Abstract
We have isolated TINC as a NIMA-interacting protein by using the yeast two-hybrid system and have confirmed that TINC interacts with NIMA in Aspergillus nidulans. The TINC-NIMA interaction is stabilized in the absence of phosphatase inhibitors and in the presence of kinase-inactive NIMA, suggesting that the interaction is enhanced when NIMA is not fully activated. TINC is a cytoplasmic protein. TINC homologues and a TINC-like protein (A. nidulans HETC) are conserved in other filamentous fungi. Neither deletion of tinC nor deletion of both tinC and A. nidulans hetC is lethal, but deletion of tinC does produce cold sensitivity as well as osmotic sensitivity. Expression of an amino-terminal-truncated form of TINC (ΔN-TINC) inhibits colony growth in Aspergillus and localizes to membrane-like structures within the cell. Examination of cell cycle progression in these cells reveals that they progress through multiple defective mitoses. Many cells contain large polyploid single nuclei, while some appear to have separated masses of DNA. Examination of the nuclear envelopes of cells containing more than one DNA mass reveals that both DNA masses are contained within a single nuclear envelope, indicating that nuclear membrane fission is defective. The ability of these cells to separate DNA segregation from nuclear membrane fission suggests that this coordination is normally a regulated process in A. nidulans. Additional experiments demonstrate that expression of ΔN-TINC results in premature NIMA disappearance in mitotic samples. We propose that TINC's interaction with NIMA and the cell cycle defects produced by ΔN-TINC expression suggest possible roles for TINC and NIMA during nuclear membrane fission.
Entry into mitosis represents the irreversible commitment by a cell to segregate duplicated DNA equally to form two independent nuclei. Entry into mitosis with damaged DNA or incompletely replicated DNA, failure to establish a proper bipolar spindle, and premature exit from mitosis can all have disastrous consequences for the cell. As such, entry into, progression through, and exit from mitosis are all highly regulated cell cycle transitions. In all eukaryotes, one of the central triggers for mitotic entry is the activation of the cyclin-dependent kinase CDC2 (12, 21, 30). In Aspergillus nidulans, the NIMA (never in mitosis A) kinase is also required for mitotic entry, as cells with active CDC2 and inactive NIMA are unable to enter mitosis (32).
Two lines of evidence suggest that a NIMA pathway of cell cycle regulation exists in a wide range of organisms. Ectopic expression of NIMA in metazoan cells can induce some of the hallmarks of mitotic onset, including DNA condensation and germinal vesicle breakdown (25). Also, NIMA-related kinases (Nek) have been identified in a range of organisms, including yeast, flies, frogs, mice, and humans (29). Additionally, mitotic regulatory roles have been identified for the Fin1p kinase (15, 16, 22, 23) from Schizosaccharomyces pombe as well as for a number of mammalian Nek kinases, including Nek2 (6, 13), Nek6 (50), and Nek9 (3, 39).
NIMA is tightly cell cycle regulated both at the level of transcription (34) and by posttranslational mechanisms, including phosphorylation (49) and proteolysis (37, 48). The coordinate effects of these regulatory mechanisms keep NIMA levels low throughout much of interphase, which is followed by a steady increase in NIMA protein levels and activity during late interphase until they reach maximal levels at the G2/M transition (33). Finally, NIMA undergoes rapid proteolysis preceding mitotic exit (37). Interestingly, while NIMA activity is required for mitotic entry, NIMA degradation is required for mitotic exit, since expression of versions of NIMA lacking sequences required for NIMA instability results in the inability of these cells to exit mitosis (37).
Recent work has helped to further define the role of NIMA at mitotic entry. A mutant allele of sonAGle2/Rae1, which encodes a component of the nuclear pore complex, was isolated as a suppressor of the nimA1 mutation (46). At restrictive temperatures nimA1 cells exhibit a G2 arrest with NIMXCDC2/NIMECyclinB localized predominantly in the cytoplasm (46). The sonA1 mutation suppresses the cell cycle arrest exhibited by nimA1 strains and allows for nuclear accumulation of NIMXCDC2/NIMECyclinB (46). These data suggest a role for NIMA in regulating the subcellular localization of NIMXCDC2/NIMECyclinB at mitotic onset. A role for NIMA in the nuclear pore complex was further strengthened by the identification of a second nuclear pore component, SONBNUP98, as a NIMA-interacting protein (9). Together, these data suggest that NIMA controls mitotic onset by regulating the localization of mitosis-specific regulatory proteins, including NIMXCDC2/NIMECyclinB, through interaction with the nuclear pore complex at mitosis (9).
In vertebrate cells, one of the hallmarks of mitosis is the breakdown of the nuclear envelope at prometaphase to allow for the interaction between chromosomes with cytoplasmic regulators, including capture of kinetochores by mitotic spindle microtubules. During late anaphase and telophase the nuclear envelope is reestablished through a stepwise process involving association of nucleoporins and membrane components around decondensing chromosomes (2, 17, 45). The spatial separation of sister chromatids on the mitotic spindle and subsequent reforming of the nuclear envelope result in the formation of two distinct daughter nuclei.
In contrast to the open mitosis exhibited by vertebrate cells, many fungi, including A. nidulans, undergo a closed mitosis, in which the nuclear envelope remains intact throughout the cell cycle (38). The presence of this barrier throughout the nuclear division process requires that mitotic regulators be able to traverse this barrier to gain access to chromosomes and establish the mitotic spindle within the confines of the nucleus.
Additionally, the maintenance of the nuclear envelope throughout mitosis necessitates a process to cleave and rejoin the nuclear envelope following chromosome separation to allow for the formation of two distinct nuclei. In Saccharomyces cerevisiae, nuclear membrane fission is linked to cytokinesis (24). Mutants defective in cytokinesis display a defect in nuclear membrane fission, since 70% of such cells initiate a second round of budding without undergoing nuclear membrane fission (24). In these cells, DNA present in the mother and bud cells are contained within a single nuclear envelope (24). It has therefore been suggested that nuclear membrane fission may be activated by components of, or the mechanical action of, cytokinesis (24). In A. nidulans, however, several rounds of mitotic division occur during spore germination in the absence of cytokinesis, resulting in the production of a multinucleate cell (14, 18, 19, 44). This disconnection between nuclear division and cytokinesis requires that nuclear membrane fission be mediated through mechanisms that are not linked to cytokinesis. We report here on the TINC (two-hybrid interactor with NIMA C) protein which interacts with NIMA in A. nidulans and that may function during nuclear membrane fission through its interaction with NIMA.
MATERIALS AND METHODS
Aspergillus genetics, immunofluorescence, and protein analysis.
A. nidulans-specific medium was used, and culture and genetic methods and analysis were performed as described previously (36). Protein preparation, immunofluorescence, and transformation of A. nidulans were performed as previously described (28, 34, 49).
For NIMA-TINC coimmunoprecipitation experiments, strain R153 was germinated to mid-log phase in yeast extract glucose (YG) medium. Protein was prepared in HK or HK buffer lacking all phosphatase inhibitors. NIMA was immunoprecipitated from 6 mg of total protein using 30 μl of affinity-purified anti-NIMA antibodies raised against the ANYRED peptide (47). Immunoprecipitates were incubated with biotinylated donkey anti-sheep antibodies followed by addition of Streptavidin MagneSphere Paramagnetic particles (Promega). Following extensive washing in HK and radioimmunoprecipitation assay buffers, immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Proteins were visualized using either E18 anti-NIMA antibodies or α-TINC antibodies (generated against the TINC-specific peptide with the sequence KGEYEPQGYERQGSQL). Coimmunoprecipitation experiments conducted with strains carrying the nimA7 mutation were performed in an identical manner with the following exceptions. Strain SO117 was germinated in YG medium to early log phase and shifted to 42°C for 3 h to inactivate nimA7. Subsequent analysis was performed as described above.
For NIMA stability experiments, strains D23C and JD161 were grown in minimal medium supplemented with 5 g of yeast extract/liter, 20 g of lactose/liter, and 40 mM threonine and grown to early log phase at a permissive temperature. G2 samples were generated by shifting the cultures to 42°C by shaking the culture flasks in a 55°C water bath. Cultures were incubated at 42°C for 3 h to allow inactivation of nimT23cdc25 and arrest of cells in G2. To generate mitotic samples, cultures were grown to early log phase and then shifted to 42°C for 3 h. At this point, nocodazole was added to a final concentration of 5 μg/ml (to depolymerize microtubules) with continued incubation at 42°C for 10 min. Cultures were downshifted to a permissive temperature of 30°C in an ice bath to allow release of the sample into pseudometaphase due to activation of the spindle assembly checkpoint. Time course samples were removed at 20, 40, and 60 min following the downshift to permissive temperature. Protein samples were prepared in HK buffer plus microcystin. NIMA was immunoprecipitated from 5 mg of total protein using 25 μl of affinity-purified anti-NIMA antibodies raised against the ANYRED peptide. Immunoprecipitates were subjected to SDS-PAGE and Western blotting with the E15 NIMA-specific antibodies. For Western blotting of NIME and tubulin, 200 μg of sample was subjected to SDS-PAGE and Western blotting with E8 α-NIME antibodies or B512 α-tubulin antibodies (Sigma).
