Summary
Wnt signaling plays a central role in many processes during embryonic development and adult homeostasis. At least 19 types of Wnt ligands, receptors, transducers, transcription factors, and antagonists have been identified in mammals. Two distinct Wnt signaling pathways, the canonical signaling pathway and the noncanonical signaling pathway, have been described. Some Wnt signaling pathway components are expressed in the dental epithelium and mesenchyme during tooth development in humans and mice. Functional studies and experimental analysis of relevant animal models confirm the effects of Wnt signaling pathway on the regulation of developing tooth formation and adult tooth homeostasis. Mutations in some Wnt signaling pathway components have been identified in syndromic and non-syndromic tooth agenesis. This review provides an overview of progress in elucidating the role of Wnt signaling pathway components in the tooth and the resulting possibilities for therapeutic development.
Keywords: Tooth, Periodontal tissue, Wnt signaling, Canonical Wnt, Tooth development, Tooth agenesis, Hypodontia, Oligodontia
1. Introduction
The first Wnt gene Wnt1, originally named integration site-1 (int-1), was identified by Nusse and Varmus in 1982 as a gene activated by the integration of mouse mammary tumor virus (MMTV) in virally induced breast tumors [1]. Int-1 encodes a secreted, cysteine-rich protein that is difficult to purify in its biologically active form. The initial identification of the signaling pathway associated with this protein was based on genetic systems. In 1987, the fly Wingless gene, which controls segment polarity during larval development in Drosophila melanogaster [2], was shown to be a homolog of int-1 [3]. Because of the homology between int-1 and Wingless, the gene was renamed Wnt1 (Wingless plus int1) [4] and was eventually recognized as the founding member of a large Wnt gene family. The Wnt proteins of molecular weight approximately 40,000 are cysteine-rich, glycosylated lipid-modified secreted proteins that regulate embryonic development, cell proliferation, differentiation, and migration [5], [6].
The first direct connection between the Wnt signaling pathway and tooth formation was reported in the late 1990s [7]. In this review, we discuss our current understanding of Wnt signaling pathway components and functions in tooth development and homeostasis.
2. Wnt signaling pathway and their components
At least 19 Wnt proteins have been identified in mammals [8]. Two distinct Wnt signaling pathways, the canonical signaling pathway and the noncanonical signaling pathway, have been characterized. Two types of Wnt proteins have been identified. One class of Wnt proteins comprises the β-catenin-dependent canonical Wnts, such as Wnt1, Wnt2, Wnt3, Wnt3a, and Wnt7a. The other class comprises the noncanonical Wnts, such as Wnt4, Wnt5a, Wnt5b, Wnt6, and Wnt11, which act independently of or inhibit the canonical Wnt signaling pathway [8]. Single knockout of Wnt2b, Wnt5b, Wnt6, Wnt8b, and Wnt16 in mice resulted in no detectable phenotype [9]. Functional redundancy of some Wnts has been described in reports of double knockout mice [9], [10]. For example, Wnt1–Wnt3a double knockout mice show a more severe phenotype of defects in neural crest development and somite patterning that are not observed in either single-mutant animals [10].
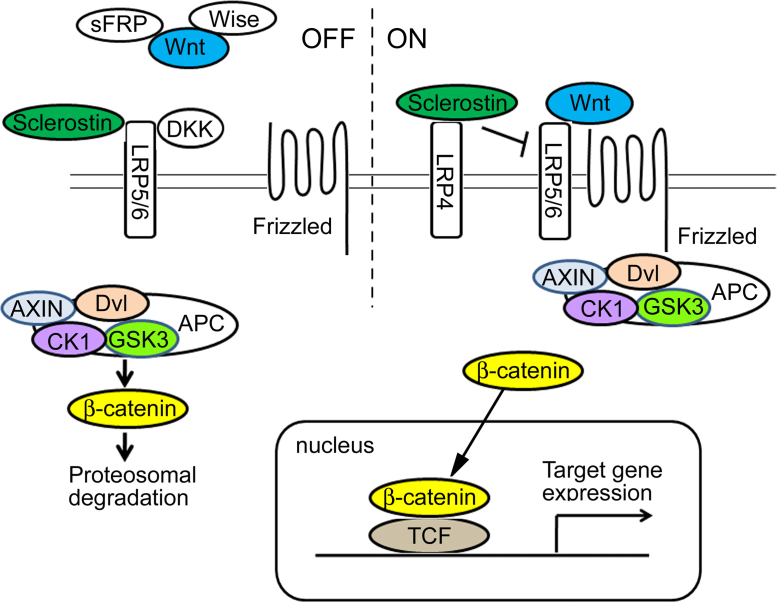
Canonical Wnt signaling is initiated by the binding of Wnt proteins to the receptors of the seven-transmembrane domain-spanning Frizzled (Fz) family (Fz1–10) as well as to the co-receptors lipoprotein receptor-related proteins (LRP) 4, 5, and 6 [11], [12]. In the absence of Wnt ligands, a complex between Axin, adenomatous polyposis coli (APC) tumor suppressor protein, casein kinase (CK) 2, glycogen synthase kinase (GSK) 3β, and β-catenin causes the phosphorylation of β-catenin by GSK3β and targets it for subsequent degradation by the proteasome [13], [14] (Fig. 1). Axin is a negative regulator of the canonical Wnt signaling pathway and a scaffold protein that brings together GSK-3β, APC, and β-catenin to form a complex [11], [15], [16]. The binding of Wnt to receptor Fz leads to the activation of Dishevelled (DVL) and causes inactivation of a complex of proteins that degrades cytoplasmic β-catenin. β-Catenin accumulates in the cytoplasm, translocates to the nucleus, and forms active transcriptional complexes with the transcription factor T-cell-specific factor/lymphoid enhancer binding factor 1 (TCF/Lef1) and with transcriptional coactivators that regulate the expression of certain target genes, including cyclin D1 and osteoprotegrin (Fig. 1) [16], [17], [18], [19], [20].
