Abstract
The Rad51 protein from the methylotrophic yeast Pichia angusta (Rad51Pa) of the taxonomic complex Hansenula polymorpha is a homolog of the RecA-RadA-Rad51 protein superfamily, which promotes homologous recombination and recombination repair in prokaryotes and eukaryotes. We cloned the RAD51 gene from the cDNA library of the thermotolerant P. angusta strain BKM Y1397. Induction of this gene in a rad51-deficient Saccharomyces cerevisiae strain partially complemented the survival rate after ionizing radiation. Purified Rad51Pa protein exhibited properties typical of the superfamily, including the stoichiometry of binding to single-stranded DNA (ssDNA) (one protomer of Rad51Pa per 3 nucleotides) and DNA specificity for ssDNA-dependent ATP hydrolysis [poly(dC) > poly(dT) > φX174 ssDNA > poly(dA) > double-stranded M13 DNA]. An inefficient ATPase and very low cooperativity for ATP interaction position Rad51Pa closer to Rad51 than to RecA. Judging by thermoinactivation, Rad51Pa alone was 20-fold more thermostable at 37°C than its S. cerevisiae homolog (Rad51Sc). Moreover, it maintained ssDNA-dependent ATPase and DNA transferase activities up to 52 to 54°C, whereas Rad51Sc was completely inactive at 47°C. A quick nucleation and an efficient final-product formation in the strand exchange reaction promoted by Rad51Pa occurred only at temperatures above 42°C. These reaction characteristics suggest that Rad51Pa is dependent on high temperatures for activity.
Pichia angusta, one of the species of the taxonomic complex Hansenula polymorpha (25), is a methylotrophic yeast which has attracted considerable attention as a promising host for the production of recombinant proteins because of its powerful promoter elements, its ability to grow at high density on inexpensive substrates, and the unusual properties of its homologous and nonhomologous recombination systems promoting a multiple tandem integration of a nonlinearized plasmid in the host chromosome (for reviews, see references 11, 28, and 31). One additional characteristic that might have a biotechnological advantage is its thermotolerance, an ability to grow at temperatures (42°C and higher) which are not acceptable for other yeast strains.
Among 2,500 P. angusta novel protein-encoding genes now identified, 6% have no homologs in Saccharomyces cerevisiae (3). Little is known about the recombination or DNA repair proteins in P. angusta, such as the homologous DNA transferase Rad51 and its paralogs. This recombinase is a RecA-RadA-like protein, found in all three domains of life (4), which forms filaments on single-stranded DNA (ssDNA) in the presence of ATP (and thus possesses ATPase activity) and promotes homologous pairing and strand exchange, which are the two main steps in the initiation of homologous recombination and recombination repair, as well (6, 16, 27). All members of the RecA-RadA-Rad51 recombinase superfamily form nucleoprotein filaments of similar structures and stoichiometries, and they display similar preferences in DNA substrates for DNA-dependent ATP hydrolysis (26, 30, 32). Although similar in ATP-dependent recombination functions, these members are divided into two subfamilies: RecA-like and RadA-Rad51-like proteins. The latter group displays weak ATP hydrolysis activity and lacks the magnitude of the ATP-induced cooperativity displayed by RecA (8, 19, 35).
Here, we characterize the basic recombination activities of Rad51Pa, a new member of the Rad51 family from P. angusta. Some properties were compared with those of Rad51 from S. cerevisiae (Rad51Sc), mainly in the context of protein thermostability. In two principle recombination activities analyzed here, a quick nucleation and an efficient final-product (FP) formation in a φX174 DNA strand exchange reaction, Rad51Pa demonstrated thermodependence rather than thermotolerance.
MATERIALS AND METHODS
Strains, plasmids, and media.
Thermotolerant P. angusta strain BKM Y1397 was kindly supplied by the Russian Collection of Microorganisms. The strain was grown in yeast extract-peptone-dextrose (YEPD) medium (1% peptone, 0.5% yeast extract, 2% glucose) at 30, 37, 40, 42, 46, and 49°C during the 3 days after an initial inoculation of not more than 1,000 cells per ml. The growth curves (data not shown) revealed that 37, 40, and even 42°C appeared to be optimal growth temperatures, whereas 30 and 46°C markedly inhibited cell growth, though the cells not only maintained survival but even grew slightly at 49°C.
S. cerevisiae diploid strain D4004 (MATa/MATα ade2Δ-248/ade2Δ-248 leu2-3,112/leu2-3,112 ura3-160,288/ura3-160,288 trp1/trp1 RAD51/RAD51) and its rad51-1 derivate D3164 (MATa/MATα ade2Δ-248/ade2Δ-248 leu2-3,112/leu2-3,112 ura3-160,288/ura3-160,288 trp1/trp1 rad51-1/rad51-1) were used to study complementation of the ionizing radiation (IR) sensitivity of the S. cerevisiae rad51-1 diploid mutant with the cloned RAD51Pa gene.
cDNA library construction and RAD51Pa cloning procedure.
The P. angusta cDNA library was constructed in a Uni-ZAP XR vector (Stratagene) with poly(A) RNA purified from BKM Y1397 cells. A fragment of the RAD51Pa gene was amplified by PCR using degenerate primers designed from conserved regions of the Rad51 protein family. The resulting PCR product was used as a probe for subsequent library screening. A positive clone containing RAD51Pa cDNA was selected and sequenced. For protein expression, the RAD51Pa gene was recloned into a pET21b vector (Novagen). The pYES2::RAD51Pa plasmid was constructed by recloning the RAD51Pa gene into the EcoRI-XhoI sites of the pYES2 plasmid under a GAL1 promoter. Both pYES2 and pYES2::RAD51Pa were used in comparative analysis of IR sensitivity.
