Our first observation suggests that fitness does not strongly predict the heart rate (HR) response to a volitional handgrip task in middle- to older-aged adults. Second, the BOLD response associated with the handgrip task, and with the HR time course, was associated with response patterns in the cortical autonomic network. Finally, whereas high cardiorespiratory fitness failed to affect absolute HR responses to isometric handgrip in this age range, a select effect was observed in cortical regions known to be associated with cardiovascular arousal, beyond that achieved through healthy active living.
Keywords: cortical autonomic network, handgrip exercise, cardiorespiratory fitness, medial prefrontal cortex, age
Abstract
This study tested the hypothesis that high cardiorespiratory fitness (peak oxygen uptake) preserves the cortical circuitry associated with cardiac arousal during exercise in middle- to older-aged individuals. Observations of changes in heart rate (HR) and in cortical blood oxygenation level-dependent (BOLD) images were made in 52 healthy, active individuals (45–73 yr; 16 women, 36 men) across a range of fitness (26–66 ml·kg−1·min−1). Seven repeated bouts of isometric handgrip (IHG) at 40% maximal voluntary contraction force were performed with functional magnetic resonance imaging at 3 T, with each contraction lasting 20 s and separated by 40 s of rest. HR responses to IHG showed high variability across individuals. Linear regression revealed that cardiorespiratory fitness was not a strong predictor of the HR response (r2 = 0.09). In a region-of-interest analysis both the IHG task and the HR time course correlated with increased cortical activation in the bilateral insula and decreased activation relative to baseline in the anterior and posterior cingulate and medial prefrontal cortex (MPFC). t-Test results revealed greater deactivation at the MPFC with higher fitness levels beyond that of guideline-based activity. Therefore, whereas high cardiorespiratory fitness failed to affect absolute HR responses to IHG in this age range, a select effect was observed in cortical regions known to be associated with cardiovascular arousal.
NEW & NOTEWORTHY Our first observation suggests that fitness does not strongly predict the heart rate (HR) response to a volitional handgrip task in middle- to older-aged adults. Second, the BOLD response associated with the handgrip task, and with the HR time course, was associated with response patterns in the cortical autonomic network. Finally, whereas high cardiorespiratory fitness failed to affect absolute HR responses to isometric handgrip in this age range, a select effect was observed in cortical regions known to be associated with cardiovascular arousal, beyond that achieved through healthy active living.
rapid increases in heart rate (HR) represent a critical reactive response to physiological stress, facilitating or sustaining blood pressure and organ perfusion. The tachycardia at the onset of exercise has been linked mechanistically to a rapid reduction in vagal chronotropic control (Fagraeus and Linnarsson 1976; Hollander and Bouman 1975; Mitchell et al. 1989). However, although some interindividual variation exists (Norton et al. 2013), advancing age impairs the magnitude of the HR and cardiac output responses at the exercise onset, resulting in a larger sympathetic drive to adjust blood pressure (Lalande et al. 2014). The mechanism(s) affecting the diminished cardiac response with exercise in aging adults remains unknown but may include either changes in autonomic neural adjustments and/or intrinsic cardiac events (Astrand 1960; Bruce et al. 1963; Craft and Schwartz 1995; Dauchot and Gravenstein 1971; Kino et al. 1975; Lalande et al. 2014; Lester et al. 1968; Seliger et al. 1978; Sheffield et al. 1978; Yin et al. 1979). Diminished HR variability (Agelink et al. 2001; Bonnemeier et al. 2003; O’Brien et al. 1986; Russoniello et al. 2013; Schwartz et al. 1991) and a reduced tachycardia following atropine administration (Dauchot and Gravenstein 1971) support the conclusion that age reduces chronic parasympathetic restraint of HR, suggesting that this effect may be a fundamental determinant of impaired cardiac acceleration with exercise in aging adults.
However, the neurological determinants of age-related impairment of cardiovagal function remain an attractive contributor because of the important role of supramedullary sites on autonomic outflow. Specifically, neuroimaging techniques have enabled investigation into a network of cortical regions associated with the autonomic nervous system and cardiovascular control in conscious humans (Basnayake et al. 2012; Cechetto 2014; Critchley et al. 2000; Gianaros et al. 2004; Macey et al. 2012; Norton et al. 2013; Shoemaker et al. 2015; Williamson 2010). These regions include the bilateral insular cortex (IC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), and hippocampus (HC). Importantly, experimental studies indicate that the IC, MPFC and HC are of particular relevance to HR control (Burns and Wyss 1985; Cechetto and Chen 1990; Fisk and Wyss 1997; Goswami et al. 2012; Norton et al. 2013; Oppenheimer et al. 1992; Owens and Verberne 2001; Ruggiero et al. 1987; Shoemaker et al. 2015; Verberne 1996; Wong et al. 2007; Yasui et al. 1991). However, changes in regional brain activation patterns appear to be altered in older brains (Cabeza 2001; Norton et al. 2015a). Beyond the age of 40 yr, older brains tend to show more symmetrical activation, either because they have increased activation in a hemisphere that is less activated than in younger adults or because they show reduced activation in the areas most activated in younger adults. This increased symmetrical hemispheric activation is a robust finding in older adults, yet it is not clear whether this change is an attenuation of the response seen in younger subjects, an inability to recruit specific areas, or an attempt to compensate for the aging process (Cabeza 2001; Cabeza et al. 2004). Furthermore, advancing age often associates with cortical atrophy (Raz et al. 2005), changes in brain functional responses (Nyberg et al. 2010), and declines in cognitive performance (Rönnlund et al. 2005). Notably, the pattern of atrophy begins early in middle age at a rate of 5% per decade after age 40, with the actual rate of decline possibly increasing with age, particularly over age 70 (Kennedy et al. 2009; Raz et al. 1997, 2005, 2010; Raz and Rodrigue 2006). However, physical brain changes do not occur to the same extent in all regions. Preferential targets include frontal and parietal regions of the brain, including the MPFC and IC regions (Raz et al. 2005, 2010). These findings fit well with the cognitive changes seen in aging, suggesting an association between increasing age, a reduction in prefrontal cortical volume, and a decrease in executive function (Gunning-Dixon and Raz 2003). Therefore, diminished associations between cardiac and neural responses may be expected in middle- to older-aged adults in whom precipitous declines in brain structure and function have just begun and are expected to increase rapidly, especially in the frontal lobes.
