Abstract
Approximately 350 million people worldwide are infected with hepatitis C virus (HCV), which is associated with morbidity and mortality related to cirrhosis, hepatocellular carcinoma, or liver failure. Recently, vast improvements have been made with the development of direct-acting antiviral (DAA) agents, which are all-oral, are better tolerated than interferon-based treatment, and provide a sustained virologic response in more than 90% of treated patients. This article reviews the new therapies available for HCV infection, with a focus on patients who have chronic HCV with and without compensated cirrhosis. As DAA development continues, more attention will need to be given to special patient populations, specifically to patients who fail treatment due to emerging resistant strains. Considerable challenges yet to be overcome are incremental diagnosis of unidentified patients and linkage to care that is affordable and available to all patients.
Keywords: Hepatitis C virus, cirrhosis, direct-acting antiviral, agents, sustained virologic response
The introduction of the first-generation protease inhibitors telaprevir (Incivek, Vertex Pharmaceuticals) and boceprevir (Victrelis, Merck) in 2011 transformed the treatment of hepatitis C virus (HCV) infection.1 With the approval of sofosbuvir/velpatasvir (Epclusa, Gilead) in June 2016, clinicians now have treatment options that achieve a high rate of sustained virologic response (SVR) in patients with any HCV genotype and that consist of an all-oral regimen that, for most patients, has a short duration (12 weeks) and a low pill burden. Additional regimens are currently under development, but much work remains to further lower the barriers to effective, large-scale treatment.
Hepatitis C Virus Epidemiology
The World Health Organization estimates that there are 130 to 150 million cases of HCV infection worldwide, totaling 3% of the world’s population, with an average of 3 to 4 million new infections occurring each year.2 HCV infection causes substantial morbidity, ranging from cirrhosis (and its related complications) to hepatocellular carcinoma (HCC), liver failure, and death. HCV infection and HCC were the leading indications for liver transplantation in the United States in 2014; however, the spectrum for transplantation is expected to shift as more patients with HCV infection are identified and successfully treated.3 According to estimates in 2013, 3.2 million Americans have chronic HCV infection, yet only 50% of patients infected with HCV know of their viral status; of those, 7% to 11% are treated, and 5% to 6% have successful clearance of the virus.4 Furthermore, it is predicted that the burden of cirrhosis due to HCV infection could reach 37.2% by 2020 in infected patients.5
In order to identify patients infected with HCV, recommendations have been made for routine testing in people born between 1945 and 1965; in people who have injected illegal drugs, received blood transfusion or organ transplantation prior to July 1992, or received clotting factor concentrates before 1987; in patients on long-term dialysis; in children born to HCV-positive mothers; in health care workers who have been exposed to HCV infection; and in patients with evidence of chronic liver disease.6
The overriding principles of treatment guidelines are that HCV infection is curable and all HCV-infected patients should receive treatment.7 Patients with cirrhosis, patients who are still HCV-positive after transplantation, patients with HIV coinfection, and patients with extrahepatic manifestations of HCV infection derive the most immediate benefit. Additionally, consideration should be given to patients who have a high risk of transmitting the disease to the community, including active intravenous drug users.8 Only patients with life-threatening illnesses who are not expected to survive beyond 1 year are not recommended for treatment.
Direct-Acting Antiviral Agents
The current understanding of the HCV viral life cycle has led to the development of pharmacologic direct targets to inhibit the replication cycle. These pharmaceutical agents, the direct-acting antiviral (DAA) agents, currently work against specific targets of the HCV replication cycle to achieve 2 goals: block virus replication and induce progressive clearance of the virus through cellular death of infected cells. Progressive clearance of infected cells is achieved by avoidance of resistant viruses being selected and induced.9
Existing strategies to address the avoidance of resistance include combining agents that work on different viral targets to decrease the likelihood of selecting resistant strains, increasing the duration of treatment in challenging settings, and adding ribavirin to the treatment regimen.9
Classes of Drugs
Nonstructural 3/4A Protease Inhibitors
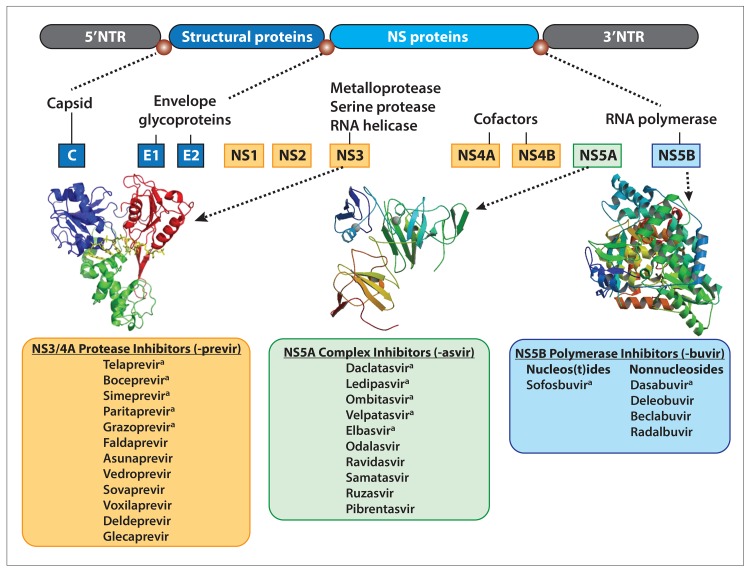
