Abstract
Significant interest in recent years has focused on gut microbiota-host interaction because accumulating evidence has revealed that intestinal microbiota play an important role in human health and disease, including cardiovascular diseases. Changes in the composition of gut microbiota associated with disease, referred to as dysbiosis, have been linked to pathologies such as atherosclerosis, hypertension, heart failure, chronic kidney disease, obesity and type 2 diabetes mellitus. In addition to alterations in gut microbiota composition, the metabolic potential of gut microbiota has been identified as a contributing factor in the development of diseases. Recent studies revealed that gut microbiota can elicit a variety of effects on the host. Indeed, the gut microbiome functions like an endocrine organ, generating bioactive metabolites, that can impact host physiology. Microbiota interact with the host through a number of pathways, including the trimethylamine (TMA)/ trimethylamine N-oxide (TMAO) pathway, short-chain fatty acids pathway, and primary and secondary bile acids pathways. In addition to these “metabolism dependent” pathways, metabolism independent processes are suggested to also potentially contribute to CVD pathogenesis. For example, heart failure associated splanchnic circulation congestion, bowel wall edema and impaired intestinal barrier function are thought to result in bacterial translocation, the presence of bacterial products in the systemic circulation and heightened inflammatory state. These are believed to also contribute to further progression of heart failure and atherosclerosis. The purpose of the current review is to highlight the complex interplay between microbiota, their metabolites and the development and progression of cardiovascular diseases. We will also discuss the roles of gut microbiota in normal physiology and the potential of modulating intestinal microbial inhabitants as novel therapeutic targets.
Keywords: Gut microbiota, Cardiovascular disease, Trimethylamine N-oxide
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of death and disability in developed countries. CVD is responsible for approximately one of every three deaths in the United States and one of every four deaths in Europe.1 Further, the steady increase of common risk factors for CVD, such as obesity, type 2 diabetes (T2DM) and the metabolic syndrome, compels the search for more effective strategies to prevent and modify the course of these cardiometabolic disorders.2
Significant interest has recently focused on the role of human gut microbiota in CVD and metabolic disorders. Microbial sequencing analysis has provided a wealth of information about the presence of characteristic gut microbiota associated with CVD.3–5 Also, a growing body of evidence shows that manipulation of the composition of gut microbiota affects host metabolism.6, 7 Furthermore, recent studies suggest that gut microbiota produce numerous metabolites, some of which are absorbed into the systemic circulation and are biologically active, whereas others are further metabolized by host enzymes, and then serve as a mediator of microbial influence on the host.8–13 Thus, the gut microbiome, functioning as a virtual endocrine system, communicates with distal organs through metabolism-dependent pathways.8–11, 14–22 This review will discuss the roles of gut microbiota in normal physiology, their associations with disease settings, and the potential of modulating gut microbiota as novel therapeutic targets with particular emphasis on the complex interplay between microbiota, their metabolites and CVD.
THE ROLE OF GUT MICROBIOTA IN HOST PHYSIOLOGY
The human body is inhabited by a huge number of bacteria, archaea, viruses, and unicellular eukaryotes.23 The collection of microorganisms that live in coexistence with their hosts has been referred to as the microbiota. The microbiota colonize mainly in the gastrointestinal tract, especially in the colon, that is primarily anaerobic, and has a rich nutrient environment serving as a preferred site for intestinal microbial colonization.
Gut microbiota participate in food digestion through two main catabolic pathways categorized as saccharolytic or proteolytic.24 In the saccharolytic pathway, gut microbiota break down sugars and are responsible for the majority of short-chain fatty acid (SCFA) production. The second catabolic pathway is represented by protein fermentation, which also induces SCFA formation, but leads to other co-metabolites such as ammonia, various amines, thiols, phenols, and indoles. Some of these metabolites are potentially toxic, and because they are predominantly renally cleared, their accumulation are often considered microbial uremic toxins.25
Gut microbiota perform multiple functions and interact with the host beyond its role in supporting physiological functions in food digestion. Gut microbiota constitute and regulate the intestinal mucosal barriers, control nutrient uptake and metabolism, assist with maturation of immunological tissues, and prevent propagation of pathogenic microorganisms.26–30 Under physiological conditions, gut microbiota continue to stimulate the immune system, which is a rapid and effective mechanism for defending against pathogens.31 Collectively, the microbiota exert a fundamental influence on systemic immunity and metabolism, and healthy gut microbiota are largely responsible for the overall health of the host.23
PATHOGENIC MECHANISM OF GUT MICROBIOTA AND METABOLITES IN CARDIOMETABOLIC DISEASES
Altered composition of gut microbiota
The majority of the gut microbial community is composed of only five phyla (Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria and Cerrucomicrobia).32 However, there is considerable diversity on the species level and their relative abundance. In the healthy gut, anaerobic Bacteroidetes and Firmicutes contribute more than 90 % of the total bacterial species.32 However, the ratio of the Firmicutes to Bacteroidetes is not the same in all individuals. Inter-individual variation in bacterial diversity is caused by differences in host genomes and also by environmental factors, such as antibiotic use, lifestyle, hygiene, and diet. 33, 34 Recently, as developments in genome sequencing technologies and bioinformatics have enabled us to identify and characterize in detail these microorganisms, the composition and potential roles of the bacteria in the pathogenesis of cardiometabolic disorders have been intensely studied.35–38
Gut microbiota-derived signaling molecules
Gut microbiota can elicit effects on the host through a variety of processes. To communicate with distant organs, gut microbial signals first need to be transmitted across the intestinal epithelium. In some cases, these signalling molecules are a structural component of microbiota such as lipopolysaccharide (LPS) and peptidoglycans that interact with host mucosal surface cells, often through so called pattern recognition receptors (PRR).39 PRR recognize pathogen-associated molecular patterns (PAMPs), which stimulate and instruct host immune response.40 Thus, LPS and peptidoglycans can trigger numerous downstream signalling processes with host receptors both at the epithelial cell border, as well as within vasculature, particularly under conditions when gut wall barrier function is impaired.41, 42 Gut microbiota can also impact host processes via bioactive metabolites that can affect distal organs directly or indirectly.43 Gut microbiota interact with the host through a number of pathways, including the trimethylamine (TMA)/ trimethylamine N-oxide (TMAO) pathway, SCFAs pathway, and primary and secondary bile acid (BAs) pathways.8–11, 14–22 Some of these molecules have been shown to functionally interact with other endocrine hormones, including ghrelin, leptin, glucagon-like peptide 1 (GLP-1), and peptide YY (PYY).44, 45 Others have been reported to stimulate the parasympathetic nervous system, thereby impacting glucose homeostasis and other metabolic processes linked to development of metabolic syndrome.46
Atherosclerosis, Coronary Artery Disease and Myocardial Infarction
Atherosclerotic plaques contain bacterial DNA, and the bacterial taxa observed in atherosclerotic plaques were also present in the gut of the same individuals.3, 38 These observations suggest the possibility that the microbial communities at these sites may be a source of bacteria in the plaque, which may impact plaque stability and development of CVD. In addition to gut microbiota, taxa characteristics of oral microbiota have also been detected in atherosclerotic plaque in humans.3 Given the many epidemiological links between periodontal disease and CVD,47–49 a role for oral microbiota in the pathophysiology of CVD has also been studied.3, 49, 50 Metagenomic sequencing of stool microbiota revealed that the microbial composition is altered in patients with unstable versus stable plaques, with unstable plaque associated with reduced fecal levels of the genus Roseburiam and both increased theoretical capacity of the microbiome to produce pro-inflammatory peptidoglycans and reduced production of anti-inflammatory carotenes.4 The gut microbiome of patients with CVD may thus be fostering inflammation by producing more pro-inflammatory molecules.
