Abstract
Background
Within an enhanced recovery pathway (ERP), the approach to treating pain should be multifaceted and the goal should be to deliver “optimal analgesia,” which we define in this paper as a technique that optimizes patient comfort and facilitates functional recovery with the fewest medication side effects.
Methods
With input from a multi-disciplinary, international group of clinicians, and through a structured review of the literature and use of a modified Delphi method, we achieved consensus surrounding the topic of optimal analgesia in the perioperative period for colorectal surgery patients.
Discussion
As a part of the first Perioperative Quality Improvement (POQI) workgroup meeting, we sought to develop a consensus document describing a comprehensive, yet rational and practical, approach for developing an evidence-based plan for achieving optimal analgesia, specifically for a colorectal surgery ERP. The goal was two-fold: (a) that application of this process would lead to improved patient outcomes and (b) that investigation of the questions raised would identify knowledge gaps to aid the direction for research into analgesia within ERPs in the years to come. This document details the evidence for a wide range of analgesic components, with particular focus from the preoperative period to the post-anesthesia care unit. The overall conclusion is that the combination of analgesic techniques employed in the perioperative period is not important as long as it is effective in delivering the goal of optimal analgesia as set forth in this document.
Keywords: Enhanced recovery pathway, Colorectal, Optimal analgesia, Pain management, Multimodal, Non-opioid adjuncts, Perioperative, Outcomes, Quality
Introduction
Pain after major abdominal surgery is severe and is a major component of the stress response if not adequately treated (Schricker and Lattermann 2015). Pain is triggered as a combination of neural and inflammatory pathways with injury to the viscera, muscle, and skin. The intensity and duration of each of these triggers varies according to the type of surgical procedure performed and which surgical approach is used (laparoscopic, robotic assisted, or open) (Reza et al. 2006). Additionally, patients respond differently to pain in the perioperative period and patients with chronic pain conditions often experience a greater amount of suffering in the immediate perioperative period. Thus, it is not only important to treat pain effectively from a humane point of view but also because this is a major factor in reducing the stress response to surgery and restoring function thereafter.
The concept of an enhanced recovery pathway (ERP) is a multi-component approach aimed at reducing the stress of surgery experienced by the patient, improving the metabolic response, and thereby speeding the return of functional recovery (Kehlet and Wilmore 2008). Within an ERP, the approach to treating pain should be multifaceted, including a combination of techniques such as neural blockade, intravenous, and multimodal oral analgesia. The goal should be to deliver “optimal analgesia,” which we define in this paper as a technique that optimizes patient comfort and facilitates functional recovery with the fewest medication side effects (see Fig. 1). Of note, this may not correspond with the lowest pain perception possible. Overall, the combination of analgesic techniques employed is not important as long as it is effective in delivering this goal of optimal analgesia.
Fig. 1.
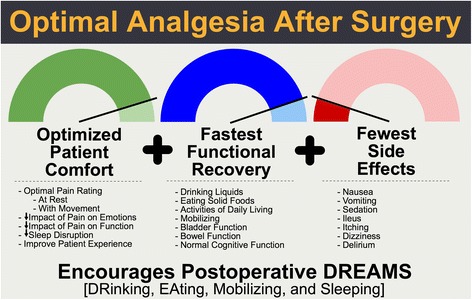
The core components of providing optimal analgesia. Pain after surgery can have profound effects on patient recovery. However, the complete elimination of pain may also have untoward effects, as listed in the figure. Optimal analgesia after surgery is an approach to pain control that facilitates a positive patient experience through optimized patient comfort that facilitates functional recovery while minimizing adverse drug events
As a part of the first Perioperative Quality Improvement (POQI) workgroup meeting, we sought to develop a consensus document addressing these questions. Our intent was to develop a comprehensive, yet rational and practical, approach for developing an evidence-based plan for achieving optimal analgesia specifically for a colorectal surgery (CRS) ERP. The goal would be two-fold: (a) that application of this process would lead to improved patient outcomes and (b) that investigation of the questions raised would identify knowledge gaps to aid the direction for research into analgesia within ERPs in the years to come. The overall vision for our working group was to encourage rigorous development and application of evidence-based perioperative medicine related to achieving optimal analgesia for patients undergoing CRS.
Methods
We used the Delphi method to achieve consensus surrounding the topic of optimal analgesia in the perioperative period for colorectal surgery patients. The Delphi method has been used in various formats to obtain the perspectives and opinions of diverse groups. The participants in the POQI consensus meeting included anesthesiologists, surgeons, and nurses who were recruited based on their expertise in the principles of enhanced recovery after surgery and perioperative medicine. For our use, the process included several iterative steps, including building consensus around the important questions related to the topic, a literature review of the topics, and sequential steps of content building and refinement until agreement is achieved and a consensus document is produced.
Expert group and process
A group of international experts was established, including viewpoints representing anesthesiology, surgery, and nursing. In this POQI I subgroup, each expert was required to submit questions related to optimal perioperative analgesia within ERPs. This first project specifically focused on colorectal surgery patients. Questions were then shared among the group for commentary and elaboration. A final list of questions was agreed upon by the end of the 2-day conference after undergoing a four-step modified Delphi process (Miller et al. 2016).
For content to be included in the paper, we searched PubMed from 1966 to April 2016. All co-authors were familiar with proper literature search protocols, and each conducted a search for at least one portion of the consensus document and shared those references with the other experts. The search was limited to human trials but not limited by language. Duplicate records were deleted. The authors screened the search results in a stepwise manner to identify the eligible studies. In the first step, we screened the titles and abstracts, and irrelevant papers were excluded. During the POQI I conference and thereafter as a writing group, reference applicability to the topic was discussed in any area where there was disagreement.
Results
Our group arrived at the following list of questions as being those most pertinent to and all-encompassing of the topic of optimal analgesia as a component of an ERP for CRS:
What is the definition of optimal analgesia for colorectal surgery?
Why should opioid use be minimized for colorectal surgery patients?
How can optimal analgesia be achieved whilst minimizing opioid use in the preoperative and intraoperative period for colorectal surgery?
How does pain vary based upon the surgical approach in colorectal surgery?
What strategies lead to successful implementation of optimal analgesia for colorectal surgery?
Q1: What is the definition of optimal analgesia for colorectal surgery?
Statement: Optimal analgesia can be defined as a technique that optimizes patient comfort and facilitates recovery of physical function including the bowel, mobilization, cough and normal sleep, while minimizing adverse effects of analgesics (see Fig. 1).
Optimal analgesia cannot be defined by simple pain intensity ratings. Although pain intensity ratings have been associated with impairments in function, there is a nonlinear relationship between types of treatment, analgesic doses, and changes in self-reported numeric pain ratings (de C Williams et al. 2000). Pain after surgery is rarely completely avoided, so “pain free” is not the primary goal of optimal analgesia. Additionally, pain over hours to days after surgery is a dynamic state that is influenced by activities such as coughing and ambulation, and thus a pain-free state is difficult to attain and sustain without medication side effects. Accordingly, the goal of pain prevention and treatment is to reduce pain interference on surgical recovery to the greatest degree possible and avoid secondary adverse outcomes evoked by inflammation, the stress response, and immobilization; hence, the emphasis of optimal analgesia on patient comfort combined with considerations of physical function, sleep, side effects, and safety.
Q2: Why should opioid use be minimized for colorectal surgery patients?
Statement: Minimizing opioid analgesia for CRS patients reduces the adverse effects of opioid use.
There are some clear governing principles that can guide pain management planning to minimize opioid use, and, as discussed above, numerous interventions exist to aid in this approach while optimizing perioperative analgesia. However, opioids have been the backbone for treating perioperative pain and are still used extensively, if not exclusively, in most surgical specialties. As such, it should be noted that the short-term side effects of opioids including nausea, vomiting, ileus, urinary retention, and somnolence can delay enteral intake and mobilization, cause patient distress, and delay hospital discharge in the colorectal surgical patient. Additionally, postoperative delirium in the elderly is a frequent complication that delays discharge and can be caused both by uncontrolled pain and its treatment with opioids (Oresanya et al. 2014; Bilotta et al. 2013; Leung et al. 2013). Finally, traditional use of an opioid-based pain management regimen is likely to be associated with developing hyperalgesia, whereas the use of non-opioid approaches may result in reduced chronic postsurgical pain, cancer recurrence, and long-term survival (Hayhurst and Durieux 2016; Maher and White 2016). This has led to the adoption of a multifaceted approach using various analgesic components to greatly reduce the need to give any opioids during the perioperative period. For all the reasons detailed above, opioid-sparing pathways should be considered a best practice.