The RNA genome of HCV is translated into proteins intracellularly in order to carry out viral function. Following RNA translation, unprocessed polyproteins are cleaved to create functional, individual proteins. A large bulk of this process is carried out by a nonstructural (NS) 3/4A serine protease.10-13 Protease inhibitors block this NS3/4A serine protease, inhibiting functional viral proteins from being created. Boceprevir and telaprevir were the first DAA agents approved in 2011 and revolutionized the way HCV infection could be treated. The initial protease inhibitors were used in combination with pegylated interferon-α and ribavirin, were only effective against HCV genotype 1 infection, and had a low barrier to resistance. Due to these factors as well as unfavorable side-effect profiles, boceprevir and telaprevir fell out of favor with the introduction of newer agents. Following boceprevir and telaprevir, simeprevir (Olysio, Janssen) became available in the United States at the end of 2013. Simeprevir has broader viral genotype coverage, but still has a low barrier to resistance, particularly in patients infected with HCV genotype 1a harboring the protease polymorphism Q80K, leading to treatment relapse.14 Simeprevir was widely used in combination with sofosbuvir (Sovaldi, Gilead) as the first viable, interferon-free regimen against HCV genotypes 1 and 4 infections. This combination, initially used off-label, was eventually approved by the US Food and Drug Administration (FDA) in 2014. Additional NS3/4A protease inhibitors include asunaprevir, paritaprevir (boosted with ritonavir), vedroprevir, grazoprevir, faldaprevir, sovaprevir, voxilaprevir, deldeprevir, and glecaprevir (Figure). As a class, these agents have high potency but limited genotype coverage and a low barrier to resistance.15,16
Figure.
The RNA genome of hepatitis C virus (HCV) is translated into proteins intracellularly to carry out viral function. A NS3/4A serine protease cleaves unprocessed polyproteins to create functional, individual proteins, a process blocked by protease inhibitors. The NS5A protein helps with HCV RNA replication regulation and viral assembly and packaging, and directly interacts with the RNA-dependent RNA polymerase (RdRp). NS5A inhibitors prevent hyperphosphorylation of the NS5A protein and alter the protein’s location from the endoplasmic reticulum. NS5B polymerase inhibitors have a high barrier to resistance and work broadly against genotypes with intermediate potency. Nucleos(t)ide inhibitors arrest RNA synthesis, while nonnucleoside inhibitors bind and disrupt the RdRp function.
3’NTR, 3’ nontranslated region; 5’NTR, 5’ nontranslated region; NS, nonstructural.
a Used in clinical practice in the United States.
Nonstructural 5A Complex Inhibitors
The NS5A complex plays a role in HCV RNA replication regulation as well as viral assembly and packaging, and directly interacts with the RNA-dependent RNA polymerase (RdRp). The exact antiviral action of NS5A inhibitors is unknown; they are theorized to inhibit hyperphosphorylation of the NS5A protein and alter the protein’s location from the endoplasmic reticulum, likely causing faulty HCV assembly.13 Ledipasvir, ombitasvir, daclatasvir (Daklinza, Bristol-Myers Squibb), elbasvir, velpatasvir, odalasvir, samatasvir, ravidasvir, ruzasvir, and pibrentasvir currently make up the class of NS5A inhibitors (Figure). Ledipasvir is one of the most potent inhibitors of the NS5A complex, but may have lower activity in HCV genotypes 2 and 3 infections.13,16,17 Ombitasvir is approved in combination with paritaprevir, ritonavir, and dasabuvir as part of the 3D regimen (Viekira Pak, AbbVie) for the treatment of HCV genotypes 1 and 4 infections, but also has a higher pill burden, which could affect compliance.18 Velpatasvir has antiviral activity against HCV replicons in genotypes 1 through 6.19 NS5A complex inhibitors have high potency, multigenotypic coverage, and generally a low barrier to resistance.13 Newer agents in this class have the promise to increase the resistance threshold.
Nonstructural 5B Polymerase Inhibitors
The RdRp is vital to HCV replication, acting to catalyze RNA synthesis and genome replication. Nucleos(t)ide inhibitors arrest RNA synthesis, while nonnucleoside inhibitors bind and disrupt the RdRp function.12
The nucleos(t)ide inhibitors are analogues that are incorporated into the viral RNA genome by the RdRp, causing termination of further replication, and competitively bind the active polymerase site. Single mutations can lead to resistance; however, there is some evidence that mutations also seem to decrease viral fitness.12 This class of NS5B polymerase inhibitors has a high barrier to resistance and works broadly against genotypes with intermediate potency.20 Sofosbuvir was the first available NS5B nucleos(t)ide inhibitor (in 2014).16
Nonnucleoside inhibitors inhibit the RdRp by binding an allosteric site in a noncompetitive fashion, which changes the biochemical activity of the polymerase. They have a low barrier of resistance. Beclabuvir, an indole derivative, binds the thumb I subdomain on the RdRp with potent activity but has reduced activity against HCV genotypes 2 and 6 infections. Dasabuvir, a benzothiadiazine derivative, binds the palm I site on the RdRp, causing changes to the active site and preventing transcription.12,21 Deleobuvir and radalbuvir are additional nonnucleoside inhibitors (Figure).
