homogenized with a Biospec Mini bead beater and centrifuged at 16,000 × g in a microcentrifuge for 20 min at 4°C. The supernatants were then centrifuged at 134,000 × g for 1 h at 4°C in a Beckman Ti50 rotor (Beckman Coulter). Total proteins (approximately 30 μg) were separated on a sodium dodecyl sulfate-12.5% polyacrylamide gel and transferred to nitrocellulose membranes (Amersham). Antigen-antibody detection was performed with the ECL Supersignal System (Pierce), following the instructions provided by the manufacturer. An anti-Pmk1 antiserum was generated at the Purdue antibody facility by injecting rabbits with a glutathione S-transferase-Pmk1 fusion protein that was purified from a bacterial extract. The anti-Pmk1 antiserum was used at a 1:5,000 dilution for Western blotting. A monoclonal anti-GFP antibody (Sigma) and a monoclonal antiactin antibody (Sigma) were diluted at 1:2,000 and used to detect GFP fusion and actin proteins, respectively.
Generating the GFP-PMK1 fusion construct.
The synthetic enhanced GFP gene (37) was amplified with primers GFPMK1F (5′ GGAAGATCTCCATGGTGAGCAAGGGCGAG 3′) and GFPMK2R (5′ GGAAGATCTTGTACAGCTCGTCCATGCC 3′), using the PFU polymerase (Stratagene). The amplified GFP fragment was cloned into the BglII site located at the 5′ end (Lys15) of the PMK1 gene on pFL1 (43). In the resulting construct, pNX45, the GFP gene was inserted in the correct orientation and confirmed by DNA sequencing to be an in-frame fusion with PMK1. The expression of the GFP-PMK1 fusion construct was under the control of the native PMK1 promoter existing on pFL1 (43). Plasmid pNX45 was cotransformed with the vector pAC905 carrying a bleomycin resistance (Bler) gene (46) into the pmk1 deletion mutant nn78 as previously described (43). Monoconidial cultures of zeocin-resistant transformants were screened for GFP expression and confirmed by Southern blot analyses.
Constructing the PMK1K53R and PMK1AEF alleles.
To generate a kinase-negative allele of PMK1, PMK1K53R, pNX45 was subjected to site-directed mutagenesis by the Kunkel method (35) with primer 5′ GTTGCCATAAGGAAGATCACCCCG 3′. The resulting plasmid, pFT36, was confirmed by sequenced analyses to carry the K (AAA)-to-R (AAG) codon change in PMK1. Changing this well-conserved lysine residue in the ATP-binding domain to arginine abolishes kinase activity in vitro and biological function in vivo of KSS1 (22). To construct a PMK1AEF allele with the tyrosine and threonine dual phosphorylation sites changed to alanine and phenylalanine, a 1.3-kb fragment amplified from the coding region of PMK1 with primers Pmk11 (5′ GCCCTGCAGCCCAAGGA 3′) and Pmk16 (5′ CAACGCGTAGCGACG ACGAATTCTGCCATG 3′) was cloned between the PstI and MluI sites on pNX45. The mutant alanine and phenylalanine codons (in bold) conferred by Pmk16 also introduced an EcoRI site (underlined). The resulting PMK1AEF construct, pFT37, was sequenced to confirm the T-to-A and Y-to-F mutations. Plasmids pFT36 and pFT37 were transformed into nn78 or the wild-type Guy11 by cotransformation with pAC905 (43).
Overexpression of the PMK1K53R and PMK1AEF alleles.
The yeast gap repair method described by Bourett and colleagues (5) was used to generate constructs for overexpressing GFP-PMK1 fusion proteins with the strong constitutive RP27 promoter (derived from the M. grisea ribosomal protein 27 gene) carried by plasmid pSM565 (5). The Bler gene amplified from pAC905 with primers BleoF (5′ TTATATCCAGATTCGTCAAGCTGTTTGATGATTTCAGTAACAAGCTTGTTACGGATTCGTG 3′) and BleoR (5′ AGTGAGCTCAGCCCGATTTCCATTCCTCAATTCAAGTCTATTTCTAGAAAGAAGGATTACC 3′) was cotransformed with NcoI-digested pSM565 into S. cerevisiae strain XK1-25 (43). Primers BleoF and BleoR contained 20 bp homologous to the left and right flanking sequences of the hygromycin resistance gene in plasmid pSM565. For plasmid pKB03 rescued from the resulting Trp+ yeast transformants, the hygromycin resistance gene in pSM565 was replaced with the Bler gene by in vivo recombination (5). The GFP-PMK1 fusion constructs were amplified from pFT36 and pFT37 with PCR primers PMK-RPF (5′ AGGAACCCAATCTTCAAAATGTCTCGCGCCAATCCACCA 3′) and PMK-RPR (5′ CTGAATGTTGAGTGGAATGATTTACCGCATAATTTCCTGGTA 3′) and then cotransformed with pKB03 into S. cerevisiae strain XK1-25. Plasmids pKB36 and pKB37 that were rescued from the resulting yeast Trp+ transformants and carried the GFP-PMK1K53R and GFP-PMK1AEF constructs, respectively, were transformed into Guy11 or nn78 by selecting for zeocin-resistant transformants.