Figure 1.
The canonical Wnt signaling pathway. The secreted Frizzled-related protein (sFRP) family or Wise bind to Wnt proteins, leading to inhibition of the interaction between Wnt and the Frizzled receptor. Sclerostin or Dickkopf (DKK) binds to lipoprotein receptor-related proteins (LRP) 5/6 to inhibit Wnt signaling. Cytosolic accumulation of β-catenin in response to a canonical Wnt signal is a crucial step in the signaling pathway. Left: In the absence of Wnt ligand, β-catenin associates with the destruction complex composed of Axin, adenomatous polyposis coli (APC), casein kinase (CK) 2, and glycogen synthase kinase (GSK)-3. GSK-3 phosphorylates β-catenin, inducing rapid degradation of β-catenin via the ubiquitin/proteasome pathway. Right: The canonical Wnt signaling is initiated by the binding of the Wnt ligand to Frizzled and LRP5/6. Subsequently, Wnt ligand blocks β-catenin degradation, allowing transportation to the nucleus, where β-catenin interacts with the transcription factor T-cell-specific factor (TCF) and activates target gene expression.
The non-canonical pathways require Fz but not LRP, β-catenin, or TCF transcription factors. Noncanonical Wnt ligands interact with alternative Wnt receptors, such as receptor tyrosine kinase-like orphan receptor (Ror) 2 or receptor tyrosine kinase Ryk [11], [21], [22]. Noncanonical Wnt signaling is mediated by at least two mechanisms. The first is the planar cell polarity (PCP) pathway, which controls tissue polarity, a process in which cells orient themselves within a plane perpendicular to the apical–basal axis [23], [24], [25]. Activation of small GTPases, such as Rho and Rac, by noncanonical Wnt/Fz is a key mechanism that regulates the Wnt-PCP pathway to promote reorganization of the actin cytoskeleton [25], [26]. The second mechanism is the Wnt/Ca2+ pathway, a pathway that promotes intracellular calcium transients to regulate cell movements [27]. In the Wnt/Ca2+ pathway, Wnt5a triggers intracellular Ca2+ release to activate protein kinase C and Ca2+/calmodulin-dependent kinase II [22]. Alternatively, binding of Wnt5a to Ror2 can activate c-Jun-N-terminal kinase and inhibit canonical Wnt signaling [21], [28], [29].
Several secreted Wnt antagonists have been reported, including secreted frizzled related protein (sFRP), Dickkopf (Dkk), Sclerostin (Sost gene product), Wnt inhibitory factor-1 (WIF-1), and Wise (Fig. 1) [30]. Members of the sFRP family and WIF-1 bind primarily to Wnt proteins, inhibiting the interaction between Wnts and Fz [31]. LRP5/6 binds to DKK and sclerostin to inhibit Wnt signaling [32], [33]. Sclerostin has also been shown to bind to LRP4 [34], and different regions of sclerostin interact with LRP5/6 and LRP4 [35]. The secreted protein Wise and its orthologs [Sostdc1, uterine sensitization-associated gene-1 (USAG-1), and Ectodin] reportedly inhibit Wnt signaling through an interaction with LRP6 [36]. Recently, Notum was identified as a secreted Wnt antagonist that belongs to the α/β hydrolase superfamily. Notum deacylates Wnt proteins to suppress signaling activity [37].
The Wnt signaling pathway is reportedly involved in regulation of bone mass and formation of bone and tooth tissues [19]. In fact, Wnt signaling plays a critical and evolutionarily conserved role in tooth development at many stages. In this review, we discuss the current understanding of Wnt signaling components and its functions in the tooth.
3. Canonical Wnt signaling pathway components in the tooth and periodontal tissue
3.1. Tooth development
Several Wnt signaling components, including Wnt ligands, receptors, transducers, transcription factors, and antagonists, are expressed in the dental epithelium and mesenchyme during tooth development in humans and mice (Table 1) [38], [39]. Wnt 3, Wnt4, Wnt6, Wnt7b, and Wnt10b are expressed in the epithelium. Wnt5a shows localized expression in the mesenchyme and dental papilla. Wnt3, Wnt5a, LRP5, Fz6, β-catenin, Lef1, and Dkk1 exhibit similar expression patterns during tooth development in humans and mice [39]. Targeting components of the canonical Wnt pathway influences tooth formation.
Table 1.
Expression of Wnt signaling pathway components in the tooth.
| Classification | Function | Name | References |
|---|---|---|---|
| Canonical | Ligand | Wnt3 | [38], [39] |
| Wnt3a | [63], [107] | ||
| Wnt4 | [38], [107], [108] | ||
| Wnt6 | [38], [107] | ||
| Wnt7a | [107] | ||
| Wnt7b | [38] | ||
| Wnt10a | [46], [107] | ||
| Wnt10b | [38], [109] | ||
| Receptor | Fz4 | [39] | |
| Fz6 | [38], [39] | ||
| Coreceptor | LRP4 | [107], [110] | |
| LRP5 | [39], [111] | ||
| LRP6 | [111] | ||
| Transducer | Axin2 | [47], [107] | |
| APC | [112], [113] | ||
| Transcription factor | β-Catenin | [39], [114] | |
| LEF1 | [107], [115] | ||
| TCF1 | [107] | ||
| TCF4 | [39] | ||
| Antagonist | DKK1 | [107], [116], [117] | |
| DKK2 | [107], [117] | ||
| DKK3 | [107], [117] | ||
| DKK4 | [107], [117] | ||
| SFRP1 | [118] | ||
| Sclerostin | [67], [68] | ||
| Wise (Sostdc1) | [111], [119] | ||
| Gpr177 | [44] | ||
| Noncanonical | Ligand | Wnt5a | [39], [72], [75], [107] |
| Wnt5b | [107] | ||
| Receptor | Ror2 | [73] | |
| YAP | [106] | ||
During tooth development, nuclear β-catenin is detected in both the dental epithelium and the underlying mesenchyme, and the canonical Wnt signaling pathway is activated at multiple stages of tooth morphogenesis [40]. The effects of canonical Wnt signaling at the early bud stage are mediated through the suppression of Msx1 and Bmp4 gene expression. Inhibition of canonical Wnt signaling results in the development of aberrantly shaped teeth, indicating an essential role of canonical Wnt signaling in early tooth development [40]. Disruption of canonical Wnt signaling either by ectopic expression of DKK1 or deletion of β-catenin or Lef1 in the oral epithelium arrests tooth morphogenesis at the early (DKK1 and β-catenin) or late bud stage (Lef1) [40], [41], [42]. Tissue-specific deletion of β-catenin in developing odontoblasts produced molars lacking roots and aberrantly thin incisors [43]. Inactivation of Gpr177, the mouse ortholog of Drosophila Wls/Evi/Srt, which is essential for proper secretion of Wnt, in the dental epithelium leads to the arrest of early tooth development, and deletion of Axin2 rescued this phenotype [44]. Tissue-specific inactivation of β-catenin in developing odontoblasts produced molars lacking roots and aberrantly thin incisors [43]. Constitutively expressing active β-catenin in the oral epithelium results in formation of multiple teeth after transplantation to the kidney capsule [45].