Examination of green fluorescent protein (GFP) fusion protein localization in living cells was performed using a Nikon Eclipse TE300 inverted microscope in conjunction with an Ultraview spinning disk confocal system (Perkin-Elmer) and an Orca ER digital camera (Hamamatsu). Examination of fixed immunofluorescence samples was performed using a Nikon Eclipse E800 microscope and an UltraPix digital camera (Life Science Resources, Ltd.). Image capture on both microscopes was performed using Ultraview image capture software (Perkin-Elmer).
A. nidulans strains.
The following A. nidulans strains were used: CB44 (pyrG89 wA3 pyroA4 alcA::tinC::pyr4+), CB45 (pyrG89 wA3 pyroA4 alcA::Δ5′tinC::pyr4+), CDS165 (pyrG89 wA3 pyroA4 GFP-sonB), D23C (diploid between SO230 and SO223), GR5 (pyrG89 wA3 pyroA4), JD148 (pabaA1 pyrG89 nimT23 alcA::pyr4+ chaA1), JD154 (pabaA1 pyrG89 nimT23 alcA::Δ5′tinC::pyr4+ chaA1), JD155 (pabaA1 pyrG89 nimT23 alcA::Δ5′tinC::pyr4+ chaA1), JD157 (pyrG89 wA3 pyroA4 GFP-sonB alcA::pyr4+), JD158 (pyrG89 wA3 pyroA4 GFP-sonB alcA::Δ5′tinC::pyr4+), JD161 (diploid between strains SO221 and JD154), JD163 (pyrG89 wA3 pyroA4 tinC-GFP::pyr4+), R153 (wA3 pyroA4), SO117 (pyrG89 wA3 nimA7 pyroA4 nicB8), SO182 (pabaA1 pyrG89 nimT23 chaA1), SO221 [pyrG89 nimT23 alcA::nimA(5C)::pyr4+ nicA2 fwA1], SO223 [pabaA1 pyrG89 nimT23 alcA::nimA(5C)::pyr4+ fwA1], and SO230 (pyrG89 nimT23 pyroA1 alcA:: pyr4+ chaA1).
Construction of two-hybrid cDNA library and nimA bait constructs.
Construction of the A. nidulans two-hybrid cDNA library and kinase-active and -inactive nimA bait constructs was performed as described previously (31).
5′ RACE PCR.
A full-length tinC cDNA clone was isolated from an AP1 adaptor-ligated A. nidulans cDNA library. Generation of the AP1-ligated cDNA library and rapid amplification of cDNA end (RACE) PCR were performed following the general guidelines provided in the Marathon cDNA amplification kit (Clontech). PCR was performed using the AP1 primer (Clontech) and a tinC-specific primer. The full-length cDNA was cloned into the PCR II vector using the TA Cloning System (Invitrogen).
TINC HA tagging.
TINC and ΔN-TINC were HA tagged at their carboxy termini following cloning into the pAL5 inducible expression vector (11). Genomic fragments corresponding to truncated and full-length tinC were PCR amplified and cloned into pAL5 as BamHI/KpnI restriction fragments. HA tagging of tinC constructs was performed using a method derived from the site-directed mutagenesis protocol outlined in the QuikChange site-directed mutagenesis kit (Stratagene) (46). The protocol introduced two consecutive copies of the HA tag at the 3′ end of the tinC constructs.
TINC antibody.
Peptide antibodies were generated against a 16-amino-acid TINC-specific peptide (KGEYEPQGYERQGSQL). Following synthesis, the peptide was linked to keyhole limpet hemocyanin, and rabbits were immunized. Peptide synthesis and production of α-TINC affinity-purified antiserum were performed by Bethyl Laboratories, Inc., Montgomery, Tex.
Deletion of tinC and A. nidulans hetC.
tinC and A. nidulans hetC were deleted as described previously (31). Briefly, an 8,802-kb genomic fragment containing tinC was cloned into the Litmus 29 plasmid vector (New England Biolabs) as an AflII/AvrII restriction fragment. This plasmid was used to generate a tinC deletion construct in which tinC was replaced by a bifunctional cassette consisting of a zeomycin resistance (Zeor) gene and A. fumigatus pyrG (5). The tinC deletion construct was linearized by digestion with AvrII and AflII and transformed into A. nidulans strain GR5. pyrG+ transformants were recovered and streaked to single colonies three times prior to confirming tinC deletion by PCR, Western blotting with α-TINC antibodies, and Southern blotting.
For deletion of A. nidulans hetC, an A. nidulans hetC-containing bacterial artificial chromosome clone (20C15) was identified from an A. nidulans genomic library as described previously (31). This bacterial artificial chromosome was used to generate a deletion construct in which A. nidulans hetC was replaced by a Zeor/pyroA+ cassette. An AgeI restriction fragment which contained the deleted A. nidulans hetC locus was transformed into A. nidulans strain GR5, and pyroA+ transformants were selected. Deletion of A. nidulans hetC was confirmed by PCR and Southern blotting.
Strains lacking both tinC and A. nidulans hetC were successfully generated by crossing ΔtinC and A. nidulans Δhet-C strains and selecting for pyroA+/pyrG+ progeny. Deletion of both tinC and A. nidulans hetC in these strains was confirmed by PCR screening and Western blotting with α-TINC antibodies.
TINC-GFP tagging.
tinC was 3′ GFP tagged using a plasmid recombination strategy described previously (5, 51). Briefly, a plasmid containing an 8.8-kb fragment of genomic DNA including tinC was transformed into the Escherichia coli strain DY331 along with a 3′-GFP-tagging cassette (51). The strain was induced for recombination, and the recombinant plasmid containing tinC-gfp was recovered and fully sequenced. Plasmid DNA was used for transformation of A. nidulans strain GR5. A. nidulans transformants were selected at random, germinated in minimal medium, and examined for GFP fluorescence by fluorescence microscopy. Expression of the TINC-GFP fusion protein was also confirmed by Western blotting with anti-GFP and α-TINC antibodies.
RESULTS
Cloning and characterization of tinC.
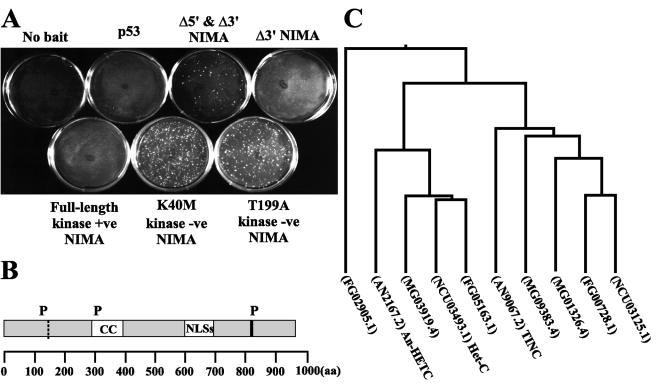
In order to identify NIMA-interacting proteins and perhaps NIMA substrates, we performed a two-hybrid screen using different forms of the NIMA kinase as bait. The screen utilized both kinase-active and -inactive forms of NIMA. The active kinase baits included a full-length NIMA and a 3′-truncated version of NIMA (Fig. 1A). The inactive NIMA constructs included two point mutations in essential residues within the catalytic domain (K40M and T199A) as well as a construct which was both 5′ and 3′ truncated (Fig. 1A). One of the proteins identified in this screen, termed TINC, interacted weakly with only kinase-inactive versions of NIMA (Fig. 1A) and is the focus of this paper. Compared to TINA, which interacts strongly with NIMA in the two-hybrid system (31), yielding between 77.5 and 19.1 U of β-galactosidase activity, the TINC-NIMA interaction generated far lower levels of β-galactosidase in the two-hybrid system (1.09 U with 5′ and 3′ ΔNIMA, 2.3 U with K40M NIMA, and 2.5 U with T199A NIMA).
FIG. 1.
TINC two-hybrid interaction and TINC sequence analysis. (A) Plasmids encoding TINC and kinase-active and -inactive forms of NIMA were cotransformed into S. cerevisiae strain CG-1945 and plated on selective synthetic defined medium. Colony formation indicates an interaction between TINC and NIMA. +ve, active; −ve, inactive. (B) TINC contains a putative coiled coil domain (CC), a cluster of nuclear localization sequences (NLSs), and three consensus NIMA phosphorylation sites (P). Indicated by a dashed vertical line is the truncation point of the tinC clone isolated in the two-hybrid screen. (C) TINC-related proteins were identified in filamentous fungal genome databases and aligned using ClustalW. A phylogenetic tree was subsequently generated from these alignments. AN, A. nidulans; NC, N. crassa; MG, M. grisea; FG, F. graminearum.
