Abstract
Background
Management of the critically bleeding patient can be encountered in many medical and surgical settings. Common for these patients is a high risk of dying from exsanguination secondary to developing coagulopathy. The purpose of this meta-analysis was to systematically review and assess randomised controlled trials (RCTs) performed on patients in acute need for blood transfusions due to bleeding to evaluate the effect of viscoelastic haemostatic assay (VHA) guidance on bleeding, transfusion requirements and mortality.
Methods
PubMed and EMBASE were searched for RCTs that 1) randomised patients into receiving transfusions based on either a VHA-guided (thromboelastography [TEG] or rotational thromboelastometry [ROTEM]) algorithm (intervention group) or at the clinician’s discretion and/or based on conventional coagulation tests (control group) and 2) adequately reported on the outcomes bleeding and/or transfusions and/or mortality. Data on bleeding, transfusions and mortality were extracted from each trial and included in a meta-analysis.
Results
Fifteen RCTs (n = 1238 patients) were included. Nine trials referred to cardiothoracic patients, one to liver transplantation, one to surgical excision of burn wounds and one to trauma. One trial was conducted with cirrhotic patients, one with patients undergoing scoliosis surgery while one trial randomised treatment in post-partum females presenting with bleeding. The amount of transfused red blood cells (RBCs), fresh frozen plasma (FFP) and bleeding volume was found to be significantly reduced in the VHA-guided groups, whereas no significant difference was found for platelet transfusion requirements or mortality.
Keywords: Bleeding, Mortality, ROTEM, TEG, Thrombelastography, Thrombelastometry
Background
Haemorrhage remains a major cause of potentially preventable deaths worldwide. Trauma and massive transfusion is associated with coagulopathy secondary to tissue injury, hypoperfusion, dilution and consumption of clotting factors and platelets [1–9]. Patients undergoing cardiac surgery accompanied by cardiopulmonary bypass (CPB) stand a high risk of dying due to microvascular bleeding and 11% have excessive bleeding after CPB – in most cases found to be nonsurgical [10, 11]. The non-surgical bleeding risk in these patients originates in coagulopathy arisen from distortion of the haemostatic system [12, 13]. Concepts of damage control surgery in trauma have evolved, prioritizing early control of the cause of bleeding by non-definitive means, while haemostatic resuscitation seeks early control of coagulopathy [14, 15]. Haemostatic resuscitation provides transfusions with fresh frozen plasma (FFP) and platelets in addition to red blood cells (RBCs) in an immediate and sustained manner as part of the transfusion protocol for critically bleeding patients. Transfusion of RBCs, FFP and platelets in a similar proportion as in whole blood prevents both hypovolemia and coagulopathy [16, 17]. Although an early and effective reversal of coagulopathy is documented [16, 18], the most effective means of preventing coagulopathy of massive transfusion remains debated. Results from recent before-and-after studies in massively bleeding patients and one randomised clinical trial (RCT) indicate that trauma exsanguination protocols involving the early administration of plasma and platelets are associated with improved survival [19–22]. Furthermore, viscoelastic haemostatic assays (VHAs), such as thrombelastography (TEG)/rotational thromboelastometry (ROTEM), appear advantageous for identifying coagulopathy in patients with severe haemorrhage, as opposed to conventional coagulation tests (CCTs) [23–25]. Current views recommend that patients with uncontrolled bleeding, regardless of its cause, should be treated with goal-directed haemostatic resuscitation involving the early administration of plasma and platelets and the use of VHAs should be considered. The aim of goal-directed therapy should be to maintain a normal haemostatic competence until surgical haemostasis is achieved, as this appears to be associated with reduced mortality [4, 6, 12, 20].
The aim of the present study was to perform a systematic review and meta-analysis of all published RCTs comparing the effect of VHAs versus CCTs on blood loss, transfusion requirements and mortality.
Materials and methods
An electronic search was conducted by one of the authors (MF) in the PubMed and EMBASE database using the following search strategy: (Thrombelastography OR Thromb?elastograph* OR thromboelastograph OR ROTEM OR TEG OR ROTEG OR Thromboelastometry OR (algorithm AND bleeding)) AND ((randomized controlled trial OR controlled clinical trial) OR (randomized OR placebo OR trial)), to identify all RCTs done on bleeding patients using treatment algorithms based on results from either TEG or ROTEM. The search identified 1245 references in PubMed and 1835 references in EMBASE. 222 duplicate findings were discarded, leaving a total of 2858 references for further assessment. References were assessed by one of the authors (MF) and discussed and consensus reached with all authors in doubt cases. Only published RCTs were eligible for this analysis. Inclusion criteria were 1) trial designs in which patients were randomly allocated to receive transfusions based on either a VHA-guided (TEG or ROTEM) algorithm (intervention group) or at the clinician’s discretion and/or based on laboratory coagulation tests (control group) and 2) references had to adequately report the outcomes bleeding and/or transfusions and/or mortality. Studies written in other languages than English were also eligible for inclusion. Trials were excluded immediately based on title or abstract, if they did not meet the inclusion criteria. Moreover, trials that were not performed on humans and paediatric studies were also excluded. The remaining studies were evaluated and assessed for relevance by all authors. Reference lists of the included studies were searched for subsequent relevant studies not identified by search engines. Corresponding authors were contacted to retrieve inadequately reported or missing data. Primary outcomes for data extraction were all-cause mortality, total amount of bleeding expressed either as bleeding at 12 h, 24 h or perioperative amount of bleeding and amount of total RBC transfusions, FFP transfusions and platelet transfusions. When amount of blood transfusions was given in mL, calculations of the corresponding number of units were done using the conversion factors illustrated in table 1. The volume per unit was an estimate of the standard volume of the given allogeneic blood product over the last years in the Capital Region Blood Bank, Rigshospitalet, Copenhagen. The latest follow up data on mortality were used in the analysis of all-cause mortality.
Table 1.
Conversion factors from mL to units
| 1U RBC | 250 mL/U |
| 1U FFP | 270 mL/U |
| 1U platelet concentrate | 340 mL/U |
Statistics
Statistical meta-analyses were conducted using Review Manager (RevMan) Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Pooled estimates and their 95% confidence intervals (CI) were calculated using the inverse variance method. The random-effects model was used in anticipation of significant heterogeneity [26]. Statistical heterogeneity was explored using the inconsistency (I2) measure [27]. For all calculations, two-tailed P values of less than 0.05 were considered statistically significant.