Wnt10a is specifically expressed in the epithelial signaling centers and the primary and secondary enamel knots [46]. The spatial and temporal distribution of Wnt10a mRNA expression shows that the expression shifts from the secondary enamel knots to the underlying preodontoblasts. In addition, Wnt10a was shown to induce dentin sialophosphoprotein expression. Axin2, which is a direct target of Wnt/β-catenin signaling activity, is also expressed in developing odontoblasts and dental pulp cells [47], indicating that canonical Wnt signaling is associated for dentinogenesis [48]. The tissue-specific inactivation of β-catenin in developing odontoblasts resulted in development of molars lacking roots [49]. Moreover, constitutive activation of β-catenin in the dental mesenchyme leads to differentiation of odontoblasts and cementoblasts and induces excessive dentin and cementum formation in mice [50]. The effects of canonical Wnt in dental pulp injury have also been described. A liposome-reconstituted Wnt3a protein protected pulp cells from cell death and stimulated proliferation of undifferentiated pulp cells, which improved pulp healing in rats [51]. On the other hand, Wnt1 negatively regulates the differentiation of dental pulp stem cells into odontoblasts by the inhibition of alkaline phosphatase (ALP) activity and the formation of mineralized nodules in dental pulp stem cells [52]. This suggests that odontogenesis is regulated through spatial and temporal expression of Wnt1 during odontogenic differentiation.
WIF1, a secreted Wnt antagonist, is strictly expressed in the enamel knot at embryonic day 14.5 (E14.5) and E16.5. Upon knockdown of WIF1, the promotion of apoptosis leads to a delay in an early event during tooth development [53]. WIF1 and Dkk2 are much more abundantly expressed in the mandibular than in the maxillary molar mesenchyme [54]. Other recent reports have claimed that noggin inhibits canonical Wnt and that canonical Wnt signaling functions primarily to control the odontogenic fate of the dental epithelium by sustaining Pitx2 expression and partly to regulate cell proliferation during early odontogenesis [55].
3.2. Periodontal tissue
The roles of canonical Wnt signaling in the development and homeostasis of periodontal tissue have been described [43], [56], [57], [58], [59]. Experiments with conditional β-catenin-stabilized mice have shown that constitutive stabilization of β-catenin in the dental mesenchyme leads to excessive formation of cementum [43], [57]. Disruption of Wnt/β-catenin signaling in odontoblasts and cementoblasts in conditional knockout mice arrests tooth root development [58]. Cells showing Wnt responsiveness are distributed adjacent to the cementum in the periodontal ligaments of continuously erupting incisor teeth and are coincident with the distribution of proliferating cells [56]. Using OCCM-30 cells, an immortalized murine cementoblast cell line, Wnt3a was shown to suppress the expression of ALP, bone sialoprotein, and osteocalcin expression [60]. Wnt3a also increases the expression of cyclin D1, a known cell cycle regulator, suggesting that Wnt3a inhibits cementoblast differentiation and promotes cell proliferation [60]. Amelogenin, an enamel matrix protein that is secreted by ameloblasts, has been implicated in cementogenesis [61]. Amelogenin activates the canonical Wnt signaling pathway in human periodontal ligament cells in culture, although the mechanism remains unclear [62].
Recently, Nemoto et al. reported Wnt3a expression in Hertwig's epithelial root sheath (HERS) cells during mouse tooth root development as well as in cultured human epithelial rest cells of Malassez, whereas Wnt3a expression could not be detected in dental mesenchymal cells in culture [63]. In immortalized murine dental follicle cells, Wnt3a induced ALP expression. Moreover, Wnt3a induced Runx2 and Osterix in these cells, suggesting a potential role of HERS in stimulating cementoblast/osteoblast differentiation of dental follicle cells via the canonical Wnt signaling pathway [63]. The activation of the canonical Wnt signaling has recently been reported to induce in vivo cementum regeneration in the rat periodontal defect model and in vitro cementoblast differentiation of human periodontal ligament cells [64]. Also, when lithium ions, which are known activators of canonical Wnt signaling [65], are released from bioactive scaffolds, they enhance the proliferation and differentiation of human periodontal ligament cells on the scaffolds [66]. Sclerostin is a canonical Wnt antagonist expressed by cementocytes [67]. Observations from sclerostin-knockout mice reveal an increased width of the cementum and concomitant moderate decrease in the periodontal space width. No significant differences were observed for the tooth and pulp chamber volume, suggesting that sclerostin alters the cementum phenotype rather than tooth development [68]. These reports indicate that regulation of the canonical Wnt signaling pathway plays critical roles in the differentiation of odontoblasts and cementoblasts and that regulation of canonical Wnt signaling may function in the formation of dentin and cementum during tooth development and tissue regeneration.