Nonetheless, substantial interindividual differences exist in age-related cortical atrophy, with some individuals showing resistance to major age-related brain pathology or neurological deficits (Nyberg et al. 2012; Pudas et al. 2013). The determinants of this variability are not known. Of the many possible options, physical activity may be one of the factors affecting this heterogeneity, as it demonstrates widespread benefits on brain health in aging individuals, including spared brain volume (Erickson et al. 2009, 2011; Niemann et al. 2014; Wood et al. 2016), improved task-related functional brain responses (Colcombe et al. 2004; Voelcker-Rehage et al. 2010), increased white matter integrity (Johnson et al. 2012; Voss et al. 2013), and improved cognitive performance (Josefsson et al. 2012). However, the functional consequences of this age-related heterogeneity on the forebrain circuitry associated with cardiovascular responses to physiological stress remain unknown. Furthermore, the dose-response relationship between cardiorespiratory fitness and healthy neural-cardiac relationships remains to be elucidated.
Therefore, the latest research shows that age-related cognitive and neural decline begins in healthy adults in the third decade of life and continues throughout advanced aging. Addressing brain health support and cognitive function early in life can dramatically support a better quality of life in later years. Given the economic, social, and personal burden associated with age-related neural deterioration, identifying cortical sites and patterns of the developing neurological changes represents a necessary step from which strategies can be developed and tested to prevent declines in structural and functional brain health before they begin.
The purpose of this study was to examine the impact of cardiorespiratory fitness on cardiac and neural responses. The present study focused on healthy, middle- to older-aged individuals to minimize the confounding variables of senescence and associated comorbidities, as well as to engage the age range in which cortical vulnerability begins to be expressed. With this approach, we tested the hypothesis that high cardiorespiratory fitness predicts a high HR response to volitional handgrip and that such response is related to preservation of the entrainment of the MPFC, IC, and HC regions of the cortical autonomic network.
METHODS
Participants.
A total of 52 healthy, active individuals participated in this study across a range of fitness and age (26–66 ml·kg−1·min−1; 45–73 yr; 16 women, 36 men). Table 1 provides group characteristics. All participants were nonsmokers, free of medications, and without diagnosed hypertension, diabetes, vascular or neurological impairments. Premenopausal females were tested during days 1–14 of the menstrual cycle, with day 1 representing the first day of menstruation. None of the postmenopausal women were on hormone replacement therapy. The University of Western Ontario Health Sciences Ethics Review Board approved this study, which adhered to the Declaration of Helsinki. Each participant provided informed, written consent.
Table 1.
Anthropometric and baseline cardiovascular data
| Average Group Data | Value Range | |
|---|---|---|
| Age, yr | 59 ± 8 | 45–79 |
| Resting MAP, mmHg | 87 ± 9 | 73–114 |
| Resting heart rate, bpm | 57 ± 9 | 41–83 |
| BMI, kg/m2 | 25 ± 3 | 19–36 |
| Cardiac output, l/min | 6 ± 2 | 3–11 |
| ΔHR (LAB) | 5 ± 3 | 1–16 |
| ΔHR (fMRI) | 6 ± 5 | −12 to 22 |
| V̇o2max, ml·kg−1·min−1 | 41 ± 11 | 21–66 |
| V̇o2max age-predicted percentile | 81 ± 25 | 25–130 |
Values are means ± SD. MAP, mean arterial pressure; BMI, body mass index; HR, heart rate; V̇o2max, maximal oxygen consumption. Age-predicted percentile based on American College of Sports Medicine guidelines. Cardiac output indexed to body surface area.
Assessment of cardiorespiratory fitness.
A graded treadmill exercise test, conducted under standard clinical observation, provided information regarding each subject’s peak oxygen uptake (V̇o2max). During this test, expired air samples were taken at 3-s intervals until the point of volitional exhaustion. According to the American College of Sports Medicine (ACSM) guidelines (ACSM 1995), V̇o2max was determined by meeting the following criteria: 1) V̇o2 ceased to increase with increasing workloads (plateau); 2) HR reached the age-predicted maximum value (220 − age); and 3) respiratory exchange ratio was >1.0.
Experimental design.
Participants completed two separate experimental sessions: 1) physiological recording (LAB session) and 2) functional magnetic resonance neuroimaging session (fMRI; Robarts Research Institute Centre for Functional and Metabolic Imaging). The sessions were performed at the same time of day and separated by a minimum period of 1 wk. Participants were familiarized with the experimental procedures before their first test session. Participants were instructed to arrive after a 12-h fast and to refrain from nicotine, alcohol, caffeine, and intense physical exertion for the same duration. Each session began with a maximal voluntary contraction (MVC) handgrip calibration, in which the participant was instructed to squeeze a nonmagnetic handgrip device connected in series to a pressure transducer (PX272, Edwards Lifesciences, Irvine, CA) to their maximal ability while in the supine position. This was repeated twice, with the larger value calibrated as 100%. All subjects were right handed and performed the isometric handgrip exercise (IHG) with their dominant hand. During each recording session, visual feedback was provided to the participant of their achieved force in real time. Baseline data were collected over 5 min of quiet supine rest. One trial (fMRI scanning run) consisted of four repeated bouts of 40% MVC force (LAB session) and seven repeated bouts of IHG at 40% MVC force (fMRI session). Each contraction bout lasted 20 s and was separated by 40 s of rest. The number of bouts was increased in the fMRI session to increase the signal-to-noise ratio. Two trial runs were performed in each the LAB and fMRI sessions. The level of perceived exertion produced by the exercise was monitored after each trial run on a scale from 6 to 20 (Borg 1982).
Physiological recording session.