Q3: How can optimal analgesia be achieved while minimizing opioid use in the preoperative and intraoperative period for colorectal surgery?
Statement: Optimal analgesia after CRS is achieved through a planned multimodal analgesia approach minimizing opioid use during all phases of perioperative care.
In order to deliver optimal analgesia, a well-structured and planned multimodal approach should be constructed that spans from the preoperative period into the post-discharge recovery phase (see Fig. 2). The components of such a plan will be discussed in detail in this manuscript. It should be noted that there are numerous successful ERPs for CRS with similar but varying analgesic components represented in Fig. 2 (Thiele et al. 2015; Miller et al. 2014; Larson et al. 2014; McEvoy et al. 2016). What is known is that reducing opioid use is of benefit, and high compliance with a variety of standardized non-opioid ERP bundles is strongly associated with reduced opioid use and improved outcomes. In this document, we will restrict the discussion to preoperative and intraoperative components. Part 2 will discuss the analgesic approach throughout all phases of postoperative care (Scott et al. 2016).
Fig. 2.
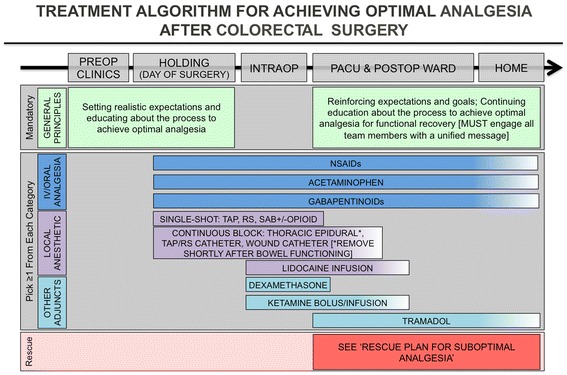
Suggested components of a multimodal approach to pain management in an ERP for colorectal surgery. Of note, the plan should be comprehensive, encompassing all phases of perioperative care from preoperative to post-discharge. However, current evidence is insufficient to determine how many components should be selected in order to maximize pain control, reduce opioid burden, and avoid the side effects of all analgesics used. (ERP enhanced recovery pathway)
Preoperative interventions
Neural blockade
Use of a single-shot spinal opioid (i.e., morphine or hydromorphone) is associated with significantly lower pain at rest and on movement, and reduced opioid requirements (Meylan et al. 2009). These benefits are more prominent in patients undergoing abdominal versus other types of surgery (e.g., cardiac). Although the dose range for this meta-analysis varies considerably (dose range, 100–4000 mcg), current practice tends toward using lower doses of intrathecal morphine (<0.3 mg) as higher dose of intrathecal morphine are associated with more episodes of respiratory depression (Gehling and Tryba 2009). Some centers use a spinal dose of bupivacaine as a carrier for the opioid to cover the incision although there is often a resulting sympathetic block.
Use of thoracic epidural anesthesia (TEA) for open CRS is associated with superior postoperative analgesia (Werawatganon and Charuluxanun 2005; Block et al. 2003), decreased pulmonary/cardiac morbidity (Popping et al. 2014), and earlier return of gastrointestinal function as compared to parenteral analgesia (Marret et al. 2007; Hughes et al. 2014). However, the overall benefits of TEA in improving recovery or decreasing length of stay in patients undergoing laparoscopic colorectal procedures are uncertain (Liu et al. 2014; Khan et al. 2013). Concern that prolonged sympathetic blockade in TEA requires patients to have further intravenous fluids to maintain intravascular volume in face of arterial hypotension were not observed in one meta-analysis of TEA versus patient-controlled analgesia (PCA) in laparoscopic colectomy (Liu et al. 2014).
Peripheral blocks: truncal, paravertebral, and surgical site
Peripheral regional analgesia options for CRS patients include transversus abdominis plane (TAP), paravertebral, or wound and peritoneal infiltration blocks/catheters. All have been shown to some extent to improve perioperative analgesia while decreasing opioid use. Paravertebral blocks and catheters for surgical anesthesia at the level of the thoracic and lumbar vertebrae are associated with less pain during the immediate postoperative period (Thavaneswaran et al. 2010).
Multiple meta-analyses indicate that TAP blocks/catheters for abdominal surgical procedures are associated with superior analgesia and decreased postoperative opioid consumption compared to opioid analgesia alone (Baeriswyl et al. 2015; Zhao et al. 2014; Johns et al. 2012; Siddiqui et al. 2011; Charlton et al. 2010). Preoperative (vs. postoperative) TAP block administration appears to have greater effects on early pain and opioid consumption compared with postoperative administration although the effect of preoperative (vs. postoperative) TAP blocks on longer-term outcomes is unknown (De Oliveira et al. 2014). TAP blocks provide comparable short-term analgesia to wound infiltration but provide superior analgesia in longer term and in the setting of a multimodal analgesic regimen (Yu et al. 2014; Guo et al. 2015a). Concerning the choice of local anesthetic, ropivacaine, bupivacaine, and liposomal bupivacaine have all been used in ERPs with good results (Hamada et al. 2016; Cohen 2012). Liposomal bupivacaine shows some promise for longer-term postoperative analgesia either as infiltration or for TAP blocks (Hutchins et al. 2015); however, there are no large-scale randomized controlled trials available in intra-abdominal surgery to guide practice or to definitively demonstrate the analgesic efficacy of this intervention (Cohen 2012; Candiotti et al. 2014). In short, while there is insufficient evidence to recommend one medication over another at this point in time, it should be noted that additives, such as dexamethasone, are needed to prolong the duration of non-liposomal mixtures (Akkaya et al. 2014).
Intraperitoneal instillation of local anesthetics during major abdominal surgery, including open and laparoscopic colectomy, is associated with significantly lower pain scores postoperatively; although one study did continue TEA in addition to intraperitoneal instillation for 2 days after surgery (Marks et al. 2012; Kahokehr et al. 2011; Park et al. 2011). Additionally, wound infiltration has been shown to be associated with a decrease in morphine consumption and significantly lower pain scores in the early postoperative period in abdominal surgery patients, but none specifically in colorectal surgery (Bamigboye and Hofmeyr 2009). A meta-analysis suggested that the use of local anesthetic wound infiltration was associated with pain scores comparable to those obtained with epidural analgesia and a slight decrease in opioid use, although the data was noted to be quite heterogeneous and significance might exist in the patients receiving each treatment (Ventham et al. 2013). The analgesic efficacy of local anesthetic infusion through wound catheters is uncertain, and meta-analyses have been conflicting in CRS (Liu et al. 2006; Gupta et al. 2011).
Oral analgesia
Major non-opioid oral analgesic agents include non-steroidal anti-inflammatory agents (NSAIDs), acetaminophen (paracetamol), gabapentinoids (gabapentin and pregabalin), and tramadol. All except oral tramadol have been examined within the setting of intra-abdominal surgery and have been found to have significant effect on reducing the opioid burden postoperatively. As such, routine, scheduled use of these agents should be considered as part of a plan to achieve optimal analgesia after colorectal surgery.
Acetaminophen (paracetamol)
Acetaminophen (paracetamol) when administered as part of a multimodal regimen is associated with a decrease in pain and decrease in opioid usage, which may result in a decrease in some opioid-related side effects (Doleman et al. 2015; De Oliveira et al. 2015; Wong et al. 2013; Apfel et al. 2013; McNicol et al. 2011; Toms et al. 2008; Remy et al. 2005). A single dose of IV acetaminophen (paracetamol), typically in a dose of 1 g, given prior to surgery (meta-analysis of 11 RCTs of 740 patients) was associated with significantly lower early pain at rest, early pain with movement, postoperative opioid consumption, and postoperative nausea and vomiting (De Oliveira et al. 2015). In a meta-analysis of 7 RCTs (n = 544 participants), 1 g or 15 mg/kg of IV acetaminophen (paracetamol) given 10–30 min before induction/incision (vs. the same dose given 10–30 min at the end of surgery/before skin closure) was associated with a reduction in 24-h opioid consumption and a lower incidence of postoperative vomiting in the preventive acetaminophen (paracetamol) group (Doleman et al. 2015). Most studies including pharmacokinetic outcomes reported higher postoperative plasma concentrations and larger proportions of patients achieving target plasma concentrations after IV dosing compared with oral dosing (Jibril et al. 2015). However, for patients who can take oral medications preoperatively, there does not appear to be evidence of a clear benefit of the intravenous formulation. Decision making should take into account of convenience and cost (Jibril et al. 2015).