4. Noncanonical Wnt signaling pathway components in tooth
Wnt5a is strongly expressed in murine and human dental papilla mesenchyme [69], [70]. Overexpression of Wnt5a inhibits the proliferation and migration of human dental papilla cells (hDPCs) [70], and Wnt5a increases mineralization-related gene expression in hDPCs, suggesting that Wnt5a promotes the differentiation of hDPCs [71]. Wnt6 overexpression also induced expression of osteogenesis-related genes while exerting no significant effect on the proliferation of hDPCs, indicating a role of Wnt6 in odontogenic differentiation [72]. The loss of the Wnt5a function experiment, which used Wnt5a-deficient mice to induce the abnormal pattern of cups due to decreased cell proliferation in dental epithelium and mesenchyme, retardation of tooth development, and smaller tooth suggests that Wnt5a regulates growth, patterning, and odontoblast differentiation during odontogenesis [73]. Conversely, the gain of Wnt5a function experiment used exogenous Wnt5a protein to induce cell death in the dental region [74]. Their observations indicate that Wnt5a regulates the balance between cell proliferation and cell death, which involves epithelial and mesenchymal interactions on tooth development.
Wnt5a expression was also detected in tooth lining cells and dental follicle cells during mouse tooth development. Knockdown of Wnt5a using siRNA enhanced the Wnt3a-induced ALP expression in SVF4 cells, an immortalized murine dental follicle cell line, indicating that Wnt5a negatively regulates canonical Wnt-mediated early dental follicle cell differentiation [75]. These results suggest the existence of a feedback mechanism between canonical and noncanonical Wnt signaling during the differentiation of dental follicle cells [75]. Other reports have suggested a similar function for Wnt5a because knockdown of Wnt5a in human PDL stem cells enhanced the ALP activity induced by osteoinductive medium, in which canonical Wnt signaling may be activated [76]. Wnt5a reportedly suppresses osteogenic-related markers of human PDL cells cultured by osteogenic induction medium [77]. Conversely, Wnt5a signaling functions as a positive regulator in dental follicle cell differentiation triggered by BMP-2 [78], suggesting that the function of Wnt5a may depend on the state of the cell. These diverse responses may be due to multiple options for the transduction of Wnt5a signals [79], including the availability of various receptors and other signaling cascade mediators [80]. The observations described above indicate that the noncanonical Wnt signaling pathway plays critical roles in tooth development and suggest that Wnt regulation may have potential uses in regenerative therapy.
5. Human diseases in tooth associated with mutations of the Wnt signaling components
5.1. Wnt10a mutation
Wnt10a of the canonical Wnt pathway is expressed in the dental epithelium and mesenchyme. WNT10a mutations have been reported in various ectodermal dysplasia syndromes, rare autosomal-recessive odonto-onycho-dermal dysplasia (OODD) [81], [82], and severe autosomal recessively inherited Schöpf–Schulz–Passarge syndrome (SPSS) [83], [84], [85]. OODD and SSPS share a common ectodermal dysplasia involving hair, teeth, nails, and skin, characterized by dry hair, hypodontia (tooth agenesis), smooth tongue, nail dysplasia, hyperkeratosis of the skin, and palmoplantar keratoderma. Currently, OODS and SSPS are considered a part of the same disorder within the Wnt10a mutations [86], [87]. Wnt10a mutations have also been described to be the most common causes of non-syndromic severe hypodontia with minor signs of ectodermal dysplasia [88] and autosomal-dominant inherited isolated hypodontia [89], [90], [91].
5.2. Axin2 mutation
Axin2, which is localized to chromosome 17q21–q25, is a negative regulator of the canonical Wnt pathway that suppresses signal transduction by promoting β-catenin degradation [92]. Axin2 mutations have been described in oligodontia [93], [94]. It also reported that three Axin2 gene variants are associated with both hypodontia and oligodontia [95]. Mutations of this gene have also been found in various human cancers, including those of skin, gastrointestinal, liver, and ovary [96]. However, the relationship between tooth agenesis and tumor is still controversial [97], [98]. Using Axin2-lacZ mice, Axin2 expression was observed in primary and secondary enamel knots and in the underlying mesenchyme at the development stage [47]. Postnatal Axin2 expression has been detected in developing odontoblasts and in the dental pulp; it has also been found concentrated around the developing roots. These observations suggest roles for Axin2 in defining the regions of enamel formation and in controlling root development during the late stages of tooth development [47].
5.3. LRP6 mutation
LRP6 encodes a transmembrane cell-surface protein that, together with members of the Fz receptor family, functions as a co-receptor in the canonical Wnt signaling pathway. Recently, Massink et al. reported four heterozygous LRP6 loss-of-function mutations in four independent families affected by non-syndromic autosomal-dominant oligodontia [99]. They also showed that the LRP6 missense variant (c.56C>T) results in altered glycosylation and improper subcellular localization of the protein, resulting in abrogated activation of canonical Wnt signaling. Thus, the disruption of canonical Wnt signaling is of importance in the etiology of tooth agenesis [99].
