Abstract
The success of Candida albicans as an opportunistic pathogen is based in part on its ability to adapt to diverse environments. The RIM101 pathway governs adaptation to neutral-alkaline environments and is required for virulence. Analysis of a genomic two-hybrid study conducted with Saccharomyces cerevisiae revealed that components involved in multivesicular bodies (MVB) transport may interact with RIM101 pathway members. Thus, we hypothesized that these proteins may function in the RIM101 pathway in C. albicans. We identified C. albicans homologs to S. cerevisiae Snf7p, Vps4p, and Bro1p and generated mutants in the cognate gene. We found that snf7Δ/Δ mutants, but not vps4Δ/Δ nor bro1Δ/Δ mutants, had phenotypes similar to, but more severe than, those of RIM101 pathway mutants. We found that the constitutively active RIM101-405 allele partially rescued snf7Δ/Δ mutant phenotypes. The vps4Δ/Δ mutant had subtle phenotypes, but these were not rescued by the RIM101-405 allele. Further, we found that the snf7Δ/Δ, vps4Δ/Δ, and bro1Δ/Δ mutants did not efficiently localize the vital dye FM4-64 to the vacuole and that it was often accumulated in an MVB-like compartment. This phenotype was not rescued by RIM101-405 or observed in RIM101 pathway mutants. These results suggest that Snf7p may serve two functions in the cell: one as a RIM101 pathway member and one for MVB transport to the vacuole.
All organisms must be able to respond to at least transient changes in environmental pH for survival. In fact, many organisms, such as fungi, can grow under a wide range of pH conditions. For example, Candida albicans, an opportunistic pathogen of humans, can grow under in vitro conditions ranging from pH 2 to 10 (7, 29). Further, C. albicans occurs as a commensal organism in most immunocompetent individuals and colonizes mucosal surfaces of the oral-pharyngeal, gastrointestinal, and urogenital tracts (5). These sites can show dramatic variation in extracellular pH. Thus, C. albicans must be able to sense and respond to changes in environmental pH to survive within the host.
The ability of fungi to sense and respond to neutral-alkaline environments is governed, at least in part, by the conserved RIM101 pathway (referred to as the PacC pathway in Aspergillus nidulans) (7, 11). In neutral-alkaline conditions, Rim101p, the zinc finger transcription factor, promotes changes in gene expression, acting as an inducer of neutral-alkaline response genes and a repressor of acidic response genes (3, 9, 20, 34, 36). Rim101p activity is governed by proteolytic processing (14, 15, 21-23). In neutral-alkaline environments, the C-terminal D/E-rich tail of Rim101p/PacC is proteolytically cleaved to yield an active N-terminal domain, which contains the three zinc fingers. In acidic environments, Rim101p/PacC is either not proteolytically processed or processed into a form distinct from that seen at neutral-alkaline pH. Processing is governed by a number of upstream gene products, including Rim13p/PalB, which encodes a calpain-like protease; Rim20p/PalA, which interacts with the D/E-rich domain; Rim8p/PalF, which has no known function; and Rim21p/PalH and Rim9p/PalI, which encode putative transmembrane domain proteins and may serve as the environmental pH sensors (9, 12, 13, 22, 24, 27, 28, 32, 35, 42).
Despite the identification of these upstream RIM101 pathway members, relatively little is known about how the pH signal is transmitted through this pathway to Rim101p. In fact, the only direct interaction between the known pathway members is between Rim20p and Rim101p. Xu and Mitchell demonstrated that in S. cerevisiae, ScRim20p interacts with the C-terminal D/E-rich domain of ScRim101p and is required for processing, leading to the hypothesis that Rim20p may promote recruitment of the likely protease Rim13p (42). Xu and Mitchell also found that C. albicans Rim20p interacts with CaRim101p by two-hybrid interaction. Finally, an interaction between PalA (Rim20p) and PacC (Rim101p) has also been observed with A. nidulans (39). Since so few interactions have been detected between the RIM101 pathway members, it seemed probable that other components may exist that bridge the gap between the known RIM101 pathway members.
S. cerevisiae is a powerful and elegant genetic system. It exists stably as a haploid or a diploid, can maintain plasmids, and was the first eukaryotic genome to be sequenced. Thus, S. cerevisiae has proved extremely amenable to the application of genomic studies. To begin to determine all the protein-protein interactions that occur within a cell, Ito et al. utilized a genomic two-hybrid strategy (18). With the results from these studies, two components of the RIM101 pathway, Rim20p and Rim13p, were found to interact with Snf7p (Fig. 1). Further, Rim20p was also found to interact with Vps4p, a protein previously found to interact with Snf7p (37). In a separate approach, Bro1p, which has homology to Rim20p, was found to interact with Vps4p and Snf7p (16).
FIG. 1.
A genomic two-hybrid screening identified proteins that may interact with members of the RIM101 pathway in S. cerevisiae (18). These studies suggested that Snf7p and Vps4p interact with each other and with Rim20p. Further, they suggest that Snf7p also interacts with Rim13p. Vps4p can be immunoprecipitated with Snf7p and the Rim20p homolog Bro1p (16). Gray lines represent a two-hybrid interaction; gray double-sided arrows represent an interaction identified by tandem affinity purification (16). Numbers in parentheses represent the total number of two-hybrid interactions observed for a given protein.
Snf7p, Vps4p, and Bro1p function in the transport of late endosomal compartments, multivesicular bodies (MVB), to the vacuole (33). Transport of MVB to the vacuole requires three protein complexes (termed ESCRTs, for “endosomal sorting complex required for transport”). Snf7p is a cytoplasmic coiled-coil protein of the ESCRT-III protein complex that is recruited to endosomal membranes (1, 2, 19). Vps4p is a cytoplasmic AAA-type ATPase that functions in all three ESCRT protein complexes to recycle components, including Snf7p, for subsequent rounds of MVB sorting (1, 2). Bro1p is a cytoplasmic protein that associates with MVB in a Snf7p-dependent manner and also requires Vps4p for dissociation (30).