Non-steroidal anti-inflammatory drugs
NSAIDs, whether non-selective or cyclooxygenase-2 inhibitors (COX-2), when administered as part of a multimodal regimen, are associated with a decrease in pain and decrease in opioid usage which may result in a decrease in some opioid-related side effects (De Oliveira et al. 2012; Marret et al. 2005; Straube et al. 2005; Maund et al. 2011; Michelet et al. 2012; Elia et al. 2005). Use of COX-2 inhibitors has minimal effect on coagulation even at supra-therapeutic doses (Leese et al. 2000). A systematic review noted that preoperative COX-2 inhibitors significantly reduced postoperative pain, analgesic consumption, and antiemetic use, and improved patients satisfaction compared with preoperative placebo (Straube et al. 2005). In the studies examining celecoxib, the doses used were 200 or 400 mg PO, and for parecoxib, they were 40 mg PO [Pandazi, 2010].
It is uncertain whether the perioperative use of NSAIDs carries a risk of harm. While it is unlikely that NSAIDs increase the risk of renal injury in euvolemic patients who do not have contraindications to receiving these medications (Myles and Power 1998), caution should be undertaken in patients who are hypotensive or thought to be hypovolemic. Additionally, there is a concern for the potential for an association with increased anastomotic leak, but the literature surrounding this question is not conclusive. As such, insufficient evidence is available to recommend against routine use of NSAIDs, especially COX-2 inhibitors, as these medications are effective in treating pain and reducing opioid use in the perioperative period (Chou et al. 2016a; Bhangu et al. 2014).
Gabapentinoids
Several meta-analyses including studies concerning intra-abdominal surgery suggest that gabapentinoids (gabapentin, pregabalin) when given as a single dose preoperatively are associated with a decrease in postoperative pain and opioid consumption at 24 h (Engelman and Cateloy 2011; Eipe et al. 2015; Hurley et al. 2006; Mishriky et al. 2015; Peng et al. 2007; Seib and Paul 2006; Zhang et al. 2011). For gabapentin, a preoperative dose of 300–1200 mg is associated with lower pain scores (both at rest and with movement) and reduced opioid consumption (Hurley et al. 2006; Peng et al. 2007; Seib and Paul 2006). It should be noted that one small RCT found that a single preoperative dose of gabapentin 600 mg PO did not significantly reduce opioid consumption or pain scores on POD 1 or 2 for patients presenting for colectomy (Siddiqui et al. 2014). However, opioid consumption and pain scores were lower at all time points in the gabapentin group compared to placebo, but there were only 36 patients per group and it was underpowered to detect any difference. As noted by the authors, continuing doses in the postoperative period may confer added benefit given the pharmacokinetics of gabapentin. This corresponds with the dosing reported in successful ERPs for CRS where gabapentin is used as one component to reduce opioid consumption in the perioperative period (Larson et al. 2014; McEvoy et al. 2016). However, the exact contribution of gabapentin to these positive outcomes is unknown. For pregabalin, a recent meta-analysis indicated that pain scores at rest were reduced with all doses of pregabalin (mostly 75–300 mg) but pain scores with movement were only reduced with the 300 mg dose and there were no significant differences in side effects between the three dose levels of pregabalin. The opioid-sparing effect of pregabalin appeared to be limited to doses 100–150 and 300 mg but not ≤75 mg at 2 h after surgery (Mishriky et al. 2015). While most of the studies in the meta-analysis involve abdominal hysterectomy and cholecystectomy, none were in CRS patients. Of note, there is a substantial cost difference at present between gabapentin and pregabalin. As such, if a gabapentinioid is to be considered as one component in an ERP, we recommend use of gabapentin as a first line agent unless the patient was prescribed pregabalin for a chronic pain condition prior to surgery or if gabapentin use resulted in significant sedation.
Intraoperative
Intravenous medications
Lidocaine
Intravenous (IV) lidocaine infusion is indicated as part of a multimodal analgesic approach for visceral surgery when other local anesthetic approaches such as regional analgesia are not possible. In open and laparoscopic abdominal surgery, IV lidocaine infusions have been shown to result in significant reduction in postoperative pain intensity at rest and with cough and movement and opioid consumption for up to 48 h postoperatively, as well as being associated with earlier return of bowel function allowing for earlier recovery and shorter length of stay (Marret et al. 2008; Vigneault et al. 2011). Lidocaine infusions are contraindicated in patients with cardiovascular instability and concomitant use of alpha agonists or beta-blockers and in patients with allergies to other amide local anesthetics (bupivacaine). Side effects are more pronounced in patients with liver dysfunction, pulmonary diseases when the predominant problem is carbon dioxide retention, and congestive heart failure. Lidocaine is typically administered as a bolus (100–150 mg or 1.5–2.0 mg/kg) followed by an infusion of 1 to 3 mg/kg/h through the end of surgery. Several meta-analyses suggest that perioperative administration of IV lidocaine is associated with a decrease in postoperative pain and opioid consumption and possibly faster return of bowel function and decreased length of hospital stay (Marret et al. 2008; Vigneault et al. 2011; Khan et al. 2016; McCarthy et al. 2010).
N -methyl- d -aspartate antagonists
Ketamine. Perioperative inhibition of N-methyl-d-aspartate (NMDA) receptors with clinically available NMDA antagonists such as ketamine may be associated with improved perioperative pain and decreased opioid use (Wang et al. 2016; Ding et al. 2014; Dahmani et al. 2011; Bell et al. 2006). Perioperative ketamine, including boluses as well as intraoperative and postoperative low-dose infusions for up to 48 h, has been shown to result in significant reductions in pain, opioid consumption, and PONV with no significant side effect profile (Zakine et al. 2008; Laskowski et al. 2011; Sami Mebazaa et al. 2008). The intraoperative boluses ranged from 0.15 to 1 mg/kg, and perioperative infusions ranged from 1 to 5 mcg/kg/min, with a postoperative infusion rate of 2 mcg/kg/min. Ketamine has also been shown to be of particular benefit in patients on chronic opioid, but this has not been specifically tested in chronic pain patients undergoing CRS (Loftus et al. 2010).
Magnesium. Systemic infusions of perioperative magnesium may reduce postoperative pain and opioid consumption (De Oliveira et al. 2013; Guo et al. 2015b; Murphy et al. 2013). The optimal dosing is uncertain as some studies include both a bolus followed by an infusion whereas others only utilize an infusion without a loading bolus. Typical boluses are 30–50 mg/kg, and the infusion rates range from 4 to 15 mg/kg/h. None of the studies in a systematic review reported clinical toxicity related to toxic serum levels of magnesium (De Oliveira et al. 2013).
Glucocorticoids
Glucocorticoid steroids may have analgesic properties possibly related to anti-inflammatory properties and should be considered as part of a multimodal perioperative pain regimen. Several meta-analyses examined perioperative dexamethasone and indicated that patients who received dexamethasone (4–10 mg or >0.1 mg/kg) had lower pain scores, used less opioids, and required less rescue analgesia (Waldron et al. 2013; Allen et al. 2012; De Oliveira et al. 2011). The concern for significant hyperglycemia (>180 mg/dL) has not been confirmed, even in bariatric patients receiving these doses of dexamethasone (Hans et al. 2006).