Results
Study characteristics
We identified a total of 2858 references. All references were screened by their title and abstract and 2812 references were found not to be relevant for this meta-analysis and were therefore excluded immediately, leaving 46 references for further scrutiny (fig. 1). Another 31 references were excluded due to the reasons explained in table 2. This left 15 RCTs with a total of n = 1238 patients to be included in this analysis. Of these trials, 9 referred to cardiothoracic patients [28–36] and one each to liver transplantation [37], surgical excision of burn wounds [38], trauma [22], cirrhotic patients [39], scoliosis surgery [40] and post-partum haemorrhage [41]. In twelve studies the intervention group was guided by TEG [22, 28, 29, 31–35, 37, 39–41] and in the remaining three by ROTEM [30, 36, 38]. Seven trials applied both results from CCTs and the discretion of the attending physician to guide the transfusions of the control group [28, 31, 32, 35, 38, 40, 41], while the control groups of eight trials were guided only by CCTs [22, 29, 30, 33, 34, 36, 37, 39] with the first transfused blood products being guided solely at the clinician’s discretion before blood analyses were available in two trials [22, 30]. Eleven trials reported on bleeding [28–35, 37, 40, 41], nine reported on mortality [22, 28, 30, 33, 34, 37, 39–41] and all studies reported on transfusion requirements. The transfusion triggers for RBCs, FFP and platelet concentrates for each study are demonstrated in table 3 and the individual study characteristics are presented in table 4.
Fig. 1.
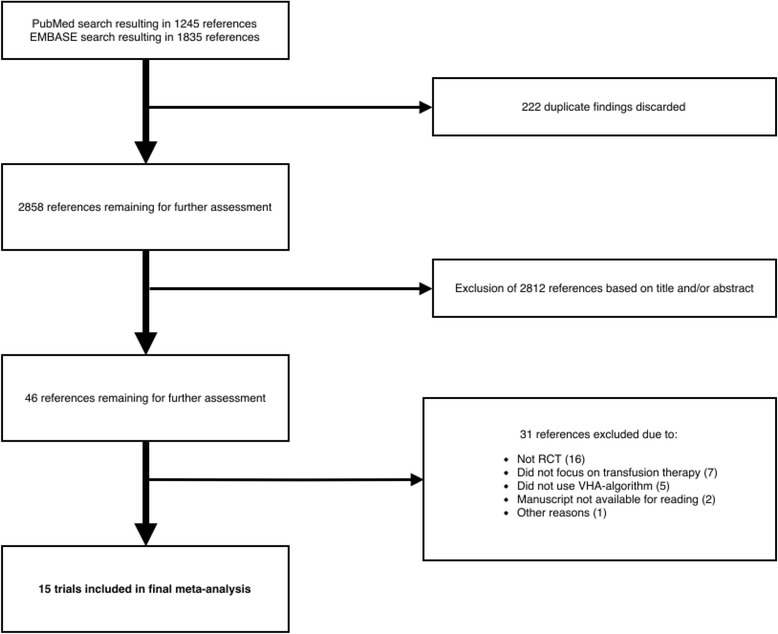
Process of inclusion of trials into meta-analysis
Table 2.
Author and year, type of patients examined and reason for exclusion in excluded scrutinized references
| Reference (Author and year) | Condition | Reason for exclusion |
|---|---|---|
| Agarwal 2015 [46] | Cardiac surgery | Focus on platelet function testing |
| Branco 2014 [47] | Trauma | Observational trial |
| Brilej 2016 [48] | Trauma | Observational trial |
| Capraro 2001 [49] | Cardiac surgery | No use of VHA |
| Despotis 1994 (a) [50] | Cardiac surgery | No use of VHA |
| Despotis 1994 (b) [51] | Cardiac surgery | No use of VHA |
| Dietrich 2008 [52] | Cardiac surgery | Focus on TXA-therapy |
| Einersen 2016 [53] | Trauma | Observational trial |
| Hajek 2010 [54] | Cardiac surgery | Intervention group is managed both with CCT and VHA-analyses |
| Hanke 2012 [55] | Aortic surgery | Not randomised – matched control group |
| Harding 1997 [56] | Liver transplantation | Observational trial |
| Helm 1998 [57] | Cardiac surgery | Not randomised – matched control group |
| Hoenicka 2015 [58] | Cardiac surgery | Focus on heparin management |
| Hopkins 1983 [59] | Acute hypotension | General treatment algorithm |
| Israelian 2009 [60] | Neuro surgery | Possibly relevant. Manuscript not available for reading. Contact information of corresponding author not available. |
| Karkouti 2016 [61] | Cardiac surgery | Stepped-Wedge Clustered RCT |
| Levin 2014 [62] | Cardiac surgery | Focus on protamine-administration |
| Lier 2009 [63] | Trauma | Review |
| Mallaiah 2015 [64] | Obstetric haemorrhage | Before-after trial |
| Manikappa 2011 [65] | Cardiac surgery | Whole blood transfusions |
| Messenger 2011 [66] | Trauma | Prospective cohort study |
| Mishra 2015 [67] | Cardiac surgery | Focus on platelet function testing |
| Naik 2015 [68] | Major spinal surgery | Non-randomised |
| Petricevic 2013 [69] | Cardiac surgery | Observational trial |
| Rahe-Meyer 2009 [70] | Aortic surgery | Non-randomised |
| Roullet 2015 [71] | Orthotopic liver transplantation | Non-randomised |
| Smart 2015 [72] | Orthotopic liver transplantation | Retrospective non-randomised trial |
| Stancheva 2011 [73] | Orthotopic liver transplantation | Observational trial |
| Tarabarin 2013 [74] | Bile duct surgery | Possibly relevant. Manuscript not available for reading. Contact information of corresponding author not available. |
| Weitzel 2012 [75] | Cardiac surgery | Focus on platelet function |
| Xu 2014 [76] | Cardiac surgery | Focus on platelet function testing |
VHA viscoelastic haemostatic assay, TXA tranexamic acid
Table 3.