Rim20p and Rim13p are predicted to act at the same point of the RIM101 pathway (on Rim101p itself) and may interact with ESCRT-III components of the MVB sorting machinery. Thus, we predicted that these components may be additional RIM101 pathway members, a hypothesis previously suggested by Xu and Mitchell with respect to S. cerevisiae (42). In this study, we identified the C. albicans homologs of S. cerevisiae Snf7p, Vps4p, and Bro1p and demonstrated through mutant analysis that Snf7p, but not Vps4p nor Bro1p, is a member of the RIM101 pathway. Further, we present evidence that suggests two distinct functions for Snf7p, the first as a RIM101 pathway member and the second in MVB transport.
METHODS AND MATERIALS
Strains and plasmids.
The C. albicans strains used in this study are derivatives of BWP17 (41) and are described in Table 1. The snf7Δ/Δ mutant (DAY534) was generated as follows. BWP17 was transformed with the snf7::ARG4 cassette, which was amplified in a PCR using the SNF7 5DR and SNF7 3DR (Table 2) primers and the pRS-ArgΔSpe template (41), to generate the heterozygous mutant DAY530. DAY530 was then transformed with the snf7::URA3-dpl200 cassette, which was amplified in a PCR using the SNF7 5DR and SNF7 3DR primers and the pDDB57 template (40), to generate the homozygous mutant DAY534. Correct integration was demonstrated by the PCR using SNF7 5detect and SNF7 3detect, which flank the site of integration (Fig. 2A).
TABLE 1.
Strains used in this study
| Strain | Background | Genotype | Reference or source |
|---|---|---|---|
| BWP17 | SC5314 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG | 41 |
| DAY5 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG rim101::ARG4/rim101::URA3 | 41 |
| DAY23 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG rim20::ARG4/rim20::URA3 | 9 |
| DAY25 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisGHIS1::hisG::his1/his1::hisG rim101::ARG4/rim101::URA3 | 8 |
| DAY61 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG rim8::ARG4/rim8::URA3 | 9 |
| DAY185 | BWP17 | ura3::λimm434/ura3::λimm434 HIS1::his1::hisG/his1::hisG ARG4::URA3::arg4::bphisG/arg4::hisG | 8 |
| DAY286 | BWP17 | ura3::λimm434/ura3::λimm434 ARG4::URA3::arg4::hisG/arg4::hisG his1::hisG/his1::hisG | 10 |
| DAY349 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG rim13::ARG4/rim13::URA3 | 22 |
| DAY530 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG snf7::ARG4/SNF7 | This study |
| DAY531 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG vps4::ARG4/VPS4 | This study |
| DAY534 | DAY530 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG snf7::ARG4/snf7::URA3-dpl200 | This study |
| DAY537 | DAY531 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG vps4::ARG4/vps4::URA3-dpl200 | This study |
| DAY544 | DAY534 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG snf7::ARG4/snf7::URA3-dpl200 RIM101::HIS1::RIM101/RIM101 | This study |
| DAY546 | DAY534 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG snf7::ARG4/snf7::URA3-dpl200 RIM101-405::HIS1::RIM101/RIM101 | This study |
| DAY568 | DAY534 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG pHIS1::RIM101-V5-Age1::his1::hisG/his1::hisG snf7::ARG4/snf7::URA3-dpl200 | |
| DAY643 | DAY349 | ura3Δ::λimm434/ura3Δ::λimm434 pHIS1::RIM101-V5-age1::his1::hisG/his1::hisG arg4::hisG/arg4::hisG rim13::URA3/rim13::ARG4 | 22 |
| DAY648 | BWP17 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG bro1::ARG4/BRO1 | This study |
| DAY653 | DAY648 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG bro1::ARG4/bro1::URA3-dpl200 | This study |
| DAY664 | DAY537 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG his1::hisG/his1::hisG vps4::ARG4/vps4::URA3-dpl200 RIM101-405:::HIS1::RIM101/RIM101 | This study |
| DAY761 | DAY534 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG HIS1::SNF7::his1::hisG/his1::hisG snf7::ARG4/snf7::URA3-dpl200 | This study |
| DAY763 | DAY534 | ura3::λimm434/ura3::λimm434 arg4::hisG/arg4::hisG HIS1::hisG::his1/his1::his1/his1::hisG snf7::ARG4/snf7::URA3-dpl200 | This study |
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| SNF7 5DR | ACTTTATTATATTAATTATAACTTTAGTTGACTGAATAAAACTTGAATAGTAAACATGTGTTTCCCAGTCACGACGTT |
| SNF7 3DR | TAATATATGTTTCTATACAAAGCTTTCGTTATTCTCCGTATTCGGTATTTCAAACACATCGTGGAATTGTGAGCGGATA |
| SNF7 5detect | AACGACAAATCAACACCAGG |
| SNF7 3detect | TGTGTTATCAAAAAACAGGG |
| VPS4 5DR | CACTATTACAAATCATATAGACTGATTTAAACTTATAAAACAATACACTTAAGCATGTCGTTTCCCAGTCACGACGTT |
| VPS4 3DR | TATAGAAATGAATATTTCTTGAATATATTTTATTCATTGGCCCCTCTTTTAATTACCTTCGTGGAATTGTGAGCGGATA |
| VPS4 5detect | GCGATTTTCAAAGATGTCGG |
| VPS4 3detect | AGAATTGATAGCGAAGACG |
| BRO1 5DR | ATGAAAACACATTTACTTGTTGTTCCAAGTAAGAAAACTGAAGAGGTTAATTGGGTCAAATTTCCCAGTCACGACGTT |
| BRO1 3DR | CTATTTACTGGAGAAAAAATTATACATATTTGGATTATAAGTTGAAGGATTATCATAAATGTGGAATTGTGAGCGGATA |
| BRO1 5′detect | TTATCCTTACATCCTTTCCG |
| BRO1 3′detect | TCAAGCTTTTTACCGTACGC |
| SNF7 5′comp | GCCTCATTGAGCAACTTGAG |
| SNF7 3′comp | TAATCGACATTAAAGGACTC |
FIG. 2.