Recently, a mechanistic link between the gut microbiota and the severity of myocardial infarction has been reported in rats.51, 52 Use of broad-spectrum antibiotics was shown to affect levels of leptin and analytes produced during aromatic amino acid catabolism, with associated reduced myocardial infarct size.51, 52 In addition, in rodent model studies, administration of Lactobacillus plantarum was associated with significant reduction in infarct size and improved left ventricular function after myocardial infarction.51 Another animal model study showed that administration of the Lactobacillus rhamnosus GR-1 attenuated left ventricular hypertrophy and heart failure after experimental myocardial infarction.53 These observations may suggest that probiotics use, in combination with standard medication, could offer additional benefits in heat failure patients, such as reducing the severity of heart failure after myocardial infarction.
In addition to the alterations in gut microbiota composition, the metabolic potential of gut microbiota has been identified as a contributing factor in CVD development. In particular, TMAO, the hepatic oxidation product of the microbial metabolite TMA, has gained considerable attention as a potential promoter of atherosclerosis and cardiometabolic diseases.40, 54 TMA is an organic compound that is generated by the gut microbiota. Specifically, microbial metabolism of dietary nutrients that possess a TMA moiety (such as choline, phosphatidylcholine, and L-carnitine) is focused primarily on obtaining carbon duel source for the microbe. TMA is then produced as a waste product by a variety of microbial enzymes (TMA lyases).55–57 TMA is rapidly oxidized into TMAO by flavin monooxygenase enzymes in the liver and then released into the circulation.58 TMAO is mainly cleared from circulation by the kidneys, and thus, renal function is also important to consider when looking at levels of TMAO in the systemic circulation.59
We recently showed the proatherogenic contribution of microbial-host TMA/TMAO generation from metabolism of dietary nutrients by using germ-free mice or short-term antibiotics for eliminating intestinal microbiota.8 ApoE−/− C57BL/6J mice, only when fed with a choline-rich diet and intact gut microbiota, led to an increase in plasma TMAO levels, macrophage foam cell formation, and enhanced aortic atherosclerotic plaque. In contrast, germ-free mice and short-term antibiotic suppression of gut microbiota eliminated TMAO generating capacity, and the latter reduced atherosclerotic burden.8 These effects were not unique to choline or phosphatidylcholine, but have similarly been observed with other dietary nutrients that can generate TMAO downstream, including L-carnitine and gamma butyrobetaine.9, 55 Comparison of both intestinal microbiota composition and function between omnivores and vegans/vegetarians revealed stark differences in gut microbial capacity to produce TMA and TMAO from dietary L-carnitine, with vegetarians and vegans having minimal capacity to form TMA from carnitine.9 Further, studies using poorly orally absorbed antibiotics coupled with the use of dietary intake of isotope-labelled phosphatidylcholine have showed a direct demonstration of an obligatory role for gut microbes in TMAO generation in humans.22
The association of TMAO levels and adverse clinical consequences has been shown in numerous independent cohorts.8, 22, 54, 59–62 The original human studies of more than 1,800 stable cardiac patients undergoing elective coronary angiography demonstrated that all TMAO-associated metabolites—choline, betaine, and L-carnitine—had a positive association with prevalent CVDs and incident cardiovascular events.8 Of these 3 compounds, circulating TMAO levels exhibited a positive correlation with atherosclerotic plaque size, whereas triglyceride, lipoproteins, fasting glucose, and hepatic triglycerides did not.8 In a subsequent study of over 4,000 subjects undergoing elective coronary angiography, elevated TMAO levels were associated with increased risk of incident major adverse cardiovascular events (MACE), including death, myocardial infarction and stroke over a 3-year follow-up period.22 Specifically, patients in the highest quartile of circulating TMAO levels had a 2.5-fold increased risk of having a MACE compared to those in the lowest quartile.22 Such prognostic value was independent of traditional cardiac risk factors, lipid parameters, C-reactive protein, and even renal function, and the hazard ratio for TMAO was much higher than for traditional risk factors such as LDL cholesterol. 22 Since these initial studies, numerous additional reports have shown associations between TMAO levels and incident CVD risks.22, 59–62 Circulating TMAO was associated with the presence of vulnerable coronary plaque, plaque rupture, and long-term risks of incident cardiovascular events in patients with acute coronary syndrome.63, 64 Mechanistic studies in animal model studies also reveal TMAO alters platelet calcium signalling, and elicits a pro-thrombotic effect in vivo.10 These observations suggest that TMAO could be a marker for coronary plaque vulnerability and progression, and a direct participant in enhanced risk for myocardial infarction. Recently, we observed that higher fasting plasma TMAO levels were associated with higher all-cause mortality over five years among 821 consecutive patients with adjudicated peripheral artery disease.62 However, although these observations highlight that plasma levels of TMAO correlate with CVD risk, there are still some questions regarding the causative effects of TMAO and the underlying mechanistic link that explains how TMAO might directly or indirectly promote CVD. In recent studies use of a small molecule inhibitor of microbial choline TMA lyase activity was shown to suppress microbial TMA and TMAO formation, macrophage foam cell formation, and atherosclerosis in vivo.65 Whether targeting this pathway elicits parallel reductions in CVD risks in humans remains unknown, but is an important area of future research.
Hypertension
Hypertension is the most prevalent modifiable risk factor for CVD. Though few studies have linked gut microbial signatures to hypertension in humans, recently, early studies showed germ-free rats have an elevated blood pressure, implicating a role for gut microbiota in blood pressure regulation.66 More recently, a limited number of studies indicate a direct association between gut microbiota and blood pressure control in animal models.67–70 Yang et al. compared alterations in the fecal microbiota in the spontaneously hypertensive rat and chronic angiotensin II infusion rat models of hypertension.67 They observed a significant dysbiosis as a result of decreases in microbial richness, diversity, evenness, and increased Firmicutes/Bacteroidetes ratio in hypertensive animals.67 Mell et al. demonstrated significant differences in cecal microbiota comparing salt-sensitive and salt-resistant strains by using Dahl rats.68 Studies employing angiotensin II-infused germ-free mice showed that gut microbiota participate in angiotensin II-induced vascular dysfunction and hypertension.71 Recent study showed a blood pressure-lowering effect in a patient with treatment-resistant hypertension when treated with a combination of antibiotics. 69 Higher abundance of the butyrate-producing genus Odoribacter was associated with lower blood pressure in overweight and obese pregnant women.72 These data suggest a strong association between gut microbial dysbiosis and hypertension pathology.