Transfusion algorithm trigger values. Table explaining individual transfusion trigger values in the respective trials included in the meta-analyses
| Reference (Author and year) | RBC | FFP | Platelets | Other | ||||
|---|---|---|---|---|---|---|---|---|
| Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | |
| Shore-Lesserson 1999 [33] | Hct < 25% (during CPB <21%) | Hct < 25% (during CPB <21%) | PT >150% of control (2U FFP) | hTEG R > 20 mm (2U FFP) | PC < 100 · 103/μL (6U PC) | PC < 100 · 103/μL AND TEG MA < 45 mm (6U PC) | Fibrinogen <100 mg/dL 10U of cryo EACA 10 g if failure |
Fibrinogen <100 mg/dL 10U of cryo LY30 > 7.5% EACA 10 g |
| Nuttall 2001 [31] | N/A | N/A | Clinician’s discretion with or without CCT | POC PT > 16.6 s and/or POC APTT > 57 s | Clinician’s discretion with or without CCT | PC < 102 · 103/mm3 and/or TEG MA <48 mm (PC or DDAVP) | Clinician’s discretion with or without CCT | Fibrinogen <144 mg/dL – cryo |
| Royston 2001 [32] | N/A | N/A | Clinician’s discretion with or without CCT | R > 14 mm < 21 mm – 1 FFP R > 21 mm < 28 mm – 2 FFP R > 28 mm – 4 FFP |
Clinician’s discretion with or without CCT | MA < 48 mm – 1 platelet pool MA < 40 mm 2 platelet pools |
Clinician’s discretion with or without CCT | LY30 > 7.5% - Aprotinin |
| Avidan 2004 [29] | Hb < 8 g/dL | Hb < 8 g/dL | If still bleeding >100 mL/h after aprotinin + desmopressin AND INR or APTT ratio > 150% control – 4U FFP | Excessive bleeding + R > 10 min – 4U FFP | Persisting excessive bleeding OR PC < 50x109/L – 1 platelet pool | PFA-100® ADP channel > 120 s, epinephrine channel > 170 s treated with DDAVP 0.4 μg/kg – if bleeding persisted 1 platelet pool | Bleeding >100 mL/h within 24 h after surgery – Aprotinin (2 Mu) + desmopressin (0.4 μg/kg) | LY30 > 7.5% + bleeding >100 mL/h – aprotinin 2Mu PFA-100® ADP channel > 120 s, epinephrine channel > 170 s – DDAVP 0.4 μg/kg |
| Ak 2009 [28] | Htc < 25% (during CPB <18%) | Htc < 25% (during CPB <18%) | PT > 14 s or APTT > 150% normal | R > 14 mm <21 mm – 1 FFP R ≥ 21 mm <28 mm – 2 FFP R ≥ 28 mm – 4 FFP |
PC < 100 · 103/μL | 40 ≤ MA < 48 mm – 1U platelets MA < 40 mm 2U platelets |
Absence of visible clots + presence of generalized oozing-type bleeding in surgical field – TXA | LY30 > 7.5% - TXA |
| Westbrook 2009 [35] | Clinician’s discretion with CCT | Hb > 70 g/L | Clinician’s discretion with CCT | 11 min < R(H) ≤ 14 min – 1U FFP 14 min < R(H) ≤ 20 min – 2U FFP 200 min < R(H) – 4U FFP |
Clinician’s discretion with CCT | MA(H) ≤ 41 mm – 5U platelets | TXA according to clinician’s discretion with CCT | LY30 > 15% - TXA |
| Girdauskas 2010 [30] | Htc < 25% (Hb 8.5 g/dL) (during CPB Htc < 20% (Hb 6.8 g/dL)) or physiologic transfusion triggers | Htc < 25% (Hb 8.5 g/dL) (during CPB Htc < 20% (Hb 6.8 g/dL)) or physiologic transfusion triggers | PT > 60s or INR >1.5 – FFP 15 mL/kg body mass | HEPTEM CT > 260 s – FFP 15 mL/kg body mass | PC < 100 · 103/μL – 1 platelet concentrate | (A) HEPTEM MCF 35-45 mm – 1 platelet concentrate (B) FIBTEM MCF >8 mm and HEPTEM MCF <35 mm – 1 platelet concentrate |
Fibrinogen <1.2 mg/dL – 2 g fibrinogen α2-Antiplasmin <80% - 3 g TXA |
FIBTEM <8 mm – 2 g fibrinogen APTEM MCF/HEPTEM MCF >1.5 – 3 g TXA APTEM CT > 120 s – 3000 IU PPSB |
| Wang 2010 [37] | Hb <8 g/dL | Hb <8 g/dL | PT and aPTT > 150% control | R > 10 min | PC < 50x109/L | MA < 55 mm – 6-8U pooled platelets | Fibrinogen <1 g/dL – cryo | α-angle < 45° - cryo |
| Paniagua 2011 [36] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Schaden 2012 [38] | Hb <8 g/dL | Hb <8 g/dL | Clinician’s discretion with or without CCT | EXTEM CT > 100 s – 4U FFP | Clinician’s discretion with or without CCT | EXTEM A10 < 45 mm and FIBTEM >12 mm – 1U platelets concentrate | TXA and fibrinogen according to clinician’s discretion with or without CCT | EXTEM A10 < 45 mm and FIBTEM A10 < 12 mm – 2 g fibrinogen Spindle shaped trace APTEM A10> > EXTEM A10 – 10 mg/kg TXA EXTEM LY30 > 10% - 10 mg/kg TXA |
| Weber 2012 [34] | Hb <8 g/dL (during CPB Hb <6 g/dL) or physiologic transfusion triggers | Hb <8 g/dL (during CPB Hb <6 g/dL) or physiologic transfusion triggers | Transfused ≥4U PRBCs without new lab results – 15 mL/kg FFP INR > 1.4 or aPTT > 50s – 20-30 IU/kg prothrombin complex concentrate or 15 mL/kg FFP |
EXTEM CT > 80s or HEPTEM >240 s – 20-30 IU/kg prothrombin complex concentrate or 15 mL/kg FFP | PC < 80000/μL | EXTEM A10 ≤ 40 mm and FIBTEM A10 > 10 mm or TRAP < 50 AU and/or ASPI <30 AU and/or ADP < 30 AU (second choice) | Fibrinogen pre-value < 200 mg/dL or currently <150 mg/dL – 25-50 mg/kg fibrinogen Suspected platelet dysfunction – 0.3 μg/kg desmopressin |
FIBTEM MCF = 0 mm – 25 mg/kg fibrinogen before protamine EXTEM A10 ≤ 40 mm and FIBTEM A10 ≤ 10 mm – 25-50 mg/kg fibrinogen TRAP < 50 AU and/or ASPI <30 AU and/or ADP < 30 AU – 0.3 μg/kg desmopressin (first choice) |
| Barinov 2015 [41] | N/A | N/A | Clinician’s discretion with CCT | N/A | Clinician’s discretion with CCT | N/A | Clinician’s discretion with CCT | N/A |
| Gonzalez 2015 [22] | First units of RBC administered according to clinician’s discretion only Hb < 10 g/dL |
First units of RBC administered according to clinician’s discretion only Hb < 10 g/dL |
First units of FFP administered according to clinician’s discretion only INR ≥ 1.5 – 2U FFP |
First units of FFP administered according to clinician’s discretion only ACT 111-139 s – 2U FFP ACT ≥ 140 s – 2U FFP, 10-pack cryo and 1U apheresis platelets ACT > 110 s – 2U FFP |
PC < 100 · 103/μL – 1U apheresis platelets | ACT ≥ 140 s – 2U FFP, 10-pack cryo and 1U apheresis platelets MA < 55 mm – 1U apheresis platelets |
Fibrinogen >150 mg/dL – 10-pack cryo Suspicion on fibrinolysis with D-dimer >0.5 μg/dL – 1 g TXA |
ACT ≥ 140 s – 2U FFP, 10-pack cryo and 1U apheresis platelets α-angle < 63° - 10-pack cryo LY30 ≥ 7.5% - 1 g TXA (after 61% of enrolment LY30 ≥ 3% - 1 g TXA) |
| De Pietri 2015 [39] | Hb <8 g/dL | Hb <8 g/dL | INR > 1.8 – 10 mL/kg ideal body weight | R > 40 min – 10 mL/kg ideal body weight | PC < 50 · 109/L – 1U PLT | MA < 30 mm – 1U apheresis platelets | ||
| Cao 2016 [40] | Hb < 70 g/L, Htc < 25% - 2U RBC | Hb < 70 g/L, Htc < 25% - 2U RBC | Clinican’s discretion | R > 8 min – FFP 15 mL/kg | PC < 50 · 109/L – 1U PLT | MA < 70 mm – 1U platelets | Fibrinogen < 0.0012 mg/L – fibrinogen 2 g | α-angle < 72° - fibrinogen 2 g |
Control group = groups managed without the use of either TEG or ROTEM. Intervention group = groups managed with the use of TEG or ROTEM. Htc haematocrit, Hb haemoglobin, PC platelet count, U units, PT prothrombin time, N/A not applicable, CCT conventional coagulation test, RBC red blood cell, FFP fresh frozen plasma, PLT platelets, INR international normalized ratio, ACT activated clotting time, MA maximal amplitude, TXA tranexamic acid, R reaction time, aPTT activated partial thromboplastin time, CPB cardiopulmonary bypass, hTEG heparinase-TEG, POC point of care
Table 4.