During the LAB session, HR was monitored by standard 3-lead electrocardiogram (ECG) techniques. Arterial blood pressure (BP) was measured continuously from the finger of the nonexercising left hand, maintained at heart level, by photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). The BP readings recorded from the Finometer were corrected against sphygmomanometrically obtained systolic (SBP) and diastolic (DBP) pressures that were made intermittently during data collection.
Physiological data analysis.
All measures were sampled at 1,000 Hz, input into a data acquisition board (PowerLab ML795, ADInstruments, Mountain View, CA) for analog-to-digital signal conversion with LabChart7 software (ADInstruments), and stored for off-line analysis. HR was calculated from successive R-R intervals obtained from the ECG signal. Beat-by-beat HR data were averaged over 2.5-s bins (the TR interval for functional scans) and time aligned to ensure a corresponding mean value for each functional scan obtained during the fMRI collection period. The HR response (ΔHR) to the IHG was determined by averaging the response over the last 20 s of each rest and the last 10 s of each IHG interval. HR responses for each participant were averaged over the four repeated blocks. Blood pressure from the Finometer was converted to mean arterial pressure (MAP) with the formula MAP = 1/3 SBP + 2/3 DBP. The Modelflow algorithm provided an index of cardiac output from the finger BP tracings (Wesseling et al. 1993). Participants’ sex, age, height, and weight were input manually into the Finometer to optimize estimation of cardiac output.
Linear regression analysis examined how cardiorespiratory fitness (V̇o2max) predicted ΔHR to the IHG task. Although a box-plot analysis revealed several outliers with either a large positive or negative change in HR, removing these individual responses did not change the significance of our regression; therefore, we chose to include them for further analysis. A threshold for significance was set at P < 0.05.
Multiple linear regression revealed an expected effect of age (P < 0.001) and sex (P < 0.05) on cardiorespiratory fitness. Therefore, we normalized all V̇o2max values to percentage of age-predicted values based on the ACSM standards for fitness (ACSM 1995). In addition, to confirm the use of age-predicted values, we performed a residuals analysis based on our own population, after regressing out the effect of age and sex. Residuals analysis revealed no clear patterns, with a symmetrical distribution and clusters around the midline and lower digits of the y-axis. All regression analyses passed the normality test, as assessed by the Shapiro-Wilk test for normal distribution.
Neuroimaging recording session.
Imaging data were collected with a whole body 3-T imaging system (Magnetom Prisma, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil (Barberi et al. 2000). A high-resolution T1-weighted structural volume was acquired with a three-dimensional MPRAGE sequence at the beginning of the scanning session (sagittal, matrix 256 × 240 mm, voxel resolution 1.0 × 1.0 × 1.0 mm, 1-mm slice thickness, no gap, flip angle 9°, TE = 2.98 ms, TI = 900 ms, TR = 2.3 ms). Transmission and detection of the blood oxygen level dependent (BOLD) contrast signal were acquired by T2-weighted gradient echo-echo planar imaging pulse sequence with the following parameters: TE = 30 ms; field of view = 240 × 240 mm, flip angle = 90°. Forty-five interleaved axial slices (3.0 × 3.0 mm in-plane voxel resolution, TR = 2.5 s) were acquired in each volume. Five volumes were acquired in the resting participant before actual data collection to allow for magnetization equilibrium; these were discarded before data analysis. Head movement was limited during the experimental session within a head cradle packed with foam padding, and each subject was instructed to avoid head movements during the scanning period. Beat-by-beat HR was calculated from the continuous signal derived from an MRI-compatible pulse Oximeter (8600FO MRI, Nonin Medical, Plymouth, MN) placed over the index finger of the nonexercising left hand. In each session, analog signals for pulse recordings and IHG contraction force were sampled at 1,000 Hz with an online data acquisition and analysis system (PowerLab, ADInstruments). Respiratory frequency was monitored continuously to prevent Valsalva maneuvers during the exercise period.
Neuroimaging data analysis.
The ΔHR to IHG was determined by averaging the response over the last 20 s of each rest and the last 10 s of each IHG interval. Individual HR time courses were determined with 2.5-s averages of the beat-by-beat HR measures to generate time-aligned data with the BOLD imaging acquisition. HR responses for each participant were averaged over the seven repeated blocks at 40% MVC.
All fMRI data were analyzed with Brain Voyager QX 2.8.4 (Brain Innovation, Maastricht, The Netherlands) (Goebel et al. 2006), following standard fMRI data analysis steps (Lindquist 2008). At the first (individual) level, preprocessing included interscan slice acquisition time correction, linear trend removal, temporal high-pass filtering to remove low-frequency drifts, and rigid-body transformation of data to the first acquired image to correct for motion. Individual functional data were coregistered to their respective T1 MPRAGE anatomical data, and subsequently transformed to Talairach space (Talairach and Tournoux 1988). The change in BOLD signal over the exercise period was modeled with a boxcar function convolved with a canonical hemodynamic response function and regressed with the individual movement parameters generated during preprocessing. This resulted in subject-specific contrast images containing whole brain information related to sites of both increased and decreased BOLD signal, relative to baseline, during the IHG task as a function of the task itself and the individual HR correlation. The general linear model was used to calculate the parameter estimates for all brain voxels (Friston et al. 1995). Corrections for multiple comparisons were made using the false discovery rate (FDR, P < 0.05), as well as a cluster level threshold estimation, such that the results represent only clusters > 10 voxels in size.
Subsequently, the individual trial effects were “collapsed” within each of the subjects before comparing the subjects within the random effects (RFX) analysis. This was performed in response to the task and the HR regression to assess the consistency of effects between individuals based on the variability of the first-level estimates across subjects. Activation clusters with a minimum size of 300 voxels were converted into voxels of interest (VOI) resulting in a list of the most significant clusters at the whole brain level. VOI details were transferred to Talairach Daemon (Research Imaging Institute, version 2.4.3) for the assignment of Talairach coordinates to the nearest gray matter voxel. Subsequently, a manual region-of-interest analysis was performed for relevant cortical autonomic network regions including the bilateral IC, ACC, PCC, MPFC, and HC, based on earlier data in young individuals performing the same IHG protocol (Norton et al. 2013; Wong et al. 2007). All fMRI data are represented in radiological convention (i.e., subject’s right appears on the left).