SCFAs, which are other important signals generated by the gut microbiota, have been recently shown to modulate blood pressure.11 SCFAs are a major product from the microbial fermentative activity in the gut, and are likely to have broad impacts on various aspects of host physiology, as well as to impact disease susceptibility.73 SCFAs can function to stimulate host G-protein coupled receptor (GPR) pathways that impact renin secretion and blood pressure regulation.11 A series of studies using the renal and vascular olfactory receptor (Olfr) 78 and GPR41 knockout mice further supports involvement of these receptors in blood pressure control. For example, stimulation of Olfr78 was observed to elevate blood pressure, whereas stimulation of GPR41 lowered blood pressure.74 Communication between the gut enteric nervous system and the central nervous system has similarly emerged as a potential link to blood pressure. 75, 76 Gut microbial products have been implicated in sympathetic activation, and maintenance of an influx of lymphocytes to intestinal tissue. 77, 78,79
Thus, gut microbiota are potentially intertwined functionally to control blood pressure, and their dysfunctions could be associated with hypertension. In fact, a beneficial role for Lactobacillus probiotics in blood pressure regulation has been reported. 80–82 Furthermore, a meta-analysis demonstrated a significant decrease in blood pressure in patients treated with probiotics. 83
Heart failure
There is a growing literature to support a role of the gut in the pathogenesis of heart failure - the so-called “gut hypothesis of heart failure.” The gut hypothesis implies that decreased cardiac output and elevated systemic congestion can lead to intestinal muscosal ischemia and/or edema, leading to increased bacterial translocation and increased circulating endotoxins that can contribute to the underlying inflammation seen in patients with heart failure.84, 85 Niebauer et al. found that heart failure patients with peripheral edema had higher plasma concentrations of endotoxin and inflammatory cytokines compared to those without edema.86 After short-term diuretic treatment, serum concentrations of endotoxin, but not cytokines decreased.86 In another study, heart failure patients with lower intestinal blood flow were shown to have higher serum concentrations of immunoglobulin A–antilipopolysaccharide, which in turn was correlated with increased growth of bacteria obtained from biopsies of colonic mucosa but not stool bacteria.87 The nature of the bacterial flora in these subjects also appeared to be different from that in the control subjects.87 Recently, Pacini et al. reported a corresponding increase in the amount of fecal intestinal bacteria and fungi with increased intestinal permeability in patients with chronic heart failure when compared to healthy controls.88 These data imply that an assessment of intestinal barrier function may lead to greater mechanistic understanding of the impact of gut-directed heart failure therapy.
In addition to the clear link between TMAO and atherosclerotic CVD risk, TMAO levels have also more recently been linked to heart failure development and poor prognosis in heart failure patients.8, 22, 60, 89 We recently observed that circulating TMAO levels are higher in patients with heart failure compared with age- and gender-matched subjects without heart failure.60 Moreover, we observed a remarkably strong adverse prognostic value associated with elevated plasma TMAO levels among a cohort of stable patients with heart failure that was incremental to traditional risk factors, cardio-renal indices and markers of systemic inflammation.60 However, the mechanism explaining why patients with heart failure have increased levels of TMAO remains to be determined.
Recent animal model studies suggest beyond association studies and adverse prognosis data in humans, the TMAO pathway may directly contribute to the development of adverse ventricular remodeling and heart failure phenotype.90 For example, using a trans-aortic constriction model of heart failure, mice fed a high choline diet had both higher TMAO levels and accelerated adverse ventricular remodeling compared to mice on a chemically defined low but sufficient choline diet.90 In addition to enhanced chamber dilation, wall thinning and reduced shortening fraction, marked increase in fibrosis was observed in mice on the high choline diet. Moreover, the pro-fibrotic TGF-B phosphor-SMAD3 pathway was shown to be enhanced in the choline diet-fed mice.59 Whether manipulation of the gut microbial TMAO pathway such as through inhibition of microbial TMA production can attenuate heart failure like phenotype from developing, or reduction in TMAO levels in subjects with heart failure improves long term outcomes, remain to be determined.
Obesity and type 2 diabetes mellitus (T2DM)
Obesity has increased worldwide and is attributed to increased energy intake and reduced energy expenditure. Obesity increases the risk of multifactorial diseases such as T2DM. Initial animal and human studies supported associations between obesity and the higher ratio of Firmicutes to Bacteroidetes.36, 91 In an intervention of calorie-restricted diets, weight loss was associated with decreases in Firmicutes to Bacteroidetes ratio both in low fat and low carbohydrate diets.36 Metagenomic analysis has revealed increased harvest of energy from indigestible carbohydrates in the microbiome of obese mice, further supporting an association of altered microbiota composition in obesity and T2DM.6, 37, 92, 93
T2DM was associated with a reduced abundance of butyrate-producing bacteria and an increased abundance of Lactobacillus spp.37, 93, 94 SCFAs, in particular butyrate, serve as energy substrates for epithelial cells of the gut. 95, 96 Microbial pathways that generate SCFAs were found to be enriched in metagenomic studies of feces recovered from obese subjects, and levels of SCFAs were elevated in overweight or obese people and animal models, consistent with these products of microbial fermentation providing extra calories to the host.6, 97, 98 Moreover, computational models based on the gut metagenome were able to predict T2DM-associated phenotype in patients with impaired glucose tolerance.93 Furthermore, vancomycin treatment in patients with metabolic syndrome reduced the abundance of butyrate-producing bacteria, leading to reduced insulin sensitivity. 99 Moreover, a study using fecal microbiota transplantation from lean donors to insulin resistant patients with metabolic syndrome demonstrated that feces from lean subjects, but not autologous transplantation, improved insulin sensitivity and was associated with enhanced numbers of butyrate-producing bacteria.100
SCFA can directly activate G-protein-coupled receptors such as GPR41 and GPR43, which affect several important processes that include inflammation and enteroendocrine regulation.101, 102 Binding of SCFAs to GPR41 induces expression of the enteroendocrine hormone PYY in gut epithelial L-cells via GPR41, leading to increase in energy harvest from the diet.102 By contrast, SCFAs can suppress insulin-mediated fat accumulation and stimulate energy expenditure in liver and muscle through the GPR43 in mouse white adipose tissue.14 SCFAs can also trigger secretion of GLP-1 by intestinal L-cells via both GPR41 and GPR43, which has a substantial impact on pancreatic function and insulin release, as well as central effects regulating appetite.103, 104 In fact, in a recent study propionate significantly increased postprandial GLP-1 and PYY while reducing calorie intake at a buffet meal, resulting in a significant reduction in weight gain after a long-term supplementation.105 Furthermore, rectal and intravenous administration of acetate was associated with increased plasma concentration of PYY and GLP-1 in humans.106 The role of SCFAs, their receptors, and their targets requires further investigation.
Bile acids (BAs) are another group of metabolites with a profound effect on human health. Secondary BAs are metabolized by the microbiota in the lower part of the small intestine and the colon.107, 108 BAs facilitate the absorption of dietary fat and fat-soluble molecules. There is an astonishing chemical diversity to the BA pool, and while the heterogeneity and function of numerous BAs are just beginning to become dissected, several species are now recognized as regulators of energy metabolism through activation of nuclear receptors such as G-protein-coupled bile acid receptor 1 (TGR5) and farnesoid X receptor (FXR).109–111
For example, gut microbiota can regulate TGR5 signalling by producing agonists and FXR signalling by metabolizing antagonists, such as tauro-β-muricholic acid (TβMCA), which is an abundant primary BA. 112, 113 Microbial metabolism of this BA relieves FXR inhibition and increases signaling.112 FXR activation in the intestine induces fibroblast growth factor 15 expression, which suppresses the expression of cholesterol 7 α-hydroxylase in the liver, an important hepatic enzyme impacting BA pool size and composition. Thus, the capacity to metabolize TβMCA has been associated with microbiota links to obesity and insulin resistance.113, 114 BAs have also been described to affect the cecal microbiota composition of rats, and a direct antimicrobial effect of BAs has been described in vitro.115, 116 Thus, a reciprocal and complex interrelationship exists between gut microbial metabolism of BAs, and BAs impact on microbial composition and function. It will be essential to identify the microbial enzymatic participants, specific BAs, and the host receptors and signaling molecules involved in the complex metaorganismal signaling pathways coordinating gut microbiota participation in human physiological processes and disease susceptibilities.