Alpha-2 agonists
A Cochrane review of dexmedetomidine infusions for pain found reduced opioid consumption but no significant difference in pain scores compared to placebo, and there was more hypotension in the dexmedetomidine group (Jessen Lundorf et al. 2016). Dexmedetomidine has been added both perineurally to nerve blocks and intravenously to prolong nerve block (Abdallah et al. 2016; Das et al. 2016). There is limited data, but dexmedetomidine does appear to prolong nerve blocks. However, the extended duration is not as long as that provided by perineural dexamethasone provides. In a recent extensive review of perioperative alpha agonists, dexmedetomidine and clonidine were compared (Blaudszun et al. 2012). Similar to dexmedetomidine, clonidine can also reduce opioid consumption. In addition, both alpha agonists appear to have a weak antiemetic effect, but as expected, both drugs had adverse effects on hemodynamics. In summary, both dexmedetomidine and clonidine when administered perioperatively can reduce morphine consumption up to 24 h and to a similar extent as acetaminophen (paracetamol), but not as much as other NSAIDs. Both clonidine and dexmedetomidine have other side effects such as sedation and hypotension that have to be considered (Garg et al. 2014). Typical doses of clonidine range from 1 to 5 mcg/kg PO, IV, or perineurally and for dexmedetomidine from 0.5 mcg/kg IV bolus followed by an infusion of 0.2–0.7 mcg/kg/h or 0.5 mcg/kg perineurally.
Acetaminophen (paracetamol)
If oral acetaminophen (paracetamol) is administered preoperatively as part of a multimodal analgesia pathway, there is typically no need to administer it again until the next scheduled dose. If the next scheduled dose is possible orally, then administration should be oral. However, if the preoperative dose has been missed or oral administration is not possible when the next dose is due, then intravenous acetaminophen (paracetamol) can be administered. Currently, there is no evidence that intravenous acetaminophen (paracetamol) is superior to oral formulations as an analgesic (Jibril et al. 2015; Fenlon et al. 2013). If a patient is not able to take oral medications, then intravenous acetaminophen (paracetamol) has been shown to be an effective opioid-sparing analgesic compared to placebo (O’Neal 2013; Smith 2011).
Non-steroidal anti-inflammatory drugs
As noted above, NSAIDs should be prescribed orally as part of routine preoperative medications for colorectal surgery. However, if a preoperative dose is not given for concerns of bleeding, parenteral options are available. Ketorolac has been used extensively, and newer preparations including intravenous ibuprofen and diclofenac are now available (De Oliveira et al. 2012; Kroll 2012). These medications can improve pain scores, reduce opioid requirements, and reduce opioid-related side effects as discussed in the section above. The optimal dose and timing of each of these medications is not yet known. There is currently no evidence of a better risk benefit profile for one intravenous NSAID over another.
Tramadol
Overall, there is limited evidence for the use of tramadol in colorectal surgery. However, one study in particular compared postoperative pain control with an opioid IV-PCA to IV tramadol and found that the patients in the non-PCA (tramadol) group needed less rescue analgesia and none of them needed to have an IV-PCA started (Choi et al. 2015). Additionally, another study included scheduled tramadol as part of an ERP (Lloyd et al. 2010). While this was only one component of the ERP, overall pain scores and other outcomes were improved. If tramadol is to be used, one study would suggest caution with its use in patients undergoing major abdominal surgery who are over 75 years, ASA 3 or 4, and have impaired mobility or frailty, as use in this setting was associated with delirium (Brouquet et al. 2010). Based on the evidence that exists in colorectal surgery, we recommend considering tramadol as an analgesic adjunct as one part of an ERP.
Opioids
The primary objective of the practitioner should be to minimize the use of opioids wherever possible. Under general anesthesia, patients are unconscious and do not perceive pain. Although there is no pain perception, surgery-induced activation of nociceptive reflex arcs has hemodynamic consequences. Using opioids to reduce the hemodynamic changes should be resisted as much as possible, as hyperalgesia can develop acutely and opioid-related side effects can occur at very low doses (Hayhurst and Durieux 2016; Rathmell et al. 2006). Control of blood pressure and heart rate at intubation and during surgery by beta-blockers, calcium channel blockers, or lidocaine should be encouraged rather than using opioids without a specific indication other than intraoperative tachycardia or hypertension. As noted above, replacing opioids with any non-opioid analgesic or esmolol intraoperatively leads to less opioid being required in the PACU and often out to 24 h postoperatively in some laparoscopic surgery (Lee and Lee 2010; Collard et al. 2007; Pranevicius and Pranevicius 2009). If an opioid must be used, we recommend using small doses of short-acting opioids such as alfentanil or fentanyl.
Q4: How does pain vary based upon the surgical approach in colorectal surgery?
Statement: The degree of pain after CRS will vary based upon the surgical approach and planned analgesic solutions will take this into account.
As the pain experience is different based on the procedure, so too should the analgesia be procedure and patient-specific. While not considered an analgesic strategy, a number of studies have reported reduction in short-term pain, analgesic use, and more rapid recovery of bowel function with laparoscopic surgery versus open (Reza et al. 2006). The difference in the pain experience between laparoscopic and open approaches for operations may be limited to the short-term management, as pain reported at 1 to 3 months did not differ between approaches (Reza et al. 2006; Lourenco et al. 2008; Murray et al. 2006). One important difference in laparoscopic CRS compared to other laparoscopic procedures is the placement of ports and size and placement of the largest incision. Different from a gallbladder or a morselized uterus, the colorectal specimens have to be removed intact. Often, the bowel anastomosis is most easily organized extra-corporeally as well. This longer incision, for specimen extraction and anastomosis, may be umbilical, transverse, upper or lower midline, or Pfannenstiel. Overall, the chosen approach/incision and its length certainly impact the best choice of analgesia plan. Additionally, as the surgeon can occasionally choose to vary approach intraoperatively as needed, a secondary plan should be in place for these circumstances.
Q5: What is the role of education in achieving optimal analgesia after CRS?
Statement: Patient and family education throughout the entire perioperative period is essential for achieving optimal analgesia after CRS.
Optimal analgesia cannot be achieved without involving the most important factor, the patient, starting with partnership, education, shared decision making, and well-coordinated transitions of care (Manary et al. 2013). Because pain is a complex and subjective biopsychosocial experience, management of patient expectations and education regarding realistic goals of pain treatment is crucial to an effective pharmacological approach. Thus, we recommend this includes analgesic treatment plans and goals for postoperative pain management (Wood 2010; Oshodi 2007a; Oshodi 2007b). While the exact timing, methods, and content of preoperative education will be locally determined, we suggest that patient education and expectation management occur through all phases of care and include information about choice and risks of analgesic technique, goals of analgesia, anticipated patient participation in recovery activities, and non-pharmacological methods that can be employed to reduce reliance on rescue analgesics (see Fig. 2) (Chou et al. 2016b; O’Connor et al. 2014). Important considerations include information about analgesia methods and goals in patient-friendly print materials with appropriate literacy levels (Ihedioha et al. 2013).
Additionally, as some patients will have had surgery prior to the institution of an ERP, and as ERPs for CRS typically utilize a multimodal analgesic approach to minimize perioperative opioid use in an attempt to decrease opioid-related side effects, it is important to discuss surgical history and pain management expectations based on prior interventions and experiences. This is especially true as some ERPs may attempt to avoid IV-PCA opioids and thus use opioids only as PRN rescue, as recommended in Part 2 (McEvoy et al. 2016; Wu et al. 2015). It should be explained to patients that opioids still remain an important option for postoperative pain management; however, the precise role of opioids in ERPs is currently not clearly defined, and expectations surrounding their use in the perioperative, along with the goals of delivering optimal analgesia, should be clarified preoperatively.
Summary and future directions
Delivering optimal analgesia is a key component of enhanced recovery pathways for colorectal surgery. Following our literature search and modified Delphi process, we conclude that the number of published studies specifically examining analgesia for CRS within an ERP with good documented compliance is limited. We have used data from these studies and extrapolated data from other published studies on analgesia for CRS to recommend a best-practice approach for creating a perioperative pain management plan that includes a number of key components (see Table 1).
Table 1.