To assess the relationship between BOLD responses with our external covariate (V̇o2max), we performed a full cortex subtraction (t-test) analysis on high-fit (≥90th percentile of age-predicted V̇o2max) vs. low-fit (<90th percentile of age-predicted V̇o2max) individuals. The 90th percentile was the chosen threshold for fitness as it accurately depicts whether individuals were meeting the expected guidelines for their age (ACSM 1995) and represents the median fitness level of our sample population. The final correlation maps were adapted by increasing the minimum threshold value (P < 0.05) and adding a cluster threshold (10 mm2).
RESULTS
Physiological results.
Baseline anthropometric and cardiovascular characteristics are provided in Table 1. The ΔHR to the 40% MVC contraction was the same during the physiological and neuroimaging sessions (Table 1).
The performance of the IHG task was monitored both by ourselves and by the participants through feedback of their achieved force in real time. A post hoc analysis of the standard deviation of these responses revealed an average variation of 3.66% around the target force. None of the participants reported feeling any significant degree of adverse emotional stress or forearm fatigue, with an average reported Borg response of 8, or very light.
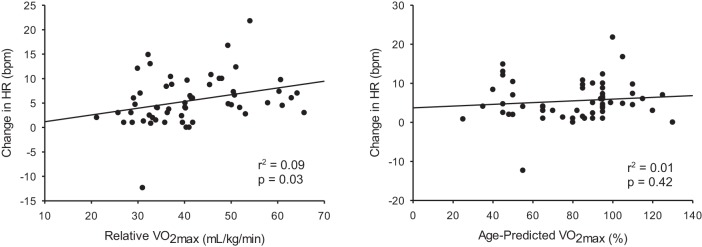
Linear regression revealed a weak, albeit significant, relationship between cardiorespiratory fitness (P = 0.03) and the change in HR in response to the IHG task (Fig. 1).
Fig. 1.
Heart rate (HR) response to isometric handgrip regressed with cardiorespiratory fitness (V̇o2max).
Functional (BOLD) imaging results: handgrip stimulus.
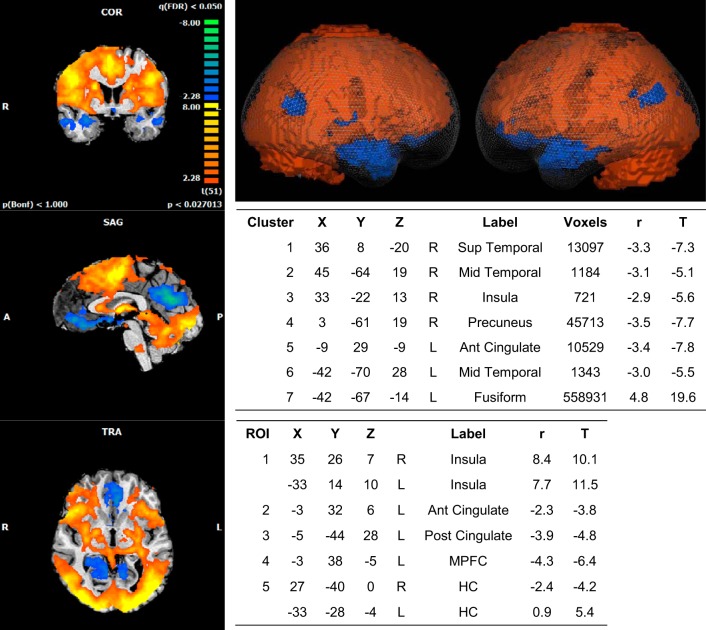
A whole brain group analysis of the cortical response to IHG revealed seven significant clusters of activation. An increase in BOLD signal relative to baseline was widespread and centered around the left fusiform gyrus, while decreases in activity were observed at the right superior and middle temporal gyrus, right insula, precuneus, left ACC gyrus, and middle temporal gyrus (Fig. 2).
Fig. 2.
BOLD imaging results: handgrip stimulus. Whole brain 2 (2D; left)- and 3 (3D, glass brain; top right)-dimensional representations of activated clusters. Peak cluster (top table) and region of interest (ROI) details (bottom table) are given with Talairach coordinates. R, right; L, left; Sup, superior; Mid, middle; Ant, anterior; Post, posterior; MPFC, medial prefrontal cortex; HC, hippocampus. r, correlation value; T, t score (β value). Spherical ROI represents nearest gray matter 257 voxels (same for all ROIs). FDR, P < 0.05. Cluster threshold = 10 voxels.
Further analysis of our a priori regions of interest revealed increased activity in the bilateral IC and left HC, while reduced activity relative to baseline was observed in the left ACC and PCC as well as the left MPFC and right HC.
The correlation coefficient, r, as per Brain Voyager’s software package, is the name given to the values that code the correlation between the BOLD contrast and the covariates specified. Therefore, in the present study, although the relative values may appear different from the traditional correlation coefficients, the relative value given to the number remains the same, i.e., a larger positive number signifies a stronger relationship than a lower or negative number.
Functional (BOLD) imaging results: heart rate.