DNA manipulation.
Standard procedures described previously (29) were used for DNA manipulation. Linear φX174 double-stranded DNA (dsDNA) was prepared from its circular form by cleavage with the PstI endonuclease, followed by extraction with phenol-chloroform, precipitation with ethanol, and resuspension in Tris-EDTA (pH 8.0) buffer. The concentrations of DNA substrates are expressed as nucleotide equivalents. The concentrations of poly(dT), poly(dC), poly(dA), ssDNA, and dsDNA were determined using the following extinction coefficients: ɛ264, 8,520 M−1 cm−1; ɛ268, 7,400 M−1 cm−1; ɛ257, 8,600 M−1 cm−1; ɛ260, 8,780 M−1 cm−1; and ɛ260, 6,500 M−1 cm−1, respectively.
Protein purifications.
The Rad51Pa protein was produced in Escherichia coli strain BL21(DE3) (F− ompT hsdSB [rB− mB−] dcm gal ΔrecA306) carrying the pET21b::RAD51Pa plasmid.  and
and  were coexpressed to facilitate the translation of the RAD51Pa minor codons (1). The primary procedures of Rad51Pa protein purification, including the selective extraction with polymin P followed by phosphocellulose chromatography, were similar to those described earlier (7). The protein fraction containing Rad51Pa was further purified by separation on Bio-Gel hydroxyapatite, Cybacron Blue (Hi-Trap Blue), and MonoQ (Hi-Trap Q) columns. Finally, Rad51Pa eluted from the MonoQ column at 400 mM KCl was stored at −70°C at a concentration of 1.2 mg/ml. Purity of the protein was more than 95%, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis followed by Coomassie blue staining. The final Rad51Pa protein fraction contained no detectable exonuclease or endonuclease activities.
were coexpressed to facilitate the translation of the RAD51Pa minor codons (1). The primary procedures of Rad51Pa protein purification, including the selective extraction with polymin P followed by phosphocellulose chromatography, were similar to those described earlier (7). The protein fraction containing Rad51Pa was further purified by separation on Bio-Gel hydroxyapatite, Cybacron Blue (Hi-Trap Blue), and MonoQ (Hi-Trap Q) columns. Finally, Rad51Pa eluted from the MonoQ column at 400 mM KCl was stored at −70°C at a concentration of 1.2 mg/ml. Purity of the protein was more than 95%, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis followed by Coomassie blue staining. The final Rad51Pa protein fraction contained no detectable exonuclease or endonuclease activities.
S. cerevisiae Rad51 protein (Rad51Sc) was purified from insect cells as described previously (21). Yeast replication protein A (RPA) was expressed in E. coli by use of plasmid pJM126 (provided by S. Brill and B. Stillman) and was purified as described previously (5).
The concentrations of proteins were determined using the following extinction coefficients at 280 nm: 1.565 · 104 M−1cm−1 for Rad51Pa, 1.26 · 104 M−1cm−1 for Rad51Sc, and 8.8 · 104 M−1cm−1 for RPA.
DNA-dependent ATP hydrolysis.
ATP hydrolysis was measured as described previously (17). Oxidation of NADH was measured as the decrease in absorbance at 340 nm by using an extinction coefficient of 6.22 mM−1 cm−1. Unless otherwise noted, the TMD reaction buffer used in all experiments contained 25 mM Tris-HCl (pH 7.5), 10 mM MgCl2, and 1 mM dithiothreitol (DTT), and the incubation temperature was 37°C. Rad51Pa and a DNA cofactor [poly(dT), poly(dA), poly(dC), or dsDNA] were added to a reaction mixture containing 1 mM ATP, 3 mM phosphoenolpyruvate, 30 U of pyruvate kinase ml−1, 0.26 mM NADH, and 30 U of lactate dehydrogenase ml−1 in TMD buffer.
The hydrolysis of ATP by the Rad51Pa protein was analyzed by using the Hill equation (9) that expresses the rate constant per monomeric Rad51Pa protein according to the formula log[v/(Vmax − v)] = nH log[ATP] − nH logKm, where [ATP] is the substrate concentration, v is the velocity of the ATPase reaction at [ATP], Vmax is its maximal velocity at the infinite ATP concentration, and nH is the Hill coefficient. All kinetic parameters were determined by fitting data to this equation at various concentrations of ATP. Microsoft Excel was used in the calculation of the parameters.
The thermodynamic parameters for the rate-determining step, changes in enthalpy (ΔH), entropy (ΔS), and activation-free energy (ΔG), were obtained from the Arrhenius plot for Rad51Pa ATPase activity with the formula ln(kcat) = ln A − Eact /RT and from respective kinetic parameter equations, namely, ΔH = Eact − RT, ΔS = R ln(Ah/ekBT), and ΔG = ΔH − TΔS, where Eact is the activation energy, A is the intercept of the Arrhenius equation, R is the gas constant, T is the absolute temperature, kB is the Boltzman constant, kcat is the turnover number, and h is the Planck constant (18).
DNA strand exchange.