Study characteristics Author and year, number of patients allocated to control or intervention group and the type of patients and/or procedures performed during the study
| Reference (Author and year) | Control/intervention (n) | Type of patients/procedures |
|---|---|---|
| Shore-Lesserson 1999 [33] | 52/53 | Cardiac surgery Moderate to high risk of microvascular bleeding (single/multiple valve replacement, combined CAB + valvular procedure, cardiac reoperation, thoracic aortic replacement). CPB performed with moderate hypothermia. |
| Nuttall 2001 [31] | 51/41 | Cardiac surgery All types of elective cardiac surgery developing abnormal bleeding after CPB. |
| Royston 2001 [32] | 30/30 | Cardiac surgery 10% in each group had heart transplantation, 50% in each group had revascularization (multiple grafts with an estimated CPB-time >100 min), 40% in each group Ross procedure, multiple valve or valve and revascularization surgery. |
| Avidan 2004 [29] | 51/51 | Cardiac surgery Routine elective first time coronary artery surgery with CPB. Cooled to 32 °C. |
| Ak 2009 [28] | 110/114 | Cardiac surgery Elective first time coronary artery bypass graft (CABG) with CPB. |
| Westbrook 2009 [35] | 37/32 | Cardiac surgery Presenting for cardiac surgery except lung transplantations. |
| Girdauskas 2010 [30] | 29/27 | Aortic surgery Patients undergoing aortic surgery with hypothermic circulatory arrest. 25 patients with acute type A dissection. |
| Wang 2010 [37] | 14/14 | Orthotopic liver transplantation |
| Paniagua 2011 [36] | 9/13 | Cardiac surgery Patients scheduled for cardiac surgery with extracorporeal circulation with major post-operative bleeding (>300 mL). |
| Schaden 2012 [38] | 16/14 | Surgical excision of burn wounds Surgical intervention performed on 3rd day after trauma. |
| Weber 2012 [34] | 50/50 | Cardiac surgery Patients scheduled for elective, complex cardiothoracic surgery (combined coronary artery bypass, graft and valve surgery, double/triple valve procedures, aortic surgery or redo surgery) with CPB. |
| Barinov 2015 [41] | 29/90 | Postpartum obstetric haemorrhage |
| Gonzalez 2015 [22] | 55/56 | Trauma patients Meeting criteria for massive transfusion protocol (MTP) activation on arrival to ED: systolic blood pressure <70 mmHg or SBP 70 – 90 mmHg with heart rate 108 beats/min, in addition to any of the following injury patterns: penetrating torso wound, unstable pelvic fracture, or abdominal ultrasound suspicious of bleeding in more than one region. |
| De Pietri 2015 [39] | 30/30 | Hepatic surgery Patients with cirrhosis + significant coagulopathy (defined as INR >1,8 and/or platelet count <50 × 109/L) undergoing invasive procedure. |
| Cao 2016 [40] | 28/32 | Scoliosis surgery Patients with an expected surgical bleeding > 1000 ml and the American Society of Anesthesiologists rating I-II in addition to a body mass index (BMI) 18 to 24 kg/m2 |
CAB coronary arterial bypass, CABG coronary artery bypass graft, CPB cardio pulmonary bypass, MTP massive transfusion protocol, ED emergency department, SBP systolic blood pressure, INR international normalised ratio
Meta-analyses
All-cause mortality
Six trials were included in the meta-analysis of all-cause mortality with a total of 579 patients of whom 291 patients were allocated to the intervention. Three trials concerned patients undergoing cardiothoracic surgery [28, 30, 34] one trial concerned orthotopic liver transplantation [37], one studied cirrhotic liver patients [39] and one studied trauma patients [22]. The meta-analysis demonstrated no difference in survival between the groups with an OR of 0.60 (95% CI 0.34 to 1.07; p = 0.08) (figure 2a).
Fig. 2.