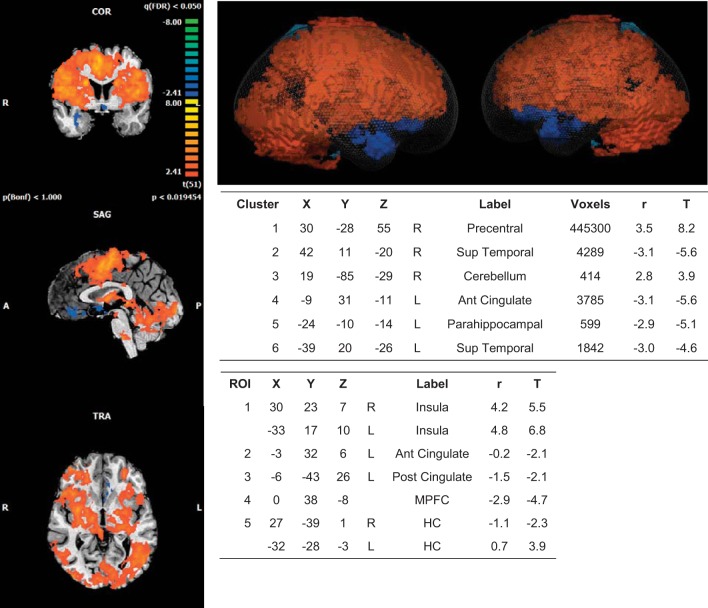
Whole brain group analysis of the cortical response regressed with the HR time course revealed six significant clusters of activation. An increase in BOLD signal relative to baseline was observed in the right precentral gyrus and cerebellum, while decreases in activity were observed in the right superior temporal gyrus as well as the left ACC, parahippocampal gyrus and superior temporal gyrus (Fig. 3).
Fig. 3.
BOLD imaging results: heart rate regressor. Whole brain 2D (left) and 3D (glass brain; top right) representations of activated clusters. Peak cluster (top table) and region of interest (ROI) details (bottom table) are given with Talairach coordinates. R, right; L, left; Sup, superior; Ant, anterior; Post, posterior; MPFC, medial prefrontal cortex; HC, hippocampus. r, correlation value; T, t score (β value). Spherical ROI represents nearest gray matter 257 voxels (same for all ROIs). FDR, P < 0.05. Cluster threshold = 10 voxels.
Region-of-interest analysis revealed similar results to the task, with increased activity in the bilateral anterior IC and left HC and reduced activity relative to baseline observed in the left ACC and PCC as well as the left MPFC and right HC.
t-Test: low V̇o2max − high V̇o2max.
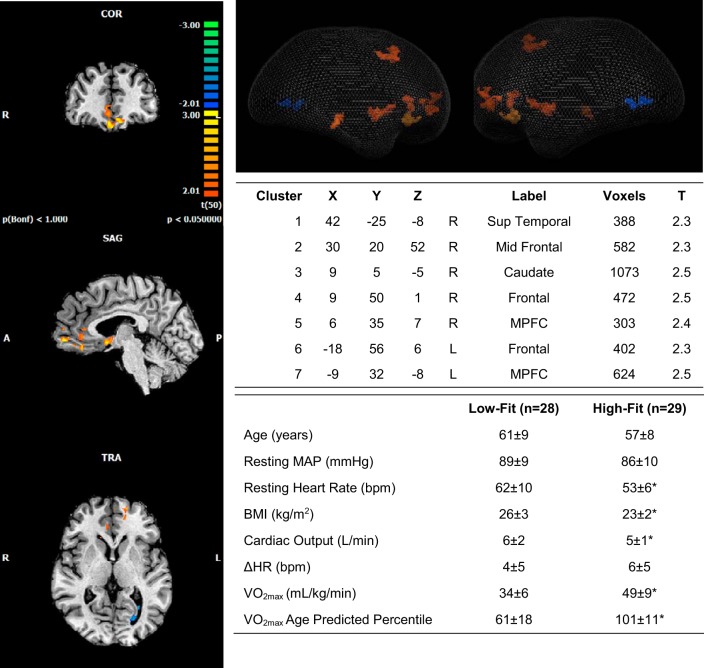
Subtraction analysis of BOLD activation patterns correlated to the HR response between the low-fit and high-fit groups revealed increased activation at the MPFC suggesting that those with a higher V̇o2max had more deactivation than those with a low V̇o2max in this region. Age and the ΔHR response to the IHG task were the same between high-fit and low-fit individuals (Fig. 4).
Fig. 4.
Subtraction analysis of low V̇o2 − high V̇o2 representing the difference in activation patterns that correlate with heart rate. Whole brain 2D (left) and 3D (glass brain; top right) representations of activated clusters are shown. Warm colors represent regions of the brain where the low-V̇o2 group has more activation/less deactivation than the high-V̇o2 group. Peak cluster details (top table) are given with Talairach coordinates. Group subject characteristics (bottom table) after assignment based on ACSM V̇o2max age-predicted percentiles. R, right; L, left; Sup, superior; Mid, middle; MPFC, medial prefrontal cortex; T, β value representing difference between groups; HR, heart rate. Cluster threshold = 10 voxels. *Different from low fit, P < 0.05.
DISCUSSION
To our knowledge, the present study represents the first exploration of the relationship between cardiorespiratory fitness and forebrain circuitry associated with cardiovascular control. Our first observation suggests that fitness does not predict the ΔHR to a volitional handgrip (IHG) task. Second, as a group, the BOLD responses associated with the IHG task, and with the HR time course, were very similar to those previously observed in young adults. Finally, in a groupwise contrast, cardiorespiratory fitness predicted the BOLD response associated with HR. We interpret these findings to suggest that higher levels of fitness positively affect cortical neurocircuitry associated with cardiovascular arousal and that the effect of such neural activity is modulated in the middle to older age range because of factors related to brain stem pathways and/or end-organ responsiveness.
Our hypothesis would predict that the change in HR should be correlated with the level of cardiorespiratory fitness. However, as shown in Fig. 1, this relationship, albeit significant, is weak, with an r2 value of 0.09. This suggests that only 9% of the variance in HR can be explained by fitness. Furthermore, when HR was normalized to age and sex (% of age-predicted values; Fig. 1), this weak relationship was no longer significant. Therefore, we believe that the strength of this relationship (or lack thereof) suggests that fitness does not adequately predict a HR response to the IHG task in this population, and that the BOLD neural signal may represent a better indicator of cardiac arousal.