DNA strand exchange was performed using the agarose gel assay described previously (22). In the reaction, a 60 μM concentration of circular φX174 ssDNA was preincubated with 20 μM Rad51Pa in the presence of TMD buffer and 2.0 mM ATP with its regenerating system for 5 min at 37°C. Then, E. coli ssDNA-binding protein and yeast RPA were added to RPA concentrations of 6 and 3 μM, respectively. After 7 min, the reaction was started by the addition of linear φX174 dsDNA to achieve a concentration of 60 μM. The reaction was terminated after 120 min by the addition of EDTA, sodium dodecyl sulfate, and proteinase K to final concentrations of 5 mM, 0.5%, and 0.1 mg/ml, respectively, and then deproteinized by incubation at 37°C for 30 min. The products of the reaction were analyzed by gel electrophoresis on 1% agarose gel in Tris-acetate-EDTA buffer and visualized by staining with ethidium bromide.
The same reaction conditions were used for DNA strand exchange promoted by Rad51Sc except that TMD buffer was replaced with MMD buffer (40 mM K-MES [K-morpholineethanesulfonic acid] [pH 6.5], 2.5 mM MgCl2, 1 mM DTT) and 5 mM spermidine was added to the reaction mixture as described previously (34). After visualization, the products of DNA strand exchange were quantified, and the amounts of FPs and joint molecules (JM) were expressed as percentages of the original amounts of dsDNA converted into these products.
Nucleotide sequence accession number.
The sequence data have been submitted to the DDBJ, EMBL, and GenBank databases under accession number AAP74362.
RESULTS
Rad51Pa amino acid sequence.
The predicted Rad51Pa amino acid sequence is presented in Fig. 1 as the sequence alignment of Rad51-related proteins from three yeast species and one Rad51 protein from a human. The choice of Rad51 proteins used for the alignment represents the obvious difference in recombination DNA repair systems operating in eukaryotes. Lower eukaryotes, such as S. cerevisiae and Schizosaccharomyces pombe, display strong DNA repair, whereas recombinational repair in higher eukaryotes, such as Homo sapiens, is significantly less efficient. This sequence alignment displays equal amino acid identities and similarities among Rad51Pa and Rad51 homologs. In a comparison of the sequences of those protein regions where comparison is possible for all four of the Rad51 proteins analyzed (between residues 24 and 317 of Rad51Pa) (Fig. 1), Rad51Pa displays about 71, 75, and 69% identity as well as 84, 87, and 82% homology to Rad51Sc and Rhp51 from S. pombe and Rad51 from H. sapiens, respectively. Thus, the Rad51Pa protein is equally similar to lower and higher eukaryotic Rad51 proteins.
FIG. 1.
Sequence alignments of three yeast Rad51 proteins (from P. angusta, S. cerevisiae, and S. pombe, designated PaRad51, ScRad51, and SpRhp51, respectively) and an H. sapiens Rad51 protein (HsRad51). A dash indicates a gap introduced into the sequences to optimize the alignment. A period represents a residue identical to that from Rad51Pa. Bold letters show similarity between amino acid residues in given positions of the alignment. Asterisks indicate boundaries of the alignment chosen for quantitative comparison of sequences (residues 24 to 317).
Rad51Pa protein partially complements the Rad51 deficiency in S. cerevisiae.
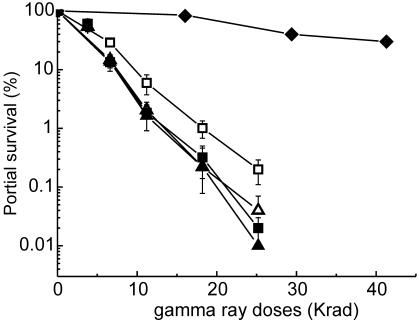
The RAD51Pa gene was inserted into the pYES2 expression vector under the galactose-inducible promoter PGAL1. Both pYES2 and pYES2::RAD51Pa plasmids were transformed into S. cerevisiae strain D3164, and the IR sensitivities of the transformed strains were compared as described in Materials and Methods (Fig. 2). At an IR dose of 25 kilorads, the yeast strain (D3164/pYES2::RAD51Pa) with induced Rad51Pa was about 10-fold more resistant than the same strain without induction (Fig. 2). However, the D4004 strain with a normal RAD51Sc gene was >1,000-fold more resistant to IR under the same conditions than the strain with Rad51Sc deficiency (D3164). Thus, Rad51Pa induced by galactose only partially complemented the survival of the DNA repair-deficient strain D3164. These data indicate that, even though Rad51Sc and Rad51Pa proteins are homologous, Rad51Pa only partially complements in vivo functions of Rad51Sc in yeast. This partial complementation indicates the complexity of the DNA repair mechanism in yeast, in which the interaction of Rad51 with other proteins plays a significant role.
FIG. 2.
Compensation of the Rad51Sc protein deficiency with Rad51Pa as measured by the resistance to γ-irradiation of strain D3164/pYES2::RAD51Pa, in which the expression of the Rad51Pa protein was induced by galactose (0.2%). Controls were D4004 (Rad51Sc+ phenotype) (filled diamonds), D3164/pYES2 without galactose in the growth medium after irradiation (Rad51Sc− phenotype) (filled triangles), D3164/pYES2 with galactose after irradiation (Rad51Sc− phenotype) (open triangles), and D3164/pYES2::RAD51Pa without galactose after irradiation (Rad51Pa− phenotype) (filled squares). The experiment parameter was D3164/pYES2::RAD51Pa with galactose after irradiation (Rad51Pa+ phenotype) (open squares).
Characteristics of ssDNA-dependent ATP hydrolysis catalyzed by Rad51Pa.