Current Treatment Regimens
The treatment regimens discussed in this article are those currently available in the United States. The scope of this review is the management of patients infected with HCV genotypes 1 through 6, including both treatment-naive and -experienced patients. Recommendations for cirrhotic patients are made with well-compensated cirrhosis in mind (normal bilirubin and albumin levels and no evidence of hepatic encephalopathy or portal hypertension complications). The Table provides a guide for HCV infection management in both initial treatment and treatment-experienced failures.
Special groups, such as HIV/HCV-coinfected patients, patients with HCV after liver transplantation, patients with decompensated cirrhosis, and patients with end-stage renal disease, deserve the attention of specialized treatment centers and are discussed elsewhere.7,22,23
Genotype 1
Treatment-Naive, Noncirrhotic Patients
At least 6 treatment regimens are available in the United States for the treatment-naive, noncirrhotic patient infected with HCV genotype 1. All of these regimens can potentially lead to a SVR rate greater than 95%. When administered for 12 weeks, sofosbuvir/ledipasvir (Harvoni, Gilead) has an expected SVR rate of 98% to 99%.24 A post hoc analysis of the registration trial data led the FDA to approve this regimen for 8 weeks in patients who had viral titers less than or equal to 6 million IU/mL.25 Further analysis of the data from the registration trials raises caution in the use of this duration in patients who are HIV-coinfected, are African American, or harbor unfavorable interleukin-28B genotypes.26 Data outside of registration clinical trials have demonstrated that 4365 HCV genotype 1–infected, treatment-naive patients had a cure rate greater than 90%, confirming the expectations of robust response in this patient population.27 A second regimen, recently approved in the United States, is sofosbuvir/velpatasvir, which, when administered in this patient population for 12 weeks, can be expected to achieve a SVR rate of 98% to 99%.19 Sofosbuvir plus daclatasvir administered for 12 weeks can be expected to yield a SVR rate as high as 98%.28
The triple combination of paritaprevir/ritonavir/ombitasvir and dasabuvir combined with ribavirin yields a SVR rate between 97% and 99%. In HCV genotype 1b infection, ribavirin is not necessary.29 The elbasvir/grazoprevir (Zepatier, Merck) combination for 12 weeks can be expected to lead to high SVR rates, but treatment is adjusted in patients with HCV genotype 1a infection with persistence-associated variants or for patients on chronic dialysis.30,31 Patients who test positive for NS5A resistance-associated variants (RAVs) for elbasvir should be reviewed, and, in this particular population, extending treatment to 16 weeks with the addition of weight-based ribavirin has excellent response rates, with initial data reporting a SVR rate of up to 99%.32-34 The older sofosbuvir/simeprevir regimen can also be successfully used; however, caution is required if patients have HCV genotype 1a infection with expression of the Q80K polymorphism in the viral protease, and SVR rates are reduced compared to alternative regimens.35-38
Treatment-Naive, Cirrhotic Patients
In cirrhotic patients, the newly approved sofosbuvir/velpatasvir regimen is highly effective with SVR rates of 98% to 99% after 12 weeks of treatment.19,39 Sofosbuvir/ledipasvir for 12 weeks (coadministered with ribavirin in patients with decompensated cirrhosis) and the elbasvir/grazoprevir regimen should be used with caution in patients with preexistent RAVs.24,30,40
While approved for use in compensated cirrhotic patients, paritaprevir/ritonavir/ombitasvir and dasabuvir should be used with caution if there is any sign of decompensation, as the FDA has issued a warning of hepatic decompensation and liver failure in rare cases with this regimen.41 In patients with HCV genotype 1a infection, the duration of this regimen is increased from 12 weeks to 24 weeks.42
Treatment-Experienced Patients
Prior treatment in patients with HCV genotype 1 infection should be carefully assessed. The complexity of management in this setting will increase as patients who have failed DAA therapies begin to surface.