The preservation of deactivation patterns within the MPFC and HC in response to both the task and the HR time course represents an important observation of the present study. These regions were specifically chosen for their known participation in cardiac adjustments to exercise and support the discrete patterns of activation consistently shown in young, healthy subjects (Goswami et al. 2011; Norton et al. 2013; Wong et al. 2007). Furthermore, high cardiorespiratory fitness has been linked to improved HC (Erickson et al. 2011; Varma et al. 2015), MPFC (Colcombe et al. 2003, 2006; Kramer et al. 2006), and anterior IC (Peters et al. 2009) volumes. In addition, cross-sectional studies show an association between fitness and brain function (Norton et al. 2015b), particularly as it pertains to cognition (Dupuy et al. 2015; Dustman et al. 1984; Gauthier et al. 2015; Prakash et al. 2011; Voss et al. 2013). The present results add additional novel outcomes that cardiorespiratory fitness positively affects MPFC and HC functional activity patterns to effortful tasks.
Nonetheless, the impact of fitness, while present in forebrain functional patterns to IHG, was not observed in HR responses. The paradoxical observation that fitness did not affect the HR response to the IHG stimulus, but did preserve MPFC deactivation in this age group, may be due to challenges associated with the focus on middle- to older-aged adults. Middle to late middle age represents a period of physiological transition in many physiological systems, including declines in fitness (Tanaka and Seals 2003) and brain mass (Raz et al. 2010). Our recent study indicated that a reserve of cortical thickness is developed through higher levels of physical fitness in aging adults (Wood et al. 2016), although the trajectory of cortical atrophy with age persisted. The present results suggest that this cortical reserve induced by higher levels of physical activity may enhance cortical functional patterns as well. Nonetheless, advancing age often produces reductions in intrinsic HR (Craft and Schwartz 1995) and the parasympathomimetic effect of low-dose atropine (Lee et al. 2008), indicative of nonneural effects of age on cardiac function. Using independent group t-tests, and contrasting the present data with previously published data from our laboratory, we sought to further examine the age-dependent relationship between HR responses to IHG and the associated cortical activation patterns. In this retrospective analysis, we established that the present group of moderately to highly active individuals was somewhat younger (57 ± 7 yr) than a sedentary group reported previously (63 ± 11 yr, P < 0.05) (Norton et al. 2013), who generated a significantly lower HR response [2 ± 2 beats per minute (bpm)] to the same IHG intensity compared with the present active participants (6 ± 5 bpm, P < 0.05). Importantly, the older sedentary group (Norton et al. 2013) failed to show MPFC or HC deactivation. Alternatively, young individuals (25 ± 4 yr; n = 17) generate a much larger HR response (>10 bpm) (Wong et al. 2007) with robust MPFC and HC deactivation, and IC activation. Therefore, the present data are consistent with a fitness-based preservation of forebrain activation patterns, but these neurological benefits have little apparent benefit for HR responses to exercise due, likely, to a local age-related impairment of HR control.
Whole brain group analysis of the cortical response regressed with the HR time course revealed six significant clusters of activation (Fig. 3) that appear to demonstrate shared variance between the IHG and HR regressors. It is possible that these regions are in some way involved in the HR response triggered by the IHG task, but we are not assuming causation. These areas, which are largely involved in voluntary motor movements, may act as a relay center and may potentially have direct connectivity within and between functional subregions of one or more key components of the autonomic control network. We have taken measures to reduce bias, such as conservative statistical thresholding, which has been shown to produce acceptable balance between minimal type I and type II errors (FDR P < 0.05 and 10 contiguous voxels) (Lieberman and Cunningham 2009).
Limitations.
The present findings are based on cross-sectional brain imaging data. Additional prospective training studies in this age group are needed to see whether elevations in cardiovagal function and cardiorespiratory function are linked with corresponding improvements in cortical neurocircuitry related to cardiovascular arousal.
In addition, there are determinants of cardiorespiratory fitness that account for variance beyond habitual physical activity such as genetics, which may account for about half of the variance in individual differences in fitness (Bouchard et al. 1999, 2011). To our knowledge, no information exists regarding genetic linkages between brain function and cardiorespiratory function.
The present study focused solely on the HR outcomes during effortful IHG. However, measures of sympathetic nerve activity, also affected by the cortical autonomic network, may expose an additional outcome of interest. In particular, age elevates sympathetic outflow in many individuals (Davy et al. 1998; Monahan 2007; Monahan et al. 2001; Seals et al. 1994; Tanaka et al. 2000), but this change can be mitigated by exercise training (Carter and Ray 2015). Moreover, IHG performed by older adults has been characterized by a greater increase in muscle sympathetic nerve activity, compensating for a smaller HR response. Therefore, it may be that the preservation of cortical activation patterns with high fitness in the present study exerted a positive influence over sympathetic outflow, rather than HR. Unfortunately, sympathetic nerve activity was not measured in the present study.
We acknowledge that the possibility of spurious activations is a major issue with fMRI. We are aware that breathing and HR are large sources of error in fMRI. However, we are interested in the HR response itself, and we have tried to minimize all other sources of error including measuring and monitoring respiration. Breathing did not change during IHG, suggesting that it is likely not associated with the IHG response, but we acknowledge that it may still be a source of noise.
Finally, there is a possibility that regional neuronal activation measured by BOLD may be masked by an increase in cardiac output during the IHG task, which can increase brain arterial blood flow and oxygenation. In our context, with a small muscle mass being engaged for a moderate intensity and short period of time, we do not see a dramatic increase in cardiac output over the IHG task period (~200 ml). Where this increase in blood volume gets displaced throughout the body is unknown, but certainly there is potential for a small increase in cerebral blood flow to the brain.
Conclusions.
The present data indicate that high cardiorespiratory fitness sustained through middle age contributes to the preservation of cortical circuitry associated with cardiovascular control. Overall, these findings support the hypothesis of a role for cardiorespiratory fitness to positively affect the cortical autonomic network, providing new insights into the mechanisms underlying preserved autonomic function and healthy brain aging in adults who engage in long-term regular exercise.