One intervention that has significant impact on microbial community structure and function is bariatric surgery. Bariatric surgery is associated with an altered microbiota and increased circulating levels of primary and secondary BAs have been observed after bariatric surgery.117–119 Increased GLP-1 and reduced glucose and triglyceride levels have also been linked to bariatric surgery.120–122 Although the underlying mechanisms have not been fully elucidated, release of satiety-promoting gut hormones such as GLP-1 and PYY, and a shift in BA metabolism together with an increased signaling through FXR have been suggested to play a role. 44, 45, 123, 124 Similarly, TGR5 is required for improved metabolism of glucose that is observed following bariatric surgery. Germ-free mice that received a fecal transplant from people who had undergone bariatric surgery 10 years earlier gained less fat than did mice that were colonized by microbiota from obese people.45 Some of the beneficial effects of bariatric surgery might therefore be mediated by the altered microbial metabolism of BAs, which affects their capacity for signalling. Although a direct antimicrobial effect of BAs has been described in vitro,116 it is still unclear whether BA–mediated microbiota alterations are a direct effect of BAs on the bacteria or whether cross-talk with the intestinal mucosa is involved.
Dyslipidemia
Recent studies suggest that the gut microbiota can mechanistically impact host lipid levels.8, 9, 125–127 Independent of body mass index and other metabolic disturbances, associations between levels of circulating triglycerides and high density lipoprotein cholesterol with gut microbiota have been reported.125 Although the underlying biological mechanisms through which gut microbiota or their metabolites can impact host lipid metabolism has not been enumerated, secondary bile acids, which are produced by gut microbiota, have been suggested to modulate both hepatic and/or systemic lipid metabolism, as well as glucose metabolism, through FXR and GPR131.17, 18, 44 In addition, some of the proatherogenic effects of TMAO are linked to reduction in reverse cholesterol transport, alteration in tissue cholesterol and sterol metabolism, and changes in bile acid composition, pool size and transport in both the liver and intestines.9 Furthermore, genetic manipulation of the expression of host hepatic FMO3 has been shown to elicit alterations in plasma lipid levels and hepatic lipid metabolism,126, 127 suggesting a major role for FMO3 in modulating lipid homeostasis.
Chronic kidney disease (CKD)
Cardiovascular and kidney diseases are closely interrelated, and the so-called cardiorenal syndrome is associated with poor clinical outcomes.128 People with chronic kidney disease (CKD) have a greater risk of CVD-related mortality.129 The increased CVD risk is only partially explained by traditional cardiovascular risk factors, and there is increasing evidence that non-traditional risk factors such as inflammation, oxidative stress, and endothelial dysfunction play a key role. 130
It is well known that the composition of gut microbiota is markedly altered in CKD patients, leading to an influx of circulating urea and other uremic toxins into the gut lumen.131, 132 Within the intestinal tract, urea is hydrolyzed by microbial urease to form large quantities of ammonia, which is then converted to ammonium hydroxide. Ammonia and ammonium hydroxide disrupt the intestinal epithelial tight junctions.131 This is thought to be a major cause of intestinal epithelial barrier dysfunction in CKD that allows the translocation of gut bacterial DNA and uremic toxins into systemic circulation, resulting in systemic inflammation.133 Recently, the DNA of gut microbiota has been detected in the plasma of CKD patients on chronic hemodialysis sing bacterial 16S rDNA amplification and DNA pyrosequencing.134 Moreover, levels of the bacterial DNA correlated with increased plasma inflammatory marker levels. Poorly dialyzable protein-bound uremic toxins such as indoxyl sulfate and p-cresyl sulfate are associated with poor cardiovascular outcomes in patients with uremia, and p-cresyl sulfate is also associated with insulin resistance.135–137 Both of these sulfates are derived from gut microbiotic metabolism of dietary amino acids and are ineffectively cleared from the circulation in cases of renal dysfunction. In a recent study, a widely distributed family of tryptophanases in the gut commensal Bacteroides was identified as a primary and rate limiting source of indoxyl sulfate in human gut bacteria; moreover, modulating the content of the gut microbes harboring the tryptophanases was shown to substantially impact indoxyl sulfate levels in vivo.138 In clinical studies, levels of this and other uremic toxins have been shown to be associated with angiographic coronary atherosclerosis severity.139 Collectively, there is significant evidence suggesting the gut could be a target of treatment of CKD in conjunction with efforts to improve dialysis techniques to better remove microbially generated uremic toxins.
TMAO has been known to accumulate in the plasma of patients with CKD, and higher TMAO levels were associated with higher mortality and progressive loss of kidney function.59–61 Data from the Framingham Heart Study indicated that TMAO was one of the few metabolites in the plasma of healthy subjects whose levels predicted incident development of CKD.140 In addition, we observed enhanced renal fibrosis and decreased renal function with choline rich diet supplementation.59 It is clear that further studies exploring the physiology of TMAO generation and metabolism are warranted to more thoroughly define the etiology of TMAO elevations in CKD.
THERAPEUTIC INTERVENTION
The many links between the altered gut microbial community, metabolites, and susceptibility for CVD and metabolic diseases has placed a spotlight on the gut microbiome as a potential novel target for therapeutics. Currently diet modulation is the major therapeutic tool utilized in clinical practice to impact chronic metabolic diseases, and while this lifestyle interaction can clearly impact gut microbial community structure and function, there are few studies that explore the impact of dietary interventions on the gut microbiome in humans. Additional approaches to manipulate the gut microbiome that hold promise, though have yet to be realized for metabolic disorders, include prebiotics, probiotics and small molecule inhibitors of defined microbial enzyme pathways.