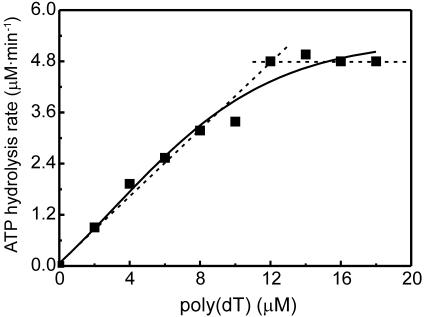
The Rad51Pa protein was purified to near homogeneity, and its ability to hydrolyze ATP was determined. Since ATP is an obligatory component of the presynaptic complex Rad51Pa::ATP::ssDNA, ssDNA-dependent ATP hydrolysis is commonly used to monitor nucleoprotein complex formation. Figure 3 shows the Rad51Pa-ssDNA-binding isotherm as monitored by poly(dT)-dependent ATP hydrolysis. Extrapolation of linear portions of this isotherm demonstrates how an estimation of Rad51Pa-ssDNA stoichiometry was done. The stoichiometry was 3.0 ± 0.2 nucleotides per one protomer of Rad51Pa filament, which is in accordance with values found for other members of the RecA-RadA-Rad51 protein family (15, 20, 35).
FIG. 3.
Rad51Pa-poly(dT)-binding isotherm monitored by ATP hydrolysis. Each point on the curves represents a result with an individual sample. The spectrophotometric assay was performed at 37°C in TMD buffer containing 4 μM Rad51Pa, 1 mM ATP with its regenerating system, the NADH-lactate dehydrogenase system, and poly(dT) at the concentration indicated. Each point in the curve (solid line) was averaged from the results of two or three repeat experiments. Broken line, extrapolation of the linear portions of the Rad51Pa-ssDNA-binding isotherm.
The levels of ability of different DNA cofactors to stimulate ATP hydrolysis promoted by Rad51Pa protein were determined by the comparison of the kcat turnover numbers (calculated as Vmax/[Rad51Pa]0) for different DNA substrates (Table 1). Rad51Pa hydrolyzed ATP only when both DNA and Mg2+ were present. The order of the levels of ability to stimulate ATP hydrolysis starts with poly(dC) as the most efficient and then poly(dT), φX174 ssDNA, poly(dA), and M13 dsDNA (the least efficient). Similar orders for different DNAs were shown previously for other RecA-like proteins (32, 36). Additionally, ssDNA-dependent ATP hydrolysis catalyzed by Rad51Pa is 25-fold less efficient than that catalyzed by E. coli RecA under similar conditions (for RecA from E. coli, kcat/ssDNA was 28 min−1). These results suggest that Rad51Pa belongs to the RadA-Rad51 subfamily of inefficient ATPases.
TABLE 1.
Comparison of the abilities of different DNA cofactors to stimulate ATP hydrolysis catalyzed by Rad51Pa as assessed by the steady-state kinetic parameter kcat
| DNA cofactor | kcat (min−1) (± SD) |
|---|---|
| Poly(dT) | 1.20 ± 0.45 |
| Poly(dC) | 1.31 ± 0.36 |
| ssDNA φX174 | 1.04 ± 0.31 |
| Poly(dA) | 0.96 ± 0.28 |
| dsDNA M13 | 0.63 ± 0.18 |
| Without MgCl2 | 0.03 ± 0.03 |
| Without DNA | 0.08 ± 0.03 |
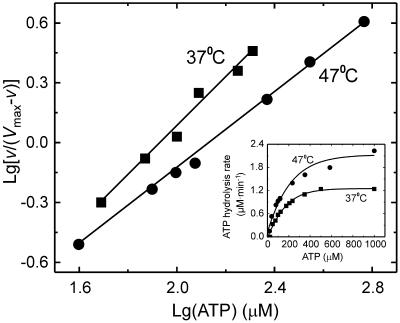
The last conclusion is further supported by the finding that Rad51Pa showed very low, if any, ATP-induced cooperativity. Cooperativity is determined by calculating the Hill constant nH (a measure of cooperativity) as a slope derived from a plot of a fractional rate [v/(Vmax − v)] versus the substrate concentration ([ATP]), presented in logarithmic coordinates (10). A Hill plot based on kinetic measurements of poly(dT)-dependent ATP hydrolysis at 37 and 47°C is shown in Fig. 4 (inset). The steady-state kinetic parameters (Km, kcat, and nH), summarized in Table 2, were determined by fitting data to the Hill equation as described in Materials and Methods. The data indicate that Rad51Pa displays a lower apparent affinity for ATP (Km) than does E. coli RecA [S0.5 is 60 μM for poly(dT) at 37° and pH 7.5; in a cooperative system, the substrate concentration where half-maximal activity occurs is S0.5] (14, 27). Furthermore, the Rad51Pa protein possesses weak cooperativity (nH is close to 1), and its catalytic efficiency (kcat/Km) is approximately 35-fold less than that of E. coli RecA. Interestingly, the Rad51Pa protein is still thermoresistant at 47°C, and its catalytic efficiency is practically equal to that observed at 37°C.
FIG. 4.
Hill plot of Rad51Pa-mediated ATP hydrolysis as a function of ATP. The inset represents the original kinetic data. The spectrophotometric ATPase assays were performed by incubating 1.0 μM Rad51Pa and 20 μM poly(dT) with the indicated amount of ATP (with its regenerating system) in TMD buffer (pH 7.5) at the indicated temperatures for 20 min. Lg, log.
TABLE 2.