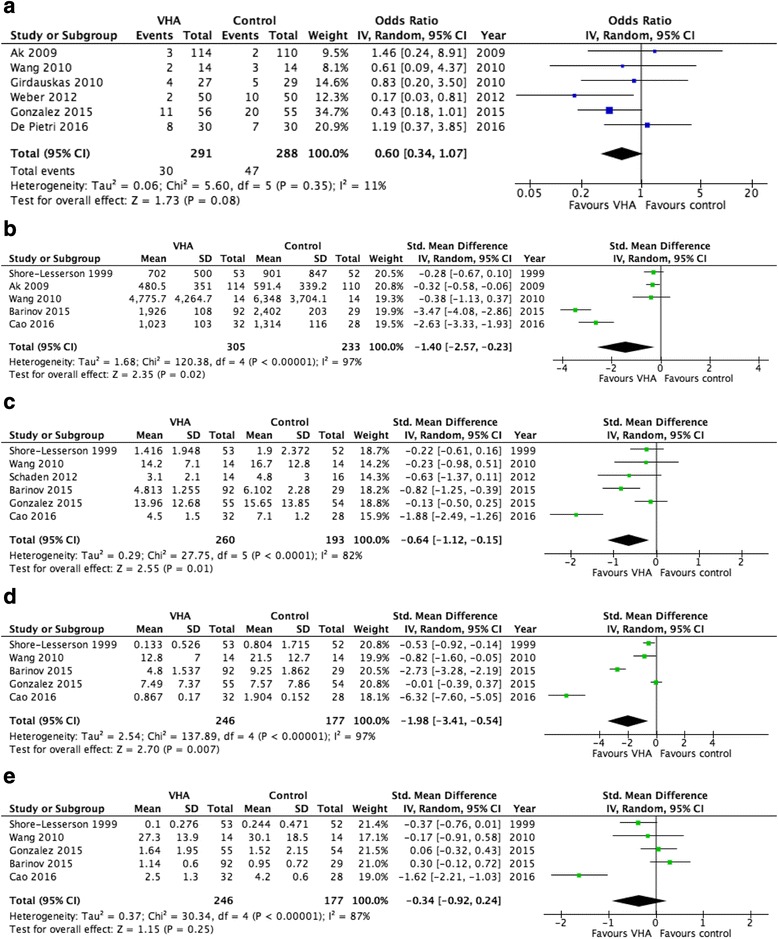
Forest plots a All-cause mortality b Perioperative, 24 h and 12 h bleeding c Total transfusion need – RBC d Total transfusion need – FFP e Total transfusion need – Platelets
Bleeding volume
Eleven RCTs reported on bleeding while only five of these studies expressed perioperative, 24 or 12-h bleeding as mean ± SD and were therefore eligible for meta-analysis [28, 37, 40–42]. Comparison of the bleeding volume in 538 patients (305 in the intervention groups) resulted in significantly reduced bleeding in the VHA treated patients (standardized mean difference −1.40 [95% CI 2.57 to −0,23]; p = 0.02) (figure 2b).
Transfusion requirements
The analysis for transfusion requirements was limited to six trials concerning RBC transfusions [22, 37, 38, 40–42] and five trials were eligible for the meta-analysis on transfusions of FFP and platelets, respectively [22, 37, 40–42]. All fifteen trials included in this analysis reported on transfusions, while only the above mentioned described the mean transfused amount per patient ± SD as required for meta-analysis. Isolating RBC-transfusion requirements, 260 out of 453 patients were in the intervention group. Random effects analysis resulted in a standardized mean difference of −0.64 (95% CI −1.12 to −0.15; p = 0.01), being statistically significant (figure 2c). Differences in FFP-transfusions were calculated in 423 patients (246 in intervention group) and resulted in a standardized mean difference of −1.98 (95% CI −3.41 to −0.54; p = 0.007), showing a significant reduction in transfused FFP in the intervention group (figure 2d). Numbers for transfused units of platelets were available from the same 423 patients as with FFP-transfusion requirements, however meta-analysis did not reach statistical significance (standardized mean difference −0.34 [95% CI −0.92 to 0.24; p = 0.25]) (figure 2e).
Discussion
We found the total bleeding volume and the amount of transfused RBCs and FFP to be significantly reduced in the VHA-guided intervention groups compared to CCT-guided control groups. Considering that most trials used the same transfusion trigger for RBCs in both groups, the difference in RBC requirements may be explained by a better haemostatic competence in TEG/ROTEM-guided groups accomplished through timely administration of plasma and platelets, further supported by the reduction of bleeding in the VHA-guided group of patients. In our meta-analysis no statistically significant difference was found between groups regarding all cause-mortality and required amounts of platelets. The sizes of the respective trial populations were small and a lack of cohesion in permission of platelet inhibitors, anticoagulants, antifibrinolytics and triggers used to guide resuscitation with blood products was observed. The control groups were managed either by clinical judgement combined with CCTs or by the sole use of algorithms applying only CCT-triggers for transfusion. The decision to transfuse potentially encompasses a bias to a greater number of transfusions between clinicians with a different background and clinical practice, in alignment with Avidan et al. [29] finding a reduction in transfusions administered with CTT-algorithm guided perioperative management versus transfusion guidance based only on the physician’s discretion. Although only a difference in amount of FFP and no statistical difference in the amount of platelets transfused between groups was detected, the timing of these transfusions may differ with VHA-analyses having shorter turn-around time than conventional coagulation tests [43]. This accentuates the importance of early administration of the appropriate blood products as also emphasized by Cotton et al. [20] who found reduced odds of mortality (74%) and transfusions in a group of trauma patients managed with early and aggressive resuscitation on admittance to the emergency department. Although 24-h transfusion requirements were reduced in patients treated according to the exsanguination protocol, amounts of intraoperative transfusions were found to be larger in this cohort in comparison with the conventionally treated controls, illustrating the importance of early resuscitation with blood products. Also Johansson et al. [21] found similar results in patients undergoing surgery for ruptured abdominal aortic aneurysm (rAAA) with a proactive intraoperative administration of platelets and FFP yielding an increase in survival in massively bleeding rAAA patients. They found a significant reduction in postoperative transfusions, indicating that early blood product administration plays a pivotal role in improving haemostasis in massive bleeders. Gonzalez et al. [22] have conducted the first RCT to evaluate VHA-guided transfusion therapy in trauma. They found a survival benefit in the TEG-guided group especially with regards to less haemorrhagic and early deaths. Additionally, they argued that the administration of more platelets and FFP does not necessarily increase survival chances but highlight the effect of the appropriate treatment being given at the optimal time rather than the amount of blood product administered. Moreover, in patients undergoing surgery with extracorporeal circulation, the use of TEG/ROTEM heparinase analyses, where coagulopathy can be identified despite patient being heparinized, may provide an even earlier assessment of coagulation status and thereby enable an earlier correction of coagulopathies, exemplified by Royston et al. [32] and Girdauskas et al. [30].
Weber et al. [34] report a notably higher mortality among their patients than usually seen in cardiac surgery. Despite this, we did not find a significant difference in mortality in the VHA-guided groups compared to conventionally treated groups. However, our meta-analysis suggested clinical difference in survival in patients having treatment based on VHA-results, in congruence with a before- and after study conducted on trauma patients by Johansson et al., showing a reduction in mortality of approximately 30% in a group resuscitated using TEG results in patients requiring massive transfusions [19]. Furthermore, a Cochrane review from Wikkelsø et al. [44] found the use of TEG or ROTEM in guiding resuscitation of bleeding patients to reduce all-cause mortality and the number of patients transfused with blood products, although no difference was found with regard to excessive bleeding events and proportion of massively transfused, in agreement with our results. Also, NICE-report done by Whiting et al. [45] finds a tendency to fewer transfusions of allogeneic blood products being administered in cardiac surgery patients treated according to VHA-results when comparing to patients managed with CCT-results, while no difference was found with regard to trauma patients and post-partum bleeding. The discrepancies in study selection with the review from Whiting et al. [45] are explained in table 5.