Fecal microbiota transplantation
Gut microbial modulation by fecal microbiota transplantation (FMT) is a possible therapeutic intervention designed to displace intestinal pathogens by introducing fecal contents from healthy subjects into the gastrointestinal tract of patients. This therapeutic approach has been growing and has caught much attention specifically in its utility to treat intestinal diseases.141 FMT is demonstrated to be effective in the treatment of antibiotic resistant Clostridium difficile infection in humans, where fecal transplantation induces an 80% remission rate. 142, 143
Recently, FMT has also been tested as an emerging therapy to manage cardiometabolic disorders.144, 145 Overweight patients with metabolic syndrome were transferred microbiota from either their own feces (autologous transfer) or from lean healthy controls (allogeneic transfer). After 6 weeks of follow-up, the allogeneic fecal transfer had improved hepatic and peripheral insulin sensitivity by 119% and 176%, respectively, as shown by a euglycemic–hyperinsulinemic clamp technique.144 This metabolic improvement was independent of any weight variations. The allogeneic fecal transfer induced an increase in overall gut microbial richness, and more specifically, increased the abundance of butyrate-producing bacteria, such as Roseburia, confirming previous results that showed an association between Roseburia and glucose homeostasis.37, 93
However, the use of FMT is currently limited due its associated risks including possible transfer of endotoxins or infectious agents that could cause new GI complications.146, 147 Further studies are needed to test if FMT could be extended to other facets of cardiometabolic disorders. Instead of fecal contents, the transplantation of only a defined group of bacteria may be a rational alternative to FMT.148
Diet intervention
A dietary approach to nutritional interventions in CVD had proved to be an effective strategy in reducing cardiovascular risk.149, 150 In a comprehensive study involving more than 900 participants, diet-dependent postprandial blood glucose levels were correlated with individual gut microbiota composition.151 Although the composition of the microbiota is quite resilient over an individual’s life span,152, 153 dietary interventions that induce rapid changes in certain nutrients can modify the microbiota composition.154, 155 Changes in Roseburia and E. rectale have been observed with changes in the proportion of carbohydrate content in the diet.156, 157 Fiber-rich diets promote the growth of beneficial commensal bacteria and limit the growth of known opportunistic pathogens.158 A high fiber diet was reported to increase acetate-producing microbiota, lower blood pressure and decrease cardiac hypertrophy and fibrosis.159 A recent study has shown that dietary intervention with whole grains, traditional Chinese medicinal foods and prebiotics, resulted in improvement in insulin sensitivity and lipid profile with concomitant reduction of opportunistic pathogen of the Enterobacteriaceae family and increase in the family Bifidobacteriaceae, a taxa generally regarded as gut barrier-protecting.160 In addition, bacterial fermentation of prebiotic soluble fiber generates SCFA, which is thought to exert several beneficial effects including potential amelioration of CVD risk factors.12, 161, 162
Given that an alteration in gut microbiota composition has been linked to different diseases, modulation of gut microbiota composition through dietary intervention represents a promising therapeutic target. However, little is known about the mechanistic interplay between the diet intervention and the relevant gut microbial metabolism. By using metagenomic sequence analysis, representative species from the dominant phyla can be extracted and an algorithm (known as CASINO53) was reported to be able to predict metabolite production that reflects the interaction between the gut microbiota composition and the consumed diet of the individual. In one study, this algorithm was validated with a diet intervention study coupled with metabolomic analyses of fecal and blood samples. 163
Probiotic, Prebiotic and Antibiotic intervention
Probiotics are live “beneficial” bacteria administered to re-establish an appropriate intestinal balance. Probiotics may potentially act through different mechanisms including pH modulation, antibacterial compound production, and competition with pathogens. 164
Administration of Christensenella minuta was reported to alter the microbial ecology and protect mice from obesity.165 In a recent study, administration of Lactobacillus reuteri was reported to increase insulin secretion by promoting incretin release in obese glucose-tolerant subjects.166 Similarly, administration of Lactobacillus sp. was associated with significant reduction of toxins produced by the small intestine, such as dimethylamine and nitrosodimethylamine, in patients with CKD,167 and changes in colon levels of certain SCFAs in patients with carotid atherosclerosis.168 In some studies, engineered probiotic bacteria are genetically modified to enhance their potential beneficial effects. For example, Davies and colleagues formed N-acylphosphatidylethanolamines-expressing Escherichia coli Nissle 1917 to alleviate high fat diet-induced obesity, insulin resistance and hepatosteatosis in mice.169 Among other therapeutic targets of interest, the intestinal alkaline phosphatase is well-known to detoxify bacterial LPS by de-phosphorylating its lipid A moiety.170 In a study using genetically engineered NAPE-expressing E. Coli Nissele 1917, a reduction in adiposity, insulin resistance and liver lipid accumulation in mice was reported.171 However, the mechanism of action and how these microbes affect cardiometabolic risks in humans are yet to be determined.
Another strategy for modulating intestinal microbiota is the use of prebiotics, which are non-microbial entities provided to elicit a favorable impact on microbial community composition and function. Typical prebiotcis are food indigestible molecules such as oligosaccharides or complex saccharides. In some studies, prebiotics administration is associated with both improved glycemic control and plasma lipid profiles. 172, 173 Three months of oligofructose supplementation in obese patients was associated with weight loss and improved glucose tolerance.174 In a preclinical study employing an insulin resistance mouse model, use of antibiotics or prebiotics was reported to reverse microbial community features associated with diabetes, improve gut permeability, reduced metabolic endotoxemia, lower inflammation, and improve glucose intolerance.175 However, in a subsequent follow-up clinical study, the prebiotic treatment in obese women did not show similar strong effects, raising questions as to the translatability of studies performed in mice compared with humans.176 It has also been found that not all humans respond to dietary changes in a similar manner, and non-responsiveness to either a fiber-rich or weight loss diet was shown to correlate with pre-intervention increased bacterial diversity.177
Therapeutic intervention focusing on the elimination of disease-causing microbiota by antibiotics is an obvious concept, but the general consensus is such non-specific anti-microbial approaches may do more harm than good. The use of antibiotics in humans during the first six months of life is associated with childhood obesity.178 Similar results were observed in mice whereby a subtherapeutic dose of antibiotics increased adiposity in young mice.179 Surprisingly, ApoE-KO mice fed a standard low cholesterol diet and maintained in germ-free conditions develop severe atherosclerosis compared to their conventionally-housed counterparts.180 These results suggest that therapeutic alteration in gut microbial composition should also focus on the preservation of the beneficial microbiota that are central in maintaining well-being because those microbiota or their metabolites may mediate cardiovascular protective effects. Taken together, these findings suggest that individualized treatment programs based on the microbiota may provide novel treatment strategies for cardiometabolic disorders.
Small molecule antimicrobial enzyme therapeutics
The recent discovery of the TMAO pathway, its numerous clinical links to adverse CVD outcomes, and its adverse effects on host cardiometabolic phenotypes, has raised the exciting possibility of selectively targeting microbial synthetic enzymes responsible for TMA generation from nutrient precursors as a potential therapeutic strategy. Proof of concept for this strategy was recently shown through development of a small molecule tool drug for the inhibition of microbial choline TMA lyase activity.65 Use of a choline structural analogue, 1,3 dimethylbutanol (DMB), that inhibited microbial generation of TMA from a variety of nutrients (eg. choline, glycerphosphocholine, phosphorylcholine, phosphatidylcholine, as well as several carnitine related nutrients), was shown to both suppress plasma levels of TMA and TMAO in mice on a high choline or carnitine containing diet, as well as inhibit both diet induced macrophage foam cell formation and aortic root atherosclerosis development.65 In a rich nutrient broth, use of DMB with multiple human commensals was shown to inhibit TMA production from choline without impacting microbial growth. Thus, with the markedly reduced selective pressures for development of resistance that exist with a targeted non-lethal microbial inhibitor strategy, there is considerable excitement about the potential for future development of microbial enzyme inhibitors for the potential treatment of cardiometabolic phenotypes in subjects.
GAPS IN KNOWLEDGE
Multiple human clinical studies reveal striking associations between either gut microbiota composition, or their derived metabolites, and both the presence and incident development of CVD. Despite these exciting and intriguing findings, by comparison, few studies have provided mechanistic or causal evidence of a direct participatory role of gut microbiota to the development of atherosclerosis or its adverse complications. Study designs using antibiotics or FMT, while compelling in terms of demonstrating mechanistic participation of gut microbiota to the monitored phenotypes, do not typically examine how specific microbiota or their products contribute to development and/or progression of diseases. A combination of both mechanistic investigations, and further prospective studies with large cohorts, are needed to understand whether (or which) gut microbiota is causally linked to host metabolism in humans. Moreover, simpy revealing a specific microbe strain can facilitate accelerated atherosclerosis in animal models and is reproducibly associated with CVD risks does not reveal how or why this association exists, nor the underlying molecular participants involved. Moreover, a better understanding of microbe-microbe interactions, and microbe-host interaction and how these are linked to the underlying molecular participants involved in disease susceptibility represent additional knowledge gaps. Studies aimed at revealing these sorts of details are needed to be able to leverage knowledge gained to develop therapeutic interventions.
CONCLUSION AND FUTURE PERSPECTIVE
Mounting evidence from animal and human studies supports that gut microbiota can influence host health and disease. The recent development of culture-independent techniques for microbiological analysis has uncovered the previously unappreciated complexity of the bacterial microbiome at various anatomic sites. In addition, the identification of bacterial metabolites which can modulate host physiological processes has opened the possibility for numerous microbial pathways as both mediators and potential pharmacological targets for the treatment of cardiometabolic diseases. Major advances are needed in our mechanistic understanding of how gut microbiota convert dietary and endogenous molecules into metabolites that communicate with peripheral organs and tissues in the host. While the advent of next-generation high-throughput sequencing technology and bioinformatics will no doubt help to further discover candidate microbial enzyme machinery involved, the business end of the pathway – the metabolite – and the host receptors that recognize them, represent the true exciting and attractive pieces of the puzzle needed for the next stage of this growing field.