6. Prospective of other Wnt signaling molecules
Yes-associated protein (YAP) and transcriptional coactivator with PDZ binding domain (TAZ) are WW domain-containing transcriptional coactivators originally identified in a proteomic screening for 14-3-3 binding proteins [100] and are well known to be regulated by the Hippo signaling pathway [101]. Azzolin et al. reported that TAZ is a downstream component of the canonical Wnt signaling pathway and a mediator of Wnt biological responses independent of the Hippo pathway [102], [103]. Recently, Park et al. reported YAP/TAZ as downstream effectors of the alternative Wnt signaling pathway [104]. The alternative Wnt-YAP/TAZ signaling axis, which consists of Wnt-Fz/Ror-Gα12/13-RhoGTPases-Lats1/2, promotes YAP/TAZ activation and TEAD-mediated transcription [104]. In transgenic mice that overexpress a constitutively active form of YAP in the dental epithelium, the resulting tooth phenotype was deformed tooth morphogenesis with widened dental lamina [105]. In contrast, YAP deficiency in the dental epithelium resulted in development of a small tooth germ with reduced epithelial cell proliferation [106]. Data from the gene expression profiles of an embryonic E14.5 YAP conditional knockout and YAP transgenic mouse tooth germs indicate that YAP regulates the expression of Hoxa1 and Hoxc13 in oral as well as dental epithelial tissues [106]. However, the relationship between Wnt signaling and YAP/TAZ in tooth formation remains to be determined. Direct interactions of YAP/TAZ in the epithelial tissues of the developing tooth may provide insights into the molecular mechanisms of Wnt signaling not only in terms of organism development but also regarding the onset and progression of human diseases.
7. Conclusion
In conclusion, several recent studies have indicated a role of both the canonical and noncanonical Wnt signaling pathways in the regulation of tooth development, maintenance, and turnover. Therefore, strategies to modulate Wnt signaling pathway components are very attractive for the treatment of tooth regeneration. Hopefully, the efforts currently underway to target Wnt signaling will be successful and generate new therapeutics in the future.
Conflict of interest
None declared.
Acknowledgements
This work was supported by Grant-in-aid for Scientific Research from Japan Society for the Promotion of Science (No. 21390552 and 22390346).
References
- 1.Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 2.Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 3.Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 4.Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., McMahon A. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64(2):231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- 5.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Ke J., Xu H.E., Williams B.O. Lipid modification in Wnt structure and function. Curr Opin Lipidol. 2013;24(2):129–133. doi: 10.1097/MOL.0b013e32835df2bf. [DOI] [PubMed] [Google Scholar]
- 7.Thesleff I., Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67(2):111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 8.Miller J. The Wnts. Genome Biol. 2002;3(1) doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Amerongen R., Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22(12):678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Ikeya M., Lee S., Johnson J., McMahon A., Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389(6654):966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 11.Gordon M., Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 12.Janda C.Y., Waghray D., Levin A.M., Thomas C., Garcia K.C. Structural basis of Wnt recognition by Frizzled. Science. 2012;337(6090):59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens J., Jerchow B.A., Würtele M., Grimm J., Asbrand C., Wirtz R. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 14.Huelsken J., Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115(Pt 21):3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 15.Zeng L., Fagotto F., Zhang T., Hsu W., Vasicek T.J., Perry W.L. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 16.Huelsken J., Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11(5):547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 17.Malbon C., Wang H. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006;72:153–166. doi: 10.1016/S0070-2153(05)72002-0. [DOI] [PubMed] [Google Scholar]
- 18.Yochum G., McWeeney S., Rajaraman V., Cleland R., Peters S., Goodman R. Serial analysis of chromatin occupancy identifies beta-catenin target genes in colorectal carcinoma cells. Proc Natl Acad Sci U S A. 2007;104(9):3324–3329. doi: 10.1073/pnas.0611576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura M., Nemoto E., Sato M.M., Nakashima A., Shimauchi H. Role of the Wnt signaling pathway in bone and tooth. Front Biosci (Elite Ed) 2010;2:1405–1413. doi: 10.2741/e201. [DOI] [PubMed] [Google Scholar]
- 20.Tamura M., Sato M., Nashimoto M. Regulation of CXCL12 expression by canonical Wnt signaling in bone marrow stromal cells. Int J Biochem Cell Biol. 2011;43(5):760–767. doi: 10.1016/j.biocel.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Green J., Kuntz S., Sternberg P. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18(11):536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Amerongen R., Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 23.Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130(19):4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- 24.Seifert J.R., Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 25.Gray R.S., Roszko I., Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21(1):120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlessinger K., Hall A., Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 27.Kühl M., Sheldahl L.C., Park M., Miller J.R., Moon R.T. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16(7):279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 28.Oishi I., Suzuki H., Onishi N., Takada R., Kani S., Ohkawara B. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 29.Minami Y., Oishi I., Endo M., Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn. 2010;239(1):1–15. doi: 10.1002/dvdy.21991. [DOI] [PubMed] [Google Scholar]