Key points for analgesia within an ERP for colorectal surgery
| • Analgesia is a key component in enhanced recovery pathways. | |
| • Optimal analgesia addresses patient pain while restoring function and minimizing side effects. | |
| • Minimizing opioid use and its side effects is a cornerstone of analgesia practice within ERPs. | |
| • Intraoperative opioid-sparing techniques and postoperative early oral multimodal analgesia are the backbone for providing analgesia within ERPs. | |
| • Open, laparoscopic, and robotic surgical approaches need different analgesic strategies. | |
| • There are many different analgesic combinations that are efficacious. | |
| • Hospitals should adopt at least two or three analgesic strategies for colorectal surgery to allow for individual patient variation or failure of the primary choice of analgesia. | |
| • Hospitals should have a troubleshooting pathway in place for breakthrough pain to minimize the negative impact of intravenous opioid use. | |
| • Audit of compliance of analgesia and restoration of function can lead to improvement of patient experience. |
There are many ways to achieve analgesia for patients undergoing CRS. The technique of choice will depend on which surgical procedure is performed and by which approach (laparoscopic, robotic assisted, or open). Additionally, patient factors and availability of experienced providers, training, and equipment within a hospital will also determine which technique can be utilized. A multifaceted approach to pain management is necessary to restore postoperative function as rapidly as possible to baseline. Importantly, this must facilitate mobility and respiratory function, enable early oral intake of diet and hydration, avoid ileus, promote normal sleep patterns, and minimize the common side effects of opiate-based analgesia. We have produced a consensus outlining a rationale and key components for approaching pain management in an ERP for CRS. Part 2 of this series will discuss the postoperative components of an optimal analgesia care plan for CRS.
Acknowledgements
Group authorship: Perioperative Quality Initiative (POQI) I Workgroup
POQI I conference directors (named authors on all manuscripts)
• Timothy E Miller, Department of Anesthesiology, Duke University Medical Center, NC, USA.
• Andrew D Shaw, Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN, USA.
• Michael G Mythen, Department of Anaesthesia, University College London, London, UK.
• Tong J Gan, Department of Anesthesiology, Stony Brook University School of Medicine, NY, USA.
Group A—analgesia
• Matthew D. McEvoy, Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN, USA (chair).
• Michael J. Scott, Department of Anaesthesia, Royal Surrey County NHS Foundation Hospital, Surrey, UK (co-chair).
• Deborah Gordon, RN, Department of Anesthesiology and Pain Medicine, University of Washington.
• Stuart Grant, Department of Anesthesiology, Duke University Medical Center, NC, USA.
• Julie K.M. Thacker, Division of Advanced Oncologic and GI Surgery, Duke University Medical Center, NC, USA.
• Christopher L. Wu, Department of Anesthesiology, The Johns Hopkins University School of Medicine, MD, USA.
Group B—fluids
• Robert H. Thiele, Departments of Anesthesiology and Biomedical Engineering, University of Virginia School of Medicine, VA, USA (chair).
• Karthik Raghunathan, Department of Anesthesiology, Duke University Medical Center, USA (co-chair).
• CS Brudney, Department of Anesthesiology, Duke University Medical Center, USA.
• Dileep N Lobo, Division of Gastrointestinal Surgery, Nottingham University Hospitals and University of Nottingham, Nottingham, UK.
• Dr. Daniel Martin, Royal free Perioperative Research Group, Royal Free Hospital, London, UK.
• Anthony Senagore, Department of Surgery, University of Texas-Medical Branch at Galveston, Galveston, TX, USA.
Group C—infection
• Stefan D Holubar, Department of Surgery, Dartmouth-Hitchcock Medical Center, NH, USA (chair).
• Traci Hedrick, Department of Surgery, University of Virginia School of Medicine, VA, USA (co-chair).
• John Kellum, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
• Ruchir Gupta, Department of Anesthesiology, Stony Brook University School of Medicine, NY, USA.
• Mark Hamilton, Department of Anaesthesia, St. George’s Hospital and Medical School, London, UK.
Group D – outcomes
• S. Ramani Moonesinghe, Department of Anaesthesia, University College London, London, UK. (chair).
• Mike PW Grocott, Department of Anesthesia and Critical Care Medicine, University of Southampton, UK (co-chair).
• Elliott Bennett-Guerrero, Department of Anesthesiology, Stony Brook University School of Medicine, NY, USA.
• Thomas J Hopkins, Department of Anesthesiology, Duke University Medical Center, NC, USA.
• Roberto Bergamaschi, Department of Surgery, Stony Brook University School of Medicine, NY, USA.
• Stuart McCluskey, Department of Anesthesia, University of Toronto, ON, Canada.
• Vijaya Gottumukkala, Department of Anesthesiology and Perioperative Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX
Funding
Funding for travel related to the work in this document was provided by the American Society of Enhanced Recovery (ASER).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the creation of this consensus document.
Authors’ contributions
All authors participated in the literature review, development of questions, modified Delphi technique, and writing of the manuscript. All authors read and approved the final manuscript.
Competing interests
MDM has funding unrelated to this topic from Edwards Lifesciences and the GE Foundation. MGM is Smiths Medical Professor of Anaesthesia and Critical Care UCL and a Consultant at UCLH. He is the Director of the UCL Centre for Anaesthesia and The UCL Discovery Lab and a resident PI at the Institute of Spots Exercise and Health. He is a paid Consultant for Edwards Lifesciences (via UCL Consulting and independently) and Deltex in the USA. He was a National Clinical Advisor for the Department of Health Enhanced Recovery Partnership until May 2013; Stock holder and advisory board for Medical Defence Technologies LLC—(“Gastrostim” patented); Director Bloomsbury Innovation Group—a community interest company owned by UCLH Charity; Co-Inventor of “QUENCH” (fluid management system) IP being exploited by UCL Business. MGM’s institution has also received charitable donations and grants from Smiths Medical Endowment and Deltex Medical. MGM was also a co-author of the GIFTASUP guidelines on perioperative fluid management; Editor in Chief of Perioperative Medicine; on the Editorial Board of the BJA and Critical Care; a member of the Improving Surgical Outcomes Group; Expert advisor to the NICE IV fluids guideline development group; Chairman of the Board of The National Institute of Academic Anaesthesia; Co-Director Xtreme Everest; Co-Chair Evidence Based Perioperative Medicine (EBPOM). ADS is a Consultant for Astute Medical and Edwards Lifesciences. He is on the Scientific Advisory Board for Thrasos and Battelle and DSMB chair for AM Pharma. TEM has received research funding for Edwards Lifesciences and is a consultant for Edwards LIfesciences and Cheetah Medical. The other authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- COX-2
Cyclooxygenase-2
- CRS
Colorectal surgery
- ERP
Enhanced recovery pathway
- IV
Intravenous
- NMDA
N-methyl-d-aspartate
- NSAIDs
Non-steroidal anti-inflammatory agents
- PACU
Post-anesthesia care unit
- PCA
Patient-controlled analgesia
- PONV
Postoperative nausea and vomiting
- POQI
Perioperative Quality Improvement
- TAP
Transversus abdominis plane
- TEA
Thoracic epidural analgesia
Contributor Information
Timothy E. Miller, Email: timothy.miller2@duke.edu
For the Perioperative Quality Initiative (POQI) I Workgroup:
Robert H. Thiele, Karthik Raghunathan, C. S. Brudney, Dileep N. Lobo, Daniel Martin, Anthony Senagore, Stefan D. Holubar, Traci Hedrick, John Kellum, Ruchir Gupta, Mark Hamilton, S. Ramani Moonesinghe, Mike P. W. Grocott, Elliott Bennett-Guerrero, Thomas J. Hopkins, Roberto Bergamaschi, Stuart McCluskey, and Vijaya Gottumukkala
References