RESULTS
GFP-PMK1 complements the pmk1 mutant.
An in-frame GFP-PMK1 translational fusion construct, pNX45, was generated by cloning a GFP gene into the BglII site of PMK1 and introduced into the pmk1 deletion mutant nn78 (43) by cotransformation with pAC905. Five zeocin-resistant transformants that formed appressoria on plastic coverslips were isolated. Transformant Xh14 was selected for further studies after Southern blot analysis revealed that it contained a single-copy integration of pNX45 (data not shown). On hydrophobic surfaces, more than 90% of the germ tubes of Xh14 and Guy11 formed melanized appressoria by 24 h (Fig. 1A). A GFP signal was detectable only in appressoria formed by Xh14 (Fig. 1A). The original pmk1 mutant, nn78, failed to form appressoria under the same conditions (Fig. 1A).
FIG. 1.
Complementation of the pmk1 mutant with the GFP-PMK1 fusion construct. (A) Appressorium formation assay. Germ tubes from the wild-type strain (Guy11) developed appressoria by 24 h, but no appressorium formation was observed in the pmk1 mutant (nn78). Under the same conditions, a transformant of nn78 expressing the GFP-PMK1 fusion construct (Xh14) formed melanized appressoria that had GFP signals when examined under epifluorescence microscopy (Xh14-GFP). Bar = 10 μm. (B) Rice infection assay. Left to right, rice leaves were sprayed with conidia of nn78, Guy11, or Xh14 or 0.25% gelatin solution as the control. Typical leaves were photographed at 7 days postinoculation.
To determine whether this GFP-PMK1 construct could complement the pmk1 mutant for plant infection, seedlings of the rice cultivar CO-39 were sprayed with conidia of Xh14. At 7 days postinoculation, leaves inoculated with Xh14 or Guy11 developed typical blast lesions (Fig. 1B). No lesions were observed on leaves sprayed with 0.25% gelatin or conidia of nn78 (Fig. 1B). These results indicated that the GFP-PMK1 fusion construct encoded a functional protein that complements the defects of the pmk1 mutant in appressorium formation and plant infection.
Expression and localization of GFP-Pmk1 fusion proteins.
To determine whether GFP-PMK1 expression was restricted to specific growth or developmental stages, we examined the GFP signal in Xh14 cultures grown in liquid 5× YEG medium or on oatmeal agar plates. A weak GFP signal was detectable in vegetative hyphae, conidiophores, ungerminated mature conidia, and germ tubes (data not shown). However, stronger fluorescence similar to that observed in appressoria (Fig. 1A) was observed only in young developing conidia (Fig. 2). These data indicate that the GFP-PMK1 fusion construct was expressed constitutively at a relatively low level in Xh14, but its expression was induced during appressorium formation and conidium development.
FIG. 2.
GFP-PMK1 was highly expressed in developing conidia. When blocks of Xh14 oatmeal cultures were examined in situ by epifluorescence microscropy, a strong fluorescence signal was observed in young developing conidia. Conidiophores bearing young conidia were also fluorescent, but the signal was much weaker. Mature conidia exhibited only weak fluorescence. CP, conidiophore; YC, young conidium; MC, mature conidium. Bar = 10 μm.
The expression and localization of GFP-Pmk1 was also examined during appressorium formation. At 2 h, GFP-Pmk1 fluorescence was somewhat homogeneously distributed in the conidial cells and germ tubes (Fig. 3A). At 4 and 8 h, GFP fluorescence was still visible in the germ tubes and conidium but enhanced in young appressoria (Fig. 3A). At 24 h, the fluorescence signal was observed mainly in mature appressoria. Conidia and germ tubes usually lacked or had only faint fluorescence (Fig. 3A). Interestingly, we noticed that the GFP signal was not homogeneously distributed in some appressoria (Fig. 3A). Nuclear staining with Hoechst 33258 indicated that the GFP fluorescence was concentrated in nuclei of these appressoria (Fig. 3B). At 4 h, only a small percentage (3.3% ± 2.1%) of appressoria had GFP signal coincident with the position of the nucleus. The number of appressoria with nuclear localization of GFP signal increased during appressorium maturation. At 12 and 24 h, the percentages of appressoria that had stronger GFP fluorescence in nuclei than in the cytoplasm was 17.7 ± 3.1 and 21.3 ± 3.1, respectively. We also observed nuclear localization of the GFP signal in mature appressoria beyond 24 h. However, because fluorescence over the entire appressorium became stronger during appressorium maturation, it became difficult and unreliable to count the percentage of appressoria with nuclear localization of GFP-Pmk1.