- 30.Malinauskas T., Jones E.Y. Extracellular modulators of Wnt signalling. Curr Opin Struct Biol. 2014;29:77–84. doi: 10.1016/j.sbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Bovolenta P., Esteve P., Ruiz J., Cisneros E., Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 32.Semënov M., Tamai K., He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 33.Semenov M.V., He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. 2006;281(50):38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]
- 34.Choi H.Y., Dieckmann M., Herz J., Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS ONE. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holdsworth G., Slocombe P., Doyle C., Sweeney B., Veverka V., Le Riche K. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J Biol Chem. 2012;287(32):26464–26477. doi: 10.1074/jbc.M112.350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lintern K., Guidato S., Rowe A., Saldanha J., Itasaki N. Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J Biol Chem. 2009;284(34):23159–23168. doi: 10.1074/jbc.M109.025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakugawa S., Langton P.F., Zebisch M., Howell S.A., Chang T.H., Liu Y. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519(7542):187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar L., Sharpe P. Expression of Wnt signalling pathway genes during tooth development. Mech Dev. 1999;85(1–2):197–200. doi: 10.1016/s0925-4773(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 39.Wang B., Li H., Liu Y., Lin X., Lin Y., Wang Y. Expression patterns of WNT/β-CATENIN signaling molecules during human tooth development. J Mol Histol. 2014;45(5):487–496. doi: 10.1007/s10735-014-9572-5. [DOI] [PubMed] [Google Scholar]
- 40.Liu F., Chu E., Watt B., Zhang Y., Gallant N., Andl T. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313(1):210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Genderen C., Okamura R., Fariñas I., Quo R., Parslow T., Bruhn L. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8(22):2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 42.Andl T., Reddy S., Gaddapara T., Millar S. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2(5):643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 43.Kim T.H., Bae C.H., Lee J.C., Ko S.O., Yang X., Jiang R. β-Catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013;92(3):215–221. doi: 10.1177/0022034512470137. [DOI] [PubMed] [Google Scholar]
- 44.Zhu X., Zhao P., Liu Y., Zhang X., Fu J., Ivy Yu H.M. Intra-epithelial requirement of canonical Wnt signaling for tooth morphogenesis. J Biol Chem. 2013;288(17):12080–12089. doi: 10.1074/jbc.M113.462473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Järvinen E., Salazar-Ciudad I., Birchmeier W., Taketo M., Jernvall J., Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103(49):18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luukko K., Løes S., Furmanek T., Fjeld K., Kvinnsland I.H., Kettunen P. Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev. 2003;120(3):270–276. doi: 10.1016/s0925-4773(02)00458-6. [DOI] [PubMed] [Google Scholar]
- 47.Lohi M., Tucker A.S., Sharpe P.T. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 2010;239(1):160–167. doi: 10.1002/dvdy.22047. [DOI] [PubMed] [Google Scholar]
- 48.Yamashiro T., Zheng L., Shitaku Y., Saito M., Tsubakimoto T., Takada K. Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation. 2007;75(5):452–462. doi: 10.1111/j.1432-0436.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 49.Bae C.H., Lee J.Y., Kim T.H., Baek J.A., Lee J.C., Yang X. Excessive Wnt/β-catenin signaling disturbs tooth-root formation. J Periodontal Res. 2013;48(4):405–410. doi: 10.1111/jre.12018. [DOI] [PubMed] [Google Scholar]
- 50.Kim T.H., Lee J.Y., Baek J.A., Lee J.C., Yang X., Taketo M.M. Constitutive stabilization of β-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011;412(4):549–555. doi: 10.1016/j.bbrc.2011.07.116. [DOI] [PubMed] [Google Scholar]
- 51.Hunter D.J., Bardet C., Mouraret S., Liu B., Singh G., Sadoine J. Wnt acts as a prosurvival signal to enhance dentin regeneration. J Bone Miner Res. 2015;30(7):1150–1159. doi: 10.1002/jbmr.2444. [DOI] [PubMed] [Google Scholar]
- 52.Scheller E., Chang J., Wang C. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87(2):126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee M.J., Kim E.J., Li L., Jung H.S. Roles of Wnt inhibitory factor 1 during tooth morphogenesis. Cell Tissue Res. 2015;362(1):61–68. doi: 10.1007/s00441-015-2170-3. [DOI] [PubMed] [Google Scholar]
- 54.Jia S., Zhou J., Gao Y., Baek J.A., Martin J.F., Lan Y. Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development. 2013;140(2):423–432. doi: 10.1242/dev.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan G., Yang G., Zheng Y., Zhu X., Chen Z., Zhang Z. The non-canonical BMP and Wnt/β-catenin signaling pathways orchestrate early tooth development. Development. 2015;142(1):128–139. doi: 10.1242/dev.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rooker S., Liu B., Helms J. Role of Wnt signaling in the biology of the periodontium. Dev Dyn. 2010;239(1):140–147. doi: 10.1002/dvdy.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim N.H., Kim H.S., Kim N.G., Lee I., Choi H.S., Li X.Y. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci Signal. 2011;4(197):ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R., Yang G., Wu X., Xie J., Yang X., Li T. Disruption of Wnt/β-catenin signaling in odontoblasts and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci. 2013;9(3):228–236. doi: 10.7150/ijbs.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim W.H., Liu B., Cheng D., Williams B.O., Mah S.J., Helms J.A. Wnt signaling regulates homeostasis of the periodontal ligament. J Periodontal Res. 2014;49(6):751–759. doi: 10.1111/jre.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nemoto E., Koshikawa Y., Kanaya S., Tsuchiya M., Tamura M., Somerman M. Wnt signaling inhibits cementoblast differentiation and promotes proliferation. Bone. 2009;44(5):805–812. doi: 10.1016/j.bone.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 61.Hatakeyama J., Sreenath T., Hatakeyama Y., Thyagarajan T., Shum L., Gibson C. The receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenic pathway is elevated in amelogenin-null mice. J Biol Chem. 2003;278(37):35743–35748. doi: 10.1074/jbc.M306284200. [DOI] [PubMed] [Google Scholar]