Pegylated Interferon and Ribavirin Failures in Noncirrhotic Patients Sofosbuvir-containing regimens include sofosbuvir/ledipasvir for 12 weeks, sofosbuvir/velpatasvir for 12 weeks, and sofosbuvir/daclatasvir for 12 weeks, with SVR expectations for these regimens ranging from 96% to 99%.19,43-45
Paritaprevir/ritonavir/ombitasvir and dasabuvir with weight-based ribavirin for 12 weeks is an alternative choice in patients with HCV genotype 1a infection, with an expected SVR rate of 96%. Ribavirin is not needed for HCV genotype 1b infection, and clinical trials of paritaprevir/ritonavir/ombitasvir and dasabuvir have demonstrated 100% response rates.46,47 While elbasvir/grazoprevir can be used in this patient population, patients infected with HCV genotype 1a require screening for RAVs.48
Pegylated Interferon and Ribavirin Failures in Cirrhotic Patients In treatment-experienced patients with cirrhosis, sofosbuvir/velpatasvir for 12 weeks not only yields a 99% SVR rate, but also does not require ribavirin.19 Sofosbuvir/ledipasvir can be used for 12 weeks in combination with ribavirin; alternatively, in patients who do not tolerate ribavirin, a 24-week treatment duration is a possibility.43 Elbasvir/grazoprevir yields a 96% SVR rate when used for 16 weeks.48
Sofosbuvir-Experienced Patients Patients who have failed a sofosbuvir-containing regimen should be considered for treatment with sofosbuvir/ledipasvir and ribavirin for 12 weeks; in cirrhotic patients, the duration should be extended to 24 weeks.43
Protease Inhibitor–Experienced, Noncirrhotic Patients If patients have previously failed telaprevir-, boceprevir-, or simeprevir-based regimens, they can be switched to sofosbuvir/velpatasvir,19 sofosbuvir/ledipasvir,43 or sofosbuvir/daclatasvir28 for 12 weeks, as all therapy combinations yield high SVR rates. Elbasvir/grazoprevir for 12 weeks is also adequate if there are no preexisting RAVs in patients infected with HCV genotype 1a.49
Protease Inhibitor–Experienced, Cirrhotic Patients In this patient subtype, sofosbuvir/velpatasvir for 12 weeks or sofosbuvir/ledipasvir and ribavirin for 12 weeks are alternative treatment options.19,50 Elbasvir/grazoprevir for 12 weeks is an option in patients without NS5A RAVs.49
Complex Treatment Failures Although possibly a numerical minority, failures to sofosbuvir/simeprevir combinations and any regimens containing NS5A agents constitute a difficult-to-treat population. The approach to treating complex treatment failures is nascent and will need to be addressed in future clinical trials with newergeneration agents that have higher barriers to resistance. Referral to clinical trials or expert treatment centers is recommended. If treatment is to be entertained, analysis for resistant-variant populations is necessary to avoid futile therapy.
Table.
Guide for HCV Infection Management in Both Initial Treatment and Treatment-Experienced Failures
| Genotype | Available Regimens |
|---|---|
| 1 | Sofosbuvir/velpatasvir: 12 weeks treatment |
| |
| Elbasvir/grazoprevir: 12 weeks treatment | |
| |
| Sofosbuvir/ledipasvir: 12 weeks treatment | |
| |
| Paritaprevir/ritonavir/ombitasvir+dasabuvir: 12 weeks treatment | |
| |
| Sofosbuvir/simeprevir: 12 weeks treatment | |
| |
| Sofosbuvir/daclatasvir: 12 weeks treatment | |
| |
| 2 | Sofosbuvir/velpatasvir: 12 weeks treatment |
| |
| Sofosbuvir/daclatasvir: 12 weeks treatment | |
| |
| 3 | Sofosbuvir/velpatasvir: 12 weeks treatment |
| |
| Sofosbuvir/elbasvir/grazoprevir: 12 weeks treatment | |
| |
| Sofosbuvir/daclatasvir: 12 weeks treatment | |
| |
| 4 | Sofosbuvir/velpatasvir: 12 weeks treatment |
| |
| Sofosbuvir/ledipasvir: 12 weeks treatment | |
| Elbasvir/grazoprevir: 12 weeks treatment | |
| |
| Paritaprevir/ritonavir/ombitasvir+ribavirin: 12 weeks treatment | |
| |
| 5 | Sofosbuvir/velpatasvir: 12 weeks treatment |
| Sofosbuvir/ledipasvir: 12 weeks treatment | |
| |
| 6 | Sofosbuvir/velpatasvir: 12 weeks treatment |
| Sofosbuvir/ledipasvir: 12 weeks treatment | |
|
FDA, US Food and Drug Administration; HCV, hepatitis C virus; NS, nonstructural; Q80K, hepatitis C virus polymorphism; RAVs, resistance-associated variants.
If patient is ribavirin-intolerant, extend duration of treatment to 24 weeks.
Cirrhosis refers to compensated cirrhosis unless stated as decompensated cirrhosis.
Developed by Dr Vargas and Dr Horsley-Silva, copyright of Mayo Foundation for Medical Education and Research, 2016.
Genotype 2
Treatment-Naive, Noncirrhotic Patients
Sofosbuvir/velpatasvir for 12 weeks yields SVR rates greater than 99% in treatment-naive, noncirrhotic patients infected with HCV genotype 2.51 Sofosbuvir/daclatasvir for 12 weeks is an alternative option, as prior studies have found high rates of SVR in treatment-naive patients with both 12- and 24-week regimens.28,52
Treatment-Naive, Cirrhotic Patients
In cirrhotic patients, 12 weeks of sofosbuvir/velpatasvir is an optimal treatment approach. Sofosbuvir/daclatasvir requires a longer duration of treatment (at least 16 weeks). The use of ribavirin is advisable in this setting, as additional data have demonstrated lower SVR rates in patients with advanced Child-Turcotte-Pugh class C or albumin levels less than 2.8 g/dL.19,51,53
Treatment-Experienced Patients
Pegylated Interferon and Ribavirin Failures in Noncirrhotic Patients The use of sofosbuvir/velpatasvir for 12 weeks is recommended in this patient population and yields a 99% SVR rate.51 The sofosbuvir/daclatasvir combination therapy is also likely to be successful.28,52
Pegylated Interferon and Ribavirin Failures in Cirrhotic Patients Sofosbuvir/velpatasvir for a total of 12 weeks is recommended in cirrhotic patients who have previously failed pegylated interferon and ribavirin. This combination therapy yields a SVR rate of up to 99%.51 Alternatively, patients could take sofosbuvir/daclatasvir from 16 to 24 weeks, with additional ribavirin recommended.52
Genotype 3
HCV genotype 3 infection remains the most challenging of all of the commonly encountered genotypes in the United States. The treating clinician should pay particular attention to patients who have cirrhosis and those who have previously received therapy.