FIG. 3.
Expression and cellular localization of GFP-PMK1 during appressorium formation in transformant Xh14. (A) Xh14 conidia germinated on glass coverslips were removed at indicated times and examined under differential interference contrast (left panels) and epifluorescence microscopy (right panels). Fluorescence was observed in the conidia and germ tubes at the early time points, such as 2 or 4 h. By 12 h, the majority of the GFP signal was observed in appressoria. At 24 h, conidial cells exhibited no GFP signal or only a very weak GFP signal. (B) The GFP-Pmk1 signal localized to the nucleus in appressoria. A conidium of Xh14 incubated on a glass coverslip for 24 h was stained with Calcofluor and Hoechst 33258 (middle panel) to visualize cell walls and nuclei (arrow). GFP signal (right panel) appeared to be concentrated in the appressorium at the position of the nucleus. Bar = 10 μm.
GFP-Pmk1 was detectable in infectious hyphae.
Onion epidermal cells were used to assay GFP-Pmk1 expression during appressorial penetration and infectious hyphal growth. Similar to appressoria formed on artificial hydrophobic surfaces, a majority of appressoria formed by Xh14 on onion epidermal cells exhibited strong fluorescence at 24 h (Fig. 4). Germ tubes and conidial cells had no GFP signals or very weak GFP signals (Fig. 4). As with the wild-type strain Guy11, appressoria formed by Xh14 were able to penetrate onion epidermal cells and differentiate into infectious hyphae. All infectious hyphae that were distinguishable by fluorescence microscopy contained GFP fluorescence at 48 h (Fig. 4). The GFP signal appeared to be evenly distributed throughout the cytoplasm, and no obvious preference for regions that were coincident with nuclei was observed (Fig. 4). These observations indicated that GFP-Pmk1 fusion protein was present in infectious hyphae and that expression from the PMK1 promoter persisted beyond the appressorial penetration stage.
FIG. 4.
GFP-PMK1 was expressed in infectious hyphae. Conidia from strain Xh14 were inoculated on onion epidermal cells and examined under differential interference contrast (left panels) or epifluorescence microscopy (right panels). At 24 h, the majority of appressoria formed by Xh14 had not penetrated plant cells yet and contained strong GFP fluorescence. At 48 h, appressoria of Xh14 that had successfully penetrated onion epidermal cells contained no GFP signal or weak GFP signal. Fluorescence was observed in infectious hyphae without any special cellular localization pattern. In some appressoria, fluorescent signals in underlying infectious hyphae could be observed through the collapsed appressoria. Bar = 10 μm. A, appressorium; IF, infectious hypha; C, conidium.
Kinase activity of Pmk1 is required for appressorium development and plant infection.
In yeast, KSS1 has kinase activity-independent functions in filamentous growth (2, 3, 34). To determine whether the kinase activity of the Pmk1 protein is essential for its function during plant infection processes, we generated two PMK1 mutant alleles, PMK1AEF and PMK1K53R, that were similar to the kinase-inactive KSS1AEF and nonphosphorylatable KSS1K42R alleles (22). After transformation of the pmk1 mutant nn78 with pFT36 or pFT37, zeocin-resistant transformants harboring the GFP-PMK1AEF and GFP-PMK1K53R constructs were identified by screening for transformants with green fluorescence in vegetative hyphae and germinating conidia. Transformants AEF-4 and K53R-110 were found by Southern blot analysis to contain a single copy of the transforming vector pFT36 or pFT37, respectively (data not shown). The GFP signal was detected in conidia and germ tubes of both AEF-4 and K53R-110 (Fig. 5A). However, germ tubes of AEF-4 and K53R-110 failed to form appressoria on artificial hydrophobic surfaces (Fig. 5A). We also assayed appressorium formation on onion epidermal cells. Even in the presence of a cutin monomer, 1,16-hexadecanediol, AEF-4 and K53R-110 did not form appressoria (data not shown). Similar results were obtained with the other five transformants, each harboring the GFP-PMK1AEF or GFP-PMK1K53R construct, that we examined. These data suggest that Pmk1 phosphorylation and its kinase activity are required for appressorium development on both artificial and plant surfaces.
FIG. 5.
The K53R and AEF mutations abolished the function of PMK1 in appressorium formation and infectious growth. (A) Conidia of transformants expressing GFP-PMK1AEF (AEF-4) and GFP-PMK1K53R (K53R-110) were incubated on glass coverslips for 24 h and examined under DIC (left panels) or epifluorescence microscopy (right panels). GFP-Pmk1 expression was detectable in conidia and germ tubes of AEF-4 and K53R-110, but no appressorium formation was observed. (B) Leaves of rice cultivar CO-39 injected with conidia from the pmk1 mutant (nn78) or transformants of nn78 expressing the GFP-PMK1 (Xh14), GFP-PMK1AEF (AEF-4), or GFP-PMK1K53R (K53R-110) constructs. Spreading lesions were observed only in leaves injected with Xh14 but not AEF-4 or K53R-110. Photos were taken at 7 days after inoculation. DIC, differential interference contrast.