- 62.Matsuzawa M., Sheu T., Lee Y., Chen M., Li T., Huang C. Putative signaling action of amelogenin utilizes the Wnt/beta-catenin pathway. J Periodontal Res. 2009;44(3):289–296. doi: 10.1111/j.1600-0765.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 63.Nemoto E., Sakisaka Y., Tsuchiya M., Tamura M., Nakamura T., Kanaya S. Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res. 2015 doi: 10.1111/jre.12294. [DOI] [PubMed] [Google Scholar]
- 64.Han P., Ivanovski S., Crawford R., Xiao Y. Activation of the canonical Wnt signaling pathway induces cementum regeneration. J Bone Miner Res. 2015;30(7):1160–1174. doi: 10.1002/jbmr.2445. [DOI] [PubMed] [Google Scholar]
- 65.Hedgepeth C.M., Conrad L.J., Zhang J., Huang H.C., Lee V.M., Klein P.S. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185(1):82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 66.Han P., Wu C., Chang J., Xiao Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials. 2012;33(27):6370–6379. doi: 10.1016/j.biomaterials.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 67.Jäger A., Götz W., Lossdörfer S., Rath-Deschner B. Localization of SOST/sclerostin in cementocytes in vivo and in mineralizing periodontal ligament cells in vitro. J Periodontal Res. 2010;45(2):246–254. doi: 10.1111/j.1600-0765.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 68.Kuchler U., Schwarze U.Y., Dobsak T., Heimel P., Bosshardt D.D., Kneissel M. Dental and periodontal phenotype in sclerostin knockout mice. Int J Oral Sci. 2014;6(2):70–76. doi: 10.1038/ijos.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarkar L., Sharpe P. Inhibition of Wnt signaling by exogenous Mfrzb1 protein affects molar tooth size. J Dent Res. 2000;79(4):920–925. doi: 10.1177/00220345000790040601. [DOI] [PubMed] [Google Scholar]
- 70.Peng L., Ye L., Dong G., Ren L., Wang C., Xu P. WNT5A inhibits human dental papilla cell proliferation and migration. Biochem Biophys Res Commun. 2009;390(3):1072–1078. doi: 10.1016/j.bbrc.2009.10.136. [DOI] [PubMed] [Google Scholar]
- 71.Peng L., Ren L.B., Dong G., Wang C.L., Xu P., Ye L. Wnt5a promotes differentiation of human dental papilla cells. Int Endod J. 2010;43(5):404–412. doi: 10.1111/j.1365-2591.2010.01693.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang C., Ren L., Peng L., Xu P., Dong G., Ye L. Effect of Wnt6 on human dental papilla cells in vitro. J Endod. 2010;36(2):238–243. doi: 10.1016/j.joen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Lin M., Li L., Liu C., Liu H., He F., Yan F. Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev Dyn. 2011;240(2):432–440. doi: 10.1002/dvdy.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai J., Mutoh N., Shin J.O., Tani-Ishii N., Ohshima H., Cho S.W. Wnt5a plays a crucial role in determining tooth size during murine tooth development. Cell Tissue Res. 2011;345(3):367–377. doi: 10.1007/s00441-011-1224-4. [DOI] [PubMed] [Google Scholar]
- 75.Sakisaka Y., Tsuchiya M., Nakamura T., Tamura M., Shimauchi H., Nemoto E. Wnt5a attenuates Wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp Cell Res. 2015;336(1):85–93. doi: 10.1016/j.yexcr.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Yamada A., Iwata T., Yamato M., Okano T., Izumi Y. Diverse functions of secreted frizzled-related proteins in the osteoblastogenesis of human multipotent mesenchymal stromal cells. Biomaterials. 2013;34(13):3270–3278. doi: 10.1016/j.biomaterials.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 77.Hasegawa D., Wada N., Maeda H., Yoshida S., Mitarai H., Tomokiyo A. Wnt5a induces collagen production by human periodontal ligament cells through TGFβ1-mediated upregulation of periostin expression. J Cell Physiol. 2015;230(11):2647–2660. doi: 10.1002/jcp.24950. [DOI] [PubMed] [Google Scholar]
- 78.Xiang L., Chen M., He L., Cai B., Du Y., Zhang X. Wnt5a regulates dental follicle stem/progenitor cells of the periodontium. Stem Cell Res Ther. 2014;5(6):135. doi: 10.1186/scrt525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veeman M.T., Axelrod J.D., Moon R.T. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 80.Pukrop T., Binder C. The complex pathways of Wnt 5a in cancer progression. J Mol Med (Berl) 2008;86(3):259–266. doi: 10.1007/s00109-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 81.Adaimy L., Chouery E., Megarbane H., Mroueh S., Delague V., Nicolas E. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81(4):821–828. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cluzeau C., Hadj-Rabia S., Jambou M., Mansour S., Guigue P., Masmoudi S. Only four genes (EDA1, EDAR, EDARADD, and WNT10A) account for 90% of hypohidrotic/anhidrotic ectodermal dysplasia cases. Hum Mutat. 2011;32(1):70–72. doi: 10.1002/humu.21384. [DOI] [PubMed] [Google Scholar]
- 83.Bohring A., Stamm T., Spaich C., Haase C., Spree K., Hehr U. WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet. 2009;85(1):97–105. doi: 10.1016/j.ajhg.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagy N., Wedgeworth E., Hamada T., White J.M., Hashimoto T., McGrath J.A. Schöpf–Schulz–Passarge syndrome resulting from a homozygous nonsense mutation in WNT10A. J Dermatol Sci. 2010;58(3):220–222. doi: 10.1016/j.jdermsci.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 85.Castori M., Castiglia D., Brancati F., Foglio M., Heath S., Floriddia G. Two families confirm Schöpf–Schulz–Passarge syndrome as a discrete entity within the WNT10A phenotypic spectrum. Clin Genet. 2011;79(1):92–95. doi: 10.1111/j.1399-0004.2010.01513.x. [DOI] [PubMed] [Google Scholar]
- 86.Kantaputra P., Kaewgahya M., Jotikasthira D., Kantaputra W. Tricho-odonto-onycho-dermal dysplasia and WNT10A mutations. Am J Med Genet A. 2014;164A(4):1041–1048. doi: 10.1002/ajmg.a.36388. [DOI] [PubMed] [Google Scholar]
- 87.Vink C.P., Ockeloen C.W., ten Kate S., Koolen D.A., Ploos van Amstel J.K., Kuijpers-Jagtman A.M. Variability in dentofacial phenotypes in four families with WNT10A mutations. Eur J Hum Genet. 2014;22(9):1063–1070. doi: 10.1038/ejhg.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plaisancié J., Bailleul-Forestier I., Gaston V., Vaysse F., Lacombe D., Holder-Espinasse M. Mutations in WNT10A are frequently involved in oligodontia associated with minor signs of ectodermal dysplasia. Am J Med Genet A. 2013;161A(4):671–678. doi: 10.1002/ajmg.a.35747. [DOI] [PubMed] [Google Scholar]