GRANTS
This work was funded by the Canadian Institutes for Health Research through a Team Grant in Physical Activity, Mobility and Neural Health (Grant 217532), with K. Shoemaker as the nominated Principal Investigator. K. Shoemaker is a Tier 1 Canada Research Chair in the Integrative Physiology of Exercise and Health.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
K.N.W. conceived and designed research; K.N.W. and T.A.L. performed experiments; K.N.W. analyzed data; K.N.W. and J.K.S. interpreted results of experiments; K.N.W. prepared figures; K.N.W. drafted manuscript; K.N.W., T.A.L., and J.K.S. edited and revised manuscript; K.N.W. and J.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Arlene Fleischhauer for exceptional technical support and data collection. Cortical imaging was conducted at the Robarts Centre for Functional and Metabolic Mapping, The University of Western Ontario, under the direction of Oksana Opalevych and Joseph Gati. A. Heinecke provided consultative assistance for brain segmentation in Brain Voyager software.
References
- ACSM ACSM’s Guidelines for Exercise Testing and Prescription (5th ed.). Philadelphia, PA: Williams & Wilkins, 1995, p. 160. [Google Scholar]
- Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res 11: 99–108, 2001. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl 49: 1–92, 1960. [PubMed] [Google Scholar]
- Barberi EA, Gati JS, Rutt BK, Menon RS. A transmit-only/receive-only (TORO) RF system for high-field MRI/MRS applications. Magn Reson Med 43: 284–289, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Basnayake SD, Green AL, Paterson DJ. Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol 97: 29–38, 2012. doi: 10.1113/expphysiol.2011.060848. [DOI] [PubMed] [Google Scholar]
- Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, Katus HA. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol 14: 791–799, 2003. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 3: 153–158, 1982. doi: 10.1055/s-2008-1026080. [DOI] [PubMed] [Google Scholar]
- Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Pérusse L, Leon AS, Rao DC. Familial aggregation of V̇o2max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 87: 1003–1008, 1999. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol (1985) 110: 1160–1170, 2011. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RA, Blackmon JR, Jones JW, Strait G. Exercising testing in adult normal subjects and cardiac patients. Pediatrics 32, Suppl: 742–756, 1963. [PubMed] [Google Scholar]
- Burns SM, Wyss JM. The involvement of the anterior cingulate cortex in blood pressure control. Brain Res 340: 71–77, 1985. doi: 10.1016/0006-8993(85)90774-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol 42: 277–286, 2001. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364–375, 2004. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36–43, 2015. doi: 10.1016/j.autneu.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol 99: 326–331, 2014. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Chen SJ. Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol Regul Integr Comp Physiol 258: R245–R255, 1990. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58: 176–180, 2003. doi: 10.1093/gerona/58.2.M176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61: 1166–1170, 2006. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 101: 3316–3321, 2004. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft N, Schwartz JB. Effects of age on intrinsic heart rate, heart rate variability, and AV conduction in healthy humans. Am J Physiol Heart Circ Physiol 268: H1441–H1452, 1995. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523: 259–270, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchot P, Gravenstein JS. Effects of atropine on the electrocardiogram in different age groups. Clin Pharmacol Ther 12: 274–280, 1971. doi: 10.1002/cpt1971122part1274. [DOI] [PubMed] [Google Scholar]
- Davy KP, DeSouza CA, Jones PP, Seals DR. Elevated heart rate variability in physically active young and older adult women. Clin Sci (Lond) 94: 579–584, 1998. doi: 10.1042/cs0940579. [DOI] [PubMed] [Google Scholar]
- Dupuy O, Gauthier CJ, Fraser SA, Desjardins-Crèpeau L, Desjardins M, Mekary S, Lesage F, Hoge RD, Pouliot P, Bherer L. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front Hum Neurosci 9: 66, 2015. doi: 10.3389/fnhum.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging 5: 35–42, 1984. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wójcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19: 1030–1039, 2009. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol 40: 679–682, 1976. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Pressor and depressor sites are intermingled in the cingulate cortex of the rat. Brain Res 754: 204–212, 1997. doi: 10.1016/S0006-8993(97)00076-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage 2: 45–53, 1995. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gauthier CJ, Lefort M, Mekary S, Desjardins-Crépeau L, Skimminge A, Iversen P, Madjar C, Desjardins M, Lesage F, Garde E, Frouin F, Bherer L, Hoge RD. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol Aging 36: 304–314, 2015. doi: 10.1016/j.neurobiolaging.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 41: 521–530, 2004. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 27: 392–401, 2006. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Frances MF, Shoemaker JK. Representation of somatosensory inputs within the cortical autonomic network. Neuroimage 54: 1211–1220, 2011. doi: 10.1016/j.neuroimage.2010.09.050. [DOI] [PubMed] [Google Scholar]
- Goswami R, Frances MF, Steinback CD, Shoemaker JK. Forebrain organization representing baroreceptor gating of somatosensory afferents within the cortical autonomic network. J Neurophysiol 108: 453–466, 2012. doi: 10.1152/jn.00764.2011. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia 41: 1929–1941, 2003. doi: 10.1016/S0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol 38: 272–278, 1975. [DOI] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage 59: 1514–1523, 2012. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson M, de Luna X, Pudas S, Nilsson LG, Nyberg L. Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. J Am Geriatr Soc 60: 2308–2312, 2012. doi: 10.1111/jgs.12000. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging 30: 1657–1676, 2009. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino M, Lance VQ, Shahamatpour A, Spodick DH. Effects of age on responses to isometric exercise. Isometric handgrip in noninvasive screening for cardiovascular disease. Am Heart J 90: 575–581, 1975. doi: 10.1016/0002-8703(75)90220-3. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol (1985) 101: 1237–1242, 2006. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Lalande S, Sawicki CP, Baker JR, Shoemaker JK. Effect of age on the hemodynamic and sympathetic responses at the onset of isometric handgrip exercise. J Appl Physiol (1985) 116: 222–227, 2014. doi: 10.1152/japplphysiol.01022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Picard G, Beske SD, Hwang GS, Taylor JA. Effects of fitness and age on the response to vagotonic atropine. Auton Neurosci 139: 60–67, 2008. doi: 10.1016/j.autneu.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester M, Sheffield LT, Trammell P, Reeves TJ. The effect of age and athletic training on the maximal heart rate during muscular exercise. Am Heart J 76: 370–376, 1968. doi: 10.1016/0002-8703(68)90233-0. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 4: 423–428, 2009. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA. The statistical analysis of fMRI data. Stat Sci 23: 439–464, 2008. doi: 10.1214/09-STS282. [DOI] [Google Scholar]