To determine the identity of TINC, the two-hybrid plasmid containing the tinC cDNA was recovered, and the insert was fully sequenced. 5′ RACE PCR was performed using an A. nidulans cDNA library to identify a full-length cDNA. A 2,937-bp open reading frame which encodes a protein of 978 amino acids with a projected molecular size of 110 kDa was identified (Fig. 1B). Comparison of the full-length cDNA sequence to the tinC cDNA recovered in the yeast two-hybrid screen revealed that the two-hybrid tinC clone lacked 397 bp at the 5′ end. For the remainder of the present paper, TINC refers to the protein produced by expression of full-length tinC, and ΔN-TINC refers to the protein produced by expression of 5′-truncated tinC. Sequence analysis revealed that TINC contains three consensus NIMA phosphorylation sites (FXXT/S) (26), a coiled coil region, and a cluster of predicted nuclear localization signal sequences between amino acids 596 and 709 (Fig. 1B). Two of the consensus NIMA phosphorylation sites were conserved among all TINC orthologues.
PCR screening of a sorted A. nidulans cosmid chromosome library mapped tinC to chromosome 5 and identified a tinC-containing cosmid. The tinC genomic sequence was obtained by primer walking of the tinC cosmid. Comparison of the genomic sequence to the TINC cDNA revealed the presence of three introns of 45, 47, and 62 bp, respectively.
BLAST homology searches in an A. nidulans sequence database (The Whitehead Institute) identified an uncharacterized protein, AN2167.2, which is 39.5% identical to TINC (Fig. 1C and Table 1). We have named AN2167.2 A. nidulans HETC due to the high level of sequence identity (48.3%) (Table 1) it shares with the N. crassa heterokaryon compatibility protein Het-C (Fig. 1C) (42, 43). Additional proteins which share significant homology with TINC were identified in Fusarium graminearum (FG00728.1, FG05163.1, and FG02905.1) and Magnaporthe grisea (MG01326.4, MG09383.4, and MG03919.4) (Fig. 1C and Table 1). Sequence alignments indicated that filamentous fungi contain a family of related proteins consisting of two main evolutionary branches with at least one member most similar to TINC and another more similar to N. crassa Het-C (Fig. 1C and Table 1).
TABLE 1.
Percent identity with A. nidulans TINC and N. crassa Het-C homologues among several filamentous fungia
| Organism | Protein | % Identity with:
|
|
|---|---|---|---|
| TINC | Het-C | ||
| N. crassa | NCU03125.1 | 49.6 | 34.5 |
| F. graminearum | FG00728.1 | 45.6 | 35.3 |
| M. grisea | MG01326.4 | 52.3 | 34.8 |
| M. grisea | MG09383.4 | 41.9 | 32.6 |
| A. nidulans | AN9067.2 (TINC) | NA | 34.6 |
| F. graminearum | FG05163.1 | 39.1 | 60.3 |
| N. crassa | NCU03493.1 (Het-C) | 34.6 | NA |
| M. grisea | MG03919.4 | 37.6 | 57.1 |
| A. nidulans | AN2167.2 (HETC) | 39.5 | 48.3 |
| F. graminearum | FG02905.1 | 31.8 | 29.2 |
NA, not applicable.
In N. crassa a polymorphic sequence region within Het-C has been shown to be responsible for determining heterokaryon incompatibility (42). Based on TINC's high sequence similarity to Het-C, 25 A. nidulans isolates representing 21 heterokaryon compatibility groups (1, 7, 8) were sequenced at the tinC locus to determine whether tinC was multiallelic and potentially involved in heterokaryon incompatibility reactions in Aspergillus (data not shown). This sequencing identified no significant sequence differences at the tinC locus, suggesting that TINC may not participate in heterokaryon compatibility determination in Aspergillus.
To determine whether A. nidulans hetC was multiallelic and may play a role in heterokaryon incompatibility, the same 25 isolates were sequenced at this locus. Sequencing analysis identified four point mutations within the strains compared to the Glasgow parent strain. Three of the mutations were silent; however, the fourth mutation, exhibited by nine of the strains, encoded a change from phenylalanine to leucine, both of which are neutral nonpolar amino acids (data not shown). The lack of sequence dissimilarity in these 25 isolates suggests that A. nidulans hetC is unlikely to be involved in regulating heterokaryon incompatibility in A. nidulans.
To investigate the function of TINC and A. nidulans HETC, strains in which tinC and A. nidulans hetC were deleted were generated using a bacterial recombination system to generate the deletion cassettes as described previously (31). Strains lacking tinC displayed moderate cold and osmotic sensitivity but were otherwise not significantly different from wild-type controls under all growth conditions tested. Strains lacking A. nidulans hetC were indistinguishable from wild-type controls. Furthermore, strains lacking both tinC and A. nidulans hetC were recovered as viable progeny from a meiotic cross. These data demonstrate that tinC and A. nidulans hetC fulfill a nonessential function under the conditions examined or that additional functional homologues exist.
TINC-specific antibody characterization.
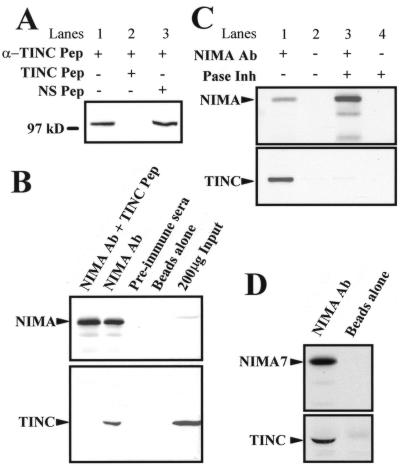
A peptide antibody (α-TINC) to the TINC-specific amino acid sequence KGEYEPQGYERQGSQL was generated. Total protein lysates were generated from an asynchronous A. nidulans culture and subjected to SDS-PAGE. Total protein blots were probed with α-TINC. The antibody specifically recognizes a single protein which migrates with mobility similar to that of a 97-kDa protein marker (Fig. 2A, lanes 1 and 3). Furthermore, addition of a TINC-specific peptide competes with α-TINC binding (Fig. 2A, lane 2), but a nonspecific peptide fails to compete (Fig. 2A, lane 3). Therefore, the α-TINC antibody specifically recognizes TINC in A. nidulans.
FIG. 2.
TINC interacts with NIMA in A. nidulans. (A) TINC antibody characterization. Western blotting of A. nidulans total cell extract confirms that the α-TINC antibody specifically recognizes a single protein which migrates near 97 kDa. Pep, peptide; NS, nonspecific. (B) NIMA coimmunoprecipitates TINC from A. nidulans total cell extracts. Protein was prepared from strain GR5 grown at 32°C to log phase. NIMA immunoprecipitates were probed for NIMA and TINC as indicated. Two hundred micrograms of total protein extract was also run as a control. Ab, antibody. (C) The NIMA-TINC interaction is phosphorylation state sensitive. Protein was prepared from strain GR5 in the absence or presence of phosphatase inhibitors (Pase Inh). NIMA immunoprecipitates were probed for TINC. (D) Protein was prepared in the presence of phosphatase inhibitors from a strain containing the nimA7 temperature-sensitive allele (SO117) grown at 30°C to mid-log phase and then shifted to 42°C for 3 h to inactivate NIMA. NIMA immunoprecipitates were probed for TINC.
NIMA interacts with TINC in A. nidulans.
In order to confirm the interaction between NIMA and TINC that we observed in the yeast two-hybrid screen, we performed coimmunoprecipitation experiments with A. nidulans protein extracts. Initial attempts to coimmunoprecipitate NIMA and TINC proved troublesome. Initially, protein lysates were made in complete HK buffer, which includes an array of phosphatase inhibitors which are essential to maintaining the kinase activity of NIMA (49). Since our two-hybrid interaction data suggested that TINC and NIMA interact more strongly when NIMA is inactive, protein lysates were prepared in HK buffer without any added phosphatase inhibitors. Under these conditions the kinase activity of NIMA is very low (49). Since only about 5% of the cells in these asynchronous cultures would be in mitosis, relatively little NIMA protein is present in these extracts, necessitating the use of a large amount of protein extract. NIMA was immunoprecipitated from 6 mg of total protein extract using a NIMA-specific peptide antibody. Immunoprecipitates were washed and then subjected to SDS-PAGE. Blots were probed with the α-TINC antibody to check for the presence of TINC in NIMA immunoprecipitates. NIMA immunoprecipitates were probed with NIMA antibody to ensure that NIMA was being successfully immunoprecipitated.