Confirmation of mutants. PCR genotyping for the snf7Δ/Δ (A), vps4Δ/Δ (B), and bro1Δ/Δ (C) mutants was performed using primers that flank the gene of interest; the results of representative PCR analysis of genomic DNA from the wild type (lanes 1; BWP17 in each case), a heterozygous mutant (lanes 2; DAY530, DAY531, and DAY648, respectively), and a homozygous mutant (lanes 3; DAY534, DAY537, and DAY653, respectively) are shown. The wild-type (*), ARG4 allele (a), and URA3 allele (u) bands are noted to the left of each sample.
The vps4Δ/Δ mutant (DAY537) were created in a similar manner. BWP17 was transformed with the vps4::ARG4 cassette, which was amplified in a PCR using the VPS4 5DR and VPS4 3DR primers and the pRS-ArgΔSpe template, to generate the heterozygous mutant DAY531. This strain was then transformed with the vps4::URA3-dpl200 cassette, which was amplified in a PCR using the VPS4 5DR and VPS4 3DR primers and the pDDB57 template, to generate the homozygous mutant DAY537. Correct integration was demonstrated by the PCR using VPS4 5detect and VPS4 3detect, which also flank the site of integration (Fig. 2B).
The bro1Δ/Δ mutant (DAY653) was generated in an analogous fashion. BWP17 was transformed with the bro1::ARG4 cassette, which was amplified in a PCR using the BRO1 5DR and BRO1 3DR primers and the pRS-ArgΔSpe template, to generate the heterozygous strain DAY648. This strain was then transformed with the bro1::URA3-dpl200 cassette, which was amplified in a PCR using the BRO1 5DR and BRO1 3DR primers and the pDDB57 template, to generate the homozygous mutant DAY653. Correct integration was demonstrated by the PCR using BRO1 5′detect and BRO1 3′detect, which flank the site of integration (Fig. 2C).
To complement the snf7Δ/Δ mutant, wild-type SNF7 sequence was amplified in a PCR using genomic DNA from BWP17 and primers SNF7 5′comp and SNF7 3′ comp. The resulting PCR product, which contains 1,000 nucleotides upstream and 721 nucleotides downstream of the SNF7 coding sequence, was cloned into pGEM-T Easy (Promega) to generate pDDB268. The SNF7 sequence was removed from pDDB268 by PvuII digestion and in vivo recombined into NotI/EcoRI-digested pDDB78. pDDB267, the resulting plasmid, was recovered into DH5α Escherichia coli by electroporation and transformed into C. albicans following NruI digestion to generate DAY761. The empty vector pDDB78 was also transformed into DAY534 following NruI digestion to yield DAY763. Plasmids pDDB61 and pDDB71 (9) were digested with PpuMI to introduce the wild-type RIM101 and constitutively active RIM101-405 alleles into DAY534 to yield DAY544 and DAY546, respectively. To test for Rim101p processing, plasmid pDDB233 (22) was transformed into DAY534 following NruI digestion.
Media and growth conditions.
C. albicans was routinely grown in YPD plus uridine (2% Bacto Peptone, 1% yeast extract, 2% dextrose, and 80 μg of uridine per ml). Selection for the Arg+ and Ura+ transformants was done on synthetic medium (0.67% yeast nitrogen base plus ammonium sulfate and without amino acids-2% dextrose-80 μg of uridine per ml was used except when selecting for URA3 and was supplemented as required in accordance with the auxotrophic needs of the cells). M199 medium (Gibco BRL) was buffered to pH 8.0 with 150 mM HEPES and supplemented with 80 μg of uridine per ml (9).
Protein preparation and Western blot analyses.
Cells were diluted 40-fold from overnight YPD plus uridine cultures into fresh M199 medium buffered with 150 mM HEPES to pH 4 or pH 7 and grown 4 h at 30°C. Cells were pelleted and stored at −80°C prior to protein extraction. Cell pellets were resuspended in ice-cold radioimmunoprecipitation assay buffer containing 1 μg of leupeptin/ml, 2 μg of aprotinin/ml, 1 μg of pepstatin/ml, 1 mM phenylmethylsulfonyl fluoride, and 10 mM dithiothreitol and transferred to glass test tubes containing acid-washed glass beads. Cells were lysed by vortexing four times for 2 min followed by 2 min on ice. Cellular debris was pelleted, and supernatants were removed and stored at −80°C. For Western blot analysis, 20 μl of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added to 20 μl of supernatant and samples were boiled for 5 min. Samples were loaded onto an SDS-8% PAGE gel and run overnight at 35 V. Proteins were transferred to nitrocellulose and blocked. Anti-V5 horseradish peroxidase antibody (Invitrogen) in 30 ml of 5% nonfat milk TBS-T solution (1:7,500 dilution; 50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.05% Tween 20) was added to the blot for 4 h at 4°C. Blots were washed in TBS-T, incubated with ECL reagent (Amersham Biosciences), and exposed to film.
FM4-64 staining.
YPD plus uridine cultures were grown overnight at 30°C. The following day, 25 μl of the overnight culture was added into 1 ml of M199 medium (pH 8) and then incubated for 3 to 3.5 h at 36.5°C. A total of 2.5 μl of 16 mM FM4-64 (Molecular Probes) in dimethyl sulfoxide was added to each culture, and the mixture was incubated on ice for 30 min. During this 30-min incubation, cells were examined and photographed (time = 0). Cells were then pelleted, washed with 1 ml of M199 medium (pH 8), and resuspended in 1 ml of M199 medium (pH 8). At this point, 80 μl of cells were taken and added to ice-cold tubes containing 10 μl of 100 mM sodium azide and 10 μl of 100 mM sodium fluoride (time = postincubation). The remainder of the culture was incubated at 37°C to more accurately reflect the mammalian host environment. At various time intervals, 80 μl of each sample was taken and stored on ice with 10 μl of 100 mM sodium azide and 10 μl of 100 mM sodium fluoride prior to microscopic examination and photographing.