Similar to the pmk1 mutant nn78, AEF-4 and K53R-110 were nonpathogenic. On seedlings of rice cultivar CO-39 sprayed with AEF-4 or K53R-110 conidia, no blast lesions were observed (data not shown). Since these transformants failed to form appressoria, we also performed injection assays with CO-39 seedlings. On rice leaves injected with Xh14 conidia, lesions were observed in and outside of the wound sites (Fig. 5B). However, AEF-4 and K53R-110, as with nn78, caused only limited necrosis at the wound sites and failed to colonize and spread through the wound sites (Fig. 5B), indicating that the GFP-PMK1AEF and GFP-PMK1K53R mutant alleles failed to complement the defect of the pmk1 mutant in colonization and infection through wounds. Therefore, both activation and kinase activity of Pmk1 are required for appressorium formation and infectious growth after penetration.
Full-length GFP-Pmk1 proteins were detectable by Western blot analyses.
In addition to examining fluorescence signals, we detected the expression of PMK1 using an anti-Pmk1 polyclonal antiserum with proteins isolated from mycelia of 2-day-old 5× YEG cultures. In the wild-type strain Guy11, a 42-kDa band was detected by the anti-Pmk1 antiserum (Fig. 6). In protein extracts from transformants Xh14, AEF-4, and K53R-110 expressing various GFP-PMK1 fusion constructs, the anti-Pmk1 antiserum detected a 68-kDa band of the predicted GFP-Pmk1 fusion protein that was not detectable with Guy11 (Fig. 6). The anti-GFP monoclonal antibody recognized the same 68-kDa band in transformants Xh14, AEF-4, and K53R-110 (Fig. 6). For Guy11 and nn78, no protein band was detected by the anti-GFP antibody. These results demonstrated that full-length GFP-Pmk1 fusion proteins were expressed in transformants Xh14, AEF-4, and K53R-110, although the expression level of the GFP-PMK1AEF allele was lower than that of GFP-PMK1 or GFP-PMK1K53R (Fig. 6). Therefore, failure of GFP-PMK1K53R or GFP-PMK1AEF to complement the pmk1 mutant for appressorium formation and plant infection was directly related to the K53R and AEF mutations, which abolished the kinase activity of Pmk1 and its ability to be phosphorylated.
FIG. 6.
Western blot analysis of the expression of PMK1 and GFP-PMK1 constructs. Total protein was isolated from mycelia of the pmk1 deletion mutant (nn78), the wild-type strain (Guy11), or transformants of nn78 expressing GFP-PMK1 (Xh14), GFP-PMK1K53R (K53R-110), or GFP-PMK1AEF (AEF-4). When probed with the anti-Pmk1 antiserum (top panel), a 42-kDa Pmk1 band was observed in Guy11. In Xh14, AEF-4, and K53R-110, a larger protein of 68 kDa corresponding to the GFP-Pmk1 fusion was detected. When probed with an anti-GFP antibody (middle), a 68 kDa band was detected in Xh14, AEF-4, and K53R-110 but not in Guy11. The bottom panel was detection with an antiactin antibody to show that a similar amount of total protein was loaded in each lane.
Overexpression of the GFP-PMKK53R allele interfered with germ tube growth during appressorium formation.
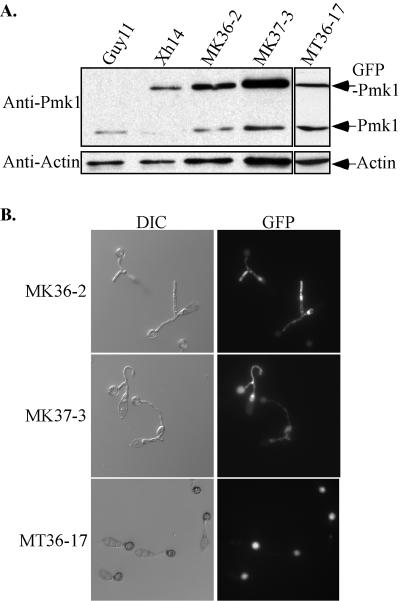
To overexpress the unphosphorylatable and kinase-negative PMK1 alleles, we cloned the GFP-PMK1AEF and GFP-PMK1K53R fusion constructs behind the RP27 promoter (see Materials and Methods). More than 12 zeocin-resistant transformants of each were isolated after transformation of the wild-type strain Guy11 with pKB36 or pKB37 and examined for appressorium formation and fluorescence. Transformants MK36-2 and MK37-3 (Table 1) were found by Southern blot analysis to contain a single copy of pKB36 or pKB37, respectively (data not shown). Overexpression of the GFP fusion proteins was determined by Western blot analysis (Fig. 7A). When probed with the anti-Pmk1 antiserum, MK36-2 and MK37-3 had two bands corresponding to the native Pmk1 and GFP-Pmk1 fusion proteins. A comparison of these two bands revealed that the GFP fusion proteins were present at a level of three to four times that of the native Pmk1 protein (Fig. 7A).