- Macey PM, Wu P, Kumar R, Ogren JA, Richardson HL, Woo MA, Harper RM. Differential responses of the insular cortex gyri to autonomic challenges. Auton Neurosci 168: 72–81, 2012. doi: 10.1016/j.autneu.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR Jr, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol 413: 433–445, 1989. doi: 10.1113/jphysiol.1989.sp017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001. [DOI] [PubMed] [Google Scholar]
- Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci 6: 170, 2014. doi: 10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KN, Badrov MB, Barron CC, Suskin N, Heinecke A, Shoemaker JK. Coronary artery disease affects cortical circuitry associated with brain-heart integration during volitional exercise. J Neurophysiol 114: 835–845, 2015a. doi: 10.1152/jn.00008.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KN, Heinecke A, Shoemaker JK. The neuroprotective effects of endurance training on the aging brain. Auton Neurosci 192: 110, 2015b. doi: 10.1016/j.autneu.2015.07.180. [DOI] [Google Scholar]
- Norton KN, Luchyshyn TA, Kevin Shoemaker J. Evidence for a medial prefrontal cortex-hippocampal axis associated with heart rate control in conscious humans. Brain Res 1538: 104–115, 2013. doi: 10.1016/j.brainres.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci 16: 292–305, 2012. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Lind J, Pudas S, Persson J, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci USA 107: 22682–22686, 2010. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J 55: 348–354, 1986. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732, 1992. doi: 10.1212/WNL.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Owens NC, Verberne AJ. Regional haemodynamic responses to activation of the medial prefrontal cortex depressor region. Brain Res 919: 221–231, 2001. doi: 10.1016/S0006-8993(01)03017-7. [DOI] [PubMed] [Google Scholar]
- Peters J, Dauvermann M, Mette C, Platen P, Franke J, Hinrichs T, Daum I. Voxel-based morphometry reveals an association between aerobic capacity and grey matter density in the right anterior insula. Neuroscience 163: 1102–1108, 2009. doi: 10.1016/j.neuroscience.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Lewis JM, Chaddock L, Malkowski E, Alves H, Kim J, Szabo A, White SM, Wójcicki TR, Klamm EL, McAuley E, Kramer AF. Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci 4: 229, 2011. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudas S, Persson J, Josefsson M, de Luna X, Nilsson LG, Nyberg L. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J Neurosci 33: 8668–8677, 2013. doi: 10.1523/JNEUROSCI.2900-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage 51: 501–511, 2010. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 7: 268–282, 1997. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15: 1676–1689, 2005. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30: 730–748, 2006. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging 20: 3–18, 2005. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mraovitch S, Granata AR, Anwar M, Reis DJ. A role of insular cortex in cardiovascular function. J Comp Neurol 257: 189–207, 1987. doi: 10.1002/cne.902570206. [DOI] [PubMed] [Google Scholar]
- Russoniello CV, Zhirnov YN, Pougatchev VI, Gribkov EN. Heart rate variability and biological age: implications for health and gaming. Cyberpsychol Behav Soc Netw 16: 302–308, 2013. doi: 10.1089/cyber.2013.1505. [DOI] [PubMed] [Google Scholar]
- Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. J Gerontol 46: M99–M106, 1991. doi: 10.1093/geronj/46.3.M99. [DOI] [PubMed] [Google Scholar]
- Seals DR, Taylor JA, Ng AV, Esler MD. Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc 26: 568–576, 1994. doi: 10.1249/00005768-199405000-00008. [DOI] [PubMed] [Google Scholar]
- Seliger V, Mácek M, Skranc O, Horák J, Piric J, Handzo P, Rous J, Jirka Z. Work capacity of the Czechoslovakian population. Eur J Appl Physiol Occup Physiol 39: 155–164, 1978. doi: 10.1007/BF00421342. [DOI] [PubMed] [Google Scholar]
- Sheffield LT, Maloof JA, Sawyer JA, Roitman D. Maximal heart rate and treadmill performance of healthy women in relation to age. Circulation 57: 79–84, 1978. doi: 10.1161/01.CIR.57.1.79. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Norton KN, Baker J, Luchyshyn T. Forebrain organization for autonomic cardiovascular control. Auton Neurosci 188: 5–9, 2015. doi: 10.1016/j.autneu.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical, 1988. [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Dynamic exercise performance in Masters athletes: insight into the effects of primary human aging on physiological functional capacity. J Appl Physiol (1985) 95: 2152–2162, 2003. doi: 10.1152/japplphysiol.00320.2003. [DOI] [PubMed] [Google Scholar]
- Varma VR, Chuang YF, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus 25: 605–615, 2015. doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am J Physiol Regul Integr Comp Physiol 270: R713–R719, 1996. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Godde B, Staudinger UM. Physical and motor fitness are both related to cognition in old age. Eur J Neurosci 31: 167–176, 2010. doi: 10.1111/j.1460-9568.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wójcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp 34: 2972–2985, 2013. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 74: 2566–2573, 1993. [DOI] [PubMed] [Google Scholar]
- Williamson JW. The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95: 1043–1048, 2010. doi: 10.1113/expphysiol.2009.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Massé N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 35: 698–708, 2007. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Wood KN, Nikolov R, Shoemaker JK. Impact of long-term endurance training vs. guideline-based physical activity on brain structure in healthy aging. Front Aging Neurosci 8: 155, 2016. doi: 10.3389/fnagi.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303: 355–374, 1991. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- Yin FC, Spurgeon HA, Greene HL, Lakatta EG, Weisfeldt ML. Age-associated decrease in heart rate response to isoproterenol in dogs. Mech Ageing Dev 10: 17–25, 1979. doi: 10.1016/0047-6374(79)90067-8. [DOI] [PubMed] [Google Scholar]