Filamentation assays.
Cultures of M199 medium (pH 8) and YPD-20% bovine calf serum were inoculated with a 1/100 dilution of an overnight YPD culture, and the cultures were incubated at 37°C for 5 and 2 h, respectively. Cells were then pelleted, washed with 1 ml of double-distilled water, and fixed in 70% ethyl alcohol for 30 min. Fixed cells were then washed and resuspended in 10 mM Tris-HCl (pH 7.4)-1 mM EDTA. Each strain was analyzed in triplicate, and at least 300 cells were counted for each sample.
RESULTS
SNF7 is required for growth at alkaline pH and on high concentrations of lithium.
We predicted that if members of the ESCRT-III complex functioned to activate Rim101p, then loss-of-function mutants in SNF7, VPS4, and BRO1 would have phenotypes similar to other RIM101 pathway mutants. To test this hypothesis with C. albicans, we utilized the TBLASTN computer algorithm (http://www-sequence.stanford.edu:8080/btcontigs6.html) to identify the C. albicans homologs of S. cerevisiae SNF7, VPS4, and BRO1. We found that orf19.6040, orf19.4339, and orf19.1670 can encode proteins 49 or 62%, 75 or 82%, and 24 or 47% identical or similar to S. cerevisiae Snf7p, Vps4p, and Bro1p, respectively. To determine whether these genes encode members of the RIM101 pathway, we generated insertion-deletion mutants by PCR product-directed gene disruption (41). Homologous integrants were identified via PCR with primers that flank the integration site (Fig. 2). We were able to recover independent homozygous mutants with all three genes, which suggests that these genes are not essential for growth.
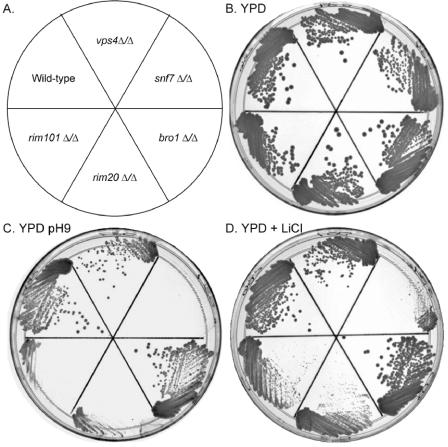
The RIM101 pathway is required for growth on alkaline medium and in the presence of high concentrations of lithium. Thus, we first analyzed the snf7Δ/Δ, vps4Δ/Δ, and bro1Δ/Δ mutants for growth on these media. On rich medium, the heterozygous mutants and the rim101Δ/Δ, rim20Δ/Δ, vps4Δ/Δ, and bro1Δ/Δ homozygous mutants grew at rates similar to those seen with the wild type (Fig. 3B and 4). However, the snf7Δ/Δ homozygous mutant appeared to have slightly reduced growth (Fig. 3B and 4A; compare the growth results for individual colonies in the most dilute lanes). Identical results were observed for independent snf7Δ/Δ, vps4Δ/Δ, and bro1Δ/Δ mutants (data not shown). On rich medium buffered to pH 9 or containing 150 mM lithium chloride, the growth seen with the heterozygous mutants and the vps4Δ/Δ and bro1Δ/Δ homozygous mutants was similar to wild-type growth results. However, the rim101Δ/Δ, rim20Δ/Δ, and snf7Δ/Δ mutants showed a severe reduction in growth on these media (Fig. 3C and D and 4A). In fact, the snf7Δ/Δ mutant showed a more severe growth defect than either the rim101Δ/Δ or the rim20Δ/Δ mutant. These results suggest that VPS4 and BRO1 do not have a significant role in growth at alkaline pH or in the presence of high concentrations of lithium. These results also suggest that SNF7 has a critical role for growth on these media.
FIG. 3.
Growth phenotypes of ESCRT-III mutants. (A) The locations of the strains analyzed were as follows: wild-type (DAY286), vps4Δ/Δ (DAY537), snf7Δ/Δ (DAY534), bro1Δ/Δ (DAY653), rim20Δ/Δ (DAY23), and rim101Δ/Δ (DAY5). (B to D) Strains were grown on YPD (B), YPD (pH 9) (C), and YPD plus LiCl (D) for 2 days at 37°C prior to photographing.
FIG. 4.
Growth phenotypes by spot dilutions. (A) His− wild-type (DAY286), rim101Δ/Δ (DAY5), snf7Δ/Δ (DAY534), and rim20Δ/Δ (DAY23) strains were serially diluted fivefold and plated on YPD, YPD at pH 9, and YPD plus LiCl and grown at 37°C. (B) Prototrophic wild-type (DAY185), rim101Δ/Δ (DAY25), snf7Δ/Δ SNF7 (DAY761), snf7Δ/Δ (DAY763), snf7Δ/Δ RIM101-405 (DAY546), and snf7Δ/Δ RIM101 (DAY544) strains were serially diluted fivefold and plated on YPD, YPD at pH 9, and YPD plus LiCl and grown at 37°C. Plates were grown for 24 h prior to photographing.
We considered the possibility that the alkaline pH and lithium growth phenotypes of the snf7Δ/Δ mutant were not due to loss of SNF7 sequence or to a specific defect in the RIM101 pathway. To test these possibilities, we first reintroduced a wild-type copy of SNF7 into the snf7Δ/Δ mutant. Introduction of wild-type SNF7 rescued the slow growth defect on rich medium and restored growth on pH 9 and 150 mM LiCl medium to levels indistinguishable from that seen with the wild type (Fig. 4B). Introduction of the empty vector into the snf7Δ/Δ mutant did not rescue these phenotypes to wild-type levels; however, we noted that these prototrophic snf7Δ/Δ strains did appear to grow better on pH 9 medium and LiCl medium than the His− auxotrophic strains (Compare Fig. 4A and B). These results demonstrate that the growth phenotypes observed with the snf7Δ/Δ mutant are due to the loss of SNF7 sequence.