NIMA was specifically immunoprecipitated by the NIMA antibody (Fig. 2B, NIMA Ab lane) but not with preimmune sera (Fig. 2B, preimmune sera lane) or streptavidin-coated paramagnetic beads alone (Fig. 2B, beads alone lane). TINC coimmunoprecipitates specifically with NIMA in these extracts (Fig. 2B, NIMA Ab lane) and is not present in immunoprecipitates where NIMA is not present (Fig. 2B, preimmune sera and beads alone lanes).
To confirm that the interaction of NIMA and TINC was sensitive to the presence of phosphatase inhibitors, a single culture was grown as previously described. Protein was produced from one half of the culture in the presence of phosphatase inhibitors, while the other half was prepared in the absence of phosphatase inhibitors. NIMA immunoprecipitates were prepared from these lysates as described above. As expected, TINC and NIMA coimmunoprecipitated in the absence but not in the presence of phosphatase inhibitors (Fig. 2C, compare lanes 1 and 3), although more NIMA was present in NIMA immunoprecipitates from lysates containing phosphatase inhibitors than in those from lysates lacking the inhibitors (Fig. 2B, top panel). These results suggest that the TINC-NIMA interaction is phosphorylation state dependent with TINC binding only with dephosphorylated and inactive NIMA.
To determine whether TINC interacted with inactive NIMA kinase in the presence of phosphatase inhibitors, a strain containing the temperature-sensitive nimA7 allele was grown to log phase and shifted to a nonpermissive temperature for 3 h to inactivate NIMA7. Total cell lysates were generated in HK buffer which included phosphatase inhibitors. NIMA was immunoprecipitated, and these immunoprecipitates were probed for TINC. TINC was detected in NIMA7 immunoprecipitates (Fig. 2D, NIMA Ab lane) but not in control immunoprecipitates prepared with streptavidin-coated beads alone (Fig. 2D, beads alone lane). Together, these results suggest that the TINC-NIMA interaction is strongest when NIMA is inactive.
TINC localization.
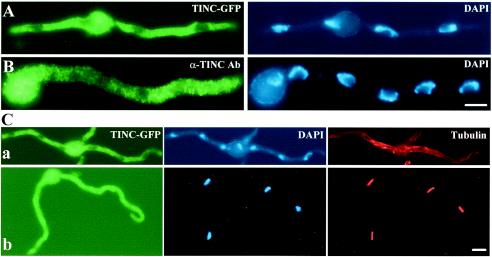
To examine TINC localization, a tinC-gfp fusion construct was created and transformed into A. nidulans. Living cells expressing TINC-GFP (JD163) were examined using confocal microscopy, and TINC-GFP was observed throughout much of the cell while some areas were devoid of TINC-GFP. The areas of the cell lacking TINC-GFP fluorescence were suggestive of nuclei based on their number, size, and general shape. To determine whether these areas of fluorescence exclusion represented nuclei, cells expressing TINC-GFP were fixed and stained with DAPI (4′,6′-diamidino-2-phenylindole) (Fig. 3A). Microscopic examination of these cells showed that these areas which lacked fluorescence did indeed correspond to nuclei (Fig. 3A). To confirm the TINC localization observed utilizing the TINC-GFP fusion protein, indirect immunofluorescence was performed using the α-TINC-specific antibody (Fig. 3B). TINC was localized throughout the cytoplasm in a punctuate fashion (Fig. 3B). Also, areas of negative staining which correspond to nuclei based on DAPI staining were observed (Fig. 3B).
FIG. 3.
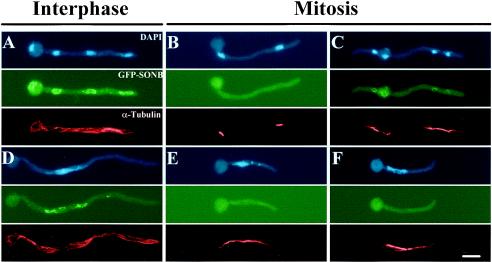
TINC is a cytoplasmic protein which enters the nucleus at mitosis. (A) Cells expressing TINC-GFP were germinated in minimal medium, fixed, DAPI stained, and examined by fluorescence microscopy. (B) Strain GR5 was germinated in YG medium at 32°C, fixed, and processed for indirect immunofluorescence microscopy. TINC was visualized with α-TINC antibody (Ab) followed by AlexaFluor 488 conjugated goat anti-rabbit immunoglobulin G (IgG). Note that nuclear exclusion of TINC is less pronounced in panel B since this image is a composite of several vertical image slices, while the TINC-GFP images are from a single focal plane. (C) Cells expressing TINC-GFP were germinated in minimal medium, fixed, and processed for indirect immunofluorescence microscopy. Microtubules were visualized with mouse monoclonal antibody TAT-1 followed by AlexaFluor 594 conjugated rat anti-mouse IgG. Cells were classed as being in either (a) interphase or (b) mitosis based on microtubule morphology. Bars, ∼5 μm.
Finally, in a small number of cells, no areas of negative fluorescence could be discerned. To investigate whether cells which displayed continuous TINC-GFP staining were mitotic, cells expressing TINC-GFP were fixed and processed for immunofluorescence utilizing an antitubulin antibody. Tubulin morphology and DNA condensation (based on DAPI staining) were used to determine whether cells were in interphase or mitosis. Examination of these cells (Fig. 3C) confirmed that (i) interphase cells contained areas of negative fluorescence corresponding to nuclei, and (ii) mitotic cells contained TINC-GFP in both the cytoplasm and nuclei. Therefore, TINC is a cytoplasmic protein throughout interphase but is also present in nuclei during mitosis.
ΔN-TINC expression produces growth inhibition.
In order to understand the function of TINC in A. nidulans, full-length TINC and a truncated form of ΔN-TINC, identical to that obtained from the yeast two-hybrid screen, were cloned into an inducible alcA expression vector. We reasoned that expression of NIMA-interacting proteins which positively impact NIMA function may induce hallmarks of premature mitosis, analogous to NIMA overexpression. Alternatively, expression of proteins which negatively impact NIMA function may display a late-interphase arrest, analogous to a lack of NIMA function.
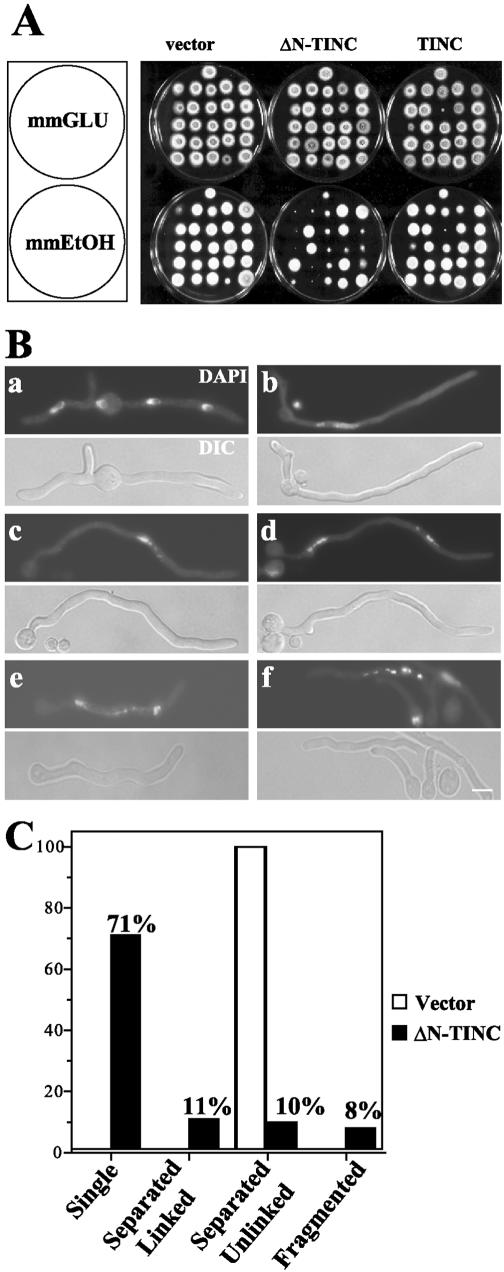
The alcA promoter allows for the selective expression of the TINC proteins on ethanol-containing medium, while TINC expression is repressed on glucose-containing medium. After transformation of alcA::tinC constructs into A. nidulans, transformants were randomly selected and tested for growth inhibition on inducing medium. Transformants expressing TINC displayed growth comparable to that of empty-vector control transformants (Fig. 4A). Note that the two TINC transformants which appear inhibited on inducing medium also appear inhibited on repressing medium (Fig. 4A). While expression of full-length TINC produced no significant colony formation defects, expression of ΔN-TINC produced varying degrees of colony growth inhibition (Fig. 4A). Southern blot analysis of these strains revealed that the severity of the colony growth inhibition phenotype correlated with the copy number of alcA-driven truncated tinC in the transformant's genome (data not shown).