Modulation of gut microbiota composition and function through diet, pre- and probiotics, and targeted non-lethal antimicrobial enzyme inhibitors may enable, in the long term, the capacity to alter host metabolic profile in a desired favorable direction. Coupled with monitoring of the gut microbial metabolite mediator level, much in the same way one can titrate diabetes regimens my monitoring glucose or glycated hemoglobin, or cholesterol levels with lipid lowering strategies, the future may hold promise for targeting a microbial pathway and titrating the intervention by monitoring blood levels of the biologically active microbial derived metabolite. Such a future would envision a more personalized and tailored therapeutic intervention to occur, with the net readout of the system, the systemic level of the microbial metabolite, serving as an integrated sensor of the myriad processes that impact community gut microbiota organization and function, host genome and environmental (eg. diet) factors that collectively can impact the host.
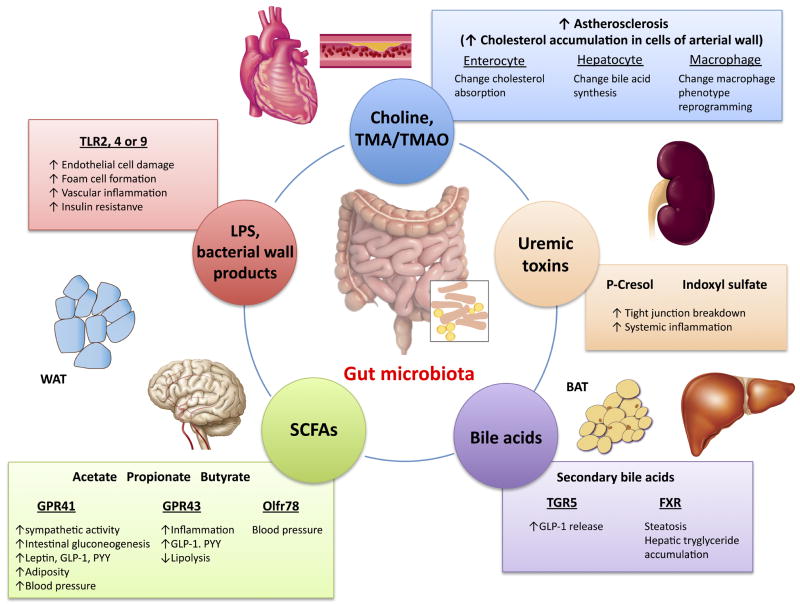
Figure 1.
Gut microbiota and possible molecular pathways linked to cardiovascular and cardiometabolic diseases.
Table 1.
| Atherosclerosis | Hypertension | Heart failure | Obesity, T2DM | Chronic kidney disease | |
|---|---|---|---|---|---|
| Alterations in gut microbiota composition | ↑Lactobacillus5 ↓Roseburiam4 | ↑Firmicutes / Bacteroides ratio67 | ↑Escherichia coli, Klebsiella pneumonia, Streptococcus viridans | ↑Firmicutes36,37,91,93,94 ↓Bacteroides36, 37, 91 | ↑Firmicutes, Proteobacteria and Actinobacteria131 |
| Alterations in gut microbiota metabolites | ↑TMAO8, 9 | ↑SCFA180 | ↑TMAO60, 61, 90 | ↑BAs, SCFA, LPS and TMAO98, 172, 182–184 | ↑Indoxyl sulfate, 185,186 p-cresol sulfate, 186,187 ammonia and urea, 188 and TMAO59 |
| Proof of concept | Dietary choline/ carnitine ↑TMAO8 DMB suppress TMA/TMAO.65 ↑TMAO associated with unstable plaque and MACE. 22,63,189 Cecal microbial transplantation transmits disease susceptibility.145 |
Infusion of AngII/TMAO associated with ↑blood pressure 190 | ↑gut permeability88 ↑TMAO was associated with LV remodelling and poor prognosis60,61,90 |
↑insulin sensitivity related to vancomycin treatment99 ↑Leptin, GLP1,103–106 PYY102, 105, 106 ↑TMAO was associated with glycemic control. 191 |
Ammonia disrupt the gut epithelial tight junction131 Elevated TMAO was associated with decreased renal function, renal fibrosis and mortality.59–61,192 |
| Interventions | Diet intervention: ↑Bacteroides and Proteobacteria, ↓Firmicutes 193 Probiotics: ↑LV hypertrophy53 Probiotics: ↑SCFA 168 |
Diet intervention: high fiber diet is associated with ↓blood pressure.159 | Diet: High fiber diet is associated with ↓cardiac hypertrophy and fibrosis.159 Probiotics: attenuate heart failure after myocardial infarction.53 |
FMT: improve insulin resistance99, 100 and Roseburia.37, 93 Bariatric surgery is associated with ↑BAs and GLP-1120–122 Probiotics: ↑insulin secretion166 ↓body weight and adipose tissue mass172–175 |
Probiotics: ↓dimethylamine and nitrosodimithylamine.167 |
T2DM = type 2 diabetes mellitus, TMAO = trymethylamine N-oxide, SCFA = short chain fatty acid, BA = bile acid, LPS = lipopolysaccharide, DMB = 1,3 dimethylbutanol, MACE = major adverse cardiac event, AngII = angiotensin II, LV = left ventricular, GLP-1 = glucagon-like peptide 1, PYY = peptide YY, FMT = fecal microbiota transplantation.
Acknowledgments
FUNDING
Drs. Tang and Hazen are supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, R01HL126827) related to the content of this paper. Dr. Hazen was partially supported by a gift from the Leonard Krieger endowment.