Next, we introduced the constitutively active RIM101-405 allele into the snf7Δ/Δ mutant, which bypasses the requirement for the upstream members of the RIM101 pathway (9). If the snf7Δ/Δ growth phenotypes are due to defects in the RIM101 pathway, then the RIM101-405 allele should rescue the snf7Δ/Δ mutant. Indeed, expression of RIM101-405 in the snf7Δ/Δ background rescued the slight growth defect on YPD medium and partially restored growth on pH 9 medium (Fig. 4), but expression of an additional wild-type copy of RIM101 in the snf7Δ/Δ background did not. However, RIM101-405 did not significantly improve snf7Δ/Δ growth on LiCl medium. These results suggest that the alkaline growth phenotypes of the snf7Δ/Δ mutant can be partially rescued by the constitutively active RIM101-405 allele, providing support for the hypothesis that SNF7 encodes an upstream member of the RIM101 pathway. Further, these results indicate that Snf7p has additional functions independent of the RIM101 pathway.
SNF7 is required for filamentation.
The RIM101 pathway positively regulates filamentation on a variety of media (9, 22, 32, 34). Thus, we asked whether SNF7, VPS4, and BRO1 are required for filamentation. On solid M199 medium (pH 8), the wild type, the heterozygous mutants, and the bro1Δ/Δ homozygous mutant formed yeast colonies with extensive peripheral filaments (Fig. 5A and data not shown). The vps4Δ/Δ homozygous mutant also formed yeast colonies with peripheral filaments; however, filamentation was not as robust as that seen with wild-type cells. Finally, the snf7Δ/Δ homozygous mutant formed yeast colonies that lacked peripheral filaments on M199 medium (pH 8) similar to those seen with the rim101Δ/Δ and the rim20Δ/Δ mutants (Fig. 5). As observed for the growth phenotypes described above, we found that introduction of a wild-type copy of SNF7 into the snf7Δ/Δ mutant completely rescued the filamentation defect but that the empty vector did not. Further, introduction of the RIM101-405 allele into the snf7Δ/Δ mutant partially restored filamentation, but an additional wild-type copy did not. Introduction of the RIM101-405 allele into the vps4Δ/Δ mutant did not rescue the partial filamentation defect (Fig. 5), suggesting that this phenotype is unrelated to the RIM101 pathway. Similar results were observed in liquid M199 medium (pH 8). These results support the hypothesis that Snf7p encodes an upstream member of the RIM101 pathway and has functions independent of the RIM101 pathway.
FIG. 5.
Alkaline-induced filamentation of ESCRT-III mutants. (A) Wild-type (DAY286), rim101Δ/Δ (DAY5), snf7Δ/Δ (DAY534), vps4Δ/Δ (DAY537), bro1Δ/Δ (DAY653), and rim20Δ/Δ (DAY23) strains were grown overnight in YPD and spotted onto M199 medium (pH 8). (B) Complementation studies of the snf7Δ/Δ mutant. Wild-type (DAY185), rim101Δ/Δ (DAY25), snf7Δ/Δ RIM101 (DAY544), snf7Δ/Δ RIM101-405 (DAY546), snf7Δ/Δ SNF7 (DAY761), and snf7Δ/Δ (DAY763) strains were grown overnight in YPD and spotted onto M199 medium (pH 8). Plates were incubated at 37°C for 5 days prior to photographing.
We next analyzed filamentation in serum. In liquid serum, wild-type, vps4Δ/Δ, and bro1Δ/Δ cells formed germ tubes and grew in the filamentous form (Table 3). As described previously, the rim101Δ/Δ mutant showed a ∼16% reduction in germ tube formation and filamentous growth compared to the wild type (P < 0.002) (22). The snf7Δ/Δ mutant had a more severe defect in germ tube formation, a ∼49% reduction compared to the wild type (P < 1 × 10−5), and a ∼39% reduction compared to the rim101Δ/Δ mutant (P < 3 × 10−4). Introduction of the RIM101-405 allele rescued the snf7Δ/Δ mutant compared to the results seen with an additional wild-type copy of RIM101 (P < 5 × 10−5). However, in similarity to the results described above, RIM101-405 only partially rescued the snf7Δ/Δ mutant, leading to a filamentation level similar to that seen with the rim101Δ/Δ mutant (P < 0.09) but not similar to that seen with wild-type cells. These results support the idea that Snf7p has Rim101p-dependent and -independent functions.
TABLE 3.
Filamentation in liquid serum medium
| Strain | Relevant genotype | % of filamentation in seruma |
|---|---|---|
| DAY286 | Wild type | 88.3 |
| DAY5 | rim101Δ/Δ | 74.1b |
| DAY534 | snf7Δ/Δ | 45.3b,c |
| DAY544 | snf7Δ/Δ RIM101 | 44.4b,c |
| DAY546 | snf7Δ/Δ RIM101-405 | 78.9b |
| DAY537 | vps4Δ/Δ | 84.7 |
| DAY653 | bro1Δ/Δ | 93.7 |
Results represent the average of three independent experiments. Standard deviation <5%.
Statistically different from the wild-type result by analysis of variance.
Statistically worse than the rim101Δ/Δ mutant result by analysis of variance.
SNF7 is required for Rim101p processing.
Rim101p is activated by proteolytic processing, which is governed by the upstream members of the RIM101 pathway. Thus, if Snf7p is an upstream member of the RIM101 pathway, then Rim101p processing should be altered in a snf7Δ/Δ mutant. To address this possibility, we introduced a V5-epitope-tagged version of Rim101p, Rim101-V5p (22), into the snf7Δ/Δ mutant and analyzed Rim101p processing by Western blotting.