Table 5.
Explanation for discrepancies with RTCs included by Whiting et al. [45] (NICE-report)
| Reference (author and year) | Reason for exclusion from this meta-analysis |
|---|---|
| Kultufan Turan et al. 2006 | Not possible to identify in PubMed or EMBASE |
| Rauter et al. 2007 | Not possible to identify in PubMed or EMBASE |
| Messenger et al. 2011 | Prospective cohort study, not randomised |
Limitations
A limited number of adequately reported trials were eligible for our meta-analyses. Out of the 15 included trials in this analysis, five did not report sufficient information to be included in any of the meta-analyses performed [29, 31, 32, 35, 36]. This meta-analysis has an overweight of trials concerning cardio-thoracic patients, while other patient groups are only represented by a single RCT each, limiting comparability of results. Furthermore, the studies included present patients with bleeding originating from different aetiologies. This can potentially be problematic in that the severity of bleeding may vary.
Conclusions
In conclusion, the performed meta-analyses demonstrated trends towards the superiority of treating haemorrhaging patients under the guidance of VHA-algorithms. There is, however, a need for larger RCTs, such as the ongoing trials “implementing Treatment Algorithms for the Correction of Trauma Induced Coagulopathy (iTACTIC)” NCT02593877.
Acknowledgements
MF would like to thank the investigators Drs. Eva Schaden, Sergey V. Barinov, Andrew Westbrook, Angela Sauaia, Ernest Moore and Koray Ak for kindly providing additional data on request.
Funding
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Authors’ contributions
MF collected, analysed and interpreted patient data and drafted, reviewed and approved the manuscript. RSO conducted calculations and statistics for meta-analysis and reviewed and approved the manuscript. PIJ contributed with guidance and discussion of results and reviewed and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CCT
Conventional coagulation test
- CI
Confidence intervals
- FFP
Fresh frozen plasma
- iTACTIC
Implementing Treatment Algorithms for the Correction of Trauma Induced Coagulopathy
- rAAA
Ruptured abdominal aorta aneurism
- RBCs
Red blood cells
- RCT
Randomised controlled trial
- ROTEM
Thromboelastometry
- SD
Standard deviation
- TEG
Thromboelastography
- VHA
Viscoelastic haemostatic assay
Contributor Information
Mathilde Fahrendorff, Email: mathilde.fahrendorff@regionh.dk.
Roberto S. Oliveri, Email: roberto.s.oliveri@gmail.com
Pär I. Johansson, Email: Per.Johansson@regionh.dk
References
- 1.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–231. doi: 10.1016/S0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 2.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42(5):857–861. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 4.Johansson PI, Ostrowski SR, Secher NH. Management of major blood loss: an update. Acta Anaesthesiol Scand. 2010;54(9):1039–1049. doi: 10.1111/j.1399-6576.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 5.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 6.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251(4):604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 7.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. Early coagulopathy in multiple injury: an analysis from the German trauma registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 9.Simmons JW, Pittet JF, Pierce B. Trauma-induced coagulopathy. Curr Anesthesiol Rep. 2014;4(3):189–199. doi: 10.1007/s40140-014-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shander A, Moskowitz D, Rijhwani TS. The safety and efficacy of “bloodless” cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9(1):53–63. doi: 10.1177/108925320500900106. [DOI] [PubMed] [Google Scholar]
- 11.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg. 2001;72(5):S1821–S1831. doi: 10.1016/S0003-4975(01)03211-8. [DOI] [PubMed] [Google Scholar]
- 12.Johansson PI, Solbeck S, Genet G, Stensballe J, Ostrowski SR. Coagulopathy and hemostatic monitoring in cardiac surgery: an update. Scand Cardiovasc J. 2012;46(4):194–202. doi: 10.3109/14017431.2012.671487. [DOI] [PubMed] [Google Scholar]
- 13.Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951–960. doi: 10.1097/TA.0b013e318187e15b. [DOI] [PubMed] [Google Scholar]
- 14.Lamb CM, MacGoey P, Navarro AP, Brooks AJ. Damage control surgery in the era of damage control resuscitation. Br J Anaesth. 2014;113(2):242–249. doi: 10.1093/bja/aeu233. [DOI] [PubMed] [Google Scholar]
- 15.Undurraga Perl VJ, Leroux B, Cook MR, Watson J, Fair K, Martin DT, et al. Damage-control resuscitation and emergency laparotomy: findings from the PROPPR study. J Trauma Acute Care Surg. 2016;80(4):568–574. doi: 10.1097/TA.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchesne JC, Islam TM, Stuke L, Timmer JR, Barbeau JM, Marr AB, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67(1):33–37. doi: 10.1097/TA.0b013e31819adb8e. [DOI] [PubMed] [Google Scholar]
- 18.Johansson PI, Oliveri RS, Ostrowski SR. Hemostatic resuscitation with plasma and platelets in trauma. J Emerg Trauma Shock. 2012;5(2):120–125. doi: 10.4103/0974-2700.96479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96(2):111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64(5):1177–1182. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 21.Johansson PI, Stensballe J, Rosenberg I, Hilslov TL, Jorgensen L, Secher NH. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47(4):593–598. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levrat A, Gros A, Rugeri L, Inaba K, Floccard B, Negrier C, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100(6):792–797. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 24.Murray D, Pennell B, Olson J. Variability of prothrombin time and activated partial thromboplastin time in the diagnosis of increased surgical bleeding. Transfusion. 1999;39(1):56–62. doi: 10.1046/j.1537-2995.1999.39199116895.x. [DOI] [PubMed] [Google Scholar]