FIG. 4.
ΔN-TINC expression causes growth inhibition characterized by an underlying cell cycle defect. (A) Strains containing empty-vector control, alcA-driven TINC, or alcA-driven ΔN-TINC were tested on repressing (mmGLU) and inducing (mmEtOH) medium for growth defects. (B) A control strain (JD148) (a) and an ΔN-TINC-expressing strain (JD155) (b to f) were germinated at 32°C in ethanol minimal medium. Cells were fixed and stained with DAPI and examined by fluorescence microscopy. DIC, differential-interference contrast. (C) The percentage of cells displaying various DNA morphologies was determined for the control and ΔN-TINC-expressing cells after DAPI staining. Bar, ∼5 μm.
ΔN-TINC expression causes cell cycle defects.
The fact that TINC interacts with NIMA in A. nidulans suggested that the underlying defect in these strains may be cell cycle specific. To determine whether this was indeed the case, conidia obtained from a strain expressing ΔN-TINC under the control of the alcA promoter (JD155) were germinated in inducing medium and stained with DAPI to visualize nuclear morphology (Fig. 4B, panels b to f). Strains which express ΔN-TINC grow at a reduced rate compared to wild-type strains. Therefore, in order to normalize the characterization of nuclei in empty-vector control (JD148) and ΔN-TINC germlings, ΔN-TINC germlings were allowed to germinate for a longer period than control cells, and only germlings which were at least 30 μm in length were considered for the following analysis. At this size, control cells had undergone at least one mitosis (Fig. 4B, panels a).
Seventy-one percent of ΔN-TINC-expressing germlings contained a single nucleus (Fig. 4B, panels b and c, and C). Of these cells, 68% appeared to be exhibiting a nim phenotype (Fig. 4B, panels b), typical of cells which lack functional NIMA. These cells displayed polarized growth in the absence of nuclear division. Their nuclei were typically large with a visible nucleolus, which is characteristic of late-interphase nuclei (Fig. 4B, panels b), and many appeared polyploid. Indeed, quantitative analysis indicated that the nuclei of ΔN-TINC-expressing cells contained 2.60-fold (standard deviation, ±0.098-fold) more DNA than comparable wild-type nuclei. Additionally, of cells which contained a single mass of DNA, about 3% contained apparently condensed DNA and lacked discernible nucleoli (Fig. 4B, panels c). The lack of visible nucleoli and the presence of condensed DNA in these cells indicated that they were in mitosis. The remaining 29% of germlings also exhibited DNA morphologies which suggested that these cells had not merely undergone arrest in late interphase (Fig. 4B, panels c to f, and C). Twenty-one percent of cells appeared to have undergone mitosis based on the presence of two discernible DNA masses (Fig. 4B, panels d and e), although the DNA masses were frequently of unequal size. In 11% of the total number of germlings (Fig. 4C), these separate masses of DNA were linked by intervening DNA (Fig. 4B, panels e), while an additional 10% of cells contained separated DNA which did not appear to be linked by a DNA bridge (Fig. 4B, panels d). Finally, 8% of ΔN-TINC germlings (Fig. 4C) displayed fragmented DNA (Fig. 4B, panels f). Together these phenotypes suggest that rather than undergoing arrest in interphase prior to mitotic entry, these cells may be undergoing faulty mitoses.
ΔN-TINC-expressing cells undergo faulty mitoses.
To further characterize the cell cycle defects produced by expression of ΔN-TINC, this protein was expressed in a strain containing GFP-SONB, a nuclear pore complex protein which localizes to the nuclear envelope (9). GFP-SONB also acts as a marker of cell cycle progression, since GFP-SONB is dispersed from the nuclear pore complex as cells enter mitosis. As cells exit mitosis, GFP-SONB returns to the nuclear pore complex, again enabling visualization of the nuclear envelope (10a).
As expected, approximately 95% of control cells (JD157) displayed interphase cytoplasmic microtubules and nuclei with GFP-SONB at their peripheries (Fig. 5A). GFP-SONB was dispersed in control cells at metaphase (Fig. 5B) and reassociated at telophase (Fig. 5C). ΔN-TINC cells (JD158) largely exhibited interphase microtubule arrays with GFP-SONB distributed around the nuclei (Fig. 5D). Those cells in mitosis, based on microtubule morphology, had dispersed GFP-SONB (Fig. 5E and F). Note that the mitotic spindles present in these mitotic cells were generally longer and displayed more prominent astral microtubules (Fig. 5E and F) than control cells (Fig. 5B and C). The aberrant spindle morphology exhibited by these cells made it difficult to characterize their mitotic stage; however, the ability to generate spindles, undergo chromosome condensation, and disperse GFP-SONB during mitosis confirmed that these cultures are likely undergoing some mitotic events.
FIG. 5.
Cells expressing ΔN-TINC undergo faulty mitoses. Cells expressing either GFP-SONB (JD157) (A, B, and C) or GFP-SONB and ΔN-TINC (JD158) (D, E, and F) were germinated at 32°C in minimal medium-ethanol, fixed, and processed for immunofluorescence. Tubulin was visualized with TAT1 mouse monoclonal antibody followed by AlexaFluor 594 IgG. Interphase cells displayed uncondensed DNA, GFP-SONB present around nuclei, and interphase microtubules (A and D). Mitotic cells contained condensed DNA, lacked nuclear-rim GFP-SONB until telophase (C), and contained mitotic spindles (B, C, E, and F). Bar, ∼5 μm.
ΔN-TINC expression produces defects in nuclear envelope fission.
The presence of linking DNA between separated DNA masses in cells which expressed ΔN-TINC suggested that these cells are not undergoing nuclear division properly. During closed mitosis, the production of two independent nuclei is accomplished though a fission of the nuclear envelope. The presence of intervening DNA suggested that cells expressing ΔN-TINC may be defective for nuclear membrane fission.
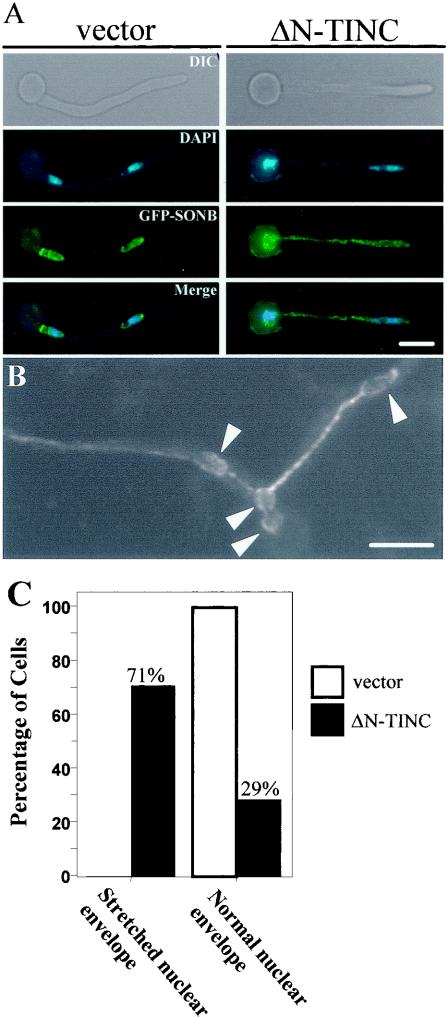
To explore this possibility, the nuclear envelope was studied by examining GFP-SONB localization in control cells or cells which expressed ΔN-TINC. Cells which were shown by DAPI staining to contain two or more DNA masses were identified. In some cases, DNA was visible trailing between the nuclei, but in some cases it was not. GFP-SONB localization in these cells was examined to determine whether apparently separate DNA masses were contained within a single nuclear envelope. Cells in which each nuclear envelope (determined by GFP-SONB localization) contained a single DNA mass were classified as containing normal nuclear envelopes. Cells in which two or more masses of DNA were present within a single nuclear envelope were classified as containing stretched nuclear envelopes.
In control cells, each separate DNA mass was contained within an individual nuclear envelope (Fig. 6A, vector panels, and C, normal nuclear envelope). However, in cells which expressed ΔN-TINC, nuclear membrane fission was defective. Specifically, 71% of cells which were shown by DAPI staining to contain separated DNA masses had failed to undergo nuclear membrane fission (Fig. 6A, ΔN-TINC panels, and C, stretched nuclear envelope). In these cells, two or more DNA masses were contained within a single nuclear envelope. In some cases this linkage between DNA masses was maintained over long distances (Fig. 6B). Figure 6B is an image of GFP-SONB localization in a living cell expressing ΔN-TINC. Bulges within the nuclear membrane presumably correspond to separated DNA masses. In the figure, four DNA masses are present within a single nuclear envelope which extends ∼40 μm.