- 89.Kantaputra P., Sripathomsawat W. WNT10A and isolated hypodontia. Am J Med Genet A. 2011;155A(5):1119–1122. doi: 10.1002/ajmg.a.33840. [DOI] [PubMed] [Google Scholar]
- 90.van den Boogaard M.J., Créton M., Bronkhorst Y., van der Hout A., Hennekam E., Lindhout D. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J Med Genet. 2012;49(5):327–331. doi: 10.1136/jmedgenet-2012-100750. [DOI] [PubMed] [Google Scholar]
- 91.Mostowska A., Biedziak B., Zadurska M., Dunin-Wilczynska I., Lianeri M., Jagodzinski P.P. Nucleotide variants of genes encoding components of the Wnt signalling pathway and the risk of non-syndromic tooth agenesis. Clin Genet. 2013;84(5):429–440. doi: 10.1111/cge.12061. [DOI] [PubMed] [Google Scholar]
- 92.Jho E., Zhang T., Domon C., Joo C., Freund J., Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lammi L., Arte S., Somer M., Jarvinen H., Lahermo P., Thesleff I. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74(5):1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong S., Liu H., Bai B., Chang H., Zhao H., Wang Y. Novel missense mutations in the AXIN2 gene associated with non-syndromic oligodontia. Arch Oral Biol. 2014;59(3):349–353. doi: 10.1016/j.archoralbio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Mostowska A., Biedziak B., Jagodzinski P.P. Axis inhibition protein 2 (AXIN2) polymorphisms may be a risk factor for selective tooth agenesis. J Hum Genet. 2006;51(3):262–266. doi: 10.1007/s10038-005-0353-6. [DOI] [PubMed] [Google Scholar]
- 96.Salahshor S., Woodgett J.R. The links between axin and carcinogenesis. J Clin Pathol. 2005;58(3):225–236. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonds J., Pollan-White S., Xiang L., Mues G., D'Souza R. Is there a link between ovarian cancer and tooth agenesis? Eur J Med Genet. 2014;57(5):235–239. doi: 10.1016/j.ejmg.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindor N.M., Win A.K., Gallinger S., Daftary D., Thibodeau S.N., Silva R. Colorectal cancer and self-reported tooth agenesis. Hered Cancer Clin Pract. 2014;12(1):7. doi: 10.1186/1897-4287-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Massink M.P., Créton M.A., Spanevello F., Fennis W.M., Cune M.S., Savelberg S.M. Loss-of-function mutations in the WNT co-receptor LRP6 cause autosomal-dominant oligodontia. Am J Hum Genet. 2015;97(4):621–626. doi: 10.1016/j.ajhg.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanai F., Marignani P.A., Sarbassova D., Yagi R., Hall R.A., Donowitz M. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19(24):6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Piccolo S., Dupont S., Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 102.Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 103.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 104.Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.S., Plouffe S.W. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162(4):780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu M., Zhao S., Wang X.P. YAP overexpression affects tooth morphogenesis and enamel knot patterning. J Dent Res. 2014;93(5):469–474. doi: 10.1177/0022034514525784. [DOI] [PubMed] [Google Scholar]
- 106.Liu M., Zhao S., Lin Q., Wang X.P. YAP regulates the expression of Hoxa1 and Hoxc13 in mouse and human oral and skin epithelial tissues. Mol Cell Biol. 2015;35(8):1449–1461. doi: 10.1128/MCB.00765-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suomalainen M., Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 2010;239(1):364–372. doi: 10.1002/dvdy.22106. [DOI] [PubMed] [Google Scholar]
- 108.Kettunen P., Løes S., Furmanek T., Fjeld K., Kvinnsland I.H., Behar O. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial–mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development. 2005;132(2):323–334. doi: 10.1242/dev.01541. [DOI] [PubMed] [Google Scholar]
- 109.Nadiri A., Kuchler-Bopp S., Haikel Y., Lesot H. Immunolocalization of BMP-2/-4, FGF-4, and WNT10b in the developing mouse first lower molar. J Histochem Cytochem. 2004;52(1):103–112. doi: 10.1177/002215540405200110. [DOI] [PubMed] [Google Scholar]
- 110.Ohazama A., Johnson E.B., Ota M.S., Choi H.Y., Choi H.J., Porntaveetus T. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS ONE. 2008;3(12):e4092. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahn Y., Sanderson B.W., Klein O.D., Krumlauf R. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 2010;137(19):3221–3231. doi: 10.1242/dev.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang M., Dobeck J.M., Sorkin B.C., Skobe Z. Adenomatous polyposis coli protein is expressed in alternate stages of the ameloblast life cycle. J Dent Res. 1998;77(12):1979–1982. doi: 10.1177/00220345980770120501. [DOI] [PubMed] [Google Scholar]
- 113.Wang X.P., O’Connell D.J., Lund J.J., Saadi I., Kuraguchi M., Turbe-Doan A. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136(11):1939–1949. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Obara N., Suzuki Y., Takeda M. Gene expression of beta-catenin is up-regulated in inner dental epithelium and enamel knots during molar tooth morphogenesis in the mouse. Cell Tissue Res. 2006;325(1):197–201. doi: 10.1007/s00441-005-0136-6. [DOI] [PubMed] [Google Scholar]
- 115.Sasaki T., Ito Y., Xu X., Han J., Bringas P., Maeda T. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 2005;278(1):130–143. doi: 10.1016/j.ydbio.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 116.Monaghan A.P., Kioschis P., Wu W., Zuniga A., Bock D., Poustka A. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev. 1999;87(1–2):45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 117.Fjeld K., Kettunen P., Furmanek T., Kvinnsland I.H., Luukko K. Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn. 2005;233(1):161–166. doi: 10.1002/dvdy.20285. [DOI] [PubMed] [Google Scholar]
- 118.Liu W., Singh R., Choi C.S., Lee H.Y., Keramati A.R., Samuel V.T. Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem. 2012;287(10):7213–7223. doi: 10.1074/jbc.M111.286724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munne P.M., Tummers M., Järvinen E., Thesleff I., Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development. 2009;136(3):393–402. doi: 10.1242/dev.025064. [DOI] [PubMed] [Google Scholar]