Kinetic parameters of poly(dT)-dependent ATP hydrolysis catalyzed by Rad51Paa
| Temp (°C) | Avg Km (μM) ± SD | kcat (min−1) ± SD | nH | kcat/Km (s−1 · M−1) |
|---|---|---|---|---|
| 37 | 86 ± 23 | 1.20 ± 0.45 | 1.23 | 240 |
| 47 | 141 ± 35 | 2.23 ± 0.37 | 0.97 | 264 |
At both temperatures, the DNA cofactor was poly(dT). See also Fig. 4.
Thermoinactivation of Rad51Pa compared with that of Rad51Sc.
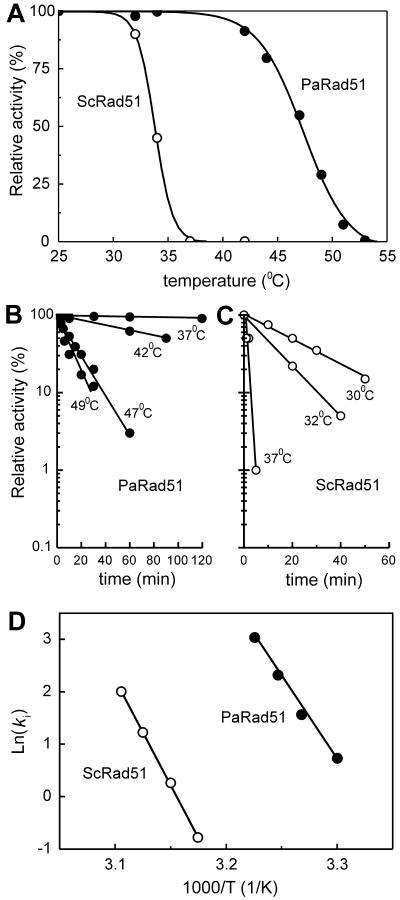
Since P. angusta is thermotolerant, it is reasonable to propose that Rad51Pa should be more thermoresistant than its homolog from bakery yeast. First, we determined the thermostability of the Rad51Pa and Rad51Sc proteins by preincubating them for 10 min at different temperatures. Levels of protein inactivation were determined by measuring residual ssDNA-dependent ATP hydrolysis activity at 37°C. Figure 5A summarizes the inactivation profiles for both proteins. Rad51Sc is completely inactivated after 10 min of incubation at 37°C. By contrast, Rad51Pa remains completely active, and preincubation at the significantly higher temperature of 54°C was required to inactivate it completely. It has been shown that the stability of Rad51 is significantly increased when protein is in the nucleoprotein complex with ssDNA (2, 12, 24). The semi-inactivation period for Rad51Pa at 47°C was 10 min, whereas the stability of Rad51Pa in the complex with poly(dT) at the same temperature was increased to 1 h (data not shown). Additionally, the inactivation kinetics of the Rad51Pa and Rad51Pa proteins at different temperatures were performed (Fig. 5B and C). Rad51Pa was more resistant to inactivation across all tested temperatures. Note that it was 20 times more thermostable at 37°C than Rad51Sc was. Moreover, Rad51Pa retained 10% of the ssDNA-dependent ATPase activity even after 30 min of preincubation at 47°C, whereas Rad51Sc was completely inactive after 5 min. These results show that Rad51Pa is more resistant to temperature inactivation than the Rad51Sc protein.
FIG. 5.
Comparative thermoinactivation of the Rad51Pa (PaRad51) and Rad51Sc (ScRad51) proteins. (A) Each protein alone was incubated in KMD buffer (20 mM potassium phosphate [pH 7.5], 4 mM MgCl2, and 1 mM DTT) with 5% glycerol for 10 min at the temperatures indicated. The residual poly(dT)-dependent ATPase activity was measured by spectrophotometric assay in the reaction mixture containing an 8 μM concentration of either Rad51Pa or Rad51Sc, 80 to 160 μM poly(dT), and 1 mM ATP with its regenerating system. Each point of the curves was averaged from the results of three separate experiments performed with different excesses of poly(dT) in order to determine a residual Vmax at the given temperature under conditions at which DNA binding sites of the protein are saturatedwith poly(dT). One hundred percent signifies the Vmax for the protein without a preliminary temperature treatment. (B and C) Time courses of the thermoinactivation of the Rad51Pa and Rad51Sc proteins. The residual poly(dT)-dependent ATPase was measured as described above. (D) Arrhenius plot. A reconstruction of the data from panels B and C is shown. T, temperature. Ki, inactivation coefficient.
The dependence of inactivation coefficient kinact (Vmax/[Rad51]0) on temperature was constructed with coordinates of an Arrhenius plot (Fig. 5D), which permitted the identification of the thermodynamic parameters for the rate-determining step of ATP hydrolysis as described in Materials and Methods (Table 3). Based on all three parameters (changes in enthalpy [ΔH], entropy of inactivation [ΔS], and inactivation-free energy [ΔG]), we conclude that the higher thermostability of Rad51Pa than that of Rad51Sc is attributed to the better structural stability of the Rad51Pa protein in the ATPase active site.
TABLE 3.
Thermodynamic parameters of poly(dT)-dependent thermoinactivation of ATP hydrolysis: comparison of Rad51Pa and Rad51Sc proteinsa
| Protein | EI (kcal · mol−1) | ΔH (kcal · mol−1) | ΔS (kcal · mol−1 · K−1) | ΔG (kcal · mol−1) |
|---|---|---|---|---|
| Rad51Pa | 71.1 ± 1.8 | 70.4 ± 2.0 | 0.15 ± 0.03 | 24.7 ± 1.6 |
| Rad51Sc | 61.6 ± 1.5 | 61.0 ± 1.7 | 0.13 ± 0.02 | 21.4 ± 1.65 |
Values are means ± standard deviations. See also Fig. 5D. EI, inactivation energy.