In wild-type cells at pH 4, Rim101-V5p was found in two forms of 85 and 65 kDa (Fig. 6). In wild-type cells at pH 7, Rim101-V5p was found primarily in two forms of 85 and 74 kDa; however, the 65-kDa form could also be observed. As reported previously, the rim13Δ/Δ mutant lacked the 74- and 65-kDa forms and only the 85-kDa form was observed (Fig. 6) (22). With the snf7Δ/Δ mutant, only the 85-kDa form of Rim101-V5p was observed at pH 4 and pH 7, in similarity to the results seen with the rim13Δ/Δ mutant. These results demonstrate that Snf7p is required for both Rim101p processing events in C. albicans and support the model that Snf7p is an upstream member of the RIM101 pathway.
FIG. 6.
Western blot analysis for Rim101p processing. Wild-type (DAY492), rim13Δ/Δ (DAY643), and snf7Δ/Δ (DAY568) strains that express Rim101-V5p were grown 4 h at pH 4 and 7. Protein was purified and separated by SDS-8% PAGE. Gels were transferred to nitrocellulose and probed with anti-V5 horseradish peroxidase (Invitrogen).
SNF7, VPS4, and BRO1 are required for endocytosis.
In S. cerevisiae, SNF7, VPS4, and BRO1 encode proteins that function in MVB transport to the vacuole (33). To determine whether the SNF7, VPS4, and BRO1 gene products identified in C. albicans carry out a similar function, we analyzed endocytic transport from the plasma membrane to the vacuole by use of the fluorescent marker FM4-64 (38). In wild-type cells, FM4-64 was initially seen at the plasma membrane (Fig. 7). However, FM4-64 was rapidly internalized into endocytic vesicles and was localized to the vacuolar membrane within 45 min. After 90 min, essentially only vacuolar membrane staining remained. In snf7Δ/Δ, vps4Δ/Δ, and bro1Δ/Δ mutant cells, FM4-64 was initially seen at the plasma membrane and was rapidly found within endocytic vesicles. However, unlike wild-type cell results, FM4-64 was delayed in progression to the vacuole. After 45 min, vacuolar membrane staining was not apparent, although the vacuole was often surrounded by diffuse staining. After 90 min, the vacuole could be visualized in the vps4Δ/Δ and bro1Δ/Δ mutants, although cytoplasmic staining by endocytic vesicles remained. However, the snf7Δ/Δ mutant still had poorly stained vacuolar membranes and primarily revealed diffuse staining. After 150 min, these three mutants still maintained diffuse staining in the cytoplasm, although the snf7Δ/Δ mutant was clearly the most defective. Although vacuolar staining could be observed in the vps4Δ/Δ, bro1Δ/Δ, and (to a lesser extent) snf7Δ/Δ mutants, we often observed brighter staining caps of FM4-64 on one side of the vacuole (Fig. 7). This phenomenon has also been described for S. cerevisiae and likely reflects FM4-64 localization to a late endocytic-prevacuolar (MVB-like) compartment (30, 38). Thus, these results suggest that Snf7p, Vps4p, and Bro1p play a role in MVB transport to the vacuole and that Snf7p has a critical role in this process.
FIG. 7.
FM4-64 staining in ESCRT-III mutants. Wild-type (DAY286), snf7Δ/Δ (DAY534), vps4Δ/Δ (DAY537), and bro1Δ/Δ (DAY653) strains were incubated 30 min on ice in M199 medium (pH 8) containing 16 mM FM4-64 (time = 0). Cells were then pelleted, washed, and resuspended in warm M199 medium (pH 8) lacking FM4-64 (time = postincubation). Cells were incubated at 37°C, and at 45-, 90-, and 150-min intervals aliquots were taken and transferred to ice-cold tubes containing sodium azide and sodium fluoride. Photographs are representative of three independent samples. pi, postincubation.
Since SNF7 appears to function in the RIM101 pathway, we asked whether the RIM101 pathway functions in the MVB transport. To address this possibility, we first asked whether the RIM101-405 allele rescues the snf7Δ/Δ endocytic defect. While introduction of the RIM101-405 allele into the snf7Δ/Δ mutant partially rescued the growth and filamentation defects of the snf7Δ/Δ mutant, FM4-64 localization to the vacuole was not significantly improved (Fig. 8A). However, introduction of a wild-type copy of SNF7 into the snf7Δ/Δ mutant completely restored the FM4-64 localization to the vacuole. Thus, the function of Snf7p for MVB transport to the vacuole does not require activated Rim101p.
FIG. 8.
FM4-64 staining complemented snf7Δ/Δ strains and RIM101 pathway mutants. (A) Rescue of snf7Δ/Δ mutants for FM4-64 staining: snf7Δ/Δ SNF7 (DAY761), snf7Δ/Δ (DAY763), snf7Δ/Δ RIM101 (DAY544), and SNF7Δ/Δ RIM101-405 (DAY546) strains were stained with FM4-64 as described for Fig. 7. Pictures represent samples taken after 60 min. (B) RIM101 pathway mutants and FM4-64 staining: wild-type (DAY286), rim101Δ/Δ (DAY5), rim20Δ/Δ (DAY23), rim8Δ/Δ (DAY61), rim13Δ/Δ (DAY349), and snf7Δ/Δ (DAY534) strains were stained with FM4-64 as described for Fig. 7. Pictures represent samples taken after 120 min.
Finally, we tested whether other RIM101 pathway members were required for FM4-64 localization. Unlike the Snf7p, Vps4p, and Bro1p results, the four previously described RIM101 pathway members, Rim101p, Rim8p, Rim13p, and Rim20p, were dispensable for FM4-64 localization to the vacuole (Fig. 8B). Thus, the previously identified RIM101 pathway members are not required for MVB transport to the vacuole.