FIG. 7.
Overexpression of PMK1 mutant alleles resulted in morphological defects during appressorium formation. (A) Western blot analyses with total proteins extracted from the wild-type strain (Guy11) or transformants expressing the PMK1K53R (MK36-2) or GFP-PMK1AEF (MK37-3) fusion construct under the RP27 promoter. When probed with the anti-Pmk1 antiserum, MK36-2 and MK37-3 had two bands corresponding to the native Pmk1 and GFP-Pmk1 fusion proteins. A separate blot containing total protein from strain MT37-12 probed with anti-Pmk1 contains similar levels of protein between the native PMK1 and the GFP fusion. Blots probed with antiactin antibody demonstrated the relative variance in total proteins loaded in each lane. (B) Branching germ tubes formed by MK36-2 and MK37-3. Conidia from 10-day-old cultures were incubated at room temperature for 24 h and examined with differential intererence contrast (DIC) or epifluorescence microscopy. MT36-17 expressing GFP-PMK1K53R fusion under the native Pmk1 promoter had no morphological defects during appressorium formation.
Transformants MK36-2 and MK37-3 had no obvious defects in vegetative growth and conidiation. Fluorescent signals were detectable in conidia, germ tubes, and appressoria produced by these transformants (Fig. 7B). Appressoria formed by these transformants were melanized and appeared to be normal in morphology. However, about 15% of the germinating conidia of MK36-2 and MK37-3 produced branching germ tubes (Fig. 7B). Since no significant difference was observed between MK36-2 and MK37-3, it is likely that the overproduction of the GFP-Pmk1K53R or GFP-Pmk1AEF protein had a similar effect on germ tube growth during appressorium formation by interfering with the normal function of wild-type PMK1. To further confirm that these defects resulted from overexpression of PMK1 mutant alleles, we also transformed pFT36 and pFT37 carrying the PMK1 gene under the control of its native promoter into the wild-type strain Guy11. The resulting transformants, MT36−17 and MT37-12 (Table 1), contained a single copy of pFT36 or pFT37, respectively. The expression level of GFP-PMK1K53R fusion in MT36-17 was similar to that of the native Pmk1 protein (Fig. 7A). MT36−17 and MT37-12 and all other transformants harboring pFT36 or pFT37 were normal in germ tube growth and appressorium formation (Fig. 7B). These data suggest that overexpression of the PMK1K53R or PMK1AEF mutant allele might interfere with the function of wild-type PMK1 in Guy11 and cause abnormal germ tube branching.
DISCUSSION
To examine the expression and localization of PMK1 in M. grisea, we generated a GFP-PMK1 fusion construct that was under the control of the native PMK1 promoter and terminator sequences. Since the pmk1 mutant expressing this fusion construct was able to form appressoria and infect rice plants, the fusion of GFP had no obvious detrimental effect on the function of PMK1 MAP kinase in vivo. Although a weak GFP signal was detectable in vegetative hyphae, conidiophores, conidia, and germ tubes of Xh14, stronger fluorescence from the GFP-Pmk1 fusion protein was observed only in appressoria and developing conidia. PMK1 is likely expressed constitutively at a relatively low level, but its transcription or translation is increased during appressorium formation and conidium development in M. grisea. The expression of PMK1 in vegetative hyphae is also made evident by the fact that PMK1 cDNA clones were isolated from the cDNA library constructed with RNA isolated from vegetative hyphae starved for nitrogen (43). However, Northern blot analyses failed to detect any PMK1 signals with RNA samples isolated from vegetative hyphae grown in complete medium or under nitrogen or carbon starvation conditions, suggesting that the transcription of PMK1 is at a low level. In yeast, FUS3 is expressed mainly in haploid cells and is induced by mating pheromone, while KSS1 is constitutively expressed in haploid and diploid cells (16). In U. maydis, kpp6 is also constitutively expressed in all cell types (6).
Our observations suggest that GFP-Pmk1 is distributed throughout the cytoplasm and nucleus during most stages of growth and may be translocated to the nucleus in developing appressoria. Nuclear import of MAP kinases has been observed as a response to appropriate stimuli in various organisms (1, 15, 19, 30, 32). In yeast, Fus3 is distributed in the cytoplasm and nucleus in vegetative cells (4, 9). A brief exposure to pheromone stimulates the accumulation of Fus3 in the nucleus, but nuclear localization of Fus3 decreases as cells recover from pheromone arrest after a longer exposure (4, 9). In contrast, Kss1 is concentrated in the nucleus, and its subcellular localization is unaffected by pheromone treatment (22). In M. grisea, appressorium formation is not a synchronous process. The exact time when Pmk1 is translocated to the nucleus during appressorium formation is difficult to ascertain. The lack of a uniform state in which all appressoria contain the GFP-Pmk1 signal within the nucleus indicates that nuclear localization of Pmk1 is transient and Pmk1 does not remain in the nuclei throughout the appressorium formation process.