Thermodependence of Rad51Pa protein nucleation.
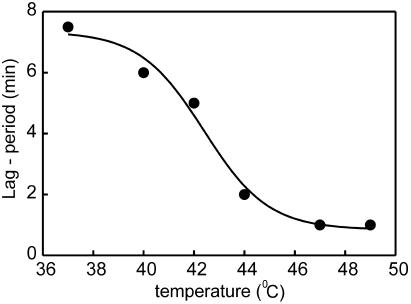
Polymerization of Rad51Sc on ssDNA in the presence of ATP results from a short lag period of nucleation or presynaptic complex formation which is ready to initiate homologous pairing from the 3′ or 5′ end (13, 21, 23, 33). The short lag period can be monitored by the ternary-complex ATPase activity. Figure 6 shows that Rad51Pa nucleation time on ssDNA was reduced from 7 min to about 1 min by increasing the temperature from 37 to 47°C. This result indicates the thermodependence in the presynaptic complex formation of homologous recombination promoted by the Rad51Pa protein.
FIG. 6.
Lag period of Rad51Pa polymerization on φX174 ssDNA depends on temperature conditions. The lag was measured as a short period of time before a constant rate of ATP hydrolysis was established at a given temperature. The reaction was performed in TMD buffer (pH 7.5) containing 4 μM Rad51Pa, 1 mM ATP with its regenerating system, 30 μM φX174 ssDNA, and all necessary additions for the ATPase spectrophotometric assay.
Thermodependence of the DNA strand exchange reaction promoted by the Rad51Pa and Rad51Sc proteins.
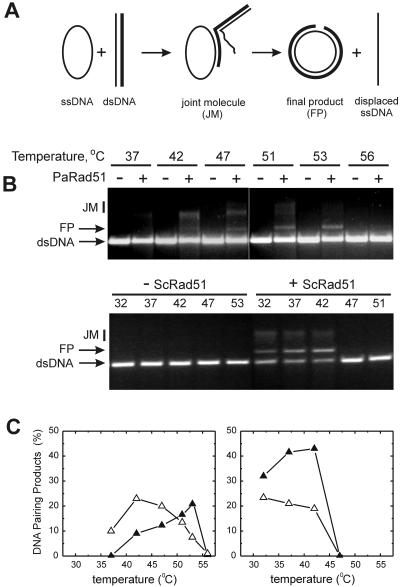
Rad51Pa and Rad51Sc were compared for their ability to promote DNA strand exchange between circular ssDNA and homologous linear duplex DNA at different temperatures (Fig. 7A). Rad51Sc promoted DNA strand exchange equally well in the temperature range between 32 and 42°C, but no products were detected at temperatures above 47°C (Fig. 7B and C). About 50% of linear DNA was converted into JM and nicked circular dsDNA (FP) in 2 h at optimal temperatures, and formation of the latter was maximal at 42°C. In contrast, Rad51Pa promoted strand exchange most efficiently at temperatures above 42°C. JM were major products of the reaction after incubation at 42°C, and incubation at 52°C was required for the accumulation of nicked circular dsDNA. These data indicate that thermodependence is as critical an in vitro property of Rad51Pa as the ability to exchange extended DNA molecules.
FIG. 7.
Strand exchange reactions promoted by the Rad51Pa (PaRad51) or Rad51Sc (ScRad51) proteins depend on temperature conditions, although in different ways. (A) Scheme of the strand exchange reaction between φX174 ssDNA (plus strand) and the replicative-form linear dsDNA of the same phage. (B) Agarose gel assay of strand exchange reactions (for details, see Materials and Methods). JM, joint molecules; FP, final products. (C) Relative amounts of JM and FP were quantified from the gels presented in panel B, as described in Materials and Methods. Open triangles, JM; filled triangles, FP. Left, Rad51Pa; right, Rad51Sc.
DISCUSSION
The RAD51 gene from thermotolerant yeast P. angusta was cloned, and its nucleotide sequence was determined. The RAD51 gene encodes a 40-kDa protein which shows about 70% identity and more than 80% similarity with core regions of Rad51 proteins of both lower and higher eukaryotes (Fig. 1). In vivo, the protein was able to complement, though only partially, the DNA repair deficiency of S. cerevisiae γ-irradiated rad51-1 cells (Fig. 2). The Rad51Pa protein was overproduced in E. coli and purified to homogeneity. Judging by ssDNA-dependent ATP hydrolysis and strand exchange reactions, the Rad51Pa protein, in vitro, maintained its activities at temperatures up to 54°C (Fig. 4 and 7 and Table 2). This thermostability may be partially explained by the stabilization of Rad51 proteins in the structure of the presynaptic ternary complex with ATP and ssDNA (24, 37). However, Rad51Pa alone treated at different temperatures exhibited significantly better thermostability than the Rad51Pa protein. Rad51Pa was about 20-fold more thermostable at 37°C than its S. cerevisiae homolog Rad51Sc. Moreover, Rad51Sc was inactivated more than 100-fold by incubation at 37°C for 5 min, but incubation of Rad51Pa at 49°C for 30 min decreased its activity only 10-fold (Fig. 5B). The impressive qualitative difference in the thermoinactivations of these two proteins (Fig. 5A) is also supported by quantitative thermodynamic data (Fig. 5C; Table 3). Because P. angusta strain BKM Y1397 survived and could even grow slightly at 49°C, it seems reasonable to conclude that the thermoresistant potential of Rad51Pa is more than enough to support the growth of its host at 42°C.