FIG. 6.
ΔN-TINC expression separates DNA division from nuclear membrane fission. (A) Control cells (JD157) (vector) or ΔN-TINC-expressing cells (JD158) (ΔN-TINC) were germinated in inducing medium, fixed, and stained with DAPI. GFP-SONB allowed visualization of nuclear membranes within the cells. DIC, differential-interference contrast. (B) Image of GFP-SONB in a living cell expressing ΔN-TINC (JD158). Arrows indicate the predicted positions of DNA masses, illustrating that the nuclear envelope linkage between DNA masses can be quite extensive. Total length of the nuclear envelope in this image, ∼40 μm. (C) Quantification of nuclear membrane fission defects in cells expressing ΔN-TINC. GFP-SONB localization was examined in cells (vector and N-TINC) which by DAPI staining appeared to contain separated DNA. Cells containing separated DNA were classed as one of the following based on GFP-SONB localization. First, cells in which each nuclear envelope contained a single DNA mass were said to contain normal nuclear envelopes. Second, cells in which two or more DNA masses were present within a single nuclear envelope were said to contain stretched nuclear envelopes. Bars, ∼5 μm.
ΔN-TINC localization.
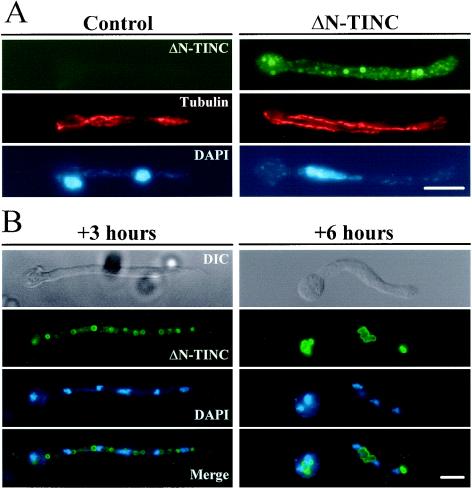
A strain expressing HA-tagged ΔN-TINC (JD155) under the control of the alcA promoter was germinated under inducing conditions in liquid medium. After allowing cells to grow to germling size, they were fixed, and ΔN-TINC localization was examined using an anti-HA antibody. HA epitopes were detected in a punctate pattern throughout the cytoplasm with concentrations at the periphery of circular membranous bodies (Fig. 7A, ΔN-TINC panels), while no anti-HA signal was detected in a control strain containing empty vector (Fig. 7A, control panels).
FIG. 7.
ΔN-TINC localization. (A) Control cells (JD148) or cells expressing HA-tagged ΔN-TINC (JD155) were germinated in inducing medium, fixed, and processed for immunofluorescence. ΔN-TINC was visualized with anti-rat HA antibody 3F10 followed by mouse anti-rat AlexaFluor 488 IgG. Tubulin was visualized as described in the legend to Fig. 5. Cells were DAPI stained to visualize DNA. (B) Cells expressing ΔN-TINC (CB45) were germinated under repressing conditions and then shifted to inducing medium. Following 3 (+3 h) and 6 (+6 h) h of induction, cells were fixed and processed for immunofluorescence. ΔN-TINC was visualized as described for panel A. Cells were DAPI stained to visualize DNA. DIC, differential-interference contrast. Bars, ∼5 μm.
An additional strain expressing higher levels of HA-tagged ΔN-TINC (CB45) was also examined for ΔN-TINC localization. These cells were germinated under repressing conditions and then shifted to inducing medium with samples processed for immunofluorescence at 1, 3, and 6 h postinduction. After 1 h of induction, HA epitopes were observed in a faint speckled pattern throughout the cytoplasm (data not shown). Following 3 h in inducing medium, ΔN-TINC was still visible throughout the cytoplasm, but the staining was dominated by large circular bodies distributed along the length of the cells (Fig. 7B, +3 hours panels). At 6 h of induction, the structures marked by ΔN-TINC localization coalesced into irregular membrane-like aggregates which were associated with nuclei (Fig. 7B, + 6 hours panels). Thus, ΔN-TINC would appear to be predominately a cytoplasmic protein that has the ability to cause accumulation of membrane-like structures, with which it is associated, that eventually concentrate in the vicinity of nuclei.
ΔN-TINC expression destabilizes NIMA in mitotic samples.
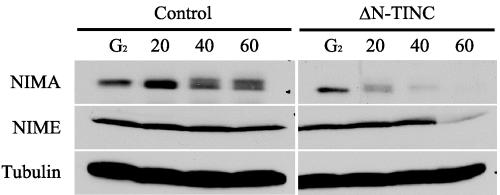
Due to the interaction between NIMA and TINC, and the cell cycle-specific defects produced by ΔN-TINC expression, we examined the level of NIMA in mitotic samples with and without ΔN-TINC expression. Interphase and mitotic samples were generated for control cells (D23C) and ΔN-TINC-expressing cells (JD161). Interphase samples were generated by incubating strains at a nonpermissive temperature for 3 h to inactivate nimT23cdc25 and arrest cells in G2. Mitotic samples were generated by addition of nocodazole followed by release of cells from G2 arrest by decreasing the temperature to a permissive temperature. Time course samples were taken at 20, 40, and 60 min postrelease to allow cells to accumulate in pseudometaphase through the activation of the mitotic spindle checkpoint. NIMA and NIMECyclinB levels were detected during progression into mitosis.
As expected, control G2 samples contained a single form of NIMA (Fig. 8, control G2 lane), and upshifted phosphorylated NIMA forms were obvious at 40 and 60 min after release into nocodazole (Fig. 8, control lanes 40 and 60) as NIMA is activated and cells undergo arrest in mitosis. ΔN-TINC G2 samples were similar to those for control cells with a single form of NIMA present (Fig. 8, ΔN-TINC G2 lane). Interestingly, NIMA disappears from the samples which were released into nocodazole (Fig. 8, ΔN-TINC lanes 20 to 60), presumably through increased protein instability. These results suggest that NIMA is prematurely destabilized during mitosis in strains expressing ΔN-TINC. Based on NIMECyclinB levels, NIMA destabilization precedes a dramatic drop in NIMECyclinB levels and exit from mitosis (Fig. 8, ΔN-TINC lanes 20 to 60). Throughout, the level of tubulin remained constant. These results suggest that cells expressing ΔN-TINC destabilize NIMA prematurely. Interestingly, this premature degradation of NIMA correlates with mitotic exit in these cells.
FIG. 8.
NIMA is destabilized in cells expressing ΔN-TINC. Cells expressing ΔN-TINC (JD161) or control cells (D23C) were germinated under mild inducing conditions at 30°C to early log phase. Cells were then shifted to 42°C for 3 h to inactivate the temperature-sensitive nimT23 allele and accumulate cells in G2 before subsequent release into mitosis. Samples were taken at 20, 40, and 60 min after release from the restrictive temperature in the presence of nocodazole. NIMA was immunoprecipitated from 5 mg of protein sample at each time point and subjected to SDS-PAGE and Western blotting with anti-NIMA (NIMA) antibodies. An additional 200 μg of each lysate was also subjected to SDS-PAGE and Western blotting with anti-NIMECyclinB (NIME) and antitubulin (Tubulin) antibodies.
Attempts to confirm the physical interaction between ΔN-TINC and NIMA in A. nidulans have not yielded definitive data. This is most likely because the ΔN-TINC/NIMA interaction is weak (Fig. 1) and because expression of ΔN-TINC can destabilize NIMA (Fig. 8).
DISCUSSION
We have conducted a two-hybrid screen to identify NIMA-interacting proteins in A. nidulans. Members of our lab have previously published the isolation of TINA, another NIMA-interacting protein isolated in this screen, which plays a role in mitotic microtubule dynamics (31). Here, we demonstrated that the TINC protein interacts with NIMA in A. nidulans and exhibits hallmarks suggestive of a protein possessing a cell cycle-specific role. Additionally, these studies suggest a role for NIMA in mitotic exit and nuclear envelope fission.
TINC is a conserved fungus-specific protein.
TINC is a member of a fungus-specific family of proteins which all share homology with Het-C of N. crassa. Of the fungi examined, all contained at least one protein highly similar to Het-C and a second protein more similar to TINC. Considering the apparent selective pressure to maintain at least two TINC-like or Het-C-like proteins, it was somewhat surprising that loss of both tinC and A. nidulans hetC function failed to produce marked growth defects under the conditions tested. It cannot, however, be ruled out that TINC serves an essential but redundant function in A. nidulans in combination with proteins lacking significant primary sequence homology to TINC or A. nidulans HETC. Additionally, the fact that neither tinC nor A. nidulans hetC appears to be multiallelic in A. nidulans suggests that neither functions in heterokaryon compatibility in the same way that N. crassa het-C does (20, 40, 42, 43). In support of this idea, hch, the Het-C sequence orthologue in P. anserina, has been shown to lack polymorphism, suggesting that it also is not involved in heterokaryon incompatibility (41).