- Abdallah FW, Dwyer T, Chan VW, Niazi AU, Ogilvie-Harris DJ, Oldfield S, Patel R, Oh J, Brull R. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. 2016;124:683–95. doi: 10.1097/ALN.0000000000000983. [DOI] [PubMed] [Google Scholar]
- Akkaya A, Yildiz I, Tekelioglu UY, Demirhan A, Bayir H, Ozlu T, Bilgi M, Kocoglu H. Dexamethasone added to levobupivacaine in ultrasound-guided tranversus abdominis plain block increased the duration of postoperative analgesia after caesarean section: a randomized, double blind, controlled trial. Eur Rev Med Pharmacol Sci. 2014;18:717–22. [PubMed] [Google Scholar]
- Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012;114:813–22. doi: 10.1213/ANE.0b013e318247f628. [DOI] [PubMed] [Google Scholar]
- Apfel CC, Turan A, Souza K, Pergolizzi J, Hornuss C. Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain. 2013;154:677–89. doi: 10.1016/j.pain.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Baeriswyl M, Kirkham KR, Kern C, Albrecht E. The analgesic efficacy of ultrasound-guided transversus abdominis plane block in adult patients: a meta-analysis. Anesth Analg. 2015;121:1640–54. doi: 10.1213/ANE.0000000000000967. [DOI] [PubMed] [Google Scholar]
- Bamigboye AA, Hofmeyr GJ. Local anaesthetic wound infiltration and abdominal nerves block during caesarean section for postoperative pain relief. Cochrane Database Syst Rev. 2009: CD006954. [DOI] [PubMed]
- Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006: CD004603. [DOI] [PubMed]
- Bhangu A, Singh P, Fitzgerald JE, Slesser A, Tekkis P. Postoperative nonsteroidal anti-inflammatory drugs and risk of anastomotic leak: meta-analysis of clinical and experimental studies. World J Surg. 2014;38:2247–57. doi: 10.1007/s00268-014-2531-1. [DOI] [PubMed] [Google Scholar]
- Bilotta F, Lauretta MP, Borozdina A, Mizikov VM, Rosa G. Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol. 2013;79:1066–76. [PubMed] [Google Scholar]
- Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–22. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA, Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–63. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, Teillet L, Nordlinger B. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251:759–65. doi: 10.1097/SLA.0b013e3181c1cfc9. [DOI] [PubMed] [Google Scholar]
- Candiotti KA, Sands LR, Lee E, Bergese SD, Harzman AE, Marcet J, Kumar AS, Haas E. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2014;76:1–6. doi: 10.1016/j.curtheres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010: CD007705. [DOI] [PubMed]
- Choi YY, Park JS, Park SY, Kim HJ, Yeo J, Kim JC, Park S, Choi GS. Can intravenous patient-controlled analgesia be omitted in patients undergoing laparoscopic surgery for colorectal cancer? Ann Surg Treat Res. 2015;88:86–91. doi: 10.4174/astr.2015.88.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CI, Shih CJ, Chen YT, Ou SM, Yang CY, Kuo SC, Chu D. Adverse effects of oral nonselective and cyclooxygenase-2-selective NSAIDs on hospitalization for acute kidney injury: a nested case-control cohort study. Medicine (Baltimore) 2016;95:e2645. doi: 10.1097/MD.0000000000002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, Griffith S, Manworren R, McCarberg B, Montgomery R, Murphy J, Perkal MF, Suresh S, Sluka K, Strassels S, Thirlby R, Viscusi E, Walco GA, Warner L, Weisman SJ, Wu CL. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Extended pain relief trial utilizing infiltration of Exparel((R)), a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–72. doi: 10.2147/JPR.S38621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, Carli F. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth Analg. 2007;105:1255–62. doi: 10.1213/01.ane.0000282822.07437.02. [DOI] [PubMed] [Google Scholar]
- Dahmani S, Michelet D, Abback PS, Wood C, Brasher C, Nivoche Y, Mantz J. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatr Anaesth. 2011;21:636–52. doi: 10.1111/j.1460-9592.2011.03566.x. [DOI] [PubMed] [Google Scholar]
- Das B, Lakshmegowda M, Sharma M, Mitra S, Chauhan R. Supraclavicular brachial plexus block using ropivacaine alone or combined with dexmedetomidine for upper limb surgery: a prospective, randomized, double-blinded, comparative study. Rev Esp Anestesiol Reanim. 2016;63:135–140. doi: 10.1016/j.redar.2015.04.012. [DOI] [PubMed] [Google Scholar]
- de C Williams AC, Davies HT, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain. 2000;85:457–63. doi: 10.1016/S0304-3959(99)00299-7. [DOI] [PubMed] [Google Scholar]
- De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–88. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- De Oliveira GS, Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114:424–33. doi: 10.1213/ANE.0b013e3182334d68. [DOI] [PubMed] [Google Scholar]
- De Oliveira GS, Jr, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:178–90. doi: 10.1097/ALN.0b013e318297630d. [DOI] [PubMed] [Google Scholar]
- De Oliveira GS, Jr, Castro-Alves LJ, Nader A, Kendall MC, McCarthy RJ. Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: a meta-analysis of randomized controlled trials. Anesth Analg. 2014;118:454–63. doi: 10.1213/ANE.0000000000000066. [DOI] [PubMed] [Google Scholar]
- De Oliveira GS, Jr, Castro-Alves LJ, McCarthy RJ. Single-dose systemic acetaminophen to prevent postoperative pain: a meta-analysis of randomized controlled trials. Clin J Pain. 2015;31:86–93. doi: 10.1097/AJP.0000000000000081. [DOI] [PubMed] [Google Scholar]
- Ding X, Jin S, Niu X, Wang T, Zhao X, Ren H, Tong Y, Li Q. Morphine with adjuvant ketamine versus higher dose of morphine alone for acute pain: a meta-analysis. Int J Clin Exp Med. 2014;7:2504–10. [PMC free article] [PubMed] [Google Scholar]
- Doleman B, Read D, Lund JN, Williams JP. Preventive acetaminophen reduces postoperative opioid consumption, vomiting, and pain scores after surgery: systematic review and meta-analysis. Reg Anesth Pain Med. 2015;40:706–12. doi: 10.1097/AAP.0000000000000311. [DOI] [PubMed] [Google Scholar]
- Eipe N, Penning J, Yazdi F, Mallick R, Turner L, Ahmadzai N, Ansari MT. Perioperative use of pregabalin for acute pain-a systematic review and meta-analysis. Pain. 2015;156:1284–300. doi: 10.1097/j.pain.0000000000000173. [DOI] [PubMed] [Google Scholar]
- Elia N, Lysakowski C, Tramer MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103:1296–304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]
- Engelman E, Cateloy F. Efficacy and safety of perioperative pregabalin for post-operative pain: a meta-analysis of randomized-controlled trials. Acta Anaesthesiol Scand. 2011;55:927–43. doi: 10.1111/j.1399-6576.2011.02471.x. [DOI] [PubMed] [Google Scholar]
- Fenlon S, Collyer J, Giles J, Bidd H, Lees M, Nicholson J, Dulai R, Hankins M, Edelman N. Oral vs intravenous paracetamol for lower third molar extractions under general anaesthesia: is oral administration inferior? Br J Anaesth. 2013;110:432–7. doi: 10.1093/bja/aes387. [DOI] [PubMed] [Google Scholar]
- Garg AX, Kurz A, Sessler DI, Cuerden M, Robinson A, Mrkobrada M, Parikh CR, Mizera R, Jones PM, Tiboni M, Font A, Cegarra V, Gomez MF, Meyhoff CS, VanHelder T, Chan MT, Torres D, Parlow J, Clanchet Mde N, Amir M, Bidgoli SJ, Pasin L, Martinsen K, Malaga G, Myles P, Acedillo R, Roshanov PS, Walsh M, Dresser G, Kumar P, Fleischmann E, Villar JC, Painter T, Biccard B, Bergese S, Srinathan S, Cata JP, Chan V, Mehra B, Wijeysundera DN, Leslie K, Forget P, Whitlock R, Yusuf S, Devereaux PJ, Investigators P Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA. 2014;312:2254–64. doi: 10.1001/jama.2014.15284. [DOI] [PubMed] [Google Scholar]
- Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. 2009;64:643–51. doi: 10.1111/j.1365-2044.2008.05817.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Li R, Wang L, Zhang D, Ma Y. Transversus abdominis plane block versus local anaesthetic wound infiltration for postoperative analgesia: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:17343–52. [PMC free article] [PubMed] [Google Scholar]