Treatment-Naive, Noncirrhotic Patients
In noncirrhotic patients, the sofosbuvir/velpatasvir combination for 12 weeks will render a 98% SVR rate.51 Sofosbuvir/daclatasvir, another FDA-approved regimen, can achieve SVR in 97% of patients.54 The regimen of sofosbuvir/elbasvir/grazoprevir for 12 weeks is likely to be highly effective in noncirrhotic, treatment-naive patients.55
Treatment-Naive, Cirrhotic Patients
The sofosbuvir/velpatasvir combination is an effective regimen in the subgroup of treatment-naive, cirrhotic, genotype 3–infected patients.51 The sofosbuvir/daclatasvir combination has a much lower SVR rate, shown to be 82% in studies, and requires 24 weeks of treatment with the addition of ribavirin.54,56
Treatment-Experienced Patients (Including Sofosbuvir- and Protease Inhibitor–Experienced)
Treatment-Experienced, Noncirrhotic Patients Sofosbuvir/daclatasvir for 12 weeks can be expected to yield a 94% SVR rate in treatment-experienced, noncirrhotic patients.54 Sofosbuvir/velpatasvir is expected to lead to a SVR in 91% of patients.51 Sofosbuvir/elbasvir/grazoprevir with ribavirin for 12 weeks has been shown to have a 95% SVR rate in this population.57
Treatment-Experienced, Cirrhotic Patients This patient population has the lowest SVR rate in the DAA era. Treatment-experienced, cirrhotic patients present a double challenge of treating in the setting of advanced fibrosis as well as potentially harboring resistant variants. Together, these challenges decrease the chances of SVR to below 90%. Sofosbuvir/velpatasvir accompanied by ribavirin for 12 weeks is an effective regimen that approximates a 90% SVR rate.51,58 If sofosbuvir/daclatasvir is the chosen treatment regimen, ribavirin is strongly recommended, and the duration should be 24 weeks, as the SVR rate is expected to be close to 90%.54
Genotype 4
Treatment-Naive, Noncirrhotic and Cirrhotic Patients
There are 4 effective treatment regimens for cirrhotic and noncirrhotic patients who are treatment-naive. Each regimen has very high efficacy and safety. The combination of ombitasvir/paritaprevir/ritonavir (Technivie, AbbVie) with ribavirin can lead to a SVR in 97% to 100% of patients.59 The number of patients treated in registration trials is limited; thus, forthcoming real-world experience will help improve the predictions of SVR. Sofosbuvir/velpatasvir can be used with expectations of a nearly 100% SVR rate, whereas sofosbuvir/ledipasvir for 12 weeks can lead to a SVR in 95% of patients.19,60,61 The elbasvir/grazoprevir regimen must be used for 12 weeks and also leads to a very high SVR rate (97%).62
Treatment-Experienced Patients
Treatment-Experienced, Noncirrhotic Patients The treatment regimens available to treatment-naive patients (described above) are also available to treatment-experienced, noncirrhotic patients. If choosing elbasvir/grazoprevir, care should be given to documenting the patient’s prior response to treatment. Patients who failed a pegylated interferon and ribavirin regimen should be treated for 16 weeks instead of 12 weeks to ensure a SVR.62
Treatment-Experienced, Cirrhotic Patients Sofosbuvir/velpatasvir and sofosbuvir/ledipasvir for 12 weeks are likely to lead to rates of 100% SVR and 95% SVR, respectively.19,60
Genotypes 5 and 6
HCV genotypes 5 and 6 infections are exceedingly rare in North America. Data backing the currently available recommendations for treatment are limited. Future updates through trials and real-world experience will provide more solid recommendations in these genotypes.
Treatment-Naive, Noncirrhotic and Cirrhotic Patients
Currently available treatment combinations include sofosbuvir/velpatasvir for 12 weeks (yielding a 99% SVR rate) and sofosbuvir/ledipasvir for 12 weeks (reaching a 95% SVR rate).19,63
Elbasvir/grazoprevir for 12 weeks has been shown in a limited number of patients to lead to a SVR rate of 80% in patients infected with HCV genotype 6.30 In patients with genotype 5 infection, elbasvir/grazoprevir with ribavirin for 12 weeks can be recommended, with SVR expectations close to 100%.64 In this difficult-to-treat population, the regimen of sofosbuvir/elbasvir/grazoprevir and ribavirin for 12 weeks is likely to lead to a greater than 95% response rate based on a recent small study.57
Drug-Drug Interactions
The effectiveness of DAA agents notwithstanding, physicians should be aware of drugs that negatively impact HCV treatment or put patients at risk. Protease inhibitors are metabolized through the cytochrome P450 3A4 (CYP3A4) isoenzyme and can lead to disruption of therapeutic regimens that may be in place when patients are receiving HCV treatment. Interactions can also occur with drug transporter proteins (eg, P-glycoprotein, breast cancer resistance protein), leading to increased elimination or altered absorption of medications.16,65,66 Many benzodiazepines, antidepressant medications, and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors can interact with DAA agents.