TINC-NIMA interaction.
Coimmunoprecipitation experiments using A. nidulans protein extracts confirmed the NIMA-TINC two-hybrid interaction. Additionally, interaction of TINC with wild-type NIMA was phosphorylation state specific, as endogenous TINC and NIMA were coimmunoprecipitated preferentially in the absence of phosphatase inhibitors. The fact that TINC was able to be coimmunoprecipitated with a kinase-inactivated allele of NIMA (nimA7) at a restrictive temperature, even in the presence of phosphatase inhibitors, suggests that the interaction is stabilized in the presence of inactive NIMA. Furthermore, since protein lysates produced in the absence of phosphatase inhibitors have been shown to exhibit highly decreased NIMA kinase activity (49), the combination of these data suggests that the TINC-NIMA interaction is highly sensitive to NIMA activation. Intriguingly, these data closely parallel those observed for the yeast two-hybrid screen, where TINC interacted weakly with only kinase-inactive forms of NIMA. The nature of the TINC-NIMA interaction suggests that TINC and NIMA may interact most strongly during G2 at a time when NIMA is not fully activated or highly phosphorylated. Also, TINC may represent a NIMA substrate. As such, a transient enzyme substrate interaction between TINC and NIMA may be stabilized in the presence of an inactive kinase, allowing for the detection of this interaction in both the two-hybrid screen and biochemical studies.
ΔN-TINC phenotype.
The specific cell cycle defects displayed by cells which expressed ΔN-TINC suggest a cell cycle regulatory role for TINC, specifically during mitotic exit and nuclear membrane fission. The localization of ΔN-TINC to apparently membranous bodies, which associate with nuclei, suggests that ΔN-TINC may affect membrane dynamics in a manner which interferes with nuclear membrane fission. However, the lack of identifiable phenotypes for either full-length TINC overexpression or loss of TINC function makes it difficult to determine with confidence whether the defects observed with ΔN-TINC expression reflect a dominant negative impact on TINC function or if expression of ΔN-TINC acts as a gain-of-function mutation. Regardless, the ability of ΔN-TINC expression to disrupt the temporal coordination of DNA segregation and nuclear membrane fission strongly suggests that the fission of nuclear envelopes at the end of the closed mitosis of A. nidulans is an actively regulated process. Previous studies in A. nidulans also support the conclusion that nuclear membrane fission is an actively regulated process. Mutations which activate CDC2 by preventing its tyrosine phosphorylation in the presence of hydroxyurea result in large polyploid nuclei, some of which appear highly stretched, similar to those seen in ΔN-TINC-expressing cells (10). Additionally, loss of function of the pot1 homologue of A. nidulans, nimU, results in premature mitotic exit and generation of large stretched polyploid nuclei (35).
As mentioned previously, the lack of discernible phenotypes for tinC deletion or TINC overexpression makes direct comment on TINC function difficult; however, the interaction between TINC and NIMA coupled with the apparent destabilization of NIMA in the presence of truncated forms of TINC does suggest a role for TINC in NIMA stabilization. As a crucial regulator of mitosis, NIMA is itself highly regulated in terms of mRNA levels, protein levels, subcellular localization, and kinase activity (34, 37, 48, 49). The redundancy present in this regulation could explain why loss of tinC function and full-length TINC overexpression fail to produce marked phenotypes. It will therefore be interesting to identify mutations which show synthetic lethality with loss of tinC/A. nidulans hetC function.
The interaction between TINC and NIMA, coupled with effects of ΔN-TINC expression on NIMA levels and accompanying nuclear membrane fission defects, suggests a role for NIMA in regulating nuclear membrane fission during mitotic exit. Previous studies have suggested a role(s) for NIMA at the nuclear envelope. Two components of the nuclear pore complex, SONAGle2/Rae1 (46) and SONBNup98 (9), have been identified as NIMA-interacting proteins. Additionally, incubation of strains containing temperature-sensitive nimA alleles at restrictive temperatures results in a G2 arrest (4, 27). However, inactivation of both nimA5 and the core anaphase-promoting complex component bimE7 by incubation at a restrictive temperature allows cells to bypass the nimA5 mutation and complete some aspects of mitosis in the absence of fully functional NIMA (33). The fact that these strains exhibit striking defects in both spindle architecture and the nuclear envelope suggests that NIMA plays crucial roles at these locations during mitosis (33). Roles at the nuclear envelope and during mitotic exit have been suggested for Fin1p, a NIMA-related kinase from Schizosaccharomyces pombe (22, 23). Specifically, Fin1p activity peaks at the metaphase-anaphase transition, and strains which lack fin1 display nuclear envelope elaborations (23).
The work presented here suggests a role for the NIMA-interacting protein TINC in contributing to NIMA stability during mitosis and further suggests a role for NIMA in regulating the active process of nuclear membrane fission at mitotic exit. Further study of the TINC-NIMA interaction, and the effects of ΔN-TINC expression on NIMA function, should provide insight into the regulation of nuclear envelope fission during closed mitosis. Finally, the closed mitosis of many filamentous fungi is unique in that rounds of nuclear division and nuclear membrane fission occur in the absence of cytokinesis to generate a multinucleate cell. These cells, therefore, cannot functionally link the process of nuclear envelope fission with cytokinesis. This fact suggests that a unique mechanism is required to accomplish nuclear membrane fission and may explain why TINC appears to be a filamentous fungus-specific protein.
Acknowledgments
We thank Fons Debets for providing us with 25 A. nidulans strains representing the range of heterokaryon compatibility groups. We also thank Kerry O'Donnell for performing the sequencing of tinC and A. nidulans hetC within these 25 strains.
This work was supported by a grant from the National Institutes of Health (GM 42564).
REFERENCES
- 1.Anwar, M. M., J. H. Croft, and R. B. Dales. 1993. Analysis of heterokaryon incompatibility between heterokaryon-compatibility (h-c) groups R and GL provides evidence that at least eight het loci control somatic incompatibility in Aspergillus nidulans. J. Gen. Microbiol. 139:1599-1603. [DOI] [PubMed] [Google Scholar]
- 2.Askjaer, P., V. Galy, E. Hannak, and I. W. Mattaj. 2002. Ran GTPase cycle and importins alpha and beta are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol. Biol. Cell 13:4355-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belham, C., J. Roig, J. A. Caldwell, Y. Aoyama, B. E. Kemp, M. Comb, and J. Avruch. 2003. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J. Biol. Chem. 278:34897-34909. [DOI] [PubMed] [Google Scholar]
- 4.Bergen, L. G., A. Upshall, and N. R. Morris. 1984. S-phase, G2, and nuclear division mutants of Aspergillus nidulans. J. Bacteriol. 159:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaveroche, M. K., J. M. Ghigo, and C. d'Enfert. 2000. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 28:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y., D. J. Riley, L. Zheng, P. L. Chen, and W. H. Lee. 2002. Phosphorylation of the mitotic regulator protein hec1 by nek2 kinase is essential for faithful chromosome segregation. J. Biol. Chem. 277:49408-49416. [DOI] [PubMed] [Google Scholar]
- 7.Dales, R. B., J. Moorhouse, and J. H. Croft. 1993. Evidence for a multi-allelic heterokaryon incompatibility (het) locus detected by hybridization among three heterokaryon-compatibility (h-c) groups of Aspergillus nidulans. Heredity 70:537-543. [DOI] [PubMed] [Google Scholar]
- 8.Dales, R. B., and J. H. Croft. 1990. Investigation of the het genes that control heterokaryon incompatibility between members of heterokaryon-compatibility (h-c) groups A and G1 of Aspergillus nidulans. J. Gen. Microbiol. 136:1717-1724. [DOI] [PubMed] [Google Scholar]
- 9.De Souza, C. P., K. P. Horn, K. Masker, and S. A. Osmani. 2003. The SONB(NUP98) nucleoporin interacts with the NIMA kinase in Aspergillus nidulans. Genetics 165:1071-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Souza, C. P. C., X. S. Ye, and S. A. Osmani. 1999. Checkpoint defects leading to premature mitosis also cause endoreplication of DNA in Aspergillus nidulans. Mol. Biol. Cell 10:3661-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.De Souza, C. P., A. H. Osmani, S. Hashmi, and S. A. Osmani. Curr. Biol., in press.