- 25.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–275. doi: 10.1097/TA.0b013e3181ae6f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Ak K, Isbir CS, Tetik S, Atalan N, Tekeli A, Aljodi M, et al. Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24(4):404–410. doi: 10.1111/j.1540-8191.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 29.Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92(2):178–186. doi: 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 30.Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140(5):1117–24.e2. doi: 10.1016/j.jtcvs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Anesthesiology. 2001;94(5):773–781. doi: 10.1097/00000542-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase-modified thrombelastography during cardiopulmonary bypass. Br J Anaesth. 2001;86(4):575–578. doi: 10.1093/bja/86.4.575. [DOI] [PubMed] [Google Scholar]
- 33.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88(2):312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Weber CF, Gorlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531–547. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 35.Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18(4):277–288. doi: 10.1016/j.hlc.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Paniagua P, Koller T, Requena T, Gil JM, Campos JM, Galan J. Randomized controled trial to evaluate postoperative coagulation management with bed-side trombelastometry (Rotem) compared with a transfusion protocol based on laboratory meausurments in bleeding patients after cardiac surgery: Preliminary data. Eur J Anaesthesiol. 2011;28:94. doi: 10.1097/00003643-201106001-00301. [DOI] [Google Scholar]
- 37.Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590–2593. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 38.Schaden E, Kimberger O, Kraincuk P, Baron DM, Metnitz PG, Kozek-Langenecker S. Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. Br J Anaesth. 2012;109(3):376–381. doi: 10.1093/bja/aes186. [DOI] [PubMed] [Google Scholar]
- 39.De Pietri L, Bianchini M, Montalti R, De Maria N, Di Maira T, Begliomini B, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63(2):566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 40.Cao X, Zhang X, Li Q. Efficacy of thromboelastography to monitor the clinical massive transfusion in scoliosis: a randomized controlled trial. Zhonghua Wai Ke Za Zhi. 2016;54(2):137–141. doi: 10.3760/cma.j.issn.0529-5815.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Barinov SV, Zhukovsky YG, Dolgikh VT, Medyannikova IV. Novel combined strategy of obstetric haemorrhage management during caesarean section using intrauterine balloon tamponade. J Matern Fetal Neonatal Med. 2015:1–21. [DOI] [PubMed]
- 42.Shore-Lesserson L, Ammar T, DePerio M, Vela-Cantos F, Fisher C, Sarier K. Platelet-activated clotting time does not measure platelet reactivity during cardiac surgery. Anesthesiology. 1999;91(2):362–368. doi: 10.1097/00000542-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Weber CF, Klages M, Zacharowski K. Perioperative coagulation management during cardiac surgery. Curr Opin Anaesthesiol. 2013;26(1):60–64. doi: 10.1097/ACO.0b013e32835afd28. [DOI] [PubMed] [Google Scholar]
- 44.Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;8:CD007871. doi: 10.1002/14651858.CD007871.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: A systematic review and cost-effectiveness analysis. Health Technol Assess. 2015;19(58):1–228. doi: 10.3310/hta19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal S, Johnson RI, Shaw M, Agarwal S, Johnson RI, Shaw M. Preoperative Point-of-Care Platelet Function Testing in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2015;29(2):333–341. doi: 10.1053/j.jvca.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Branco BC, Inaba K, Ives C, Okoye O, Shulman I, David JS, et al. Thromboelastogram evaluation of the impact of hypercoagulability in trauma patients. Shock. 2014;41(3):200–207. doi: 10.1097/SHK.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 48.Brilej D, Stropnik D, Lefering R, Komadina R. Algorithm for activation of coagulation support treatment in multiple injured patients--cohort study. Eur J Trauma Emerg Surg. 2016;22. [DOI] [PubMed]
- 49.Capraro L, Kuitunen A, Salmenperä M, Kekomäki R, Capraro L, Kuitunen A, Salmenperä M, Kekomäki R. On-site coagulation monitoring does not affect hemostatic outcome after cardiac surgery. Acta Anaesthesiol Scand. 2001;45(2):200–206. doi: 10.1034/j.1399-6576.2001.450211.x. [DOI] [PubMed] [Google Scholar]
- 50.Despotis GJ, Grishaber JE, Goodnough LT, Despotis GJ, Grishaber JE, Goodnough LT. The effect of an intraoperative treatment algorithm on physicians' transfusion practice in cardiac surgery. Transfusion. 1994;34(4):290–296. doi: 10.1046/j.1537-2995.1994.34494233575.x. [DOI] [PubMed] [Google Scholar]
- 51.Despotis GJ, Santoro SA, Spitznagel E, Kater KM, Cox JL, Barnes P, et al. Prospective evaluation and clinical utility of on-site monitoring of coagulation in patients undergoing cardiac operation. Radiol Med. 1994;87(3):219–228. [PubMed] [Google Scholar]
- 52.Dietrich W, Spannagl M, Boehm J, Hauner K, Braun S, Schuster T, et al. Tranexamic acid and aprotinin in primary cardiac operations: an analysis of 220 cardiac surgical patients treated with tranexamic acid or aprotinin. Anesth Analg. 2008;107(5):1487–1495. doi: 10.1213/ane.0b013e318182252b. [DOI] [PubMed] [Google Scholar]
- 53.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, et al. Rapid-thrombelastography (r-TEG) thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2016;31. [DOI] [PMC free article] [PubMed]
- 54.Hajek R, Ruzickova J, Nemec P, Fluger I, Simek M. Thrombelastography in haemostasis monitoring during cardiac surgery. Anesthesia and Analgesia.Conference:2010 Annual Meeting of the International AnesthesiaResearch Society, IARS . Honolulu, HI United States. Conference Start: 20100320. Conference End: 3. Conference Publication: (var.pagings). 110 (3 SUPPL. 1) (pp S101)
- 55.Hanke AA, Herold U, Dirkmann D, Tsagakis K, Jakob H, Görlinger K, Hanke AA, Herold U, Dirkmann D, Tsagakis K, Jakob H, Görlinger K. Thromboelastometry Based Early Goal-Directed Coagulation Management Reduces Blood Transfusion Requirements, Adverse Events, and Costs in Acute Type A Aortic Dissection: A Pilot Study. Transfus Med Hemother. 2012;39(2):121–128. doi: 10.1159/000337723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harding SA, Mallett SV, Peachey TD, Cox DJ, Harding SA, Mallett SV, Peachey TD, Cox DJ. Use of heparinase modified thrombelastography in liver transplantation. Br J Anaesth. 1997;78(2):175–179. doi: 10.1093/bja/78.2.175. [DOI] [PubMed] [Google Scholar]
- 57.Helm RE, Rosengart TK, Gomez M, Klemperer JD, DeBois WJ, Velasco F, Gold JP, Altorki NK, Lang S, Thomas S, Isom OW, Krieger KH. Comprehensive Multimodality Blood Conservation: 100 Consecutive CABG Operations Without Transfusion. Ann Thorac Surg. 1998;65(1):125–136. doi: 10.1016/S0003-4975(97)01004-7. [DOI] [PubMed] [Google Scholar]