PMK1 and its homologues have been shown to regulate infectious growth after penetration in a few plant-pathogenic fungi (11, 38, 43, 46). In Xh14, GFP signal was detectable throughout the infectious hyphae formed inside plant cells (Fig. 4), indicating that PMK1 was expressed after appressorial penetration. However, no obvious nuclear localization of GFP-Pmk1 was observed in any of the infectious hyphae examined at 48 or 72 h. While nuclei in appressoria are likely to be arrested at the G1 stage, nuclei in infectious hyphae may be in different nuclear division stages. In M. grisea, bulbous, branching infectious hyphae that develop inside plant cells are morphologically different from vegetative hyphae. It is likely that the infectious hyphae are developmentally and physiologically less uniform than unicellular appressoria formed on inert artificial surfaces. Therefore, it remains possible that GFP-Pmk1 is transiently translocated into nuclei in infectious hyphae.
In S. cerevisiae, Kss1 has a dual role in regulating filamentous growth (2, 3). The stimulatory function of Kss1 requires its activation and kinase activity. The inhibitory role of Kss1, however, depends on its binding with the Ste12 transcription factor and does not require the kinase activity (2, 3, 7, 34). Interestingly, Fus3 also has a kinase-dependent activation function and a kinase-independent inhibitory role in the pheromone response pathway (24). The active Fus3 limits the extent of Kss1 activation (34), but kinase-negative Fus3 proteins inhibit the activation of the mating pathway by Kss1 (23, 24). In M. grisea, an inhibitory mechanism such as that seen between FUS3 and KSS1 may not exist, because PMK1 is the only homologue of yeast FUS3/KSS1. In this study, we created a putative kinase-inactive allele (PMK1K53R) and a putative nonphosphorylatable allele (PMK1AEF) of PMK1 that were equivalent to Kss1K42R and Kss1pAEF (22), respectively. Both mutant alleles were created with the GFP-PMK1 construct carried on pNX45 because it encoded a fully functional GFP-Pmk1 fusion protein and its expression in M. grisea could be examined with green fluorescence. Expression of the GFP-PMK1K53R or GFP-PMK1AEF allele failed to restore appressorium formation in nn78 (Fig. 6), indicating that the K53R and AEF mutations resulted in nonfunctional Pmk1. Thus, both the kinase activity of Pmk1 and its ability to be activated by upstream MAPKK are essential for the function of PMK1 during appressorium formation and plant infection. In U. maydis, transformants expressing the nonactivatable kpp6AEF allele are more severely compromised in pathogenesis than the kpp6 deletion mutant (6), indicating that Kpp6 has a kinase-independent regulatory role in plant penetration. Kpp2 also has a kinase-independent role in regulating a gene expression, but phosphorylation and kinase activity of Kpp2 are essential for b gene expression and conjugation tube formation in response to mating pheromones (28). Since there was no obvious difference between nn78 and nn78 transformants expressing the PMK1K53R or GFP-PMK1AEF allele, it is unlikely that these PMK1 mutant alleles have any negative regulatory functions in M. grisea.
Although no obvious defect was observed in the wild-type strains expressing the GFP-PMK1K53R and GFP-PMK1AEF constructs with the native PMK1 promoter, expression of these mutant PMK1 alleles with the RP27 promoter in Guy11 often resulted in double germ tube formation or germ tube branching near the site of germ tube emergence (Fig. 7B). Overexpression of these mutant PMK1 alleles likely has a negative effect on the function of native PMK1 during appressorium formation. Since no difference was observed between transformants MK36-2 and MK37-3, the kinase-inactive and nonphosphorylatable alleles of PMK1 may have similar interference effects. One possibility is that high levels of expression of the kinase-negative K53R or unphosphorylatable alleles of PMK1 by the RP27 promoter may compete with the native Pmk1 for binding to its target proteins, blocking their activation and interfering with appressorium formation. In yeast, catalytically inactive Fus3 inhibits the ability of active Fus3 to activate Ste12 and blocks further differentiation by restoring mitotic growth (14). In M. grisea, mutants with a deletion of MST12, a homologue of STE12, are normal in appressorium formation although they are defective in plant penetration (29). However, our preliminary data indicated that overexpression of the wild-type PMK1 allele with the RP27 promoter resulted in a similar, if not more severe, defect of branching germ tubes (data not shown). Therefore, it is also possible that the defect of germ tube branching resulted from a negative effect brought on by the overabundance of the wild-type or mutant GFP-Pmk1 fusion proteins. We noticed that the percentage of abnormal germ tube branching varied between cultures of different ages, and defects caused by the overexpression of PMK1 mutant alleles usually were more severe in young cultures derived from the original transformants. In addition, we failed to isolate transformants that expressed PMK1 with the RP27 promoter at a level more than fivefold that of the native promoter, indicating that overexpression of these PMK1 mutant alleles may have a detrimental effect on growth in M. grisea.