- 11.Doonan, J. H., C. MacKintosh, S. Osmani, P. Cohen, G. Bai, E. Y. C. Lee, and N. R. Morris. 1991. A cDNA encoding rabbit muscle protein phosphatase 1α complements the Aspergillus cell cycle mutation, bimG11. J. Biol. Chem. 266:18889-18894. [PubMed] [Google Scholar]
- 12.Doree, M., and T. Hunt. 2002. From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J. Cell Sci. 115:2461-2464. [DOI] [PubMed] [Google Scholar]
- 13.Faragher, A. J., and A. M. Fry. 2003. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol. Biol. Cell 14:2876-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiddy, C., and A. P. Trinci. 1976. Mitosis, septation, branching and the duplication cycle in Aspergillus nidulans. J. Gen. Microbiol. 97:169-184. [DOI] [PubMed] [Google Scholar]
- 15.Grallert, A., and I. M. Hagan. 2002. Schizosaccharomyces pombe NIMA-related kinase, Fin1, regulates spindle formation and an affinity of Polo for the SPB. EMBO J. 21:3096-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grallert, A., A. Krapp, S. Bagley, V. Simanis, and I. M. Hagan. 2004. Recruitment of NIMA kinase shows that maturation of the S. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity. Genes Dev. 18:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hachet, V., T. Kocher, M. Wilm, and I. W. Mattaj. 2004. Importin alpha associates with membranes and participates in nuclear envelope assembly in vitro. EMBO J. 23:1526-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, S. D., L. Hamer, K. E. Sharpless, and J. E. Hamer. 1997. The Aspergillus nidulans sepA gene encodes an FH1/2 protein involved in cytokinesis and the maintenance of cellular polarity. EMBO J. 16:3474-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, S. D., J. L. Morrell, and J. E. Hamer. 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136:517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson, D. J., K. Beurkens, and K. L. Klomparens. 1998. Microscopic and ultrastructural examination of vegetative incompatibility in partial diploids heterozygous at het loci in Neurospora crassa. Fungal Genet. Biol. 23:45-56. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto, T., and E. Okumura. 1997. In vivo regulation of the entry into M-phase: initial activation and nuclear translocation of cyclin B/Cdc2. Prog. Cell Cycle Res. 3:241-249. [DOI] [PubMed] [Google Scholar]
- 22.Krien, M. J., S. J. Bugg, M. Palatsides, G. Asouline, M. Morimyo, and M. J. O'Connell. 1998. A NIMA homologue promotes chromatin condensation in fission yeast. J. Cell Sci. 111:967-976. [DOI] [PubMed] [Google Scholar]
- 23.Krien, M. J., R. R. West, U. P. John, K. Koniaras, J. R. McIntosh, and M. J. O'Connell. 2002. The fission yeast NIMA kinase Fin1p is required for spindle function and nuclear envelope integrity. EMBO J. 21:1713-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippincott, J., and R. Li. 2000. Nuclear envelope fission is linked to cytokinesis in budding yeast. Exp. Cell Res. 260:277-283. [DOI] [PubMed] [Google Scholar]
- 25.Lu, K. P., and T. Hunter. 1995. Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell 81:413-424. [DOI] [PubMed] [Google Scholar]
- 26.Lu, K. P., B. E. Kemp, and A. R. Means. 1994. Identification of substrate specificity determinants for the cell cycle-regulated NIMA protein kinase. J. Biol. Chem. 269:6603-6607. [PubMed] [Google Scholar]
- 27.Morris, N. R. 1976. Mitotic mutants of Aspergillus nidulans. Genet. Res. (Cambridge) 26:237-254. [DOI] [PubMed] [Google Scholar]
- 28.Oakley, B. R., and S. A. Osmani. 1993. Cell-cycle analysis using the filamentous fungus Aspergillus nidulans, p. 127-142. In P. Fantes and R. Brooks (ed.), The cell cycle, a practical approach. Oxford University Press, New York, N.Y.
- 29.O'Connell, M. J., M. J. Krien, and T. Hunter. 2003. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 13:221-228. [DOI] [PubMed] [Google Scholar]
- 30.Ohi, R., and K. L. Gould. 1999. Regulating the onset of mitosis. Curr. Opin. Cell Biol. 11:267-273. [DOI] [PubMed] [Google Scholar]
- 31.Osmani, A. H., J. Davies, C. E. Oakley, B. R. Oakley, and S. A. Osmani. 2003. TINA interacts with the NIMA kinase in Aspergillus nidulans and negatively regulates astral microtubules during metaphase arrest. Mol. Biol. Cell 14:3169-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osmani, A. H., S. L. McGuire, and S. A. Osmani. 1991. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell 67:283-291. [DOI] [PubMed] [Google Scholar]
- 33.Osmani, A. H., K. O'Donnell, R. T. Pu, and S. A. Osmani. 1991. Activation of the nimA protein kinase plays a unique role during mitosis that cannot be bypassed by absence of the bimE checkpoint. EMBO J. 10:2669-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osmani, S. A., G. S. May, and N. R. Morris. 1987. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J. Cell Biol. 104:1495-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt, C. W., E. Moreau, P. A. Lunness, and J. H. Doonan. 2004. The pot1+ homologue in Aspergillus nidulans is required for ordering mitotic events. J. Cell Sci. 117:199-209. [DOI] [PubMed] [Google Scholar]
- 36.Pontecorvo, G. 1953. The genetics of Aspergillus nidulans, p. 141-238. In M. Demerec (ed.), Advances in genetics, vol. 5. Academic Press, New York, N.Y. [DOI] [PubMed]
- 37.Pu, R. T., and S. A. Osmani. 1995. Mitotic destruction of the cell cycle regulated NIMA protein kinase of Aspergillus nidulans is required for mitotic exit. EMBO J. 14:995-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinow, C. F., and C. E. Caten. 1969. Mitosis in Aspergillus nidulans. J. Cell Sci. 5:403-431. [DOI] [PubMed] [Google Scholar]
- 39.Roig, J., A. Mikhailov, C. Belham, and J. Avruch. 2002. Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression. Genes Dev. 16:1640-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar, S., G. Iyer, J. Wu, and N. L. Glass. 2002. Nonself recognition is mediated by HET-C heterocomplex formation during vegetative incompatibility. EMBO J. 21:4841-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saupe, S. J., C. Clave, M. Sabourin, and J. Begueret. 2000. Characterization of hch, the Podospora anserina homolog of the het-c heterokaryon incompatibility gene of Neurospora crassa. Curr. Genet. 38:39-47. [DOI] [PubMed] [Google Scholar]
- 42.Saupe, S. J., and N. L. Glass. 1997. Allelic specificity at the het-c heterokaryon incompatibility locus of Neurospora crassa is determined by a highly variable domain. Genetics 146:1299-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saupe, S. J., G. A. Kuldau, M. L. Smith, and N. L. Glass. 1996. The product of the het-C heterokaryon incompatibility gene of Neurospora crassa has characteristics of a glycine-rich cell wall protein. Genetics 143:1589-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolkow, T. D., S. D. Harris, and J. E. Hamer. 1996. Cytokinesis in Aspergillus nidulans is controlled by cell size, nuclear positioning and mitosis. J. Cell Sci. 109:2179-2188. [DOI] [PubMed] [Google Scholar]
- 45.Wozniak, R., and P. R. Clarke. 2003. Nuclear pores: sowing the seeds of assembly on the chromatin landscape. Curr. Biol. 13:R970-R972. [DOI] [PubMed] [Google Scholar]
- 46.Wu, L., S. A. Osmani, and P. M. Mirabito. 1998. A role for NIMA in the nuclear localization of cyclin B in Aspergillus nidulans. J. Cell Biol. 141:1575-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye, X. S., R. R. Fincher, A. Tang, K. O'Donnell, and S. A. Osmani. 1996. Two S-phase checkpoint systems, one involving the function of both BIME and Tyr15 phosphorylation of p34cdc2, inhibit NIMA and prevent premature mitosis. EMBO J. 15:3599-3610. [PMC free article] [PubMed] [Google Scholar]
- 48.Ye, X. S., R. R. Fincher, A. Tang, A. H. Osmani, and S. A. Osmani. 1998. Regulation of the anaphase-promoting complex/cyclosome by BIMA (APC3) and proteolysis of NIMA. Mol. Biol. Cell 9:3019-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye, X. S., G. Xu, P. T. Pu, R. R. Fincher, S. L. McGuire, A. H. Osmani, and S. A. Osmani. 1995. The NIMA protein kinase is hyperphosphorylated and activated downstream of p34cdc2/cyclin B: coordination of two mitosis promoting kinases. EMBO J. 14:986-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin, M. J., L. Shao, D. Voehringer, T. Smeal, and B. Jallal. 2003. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J. Biol. Chem. 278:52454-52460. [DOI] [PubMed] [Google Scholar]
- 51.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]