- 58.Hoenicka M, Rupp P, Müller-Eising K, Deininger S, Kunert A, Liebold A, Gorki H, Hoenicka M, Rupp P, Müller-Eising K, Deininger S, Kunert A, Liebold A, Gorki H. Anticoagulation management during multivessel coronary artery bypass grafting: a randomized trial comparing individualized heparin management and conventional hemostasis management. J Thromb Haemost. 2015;13(7):1196–1206. doi: 10.1111/jth.12999. [DOI] [PubMed] [Google Scholar]
- 59.Hopkins JA, Shoemaker WC, Chang PC, Schluchter M, Greenfield S. Clinical trial of an emergency resuscitation algorithm. J Surg Res. 1983;35(3):227–233. doi: 10.1016/S0022-4804(83)80008-0. [DOI] [PubMed] [Google Scholar]
- 60.Israelian LA, Gromova VV, Lubnin AI. Reducing the frequency of fresh frozen donor plasma transfusion on the basis of the results of thromboelastographic study in neurosurgical patients with intraoperative blood loss. Anesteziologiia i reanimatologiia; 2009 [PubMed]
- 61.Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, Pinto R, Scales DC, Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, Pinto R, Scales DC. Point-of-Care Hemostatic Testing in Cardiac SurgeryClinical Perspective. Circulation. 2016;134(16):1152–1162. doi: 10.1161/CIRCULATIONAHA.116.023956. [DOI] [PubMed] [Google Scholar]
- 62.Levin AI, Heine AM, Coetzee JF, Coetzee A, Levin AI, Heine AM, Coetzee JF, Coetzee A. Heparinase Thromboelastography Compared With Activated Coagulation Time for Protamine Titration After Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2014;28(2):224–229. doi: 10.1053/j.jvca.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 63.Lier H, Krep H, Schochl H. Coagulation management in the treatment of multiple trauma. Anaesthesist. 2009;58(10):1010–1026. doi: 10.1007/s00101-009-1595-z. [DOI] [PubMed] [Google Scholar]
- 64.McNamara H, Mallaiah S, Barclay P, Chevannes C, Bhalla A, McNamara H, Mallaiah S, Barclay P, Chevannes C, Bhalla A. Coagulopathy and placental abruption: changing management with ROTEM-guided fibrinogen concentrate therapy. Int J Obstet Anesth. 2015;24(2):174–179. doi: 10.1016/j.ijoa.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Manikappa S, Mehta Y, Juneja R, Trehan N. Changes in transfusion therapy guided by thromboelastograph in cardiac surgery. Clin Pharmacol Ther. 2008;83(3):460–470. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 66.Messenger BM, Craft RM, Carroll RC, Daley BJ, Enderson B, Snider CG. TEG-guided massive transfusion in trauma patients. Anesthesia and Analgesia.Conference:2011 Annual Meeting of the International Anesthesia Research Society, IARS . Vancouver, BC Canada. Conference Start: 20110521. Conference End: 4. Conference Publication: (var.pagings). 112 (5 SUPPL. 1) (no pagination).
- 67.Mishra PK, Thekkudan J, Sahajanandan R, Gravenor M, Lakshmanan S, Fayaz KM, Luckraz H, Mishra PK, Thekkudan J, Sahajanandan R, Gravenor M, Lakshmanan S, Fayaz KM, Luckraz H. The role of point-of-care assessment of platelet function in predicting postoperative bleeding and transfusion requirements after coronary artery bypass grafting. Ann Card Anaesth. 2015;18(1):45. doi: 10.4103/0971-9784.148321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naik BI, Pajewski TN, Bogdonoff DL, Zuo Z, Clark P, Terkawi AS, et al. Rotational thromboel astometry-guided blood product management in major spine surgery. Journal of Neurosurgery: Spine. 2015;23(2):239–249. doi: 10.3171/2014.12.SPINE14620. [DOI] [PubMed] [Google Scholar]
- 69.Petricevic M, Biocina B, Milicic D, Konosic S, Svetina L, Lekić A, Zdilar B, Burcar I, Milosevic M, Brahimaj R, Samardzic J, Gasparovic H, Petricevic M, Biocina B, Milicic D, Konosic S, Svetina L, Lekić A, Zdilar B, Burcar I, Milosevic M, Brahimaj R, Samardzic J, Gasparovic H. Bleeding risk assessment using whole blood impedance aggregometry and rotational thromboelastometry in patients following cardiac surgery. J Thromb Thrombolysis. 2013;36(4):514–526. doi: 10.1007/s11239-013-0868-1. [DOI] [PubMed] [Google Scholar]
- 70.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, Pichlmaier M, Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, Pichlmaier M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138(3):694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 71.Roullet S, Freyburger G, Cruc M, Quinart A, Stecken L, Audy M, Chiche L, Sztark F, Roullet S, Freyburger G, Cruc M, Quinart A, Stecken L, Audy M, Chiche L, Sztark F. Management of bleeding and transfusion during liver transplantation before and after the introduction of a rotational thromboelastometry-based algorithm. Liver Transpl. 2015;21(2):169–179. doi: 10.1002/lt.24030. [DOI] [PubMed] [Google Scholar]
- 72.Smart L, Scharpf DT, Gray NO, Traetow D, Black S, Michaels A, et al. Rotational thromboelastometry (ROTEM) versus conventional coagulation tests during orthotopic liver transplantation: Comparison of intraoperative blood loss, transfusion requirements, and cost. Hepatology.Conference:66th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting 2015. San Francisco, CA United States. Conference Start: 20151113. Conference End: 7. Conference Publication: (var.pagings). 62 (pp 832A).
- 73.Stancheva A, Spassov L, Tzatchev K. Correlation between rotation thrombelastometry ROTEM analysis and standard haemostatic parameters during liver transplantation. Clin Lab. 2011;57(5-6):407–413. [PubMed] [Google Scholar]
- 74.Tarabarin OA, Tkachenko AI, Salekh EN, Kushnir OS, Zarutskii IL, Tuchkov AV. [Diagnosis and correction of hemostasis disorder during surgeries for bile duct diseases]. Klinichna khirurhiia / Ministerstvo okhorony zdorov'ia Ukrainy, Naukove tovarystvo khirurhiv Ukrainy. 2013. [PubMed]
- 75.Weitzel NS, Weitzel LB, Epperson LE, Karimpour-Ford A, Tran ZV, Seres T, Weitzel NS, Weitzel LB, Epperson LE, Karimpour-Ford A, Tran ZV, Seres T. Platelet mapping as part of modified thromboelastography (TEG ) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia. 2012;67(10):1158–1165. doi: 10.1111/j.1365-2044.2012.07231.x. [DOI] [PubMed] [Google Scholar]
- 76.Xu L, Wang LF, Yang XC, Li KB, Sun H, Zhang DP, et al. Platelet function monitoring guided antiplatelet therapy in patients receiving high-risk coronary interventions. Chin Med J (Engl) 2014;127(19):3364–3370. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.