Two additional conclusions can be derived from our results. First, Rad51Pa is a typical representative of the Rad51 subfamily. Indeed, according to such general characteristics as the stoichiometry (Fig. 2) and DNA specificity (Table 1) of Rad51Pa-ssDNA interactions, the protein is referred to as a member of the RecA-RadA-Rad51 superfamily. However, two particular properties, the inefficient ATPase (Table 2) and very low cooperativity (Fig. 4) in Rad51Pa-ATP interactions, are typical only for RadA-Rad51 proteins (32, 35).
Second, Rad51Pa is a thermodependent protein rather than a thermoresistant protein. Indeed, the temperature of 42°C is critical for at least two protein characteristics which are thought to be important for recombination. These characteristics are a sharp enhancement of nucleation in presynaptic filament formation (Fig. 6) and the ability to form extended JM and thus φX174 FPs in the strand exchange reaction (Fig. 7). Interestingly, this critical temperature was very close to the optimal growth temperature interval (37 to 42°C) found for P. angusta strain BKM Y1397.
Acknowledgments
We thank Takehiko Shibata (RIKEN, Saitama, Japan) for kindly supplying us with the  -
- plasmid, Andrei Alexseev (PNPI) and Seiki Kuramitsu (Osaka University, Osaka, Japan) for help in the construction of the P. angusta cDNA library used in the work, Elena Sukhanova (PNPI) for assistance in some genetic experiments, and Vladimir Korolev (PNPI) and Leonid Firsov (PNPI) for fruitful discussions of some experimental results.
plasmid, Andrei Alexseev (PNPI) and Seiki Kuramitsu (Osaka University, Osaka, Japan) for help in the construction of the P. angusta cDNA library used in the work, Elena Sukhanova (PNPI) for assistance in some genetic experiments, and Vladimir Korolev (PNPI) and Leonid Firsov (PNPI) for fruitful discussions of some experimental results.
This research was supported by the RAS Presidium Program “Gene Pool Dynamics of Plants, Animals and Human Beings.”
REFERENCES
- 1.Aihara, H., Y. Ito, H. Kurumizaka, S. Yokoyama, and T. Shibata. 1999. The N-terminal domain of the human Rad51 protein binds DNA: structure and a DNA binding surface as revealed by NMR. J. Mol. Biol. 290:495-504. [DOI] [PubMed] [Google Scholar]
- 2.Alexseyev, A. A., D. M. Baitin, S. Kuramitsu, T. Ogawa, H. Ogawa, and V. A. Lanzov. 1997. A recombinational defect in the C-terminal domain of Escherichia coli RecA2278-5 protein is compensated by protein binding to ATP. Mol. Microbiol. 23:255-265. [DOI] [PubMed] [Google Scholar]
- 3.Blandin, G., B. Llorente, A. Malpertuy, P. Wincker, F. Artiguenave, and B. Dujon. 2000. Genomic exploration of the hemiascomycetous yeasts: 13. Pichia angusta. FEBS Lett. 487:76-81. [DOI] [PubMed] [Google Scholar]
- 4.Brendel, V., L. Brocchieri, S. J. Sandler, A. J. Clark, and S. Karlin. 1997. Evolutionary comparisons of RecA-like proteins across all major kingdoms of living organisms. J. Mol. Evol. 44:528-541. [DOI] [PubMed] [Google Scholar]
- 5.Brill, S. J., and B. Stillman. 1989. Yeast replication factor-A functions in the unwinding of the SV40 origin of DNA replication. Nature 342:92-95. [DOI] [PubMed] [Google Scholar]
- 6.Cox, M. M. 2003. The bacterial RecA protein as a motor protein. Annu. Rev. Microbiol. 57:551-577. [DOI] [PubMed] [Google Scholar]
- 7.Cox, M. M., K. McEntee, and I. R. Lehman. 1981. A simple and rapid procedure for the large scale purification of the RecA protein of Escherichia coli. J. Biol. Chem. 256:4676-4678. [PubMed] [Google Scholar]
- 8.De Zutter, J. K., and K. L. Knight. 1999. The hRad51 and RecA proteins show significant differences in cooperative binding to single-stranded DNA. J. Mol. Biol. 293:769-780. [DOI] [PubMed] [Google Scholar]
- 9.Fersht, A. 1985. Enzyme structure and mechanism, 2nd ed. W. H. Freeman, New York, N.Y.
- 10.Fersht, A. 1999. Structure and mechanism in protein science. A guide to enzyme catalysis and protein folding. W. H. Freeman and Co., New York, N.Y.