Every DAA regimen has the potential for drug interactions with HIV antiretroviral combinations, and most can be continued with careful monitoring or dosing modifications. However, there are a few contraindicated combinations. Consultation with an HIV treatment center is paramount if patients with HCV/HIV coinfection are treated in the primary care setting.
Immunosuppressive agents also are metabolized through some of the same pathways as DAA agents and can alter their drug levels. Simeprevir is contraindicated with cyclosporine and should be monitored closely when administered with tacrolimus.66 Mammalian target of rapamycin inhibitors, including sirolimus and everolimus, should be monitored carefully with DAA agents. Because the 3D regimen contains ritonavir, which inhibits the CYP3A4 isoenzyme, this regimen should be monitored closely with cyclosporine and tacrolimus. Treatment of HCV infection in posttransplant patients should be carried out in coordination with the transplant center to ensure the safety of this patient population.
Specific drug regimen cautions exist that should be understood by the treating clinician. Sofosbuvir-containing regimens have been noted to induce severe and life-threatening bradyarrhythmias in patients with concurrent amiodarone administration. This cannot be overlooked, particularly in view of the long half-life of amiodarone.67,68 Paritaprevir/ritonavir/ombitasvir and dasabuvir, while studied and approved for use in compensated cirrhotic patients, has had a warning issued postapproval by the FDA alerting clinicians to the possibility of decompensation. Although the mechanics are difficult to explain, protease inhibitors have been reported to cause hepatic decompensation and as a class should be used with caution and without hesitation to discontinue if signs of decompensation are present.41
Another easily avoided dosing issue is the decrease of ledipasvir absorption when used concurrently with strong acid inhibitors such as proton pump inhibitors. Guidelines exist for dosing acid-lowering agents in this setting, although avoidance of these agents is strongly encouraged when using the sofosbuvir/ledipasvir coformulated regimen.16
As DAA prescriptions increase, physicians should be aware of the potential complications created by drug-drug interactions. An authoritative, easy-to-use online reference provides the latest updates and interactions.69 The Department of Health and Human Services also provides treatment guidelines, including drug interactions of DAA agents and antiretroviral agents.70 Patients should avoid herbal and dietary supplements while taking DAA agents to avoid any unforeseen interactions.66
Challenges
Despite great advances in treatment options for HCV infection, patients may still not have access to effective treatments. Highly efficacious treatments are important to the success of managing HCV infection, but if more than half of affected individuals remain undiagnosed, the improvement of the prevalence of this disease at the national level will remain marginal. Recognition of HCV infection and linkage to care should be a high health care delivery imperative. Additionally, efforts to deliver these medications at a reasonable cost to patients and health care systems are limited and form the foundation of the linkage to care.
Clinicians should make the diagnosis, direct prescriptions to correct third-party payers with appropriate documentation to avoid coverage denials, and have a rebuttal strategy. It is important for clinicians to make a clear therapeutic plan with patients based upon prior therapy history, hepatic fibrosis staging, and viral factors such as genotype and RAV analysis. Ultimately, patient selection and education can lead to maximal success rates.
Certain patient populations should be evaluated and receive therapy at an experienced treatment center. These populations include patients with decompensated cirrhosis and patients who previously failed sofosbuvir/simeprevir or sofosbuvir/ledipasvir combinations, or any regimens containing NS5A agents. Careful evaluation should also occur in populations not covered in this review, including patients with renal impairment or failure, patients with HIV coinfection, patients with HCV infection after liver transplantation, and children with HCV infection.
Summary
Knowledge of treatment regimens and expected results facilitates high rates of response to treatment. There are very few contraindications to treating patients infected with HCV. As more data on the tolerability and effectiveness of DAA agents emerge, the barriers to treatment shift toward cost and identifying patients who are actively infected with HCV. Future directions should focus on medical policies and advancement of physician knowledge on diagnosing the HCV-infected population. Ultimately, once HCV infection is identified, appropriate treatment can be selected based upon genotype, clinical situation, contraindication, and drug-drug interactions.
References
- 1.Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol. 2015;62(1 suppl):S87–S99. doi: 10.1016/j.jhep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. [Accessed December 13, 2016]. http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/ Published April 2016. [PubMed]
- 3.Kim WR, Lake JR, Smith JM, et al. Liver. Am J Transplant. 2016;16(2):69–98. doi: 10.1111/ajt.13668. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–521. 521.e1–e6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Viral hepatitis—hepatitis C information. [Accessed December 13, 2016]. http://www.cdc.gov/hepatitis/hcv/ Updated May 31, 2015.