Footnotes
DISCLOSURES
Dr. Hazen is named as inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen is a paid consultant for Esperion Therapeutics and Procter & Gamble. Dr. Hazen has received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Siemens Healthcare, Esperion Therapeutics, and Frantz Biomarkers LLC. Drs. Tang and Kitai have no relationships relevant to the contents of this paper to disclose.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Backhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. doi: 10.1371/journal.pone.0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 7.Fak F, Backhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in apoe−/− mice. PLoS One. 2012;7:e46837. doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 13.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine n-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappab. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor gpr43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. Tgr5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, Rizzo G, Gioiello A, Adorini L, Pellicciari R, Auwerx J, Schoonjans K. Tgr5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma K, Saha PK, Chan L, Moore DD. Farnesoid x receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer JL, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JG, Noel JP, Nicolaou KC, Evans RM. A chemical, genetic, and structural analysis of the nuclear bile acid receptor fxr. Mol Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 25.Nallu A, Sharma S, Ramezani A, Muralidharan J, Raj D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl Res. 2017;179:24–37. doi: 10.1016/j.trsl.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 27.Savage DC. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am J Clin Nutr. 1970;23:1495–1501. doi: 10.1093/ajcn/23.11.1495. [DOI] [PubMed] [Google Scholar]
- 28.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Wei H, Chen Y, Lu H, Zuo J, Su M, Qiu Y, Jia W, Xiao C, Smith LM, Yang S, Holmes E, Tang H, Zhao G, Nicholson JK, Li L, Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: Implications for health outcomes. Nat Med. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 34.Carmody RN, Gerber GK, Luevano JM, Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 36.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 37.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 38.Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A, Kuhbacher T, Nikolaus S, Namsolleck P, Blaut M, Hampe J, Sahly H, Reinecke A, Haake N, Gunther R, Kruger D, Lins M, Herrmann G, Folsch UR, Simon R, Schreiber S. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- 39.Larsson E, Tremaroli V, Lee YS, Koren O, Nookaew I, Fricker A, Nielsen J, Ley RE, Backhed F. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through myd88. Gut. 2012;61:1124–1131. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JM, Hazen SL. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving glp-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 44.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. Fxr is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremaroli V, Karlsson F, Werling M, Stahlman M, Kovatcheva-Datchary P, Olbers T, Fandriks L, le Roux CW, Nielsen J, Backhed F. Roux-en-y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesaniemi YA, Syrjala SL, Jungell PS, Isoluoma M, Hietaniemi K, Jokinen MJ. Association between dental health and acute myocardial infarction. BMJ. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyvarinen K, Mantyla P, Buhlin K, Paju S, Nieminen MS, Sinisalo J, Pussinen PJ. A common periodontal pathogen has an adverse association with both acute and stable coronary artery disease. Atherosclerosis. 2012;223:478–484. doi: 10.1016/j.atherosclerosis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Fak F, Tremaroli V, Bergstrom G, Backhed F. Oral microbiota in patients with atherosclerosis. Atherosclerosis. 2015;243:573–578. doi: 10.1016/j.atherosclerosis.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 50.Serra e Silva Filho W, Casarin RC, Nicolela EL, Jr, Passos HM, Sallum AW, Goncalves RB. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS One. 2014;9:e109761. doi: 10.1371/journal.pone.0109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS One. 2016;11:e0160840. doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, Karmazyn M. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 54.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gamma-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to tmao. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Patel NA, Crombie A, Scrivens JH, Murrell JC. Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc Natl Acad Sci U S A. 2011;108:17791–17796. doi: 10.1073/pnas.1112928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine n-oxide (tmao) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-n-oxide in patients with heart failure: Refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WH. Trimethylamine n-oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu Q, Zhao M, Wang D, Hu H, Guo C, Chen W, Li Q, Zheng L, Chen B. Coronary plaque characterization assessed by optical coherence tomography and plasma trimethylamine-n-oxide levels in patients with coronary artery disease. Am J Cardiol. 2016;118:1311–1315. doi: 10.1016/j.amjcard.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 64.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Luscher TF. Gut microbiota-dependent trimethylamine n-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017:11. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honour J. The possible involvement of intestinal bacteria in steroidal hypertension. Endocrinology. 1982;110:285–287. doi: 10.1210/endo-110-1-285. [DOI] [PubMed] [Google Scholar]
- 67.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension - a case report. Int J Cardiol. 2015;201:157–158. doi: 10.1016/j.ijcard.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, Schuler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schafer K, Munzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin ii-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 73.Evans JM, Morris LS, Marchesi JR. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–47. doi: 10.1530/JOE-13-0131. [DOI] [PubMed] [Google Scholar]
- 74.Pluznick JL. Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol. 2013;305:F439–444. doi: 10.1152/ajprenal.00252.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharkey KA, Savidge TC. Role of enteric neurotransmission in host defense and protection of the gastrointestinal tract. Auton Neurosci. 2014;181:94–106. doi: 10.1016/j.autneu.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–323. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palsson J, Ricksten SE, Delle M, Lundin S. Changes in renal sympathetic nerve activity during experimental septic and endotoxin shock in conscious rats. Circ Shock. 1988;24:133–141. [PubMed] [Google Scholar]
- 78.Straub RH, Pongratz G, Weidler C, Linde HJ, Kirschning CJ, Gluck T, Scholmerich J, Falk W. Ablation of the sympathetic nervous system decreases gram-negative and increases gram-positive bacterial dissemination: Key roles for tumor necrosis factor/phagocytes and interleukin-4/lymphocytes. J Infect Dis. 2005;192:560–572. doi: 10.1086/432134. [DOI] [PubMed] [Google Scholar]
- 79.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83:255–263. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 81.Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of lactobacillus johnsonii la1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005;389:109–114. doi: 10.1016/j.neulet.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 82.Gomez-Guzman M, Toral M, Romero M, Jimenez R, Galindo P, Sanchez M, Zarzuelo MJ, Olivares M, Galvez J, Duarte J. Antihypertensive effects of probiotics lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:2326–2336. doi: 10.1002/mnfr.201500290. [DOI] [PubMed] [Google Scholar]
- 83.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 84.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Krack A, Richartz BM, Gastmann A, Greim K, Lotze U, Anker SD, Figulla HR. Studies on intragastric pco2 at rest and during exercise as a marker of intestinal perfusion in patients with chronic heart failure. Eur J Heart Fail. 2004;6:403–407. doi: 10.1016/j.ejheart.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet. 1999;353:1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 87.Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64:1092–1102. doi: 10.1016/j.jacc.2014.06.1179. [DOI] [PubMed] [Google Scholar]
- 88.Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-n-oxide. Eur Heart J. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, Lefer DJ. Choline diet and its gut microbe-derived metabolite, trimethylamine n-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail. 2016;9:e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F. Gut metagenome in european women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 94.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sorensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donohoe DR, Wali A, Brylawski BP, Bultman SJ. Microbial regulation of glucose metabolism and cell-cycle progression in mammalian colonocytes. PLoS One. 2012;7:e46589. doi: 10.1371/journal.pone.0046589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meyer TW, Hostetter TH. Uremic solutes from colon microbes. Kidney Int. 2012;81:949–954. doi: 10.1038/ki.2011.504. [DOI] [PubMed] [Google Scholar]
- 98.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and scfa in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 99.Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, van Nood E, Holleman F, Knaapen M, Romijn JA, Soeters MR, Blaak EE, Dallinga-Thie GM, Reijnders D, Ackermans MT, Serlie MJ, Knop FK, Holst JJ, van der Ley C, Kema IP, Zoetendal EG, de Vos WM, Hoekstra JB, Stroes ES, Groen AK, Nieuwdorp M. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 100.Sharma M, Thandassery RB, Bhargava N. Double pylorus: An optical illusion or reality? Gastroenterology. 2012;143:e7–8. doi: 10.1053/j.gastro.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 101.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor gpr43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding g protein-coupled receptor, gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor ffar2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fava S. Glucagon-like peptide 1 and the cardiovascular system. Curr Diabetes Rev. 2014;10:302–310. doi: 10.2174/1573399810666141030125830. [DOI] [PubMed] [Google Scholar]
- 105.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Freeland KR, Wolever TM. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide yy, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010;103:460–466. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 107.Midtvedt T. Microbial bile acid transformation. Am J Clin Nutr. 1974;27:1341–1347. doi: 10.1093/ajcn/27.11.1341. [DOI] [PubMed] [Google Scholar]