Acknowledgments
We thank Larry Dunkle and Charles Woloshuk for critical reading of the manuscript, Christopher Staiger for fruitful discussions, and Xinhua Zhao for rice infections.
This work was supported by a grant (to J.X.) from the U.S. Department of Agriculture National Research Initiative (2001-35319-09924).
REFERENCES
- 1.Adachi, M., M. Fukuda, and E. Nishida. 1999. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 18:5347-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardwell, L., J. G. Cook, D. Voora, D. M. Baggott, A. R. Martinez, and J. Thorner. 1998. Repression of yeast Ste12 transcription factor by direct binding of unphosphorylated Kss1 MAPK and its regulation by the Ste7 MEK. Genes Dev. 12:2887-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardwell, L., J. G. Cook, J. X. Zhu-Shimoni, D. Voora, and J. Thorner. 1998. Differential regulation of transcription: repression by unactivated mitogen-activated protein kinase Kss1 requires the Dig1 and Dig2 proteins. Proc. Natl. Acad. Sci. USA 95:15400-15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell, E., I. M. Halatek, H. J. N. Kim, A. T. Ellicott, A. A. Obukhov, and D. E. Stone. 2003. Effect of the pheromone-responsive G(alpha) and phosphatase proteins of Saccharomyces cerevisiae on the subcellular localization of the Fus3 mitogen-activated protein kinase. Mol. Cell. Biol. 23:1135-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourett, T. M., J. A. Sweigard, K. J. Czymmek, A. Carroll, and R. J. Howard. 2002. Reef coral fluorescent proteins for visualizing fungal pathogens. Fungal Genet. Biol. 37:211-220. [DOI] [PubMed] [Google Scholar]
- 6.Brachmann, A., J. Schirawski, P. Muller, and R. Kahmann. 2003. An unusual MAP kinase is required for efficient penetration of the plant surface by Ustilago maydis. EMBO J. 22:2199-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitkreutz, A., L. Boucher, and M. Tyers. 2001. MAPK specificity in the yeast pheromone response independent of transcriptional activation. Curr. Biol. 11:1266-1271. [DOI] [PubMed] [Google Scholar]
- 8.Chao, C. C. T., and A. H. Ellingboe. 1991. Selection for mating competence in Magnaporthe grisea pathogenic to rice. Can. J. Bot. 69:2130-2134. [Google Scholar]
- 9.Choi, K. Y., J. E. Kranz, S. K. Mahanty, K. S. Park, and E. A. Elion. 1999. Characterization of Fus3 localization: active Fus3 localizes in complexes of varying size and specific activity. Mol. Biol. Cell 10:1553-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook, J. G., L. Bardwell, and J. Thorner. 1997. Inhibitory and activating functions for MAPK Kss1 in the Saccharomyces cerevisiae filamentous-growth signaling pathway. Nature 390:85-88. [DOI] [PubMed] [Google Scholar]
- 11.Di Pietro, A., F. I. Garcia-Maceira, E. Meglecz, and M. I. G. Roncero. 2001. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39:1140-1152. [PubMed] [Google Scholar]
- 12.Dixon, K. P., J. R. Xu, N. Smirnoff, and N. J. Talbot. 1999. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11:2045-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohlman, H. G., and J. W. Thorner. 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70:703-754. [DOI] [PubMed] [Google Scholar]
- 14.Farley, F. W., B. Satterberg, E. J. Goldsmith, and E. A. Elion. 1999. Relative dependence of different outputs of the Saccharomyces cerevisiae pheromone response pathway on the MAP kinase Fus3p. Genetics 151:1425-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrigno, P., and P. A. Silver. 1999. Regulated nuclear localization of stress-responsive factors: how the nuclear trafficking of protein kinases and transcription factors contributes to cell survival. Oncogene 18:6129-6134. [DOI] [PubMed] [Google Scholar]
- 16.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, S. D., J. L. Morrell, and J. E. Hamer. 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136:517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenczmionka, N. J., F. J. Maier, A. P. Losch, and W. Schafer. 2003. Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head-blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr. Gen. 43:87-95. [DOI] [PubMed] [Google Scholar]
- 19.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605-615. [DOI] [PubMed] [Google Scholar]
- 20.Leung, H., E. S. Borromeo, M. A. Bernardo, and J. L. Notteghem. 1988. Genetic analysis of virulence in the rice blast fungus Magnaporthe grisea. Phytopathology 78:1227-1233. [Google Scholar]