DISCUSSION
The ability of C. albicans to respond appropriately to changes in environmental pH is required for pathogenesis (7). This ability is governed in part by the RIM101 pathway at neutral-alkaline pH. Here, we have identified Snf7p, an ESCRT-III component, as a new member of the RIM101 pathway in C. albicans. This finding is supported by several lines of evidence. First, the snf7Δ/Δ mutant has growth defects in environments that require the RIM101 pathway, including alkaline pH and high lithium concentrations. Second, the snf7Δ/Δ mutant has filamentation defects under conditions of stimuli that require the RIM101 pathway. Third, growth and filamentation defects associated with the snf7Δ/Δ mutant are partially restored by expression of the constitutively active RIM101-405 allele. However, Vps4p and Bro1p, two additional ESCRT-III components, do not function in the RIM101 pathway. Loss of Vps4p did result in a slight defect in filamentation; however, unlike the results seen with the snf7Δ/Δ mutant, the RIM101-405 allele did not rescue this phenotype in the vps4Δ/Δ mutant. Further, we have found that Rim101p processing is absolutely dependent on Snf7p but not Vps4p (unpublished data). Further, during the revision process for the work described here, a related study also demonstrated a link between MVB transport and the RIM101 pathway in S. cerevisiae and C. albicans (43). Thus, in total these results strongly suggest that Snf7p is an upstream member of the RIM101 pathway.
As described for S. cerevisiae and higher eukaryotes, we found that Snf7p, Vps4p, and Bro1p are required for MVB for vacuole transport in C. albicans. However, this function is independent of the RIM101 pathway. Again, this idea is supported by several lines of evidence. First, snf7Δ/Δ, vps4Δ/Δ, and bro1Δ/Δ mutant cells all fail or have a significant delay in FM4-64 localization to the vacuole compared to wild-type cells. Further, these mutants appear to accumulate MVB-like structures within the cell. Second, the previously identified RIM101 pathway members are dispensable for vacuolar localization of FM4-64 and do not accumulate MVB-like structures. Finally, the constitutively active RIM101-405 allele does not rescue vacuole FM4-64 localization in the snf7Δ/Δ mutant.
On the basis of these results, we propose that in C. albicans, Snf7p functions in the RIM101 pathway and as an ESCRT-III component and that these two functions are distinct (Fig. 9). Since Snf7p functions in both the RIM101 pathway and the MVB pathway, additive defects result in severe phenotypes. Further, this model predicts that constitutive activation of Rim101p would only partially rescue snf7Δ/Δ phenotypes, which is what we observed (Fig. 4 and 5). Analysis of the vps4Δ/Δ mutant suggests that MVB transport may be required for filamentation (Fig. 5). In fact, other studies have suggested that correct vesicle trafficking to the vacuole is required for filamentation (4, 31). However, the bro1Δ/Δ mutant did not have any significant defects in filamentation or growth. Bro1p appears to act very late in MVB transport, and it has been suggested that Bro1p acts downstream of the ESCRT-III complex (30). Thus, it is also possible that Bro1p acts downstream of the requirement for MVB transport for filamentation.
FIG. 9.
Model of Snf7p function. Snf7p has two distinct functions in C. albicans. One function is to transport MVB to the vacuole during endocytosis. The second function is as a member of the RIM101 pathway, which is activated in response to neutral-alkaline environments.
Why does Snf7p function link MVB transport and Rim101p activation? In alkaline environments, the plasma membrane H+-ATPase is inhibited to prevent disruption of the proton gradient and cells utilize Ena1p, the Na+-ATPase, to establish a sodium gradient. In both C. albicans and S. cerevisiae, ENA1 expression is positively regulated by Rim101p (3, 20, 21). However, cells also require the vacuolar H+-ATPase to maintain acidification of the vacuole, which in alkaline environments will be continually neutralized through the process of endocytosis (26). Genomic studies of S. cerevisiae have revealed that components of the endocytic machinery, including Snf7p and Vps4p, are required for wild-type rates of growth in alkaline environments, suggesting that MVB transport to the vacuole is critical under these stress conditions (17). Thus, when yeast cells encounter alkaline environments, the process of MVB transport to the vacuole is important for growth and the RIM101 pathway is activated. Therefore, it is possible that it is a switch to dependence on the endocytic pathway for growth that activates the RIM101 pathway.
In an S. cerevisiae two-hybrid screening, Vps4p was found to interact with Rim20p (18). However, our results suggest that Vps4p is not involved in the RIM101 pathway. Bro1p, a homolog of Rim20p, can be immunoprecipitated with Vps4p (Fig. 1) and requires Vps4p to dissociate from MVB (30). Thus, there appears to be a functional link between Bro1p and Vps4p. Judging on the basis of these observations, it seems likely that the two-hybrid interaction Vps4p has with Rim20p is at a region of homology with Bro1p. In fact, studies of S. cerevisiae suggest that Rim20p and Bro1p have distinct functions in activation of the RIM101 pathway and MVB transport (30, 42). Thus, while Vps4p clearly interacts with Bro1p, there does not appear to be an interaction between Vps4p and Rim20p in vivo.
The interaction between the RIM101/PacC pathway and MVB transport appears to be conserved through several distantly related ascomycetes, including C. albicans, S. cerevisiae, and A. nidulans. We have shown that the RIM101 pathway is required for virulence in a systemic model of infection, and we have identified Snf7p as a new member of this pathway (7). Our results also suggest that Snf7p and Vps4p have Rim101p-independent functions in filamentation. Since the ability to switch between yeast and filamentous forms is a virulence trait (6, 25), it seems likely that the process of MVB transport to the vacuole and endocytosis as a whole may also be required for virulence. Thus, the MVB transport machinery and/or endocytosis may serve as a potential new target for antifungal therapeutics, which may work on diverse fungal pathogens.
Acknowledgments
We thank Aaron P. Mitchell and Paul T. Magee for helpful discussions on this work and Samuel Martin for critical review of the manuscript. We also thank Catie Hill for help constructing the SNF7 complementation vector. We are grateful to members of the numerous groups in the Candida community involved in establishing an accessible genome database and in particular to Andre Nantel and Malcolm Whiteway at the NRC Biotechnology Research Institute.