- 7.American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. [Accessed December 13, 2016]. http://www.hcvguidelines.org/ [DOI] [PMC free article] [PubMed]
- 8.Henderson DK, Dembry L, Fishman NO, et al. Society for Healthcare Epidemiology of America. SHEA guideline for management of healthcare workers who are infected with hepatitis B virus, hepatitis C virus, and/or human immunodeficiency virus. Infect Control Hosp Epidemiol. 2010;31(3):203–232. doi: 10.1086/650298. [DOI] [PubMed] [Google Scholar]
- 9.Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: concepts in anti-HCV drug development. Semin Liver Dis. 2014;34(1):22–29. doi: 10.1055/s-0034-1371007. [DOI] [PubMed] [Google Scholar]
- 10.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436(7053):933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 11.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102(8):2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltahla AA, Luciani F, White PA, Lloyd AR, Bull RA. Inhibitors of the hepatitis C virus polymerase; mode of action and resistance. Viruses. 2015;7(10):5206–5224. doi: 10.3390/v7102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59(2):375–382. doi: 10.1016/j.jhep.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918–1929. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi: 10.1016/j.jhep.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther. 2016;43(6):674–696. doi: 10.1111/apt.13514. [DOI] [PubMed] [Google Scholar]
- 17.Bunchorntavakul C, Reddy KR. Review article: the efficacy and safety of daclatasvir in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2015;42(3):258–272. doi: 10.1111/apt.13264. [DOI] [PubMed] [Google Scholar]
- 18.Hussaini T. Paritaprevir/ritonavir-ombitasvir and dasabuvir, the 3D regimen for the treatment of chronic hepatitis C virus infection: a concise review. Hepat Med. 2016;8:61–68. doi: 10.2147/HMER.S72429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feld JJ, Jacobson IM, Hézode C, et al. ASTRAL-1 Investigators. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 20.Hong Z, Cameron CE, Walker MP, et al. A novel mechanism to ensure terminal initiation by hepatitis C virus NS5B polymerase. Virology. 2001;285(1):6–11. doi: 10.1006/viro.2001.0948. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Ng KK, Cherney MM, et al. Non-nucleoside analogue inhibitors bind to an allosteric site on HCV NS5B polymerase. Crystal structures and mechanism of inhibition. J Biol Chem. 2003;278(11):9489–9495. doi: 10.1074/jbc.M209397200. [DOI] [PubMed] [Google Scholar]
- 22.Bonacci M, Lens S, Mariño Z, Forns X. Challenges in special populations: HIV/HCV coinfection, liver transplantation and patients with end-stage renal disease. Dig Dis. 2016;34(4):317–326. doi: 10.1159/000444470. [DOI] [PubMed] [Google Scholar]
- 23.Maan R, van Tilborg M, Deterding K, et al. Safety and effectiveness of direct-acting antiviral agents for treatment of patients with chronic hepatitis C virus infection and cirrhosis. Clin Gastroenterol Hepatol. 2016;14(12):1821–1830. doi: 10.1016/j.cgh.2016.07.001. e6. [DOI] [PubMed] [Google Scholar]
- 24.Afdhal N, Zeuzem S, Kwo P, et al. ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 25.Kowdley KV, Gordon SC, Reddy KR, et al. ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 26.Wilder JM, Jeffers LJ, Ravendhran N, et al. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology. 2016;63(2):437–444. doi: 10.1002/hep.28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64(2):405–414. doi: 10.1002/hep.28625. [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 29.Ferenci P, Bernstein D, Lalezari J, et al. PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 30.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 31.Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1087–1097. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 32.Zeuzem S, Mizokami M, Pianko S, et al. Prevalence of pre-treatment NS5A resistance associated variants in genotype 1 patients across different regions using deep sequencing and effect on treatment outcome with LDV/SOF. Hepatology. 2015;62(1 suppl):254A–255A. [Google Scholar]
- 33.Zeuzem S, Rockstroh JK, Kwo PY, et al. Predictors of response to grazoprevir/elbasvir among HCV genotype 1 (GT1)-infected patients: integrated analysis of phase 2-3 trials. Hepatology. 2015;62:554A–555A. [Google Scholar]
- 34.Jacobson IM, Asante-Appiah E, Wong P, et al. Prevalence and impact of baseline NS5A resistance-associated variants (RAVs) on the efficacy of elbasvir/grazoprevir (EBR/GZR) against GT1a infection—16 weeks vs 12 weeks; Paper presented at: 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13-17, 2015; Boston, MA. [Google Scholar]
- 35.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 36.Kwo P, Gitlin N, Nahass R, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64(2):370–380. doi: 10.1002/hep.28467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. HCV-TARGET Study Group. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016;150(2):419–429. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawitz E, Matusow G, DeJesus E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2) Hepatology. 2016;64(2):360–369. doi: 10.1002/hep.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curry MP, O’Leary JG, Bzowej N, et al. ASTRAL-4 Investigators. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 40.Lawitz E, Gane E, Pearlman B, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1075–1086. doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed] [Google Scholar]
- 41.US Food and Drug Administration. FDA drug safety communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. [Accessed December 13, 2016]. http://www.fda.gov/drugs/drugsafety/ucm468634.htm Published October 22, 2015. Updated August 24, 2016.