- 108.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 109.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 110.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 112.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring fxr antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid x receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid x receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 116.Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013;37:1553–1559. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by roux-en-y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: Potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 121.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after roux-en-y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, Laakso M, Gylling H, Patti ME, Auwerx J, Pihlajamaki J. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after roux-en-y gastric bypass. Obes Surg. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.le Roux CW, Bloom SR. Why do patients lose weight after roux-en-y gastric bypass? J Clin Endocrinol Metab. 2005;90:591–592. doi: 10.1210/jc.2004-2211. [DOI] [PubMed] [Google Scholar]
- 124.Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10:575–584. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- 125.Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA, Brandsma E, Marczynska J, Imhann F, Weersma RK, Franke L, Poon TW, Xavier RJ, Gevers D, Hofker MH, Wijmenga C, Zhernakova A. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–824. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The tmao-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;14:01065–01061. doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 129.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 130.Schnabel R, Blankenberg S. Oxidative stress in cardiovascular disease: Successful translation from bench to bedside? Circulation. 2007;116:1338–1340. doi: 10.1161/CIRCULATIONAHA.107.728394. [DOI] [PubMed] [Google Scholar]
- 131.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 132.Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in esrd. Am J Nephrol. 2014;39:230–237. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi K, Wang F, Jiang H, Liu H, Wei M, Wang Z, Xie L. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. 2014;59:2109–2117. doi: 10.1007/s10620-014-3202-7. [DOI] [PubMed] [Google Scholar]
- 135.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P. P-cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–1189. doi: 10.2215/CJN.07971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO. P-cresyl sulfate promotes insulin resistance associated with ckd. J Am Soc Nephrol. 2013;24:88–99. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Devlin AS, Marcobal A, Dodd D, Nayfach S, Plummer N, Meyer T, Pollard KS, Sonnenburg JL, Fischbach MA. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe. 2016;20:709–715. doi: 10.1016/j.chom.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang CP, Lu LF, Yu TH, Hung WC, Chiu CA, Chung FM, Yeh LR, Chen HJ, Lee YJ, Houng JY. Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis severity in stable angina patients with early stage of renal failure. Atherosclerosis. 2010;211:579–583. doi: 10.1016/j.atherosclerosis.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 140.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik A, Souza AL, Farrell L, Domos C, Yeh RW, Palacios I, Rosenfield K, Vasan RS, Florez JC, Wang TJ, Fox CS, Gerszten RE. A combined epidemiologic and metabolomic approach improves ckd prediction. J Am Soc Nephrol. 2013;24:1330–1338. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 143.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 144.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 145.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brandt LJ. Fmt: First step in a long journey. Am J Gastroenterol. 2013;108:1367–1368. doi: 10.1038/ajg.2013.165. [DOI] [PubMed] [Google Scholar]
- 147.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1036–1038. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 148.Wymore Brand M, Wannemuehler MJ, Phillips GJ, Proctor A, Overstreet AM, Jergens AE, Orcutt RP, Fox JG. The altered schaedler flora: Continued applications of a defined murine microbial community. ILAR J. 2015;56:169–178. doi: 10.1093/ilar/ilv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. Dash collaborative research group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 150.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 151.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalova L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 152.Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr. 2015;113(Suppl):S1–5. doi: 10.1017/S0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012;20:738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 158.Foye OT, Huang IF, Chiou CC, Walker WA, Shi HN. Early administration of probiotic lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and smad 7 cell signaling. FEMS Immunol Med Microbiol. 2012;65:467–480. doi: 10.1111/j.1574-695X.2012.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marques FZ, Nelson EM, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High fibre diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in doca-salt hypertensive mice. Circulation. 2016;7:024545. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 160.Xiao S, Fei N, Pang X, Shen J, Wang L, Zhang B, Zhang M, Zhang X, Zhang C, Li M, Sun L, Xue Z, Wang J, Feng J, Yan F, Zhao N, Liu J, Long W, Zhao L. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol Ecol. 2014;87:357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, Bakker BM. Short-chain fatty acids protect against high-fat diet-induced obesity via a ppargamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 162.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shoaie S, Ghaffari P, Kovatcheva-Datchary P, Mardinoglu A, Sen P, Pujos-Guillot E, de Wouters T, Juste C, Rizkalla S, Chilloux J, Hoyles L, Nicholson JK, Dore J, Dumas ME, Clement K, Backhed F, Nielsen J. Quantifying diet-induced metabolic changes of the human gut microbiome. Cell Metab. 2015;22:320–331. doi: 10.1016/j.cmet.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 164.Ojetti V, Lauritano EC, Barbaro F, Migneco A, Ainora ME, Fontana L, Gabrielli M, Gasbarrini A. Rifaximin pharmacology and clinical implications. Expert Opin Drug Metab Toxicol. 2009;5:675–682. doi: 10.1517/17425250902973695. [DOI] [PubMed] [Google Scholar]
- 165.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Simon MC, Strassburger K, Nowotny B, Kolb H, Nowotny P, Burkart V, Zivehe F, Hwang JH, Stehle P, Pacini G, Hartmann B, Holst JJ, MacKenzie C, Bindels LB, Martinez I, Walter J, Henrich B, Schloot NC, Roden M. Intake of lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: A proof of concept. Diabetes Care. 2015;38:1827–1834. doi: 10.2337/dc14-2690. [DOI] [PubMed] [Google Scholar]
- 167.Simenhoff ML, Dunn SR, Zollner GP, Fitzpatrick ME, Emery SM, Sandine WE, Ayres JW. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried lactobacillus acidophilus. Miner Electrolyte Metab. 1996;22:92–96. [PubMed] [Google Scholar]
- 168.Karlsson C, Ahrne S, Molin G, Berggren A, Palmquist I, Fredrikson GN, Jeppsson B. Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: A randomized controlled trial. Atherosclerosis. 2010;208:228–233. doi: 10.1016/j.atherosclerosis.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 169.Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, Coulon D, McGuinness OP, Niswender KD, Davies SS. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest. 2014;124:3391–3406. doi: 10.1172/JCI72517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lalles JP. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68:323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 171.Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by lactobacillus reuteri ncimb 30242: A randomized controlled trial. Eur J Clin Nutr. 2012;66:1234–1241. doi: 10.1038/ejcn.2012.126. [DOI] [PubMed] [Google Scholar]
- 172.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 173.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 174.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide yy in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, Francois P, de Vos WM, Delzenne NM, Schrenzel J, Cani PD. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, Delzenne NM. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Salonen A, Lahti L, Salojarvi J, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE, Louis P, Flint HJ, de Vos WM. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stepankova R, Tonar Z, Bartova J, Nedorost L, Rossman P, Poledne R, Schwarzer M, Tlaskalova-Hogenova H. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in apoe-deficient mice fed standard low cholesterol diet. J Atheroscler Thromb. 2010;17:796–804. doi: 10.5551/jat.3285. [DOI] [PubMed] [Google Scholar]
- 181.Pluznick J. A novel scfa receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 183.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS. P-cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lin CJ, Chen HH, Pan CF, Chuang CK, Wang TJ, Sun FJ, Wu CJ. P-cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal. 2011;25:191–197. doi: 10.1002/jcla.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S, Nakajima M, Kataoka K, Kim-Mitsuyama S, Tanaka M, Fukagawa M, Otagiri M, Maruyama T. P-cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of nadph oxidase. Kidney Int. 2013;83:582–592. doi: 10.1038/ki.2012.448. [DOI] [PubMed] [Google Scholar]
- 188.Walser M, Bodenlos LJ. Urea metabolism in man. J Clin Invest. 1959;38:1617–1626. doi: 10.1172/JCI103940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Suzuki T, Heaney LM, Jones DJ, Ng LL. Trimethylamine n-oxide and risk stratification after acute myocardial infarction. Clin Chem. 2017;63:420–428. doi: 10.1373/clinchem.2016.264853. [DOI] [PubMed] [Google Scholar]
- 190.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-n-oxide: A carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin ii in rats. Can J Cardiol. 2014;30:1700–1705. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 191.Tang WH, Wang Z, Li XS, Fan Y, Li DS, Wu Y, Hazen SL. Increased trimethylamine n-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63:297–306. doi: 10.1373/clinchem.2016.263640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS. Serum trimethylamine-n-oxide is elevated in ckd and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tachon S, Lee B, Marco ML. Diet alters probiotic lactobacillus persistence and function in the intestine. Environ Microbiol. 2014;16:2915–2926. doi: 10.1111/1462-2920.12297. [DOI] [PubMed] [Google Scholar]