- Guo BL, Lin Y, Hu W, Zhen CX, Bao-Cheng Z, Wu HH, Kaye AD, Duan JH, Qu Y. Effects of systemic magnesium on post-operative analgesia: is the current evidence strong enough? Pain Physician. 2015;18:405–18. [PubMed] [Google Scholar]
- Gupta A, Favaios S, Perniola A, Magnuson A, Berggren L. A meta-analysis of the efficacy of wound catheters for post-operative pain management. Acta Anaesthesiol Scand. 2011;55:785–96. doi: 10.1111/j.1399-6576.2011.02463.x. [DOI] [PubMed] [Google Scholar]
- Hamada T, Tsuchiya M, Mizutani K, Takahashi R, Muguruma K, Maeda K, Ueda W, Nishikawa K. Levobupivacaine–dextran mixture for transversus abdominis plane block and rectus sheath block in patients undergoing laparoscopic colectomy: a randomised controlled trial. Anaesthesia. 2016;71:411–6. doi: 10.1111/anae.13408. [DOI] [PubMed] [Google Scholar]
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth. 2006;97:164–70. doi: 10.1093/bja/ael111. [DOI] [PubMed] [Google Scholar]
- Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology. 2016;124:483–8. doi: 10.1097/ALN.0000000000000963. [DOI] [PubMed] [Google Scholar]
- Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg. 2014;149:1224–30. doi: 10.1001/jamasurg.2014.210. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31:237–47. doi: 10.1016/j.rapm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hutchins J, Delaney D, Vogel RI, Ghebre RG, Downs LS, Jr, Carson L, Mullany S, Teoh D, Geller MA. Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: a prospective randomized controlled study. Gynecol Oncol. 2015;138:609–13. doi: 10.1016/j.ygyno.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihedioha U, Vaughan S, Mastermann J, Singh B, Chaudhri S. Patient education videos for elective colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2013;15:1436–41. doi: 10.1111/codi.12348. [DOI] [PubMed] [Google Scholar]
- Jessen Lundorf L, Korvenius Nedergaard H, Moller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst Rev. 2016;2:CD010358. doi: 10.1002/14651858.CD010358.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibril F, Sharaby S, Mohamed A, Wilby KJ. Intravenous versus oral acetaminophen for pain: systematic review of current evidence to support clinical decision-making. Can J Hosp Pharm. 2015;68:238–47. doi: 10.4212/cjhp.v68i3.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns N, O'Neill S, Ventham NT, Barron F, Brady RR, Daniel T. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorectal Dis. 2012;14:e635–42. doi: 10.1111/j.1463-1318.2012.03104.x. [DOI] [PubMed] [Google Scholar]
- Kahokehr A, Sammour T, Zargar Shoshtari K, Taylor M, Hill AG. Intraperitoneal local anesthetic improves recovery after colon resection: a double-blinded randomized controlled trial. Ann Surg. 2011;254:28–38. doi: 10.1097/SLA.0b013e318221f0cf. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–98. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- Khan SA, Khokhar HA, Nasr AR, Carton E, El-Masry S. Effect of epidural analgesia on bowel function in laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc. 2013;27:2581–91. doi: 10.1007/s00464-013-2794-x. [DOI] [PubMed] [Google Scholar]
- Khan JS, Yousuf M, Victor JC, Sharma A, Siddiqui N. An estimation for an appropriate end time for an intraoperative intravenous lidocaine infusion in bowel surgery: a comparative meta-analysis. J Clin Anesth. 2016;28:95–104. doi: 10.1016/j.jclinane.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Kroll PB. Intravenous ibuprofen for postoperative pain. Pain Manag. 2012;2:47–54. doi: 10.2217/pmt.11.68. [DOI] [PubMed] [Google Scholar]
- Larson DW, Lovely JK, Cima RR, Dozois EJ, Chua H, Wolff BG, Pemberton JH, Devine RR, Huebner M. Outcomes after implementation of a multimodal standard care pathway for laparoscopic colorectal surgery. Br J Surg. 2014;101:1023–30. doi: 10.1002/bjs.9534. [DOI] [PubMed] [Google Scholar]
- Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–23. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee JN. The effect of perioperative esmolol infusion on the postoperative nausea, vomiting and pain after laparoscopic appendectomy. Korean J Anesthesiol. 2010;59:179–84. doi: 10.4097/kjae.2010.59.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol. 2000;40:124–32. doi: 10.1177/00912700022008766. [DOI] [PubMed] [Google Scholar]
- Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry. 2013;21:946–56. doi: 10.1016/j.jagp.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914–32. doi: 10.1016/j.jamcollsurg.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu X, Duan X, Wu J. Thoracic epidural analgesia (TEA) vs. patient controlled analgesia (PCA) in laparoscopic colectomy: a meta-analysis. Hepatogastroenterology. 2014;61:1213–9. [PubMed] [Google Scholar]
- Lloyd GM, Kirby R, Hemingway DM, Keane FB, Miller AS, Neary P. The RAPID protocol enhances patient recovery after both laparoscopic and open colorectal resections. Surg Endosc. 2010;24:1434–9. doi: 10.1007/s00464-009-0795-6. [DOI] [PubMed] [Google Scholar]
- Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–46. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- Lourenco T, Murray A, Grant A, McKinley A, Krukowski Z, Vale L. Laparoscopic surgery for colorectal cancer: safe and effective?—a systematic review. Surg Endosc. 2008;22:1146–60. doi: 10.1007/s00464-007-9686-x. [DOI] [PubMed] [Google Scholar]
- Maher DP, White PF. Proposed mechanisms for association between opioid usage and cancer recurrence after surgery. J Clin Anesth. 2016;28:36–40. doi: 10.1016/j.jclinane.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368:201–3. doi: 10.1056/NEJMp1211775. [DOI] [PubMed] [Google Scholar]
- Marks JL, Ata B, Tulandi T. Systematic review and metaanalysis of intraperitoneal instillation of local anesthetics for reduction of pain after gynecologic laparoscopy. J Minim Invasive Gynecol. 2012;19:545–53. doi: 10.1016/j.jmig.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102:1249–60. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- Marret E, Remy C, Bonnet F, Postoperative Pain Forum G Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg. 2007;94:665–73. doi: 10.1002/bjs.5825. [DOI] [PubMed] [Google Scholar]
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95:1331–8. doi: 10.1002/bjs.6375. [DOI] [PubMed] [Google Scholar]
- Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106:292–7. doi: 10.1093/bja/aeq406. [DOI] [PubMed] [Google Scholar]
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70:1149–63. doi: 10.2165/10898560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McEvoy MD, Wanderer JP, King AB, Geiger TM, Tiwari V, Terekhov M, Ehrenfeld JM, Furman WR, Lee LA, Sandberg WS. A perioperative consult service results in reduction in cost and length of stay for colorectal surgical patients: evidence from a healthcare redesign project. Perioper Med (Lond) 2016;5:3. doi: 10.1186/s13741-016-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol ED, Tzortzopoulou A, Cepeda MS, Francia MB, Farhat T, Schumann R. Single-dose intravenous paracetamol or propacetamol for prevention or treatment of postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2011;106:764–75. doi: 10.1093/bja/aer107. [DOI] [PubMed] [Google Scholar]
- Meylan N, Elia N, Lysakowski C, Tramer MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth. 2009;102:156–67. doi: 10.1093/bja/aen368. [DOI] [PubMed] [Google Scholar]
- Michelet D, Andreu-Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, Mantz J, Dahmani S. A meta-analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesth Analg. 2012;114:393–406. doi: 10.1213/ANE.0b013e31823d0b45. [DOI] [PubMed] [Google Scholar]
- Miller TE, Thacker JK, White WD, Mantyh C, Migaly J, Jin J, Roche AM, Eisenstein EL, Edwards R, Anstrom KJ, Moon RE, Gan TJ, Enhanced Recovery Study G Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–61. doi: 10.1213/ANE.0000000000000206. [DOI] [PubMed] [Google Scholar]
- Miller TE, Shaw AD, Mythen MG, Gan TJ, Perioperative Quality Initiative IW Evidence-based perioperative medicine comes of age: the Perioperative Quality Initiative (POQI): the 1st consensus conference of the Perioperative Quality Initiative (POQI) Perioper Med (Lond) 2016;5:26. doi: 10.1186/s13741-016-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114:10–31. doi: 10.1093/bja/aeu293. [DOI] [PubMed] [Google Scholar]