- 42.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 43.Afdhal N, Reddy KR, Nelson DR, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 44.Foster GR, Irving WL, Cheung MC, et al. HCV Research, UK. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Pol S, Bourliere M, Lucier S, et al. ANRS/AFEF HEPATHER study group. Safety and efficacy of daclatasvir-sofosbuvir in HCV genotype 1-mono-infected patients [published online September 10, 2016] J Hepatol. doi:10.1016/j.hep.2016.08.021. [DOI] [PubMed]
- 46.Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 47.Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147(2):359–365. e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 48.Kwo P, Gane E, Peng CY, et al. Efficacy and safety of grazoprevir/elbasvir +/- RBV for 12 weeks in patients with HCV G1 or G4 infection who previously failed peginterferon/RBV: C-EDGE treatment-experienced trial [EASL abstract P0886] J Hepatol. 2015;62(2):S674–S675. [Google Scholar]
- 49.Forns X, Gordon SC, Zuckerman E, et al. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015;63(3):564–572. doi: 10.1016/j.jhep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Bourlière M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15(4):397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 51.Foster GR, Afdhal N, Roberts SK, et al. ASTRAL-2 Investigators; ASTRAL-3 Investigators. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 52.Wyles DL, Ruane PJ, Sulkowski MS, et al. ALLY-2 Investigators. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):714–725. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 53.Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63(5):1493–1505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson DR, Cooper JN, Lalezari JP, et al. ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawitz E, Poordad F, Gutierrez JA, et al. Short-duration treatment with elbasvir/grazoprevir and sofosbuvir for hepatitis C: a randomized trial [published online October 22, 2016] Hepatology. doi:10.1002/hep.28877. [DOI] [PubMed]
- 56.Hezode C, De Ledinghen V, Fontaine H, et al. Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV genotype 3 infection: interim analysis of a French multicenter compassionate use program [EASL abstract LP05] J Hepatol. 2015;62(2):S265–S266. [Google Scholar]
- 57.Foster GR, Agarwal K, Cramp M, et al. C-ISLE: Grazoprevir/elbasvir plus sofosbuvir in treatment-naive and treatment-experienced HCV GT3 cirrhotic patients treated for 8, 12 or 16 weeks; Paper presented at: 67th Annual Meeting of the American Association for the Study of Liver Diseases; November 11-15, 2016; Boston, MA. [Google Scholar]
- 58.Pianko S, Flamm SL, Shiffman ML, et al. Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection: a randomized trial. Ann Intern Med. 2015;163(11):809–817. doi: 10.7326/M15-1014. [DOI] [PubMed] [Google Scholar]
- 59.Hézode C, Asselah T, Reddy KR, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502–2509. doi: 10.1016/S0140-6736(15)60159-3. [DOI] [PubMed] [Google Scholar]
- 60.Kohli A, Kapoor R, Sims Z, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15(9):1049–1054. doi: 10.1016/S1473-3099(15)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abergel A, Metivier S, Samuel D, et al. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology. 2016;64(4):1049–1056. doi: 10.1002/hep.28706. [DOI] [PubMed] [Google Scholar]
- 62.Asselah T, Reesink H, Gerstoft J, et al. High efficacy of elbasvir and grazoprevir with or without ribavirin in 103 treatment-naive and experienced patients with HCV genotype 4 infection: a pooled analysis; Paper presented at: 66th Annual Meeting of the American Association for the Study of Liver Diseases; November 13-17, 2015; Boston, MA. [Google Scholar]
- 63.Abergel A, Asselah T, Metivier S, et al. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis. 2016;16(4):459–464. doi: 10.1016/S1473-3099(15)00529-0. [DOI] [PubMed] [Google Scholar]
- 64.Brown A, Hezode C, Zuckerman E, et al. C-SCAPE: efficacy and safety of 12 weeks of grazoprevir +/-elbasvir +/-ribavirin in patients with HCV GT2, 4, 5 or 6 infection [EASL abstract P0771] J Hepatol. 2015;62(2):S619. [Google Scholar]
- 65.Smolders EJ, de Kanter CT, de Knegt RJ, van der Valk M, Drenth JP, Burger DM. Drug-drug interactions between direct-acting antivirals and psychoactive medications. Clin Pharmacokinet. 2016;55(12):1471–1494. doi: 10.1007/s40262-016-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dick TB, Lindberg LS, Ramirez DD, Charlton MR. A clinician’s guide to drug-drug interactions with direct-acting antiviral agents for the treatment of hepatitis C viral infection. Hepatology. 2016;63(2):634–643. doi: 10.1002/hep.27920. [DOI] [PubMed] [Google Scholar]
- 67.US Food and Drug Administration. FDA drug safety communication: FDA warns of serious slowing of the heart rate when antiarrhythmic drug amiodarone is used with hepatitis C treatments containing sofosbuvir (Harvoni) or Sovaldi in combination with another direct acting antiviral drug. [Accessed December 13, 2016]. http://www.fda.gov/drugs/drugsafety/ucm439484.htm Published March 24, 2015. Updated January 16, 2016.
- 68.Back DJ, Burger DM. Interaction between amiodarone and sofosbuvir-based treatment for hepatitis C virus infection: potential mechanisms and lessons to be learned. Gastroenterology. 2015;149(6):1315–1317. doi: 10.1053/j.gastro.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 69.University of Liverpool. HEP drug interaction checker. [Accessed December 13, 2016]. http://www.hepdruginteractions.org/ Updated December 13, 2016.
- 70.AIDSinfo. AIDSinfo drug database. [Accessed December 13, 2016]. https://aidsinfo.nih.gov/drugs Updated December 13, 2016.