- 21.Lev, S., A. Sharon, R. Hadar, H. Ma, and B. A. Horwitz. 1999. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc. Natl. Acad. Sci. USA 96:13542-13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, D., J. G. Cook, and J. Thorner. 1995. Phosphorylation and localization of Kss1, a MAP kinase of the Saccharomyces cerevisiae pheromone response pathway. Mol. Biol. Cell 6:889-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhani, H. D., and G. R. Fink. 1998. The riddle of MAP kinase signaling specificity. Trends Genet. 14:151-155. [DOI] [PubMed] [Google Scholar]
- 24.Madhani, H. D., C. A. Styles, and G. R. Fink. 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91:673-684. [DOI] [PubMed] [Google Scholar]
- 25.Mayorga, M. E., and S. E. Gold. 1999. A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 34:485-497. [DOI] [PubMed] [Google Scholar]
- 26.Mey, G., B. Oeser, M. H. Lebrun, and P. Tudzynski. 2002. The biotrophic, non-appressorium-forming grass pathogen Claviceps purpurea needs a Fus3/Pmk1 homologous mitogen-activated protein kinase for colonization of rye ovarian tissue. Mol. Plant-Microbe Interact. 15:303-312. [DOI] [PubMed] [Google Scholar]
- 27.Muller, P., C. Aichinger, M. Feldbrugge, and R. Kahmann. 1999. The MAP kinase Kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol. Microbiol. 34:1007-1017. [DOI] [PubMed] [Google Scholar]
- 28.Muller, P., G. Weinzierl, A. Brachmann, M. Feldbrugge, and R. Kahmann. 2003. Mating and pathogenic development of the smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot. Cell 2:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, G., C. Y. Xue, L. Zheng, S. Lam, and J. R. Xu. 2002. MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 15:183-192. [DOI] [PubMed] [Google Scholar]
- 30.Pouyssegur, J., and P. Lenormand. 2003. Fidelity and spatio-temporal control in MAP kinase (ERKs) signaling. Eur. J. Biochem. 270:3291-3299. [DOI] [PubMed] [Google Scholar]
- 31.Raman, M., and M. H. Cobb. 2003. MAP kinase modules: many roads home. Curr. Biol. 13:886-888. [DOI] [PubMed] [Google Scholar]
- 32.Reiser, V., H. Ruis, and G. Ammerer. 1999. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell 10:1147-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Roldan, M. C., F. J. Maier, and W. Schafer. 2001. PTK1, a mitogen-activated protein kinase gene, is required for conidiation, appressorium formation, and pathogenicity of Pyrenophora teres on barley. Mol. Plant-Microbe Interact. 14:116-125. [DOI] [PubMed] [Google Scholar]
- 34.Sabbagh, W., L. J. Flatauer, A. J. Bardwell, and L. Bardwell. 2001. Specificity of MAP kinase signaling in yeast differentiation involves transient versus sustained MAPK activation. Mol. Cell 8:683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sievers, N., E. Bertsch, and R. Fischer. 1999. Isolation of nuclear migration mutants of Aspergillus nidulans using GFP expressing strains. Mycol. Res. 103:961-966. [Google Scholar]
- 38.Takano, Y., T. Kikuchi, Y. Kubo, J. E. Hamer, K. Mise, and I. Furusawa. 2000. The Colletotrichum lagenarium Map kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant-Microbe Interact. 13:374-383. [DOI] [PubMed] [Google Scholar]
- 39.Urban, M., E. Mott, T. Farley, and K. Hammond-Kosack. 2003. The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Mol. Plant Pathol. 4:347-359. [DOI] [PubMed] [Google Scholar]
- 40.Valent, B. 1990. Rice blast as a model system for plant pathology. Phytopathology 80:33-36. [Google Scholar]
- 41.Valent, B., and F. G. Chumley. 1991. Molecular genetic analysis of the rice blast fungus Magnaporthe grisea. Annu. Rev. Phytopathol. 29:443-467. [DOI] [PubMed] [Google Scholar]
- 42.Xu, J. R. 2000. MAP kinases in fungal pathogens. Fungal Genet. Biol. 31:137-152. [DOI] [PubMed] [Google Scholar]
- 43.Xu, J. R., and J. E. Hamer. 1996. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10:2696-2706. [DOI] [PubMed] [Google Scholar]
- 44.Xu, J. R., C. J. Staiger, and J. E. Hamer. 1998. Inactivation of the mitogen-activated protein kinase MPS1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA 95:12713-12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, J. R., M. Urban, J. A. Sweigard, and J. E. Hamer. 1997. The CPKA gene of Magnaporthe grisea is essential for appressorial penetration. Mol. Plant-Microbe Interact. 10:187-194. [Google Scholar]
- 46.Zheng, L., M. Campbell, J. Murray, S. Lam, and J. R. Xu. 2000. The BMP1 gene is essential for pathogenicity in the gray mold fungus Botrytis cinerea. Mol. Plant-Microbe Interact. 13:724-732. [DOI] [PubMed] [Google Scholar]