- Murphy JD, Paskaradevan J, Eisler LL, Ouanes JP, Tomas VA, Freck EA, Wu CL. Analgesic efficacy of continuous intravenous magnesium infusion as an adjuvant to morphine for postoperative analgesia: a systematic review and meta-analysis. Middle East J Anaesthesiol. 2013;22:11–20. [PubMed] [Google Scholar]
- Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, Krukowski Z, Vale L, Grant A. Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2006;10:1–141. doi: 10.3310/hta10450. [DOI] [PubMed] [Google Scholar]
- Myles PS, Power I. Does ketorolac cause postoperative renal failure: how do we assess the evidence? Br J Anaesth. 1998;80:420–1. doi: 10.1093/bja/80.4.420. [DOI] [PubMed] [Google Scholar]
- O'Connor G, Coates V, O'Neill S. Randomised controlled trial of a tailored information pack for patients undergoing surgery and treatment for rectal cancer. Eur J Oncol Nurs. 2014;18:183–91. doi: 10.1016/j.ejon.2013.10.011. [DOI] [PubMed] [Google Scholar]
- O'Neal JB. The utility of intravenous acetaminophen in the perioperative period. Front Public Health. 2013;1:25. doi: 10.3389/fpubh.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311:2110–20. doi: 10.1001/jama.2014.4573. [DOI] [PubMed] [Google Scholar]
- Oshodi TO. The impact of preoperative education on postoperative pain. Part 2. Br J Nurs. 2007;16:790–7. doi: 10.12968/bjon.2007.16.13.24244. [DOI] [PubMed] [Google Scholar]
- Oshodi TO. The impact of preoperative education on postoperative pain. Part 1. Br J Nurs. 2007;16:706–10. doi: 10.12968/bjon.2007.16.12.23719. [DOI] [PubMed] [Google Scholar]
- Pandazi A1, Kapota E, Matsota P, Paraskevopoulou P, Dervenis C, Kostopanagiotou G. Preincisional versus postincisional administration of parecoxib in colorectal surgery: effect on postoperative pain control and cytokine response. A randomized clinical trial.World J Surg. 2010;34:2463–9. [DOI] [PubMed]
- Park YH, Kang H, Woo YC, Park SG, Baek CW, Jung YH, Kim JY, Koo GH, Kim SD, Park JS. The effect of intraperitoneal ropivacaine on pain after laparoscopic colectomy: a prospective randomized controlled trial. J Surg Res. 2011;171:94–100. doi: 10.1016/j.jss.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Peng PW, Wijeysundera DN, Li CC. Use of gabapentin for perioperative pain control—a meta-analysis. Pain Res Manag. 2007;12:85–92. doi: 10.1155/2007/840572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, Wenk M, Tramer MR. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259:1056–67. doi: 10.1097/SLA.0000000000000237. [DOI] [PubMed] [Google Scholar]
- Pranevicius M, Pranevicius O. Non-opioid anesthesia with esmolol avoids opioid-induced hyperalgesia and reduces fentanyl requirement after laparoscopy. Anesth Analg. 2009;108:1048. doi: 10.1213/ane.0b013e3181938f3f. [DOI] [PubMed] [Google Scholar]
- Rathmell JP, Wu CL, Sinatra RS, Ballantyne JC, Ginsberg B, Gordon DB, Liu SS, Perkins FM, Reuben SS, Rosenquist RW, Viscusi ER. Acute post-surgical pain management: a critical appraisal of current practice, December 2-4, 2005. Reg Anesth Pain Med. 2006;31:1–42. doi: 10.1016/j.rapm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–13. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. 2006;93:921–8. doi: 10.1002/bjs.5430. [DOI] [PubMed] [Google Scholar]
- Sami Mebazaa M, Mestiri T, Kaabi B, Ben Ammar MS. Clinical benefits related to the combination of ketamine with morphine for patient controlled analgesia after major abdominal surgery. Tunis Med. 2008;86:435–40. [PubMed] [Google Scholar]
- Schricker T, Lattermann R. Perioperative catabolism. Can J Anaesth. 2015;62:182–93. doi: 10.1007/s12630-014-0274-y. [DOI] [PubMed] [Google Scholar]
- Scott MJ MM, Gordon DB, Grant S, Thacker JKM, Wu CL, Gan TJ, Mythen MG, Shaw AD, Miller TE, for the Perioperative Quality Initiative (POQI) I Workgroup: American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 2—from PACU to the transition home. Perioper Med (Lond) 2016. doi:10.1186/s13741-017-0063-6. [DOI] [PMC free article] [PubMed]
- Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth. 2006;53:461–9. doi: 10.1007/BF03022618. [DOI] [PubMed] [Google Scholar]
- Siddiqui MR, Sajid MS, Uncles DR, Cheek L, Baig MK. A meta-analysis on the clinical effectiveness of transversus abdominis plane block. J Clin Anesth. 2011;23:7–14. doi: 10.1016/j.jclinane.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Siddiqui NT, Fischer H, Guerina L, Friedman Z. Effect of a preoperative gabapentin on postoperative analgesia in patients with inflammatory bowel disease following major bowel surgery: a randomized, placebo-controlled trial. Pain Pract. 2014;14:132–9. doi: 10.1111/papr.12058. [DOI] [PubMed] [Google Scholar]
- Smith HS. Perioperative intravenous acetaminophen and NSAIDs. Pain Med. 2011;12:961–81. doi: 10.1111/j.1526-4637.2011.01141.x. [DOI] [PubMed] [Google Scholar]
- Straube S, Derry S, McQuay HJ, Moore RA. Effect of preoperative Cox-II-selective NSAIDs (coxibs) on postoperative outcomes: a systematic review of randomized studies. Acta Anaesthesiol Scand. 2005;49:601–13. doi: 10.1111/j.1399-6576.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- Thavaneswaran P, Rudkin GE, Cooter RD, Moyes DG, Perera CL, Maddern GJ. Brief reports: paravertebral block for anesthesia: a systematic review. Anesth Analg. 2010;110:1740–4. doi: 10.1213/ANE.0b013e3181da82c8. [DOI] [PubMed] [Google Scholar]
- Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, McMurry TL, Goudreau BJ, Umapathi BA, Kron IL, Sawyer RG, Hedrick TL. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220:430–43. doi: 10.1016/j.jamcollsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 2008: CD004602. [DOI] [PMC free article] [PubMed]
- Ventham NT, Hughes M, O'Neill S, Johns N, Brady RR, Wigmore SJ. Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg. 2013;100:1280–9. doi: 10.1002/bjs.9204. [DOI] [PubMed] [Google Scholar]
- Vigneault L, Turgeon AF, Cote D, Lauzier F, Zarychanski R, Moore L, McIntyre LA, Nicole PC, Fergusson DA. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58:22–37. doi: 10.1007/s12630-010-9407-0. [DOI] [PubMed] [Google Scholar]
- Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110:191–200. doi: 10.1093/bja/aes431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Johnston B, Kaushal A, Cheng D, Zhu F, Martin J. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anaesth. 2016;63:311–325. doi: 10.1007/s12630-015-0551-4. [DOI] [PubMed] [Google Scholar]
- Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 2005: CD004088. [DOI] [PubMed]
- Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr Anaesth. 2013;23:475–95. doi: 10.1111/pan.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. Postoperative pain 2: patient education, assessment and management. Nurs Times. 2010;106:14–6. [PubMed] [Google Scholar]
- Wu CL, Benson AR, Hobson DB, Roda CP, Demski R, Galante DJ, Page AJ, Pronovost PJ, Wick EC. Initiating an enhanced recovery pathway program: an anesthesiology department’s perspective. Jt Comm J Qual Patient Saf. 2015;41:447–56. doi: 10.1016/S1553-7250(15)41058-X. [DOI] [PubMed] [Google Scholar]
- Yu N, Long X, Lujan-Hernandez JR, Succar J, Xin X, Wang X. Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14:121. doi: 10.1186/1471-2253-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakine J, Samarcq D, Lorne E, Moubarak M, Montravers P, Beloucif S, Dupont H. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: a prospective, randomized, double-blind, controlled study. Anesth Analg. 2008;106:1856–61. doi: 10.1213/ane.0b013e3181732776. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011;106:454–62. doi: 10.1093/bja/aer027. [DOI] [PubMed] [Google Scholar]
- Zhao X, Tong Y, Ren H, Ding XB, Wang X, Zong JY, Jin SQ, Li Q. Transversus abdominis plane block for postoperative analgesia after laparoscopic surgery: a systematic review and meta-analysis. Int J Clin Exp Med. 2014;7:2966–75. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the creation of this consensus document.