- 11.Gellissen, G. 2000. Heterologous protein production in methylotrophic yeasts. Appl. Microbiol. Biotechnol. 54:741-750. [DOI] [PubMed] [Google Scholar]
- 12.Glazunov, E. A., Y. Kil, and V. A. Lantsov. 2001. Two types of temperature dependence of homologous recombinases in archaea: the properties of the Desulfurococcus amylolyticus recombinase. Dokl. Biol. Sci. 379:389-392. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, R. C., E. I. Golub, M. S. Wold, and C. M. Radding. 1998. Polarity of DNA strand exchange promoted by recombination proteins of the RecA family. Proc. Natl. Acad. Sci. USA 95:9843-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz, F. S., and F. R. Bryant. 2001. Interdependence of the kinetics of NTP hydrolysis and the stability of the RecA-ssDNA complex. Biochemistry 40:11082-11089. [DOI] [PubMed] [Google Scholar]
- 15.Kil, Y. V., D. M. Baitin, R. Masui, E. A. Bonch-Osmolovskaya, S. Kuramitsu, and V. A. Lanzov. 2000. Efficient strand transfer by the RadA recombinase from the hyperthermophilic archaeon Desulfurococcus amylolyticus. J. Bacteriol. 182:130-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowalczykowski, S. C. 2000. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 25:156-165. [DOI] [PubMed] [Google Scholar]
- 17.Kreuzer, K. N., and C. V. Jongeneel. 1983. Escherichia coli phage T4 topoisomerase. Methods Enzymol. 100:144-160. [DOI] [PubMed] [Google Scholar]
- 18.McGhee, J. D., and P. H. von Hippel. 1974. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J. Mol. Biol. 86:469-489. [DOI] [PubMed] [Google Scholar]
- 19.Mikawa, T., R. Masui, and S. Kuramitsu. 1998. RecA protein has extremely high cooperativity for substrate in its ATPase activity. J. Biochem. 123:450-457. [DOI] [PubMed] [Google Scholar]
- 20.Morrical, S. W., J. Lee, and M. M. Cox. 1986. Continuous association of Escherichia coli single-stranded DNA binding protein with stable complexes of RecA protein and single-stranded DNA. Biochemistry 25:1482-1494. [DOI] [PubMed] [Google Scholar]
- 21.Namsaraev, E., and P. Berg. 1997. Characterization of strand exchange activity of yeast Rad51 protein. Mol. Cell. Biol. 17:5359-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namsaraev, E. A., D. Baitin, I. V. Bakhlanova, A. A. Alexseyev, H. Ogawa, and V. A. Lanzov. 1998. Biochemical basis of hyper-recombinogenic activity of Pseudomonas aeruginosa RecA protein in Escherichia coli cells. Mol. Microbiol. 27:727-738. [DOI] [PubMed] [Google Scholar]
- 23.Namsaraev, E. A., and P. Berg. 1998. Branch migration during Rad51-promoted strand exchange proceeds in either direction. Proc. Natl. Acad. Sci. USA 95:10477-10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namsaraev, E. A., and P. Berg. 1998. Interaction of Rad51 with ATP and Mg2+ induces a conformational change in Rad51. Biochemistry 37:11932-11939. [DOI] [PubMed] [Google Scholar]
- 25.Naumov, G. I., E. S. Naumova, V. I. Kondratieva, S. A. Bulat, N. V. Mironenko, L. C. Mendonca-Hagler, and A. N. Hagler. 1997. Genetic and molecular delineation of three sibling species in the Hansenula polymorpha complex. Syst. Appl. Microbiol. 20:50-56. [Google Scholar]
- 26.Ogawa, T., A. Shinohara, A. Nabetani, T. Ikeya, X. Yu, E. H. Egelman, and H. Ogawa. 1993. RecA-like recombination proteins in eukaryotes: functions and structures of RAD51 genes. Cold Spring Harbor Symp. Quant. Biol. 58:567-576. [DOI] [PubMed] [Google Scholar]
- 27.Roca, A. I., and M. M. Cox. 1997. RecA protein: structure, function, and role in recombinational DNA repair. Prog. Nucleic Acid Res. Mol. Biol. 56:129-223. [DOI] [PubMed] [Google Scholar]
- 28.Romanos, M. A., C. A. Scorer, and J. J. Clare. 1992. Foreign gene expression in yeast: a review. Yeast 8:423-488. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Seitz, E. M., J. P. Brockman, S. J. Sandler, A. J. Clark, and S. C. Kowalczykowski. 1998. RadA protein is an archaeal RecA protein homolog that catalyzes DNA strand exchange. Genes Dev. 12:1248-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohn, J.-H., E.-S. Choi, H. A. Kang, J.-S. Rhee, and S.-K. Rhee. 1999. A family of telomere-associated autonomously replicating sequences and their functions in targeted recombination in Hansenula polymorpha DL-1. J. Bacteriol. 181:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spies, M., Y. Kil, R. Masui, R. Kato, C. Kujo, T. Ohshima, S. Kuramitsu, and V. Lanzov. 2000. The RadA protein from a hyperthermophilic archaeon Pyrobaculum islandicum is a DNA-dependent ATPase that exhibits two disparate catalytic modes, with a transition temperature at 75 degrees C. Eur. J. Biochem. 267:1125-1137. [DOI] [PubMed] [Google Scholar]
- 33.Sung, P., and D. L. Robberson. 1995. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82:453-461. [DOI] [PubMed] [Google Scholar]
- 34.Sung, P., and S. A. Stratton. 1996. Yeast Rad51 recombinase mediates polar DNA strand exchange in the absence of ATP hydrolysis. J. Biol. Chem. 271:27983-27986. [DOI] [PubMed] [Google Scholar]
- 35.Tombline, G., and R. Fishel. 2002. Biochemical characterization of the human RAD51 protein. I. ATP hydrolysis. J. Biol. Chem. 277:14417-14425. [DOI] [PubMed] [Google Scholar]
- 36.Weinstock, G. M., K. McEntee, and I. R. Lehman. 1981. Hydrolysis of nucleoside triphosphates catalyzed by the RecA protein of Escherichia coli. Steady state kinetic analysis of ATP hydrolysis. J. Biol. Chem. 256:8845-8849. [PubMed] [Google Scholar]
- 37.Zaitseva, E. M., E. N. Zaitsev, and S. C. Kowalczykowski. 1999. The DNA binding properties of Saccharomyces cerevisiae Rad51 protein. J. Biol. Chem. 274:2907-2915. [DOI] [PubMed] [Google Scholar]