This research of Dana A. Davis was supported in part by the Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
REFERENCES
- 1.Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271-282. [DOI] [PubMed] [Google Scholar]
- 2.Babst, M., B. Wendland, E. J. Estepa, and S. D. Emr. 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17:2982-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensen, E. S., S. J. Martin, M. Li, J. Berman, and D. A. Davis. Transcriptional profiling in C. albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol., in press. [DOI] [PubMed]
- 4.Bruckmann, A., W. Kunkel, K. Augsten, R. Wetzker, and R. Eck. 2001. The deletion of CaVPS34 in the human pathogenic yeast Candida albicans causes defects in vesicle-mediated protein sorting and nuclear segregation. Yeast 18:343-353. [DOI] [PubMed] [Google Scholar]
- 5.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 6.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 7.Davis, D. 2003. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr. Genet. 44:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, D. A., V. M. Bruno, L. Loza, S. G. Filler, and A. P. Mitchell. 2002. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denison, S. H. 2000. pH regulation of gene expression in fungi. Fungal Genet. Biol. 29:61-71. [DOI] [PubMed] [Google Scholar]
- 12.Denison, S. H., S. Negrete-Urtasun, J. M. Mingot, J. Tilburn, W. A. Mayer, A. Goel, E. A. Espeso, M. A. Penalva, and H. N. Arst, Jr. 1998. Putative membrane components of signal transduction pathways for ambient pH regulation in Aspergillus and meiosis in Saccharomyces are homologous. Mol. Microbiol. 30:259-264. [DOI] [PubMed] [Google Scholar]
- 13.Denison, S. H., M. Orejas, and H. N. Arst, Jr. 1995. Signaling of ambient pH in Aspergillus involves a cysteine protease. J. Biol. Chem. 270:28519-28522. [DOI] [PubMed] [Google Scholar]
- 14.Diez, E., J. Alvaro, E. A. Espeso, L. Rainbow, T. Suarez, J. Tilburn, H. N. Arst, Jr., and M. A. Penalva. 2002. Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J. 21:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futai, E., T. Maeda, H. Sorimachi, K. Kitamoto, S. Ishiura, and K. Suzuki. 1999. The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol. Gen. Genet. 260:559-568. [DOI] [PubMed] [Google Scholar]
- 16.Gavin, A. C., M. Bosche, R. Krause, P. Grandi, M. Marzioch, A. Bauer, J. Schultz, J. M. Rick, A. M. Michon, C. M. Cruciat, M. Remor, C. Hofert, M. Schelder, M. Brajenovic, H. Ruffner, A. Merino, K. Klein, M. Hudak, D. Dickson, T. Rudi, V. Gnau, A. Bauch, S. Bastuck, B. Huhse, C. Leutwein, M. A. Heurtier, R. R. Copley, A. Edelmann, E. Querfurth, V. Rybin, G. Drewes, M. Raida, T. Bouwmeester, P. Bork, B. Seraphin, B. Kuster, G. Neubauer, and G. Superti-Furga. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415:141-147. [DOI] [PubMed] [Google Scholar]
- 17.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 18.Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori, and Y. Sakaki. 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranz, A., A. Kinner, and R. Kolling. 2001. A family of small coiled-coil-forming proteins functioning at the late endosome in yeast. Mol. Biol. Cell 12:711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamb, T. M., and A. P. Mitchell. 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 22.Li, M., S. J. Martin, V. M. Bruno, A. P. Mitchell, and D. A. Davis. 2004. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot. Cell 3:741-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, W., and A. P. Mitchell. 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccheroni, W., Jr., G. S. May, N. M. Martinez-Rossi, and A. Rossi. 1997. The sequence of palF, an environmental pH response gene in Aspergillus nidulans. Gene 194:163-167. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 26.Munn, A. L., and H. Riezman. 1994. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J. Cell Biol. 127:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negrete-Urtasun, S., S. H. Denison, and H. N. Arst, Jr. 1997. Characterization of the pH signal transduction pathway gene palA of Aspergillus nidulans and identification of possible homologs. J. Bacteriol. 179:1832-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrete-Urtasun, S., W. Reiter, E. Diez, S. H. Denison, J. Tilburn, E. A. Espeso, M. A. Penalva, and H. N. Arst, Jr. 1999. Ambient pH signal transduction in Aspergillus: completion of gene characterization. Mol. Microbiol. 33:994-1003. [DOI] [PubMed] [Google Scholar]
- 29.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailiere Tindall, London, England.
- 30.Odorizzi, G., D. J. Katzmann, M. Babst, A. Audhya, and S. D. Emr. 2003. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell Sci. 116:1893-1903. [DOI] [PubMed] [Google Scholar]
- 31.Palmer, G. E., A. Cashmore, and J. Sturtevant. 2003. Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot. Cell 2:411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raiborg, C., T. E. Rusten, and H. Stenmark. 2003. Protein sorting into multivesicular endosomes. Curr. Opin. Cell Biol. 15:446-455. [DOI] [PubMed] [Google Scholar]
- 34.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su, S. S., and A. P. Mitchell. 1993. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics 133:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Penalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uetz, P., L. Giot, G. Cagney, T. A. Mansfield, R. S. Judson, J. R. Knight, D. Lockshon, V. Narayan, M. Srinivasan, P. Pochart, A. Qureshi-Emili, Y. Li, B. Godwin, D. Conover, T. Kalbfleisch, G. Vijayadamodar, M. Yang, M. Johnston, S. Fields, and J. M. Rothberg. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623-627. [DOI] [PubMed] [Google Scholar]
- 38.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent, O., L. Rainbow, J. Tilburn, H. N. Arst, Jr., and M. A. Penalva. 2003. YPXL/I is a protein interaction motif recognized by Aspergillus PalA and its human homologue, AIP1/Alix. Mol. Cell. Biol. 23:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson, R. B., D. Davis, B. M. Enloe, and A. P. Mitchell. 2000. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast 16:65-70. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing in Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu, W., and A. P. Mitchell. 2001. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, W., F. J. Smith, Jr., R. Subaran, and A. P. Mitchell. 2004. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]