Abstract
Recent studies have focused on evidence-based interventions to prevent mobility decline and enhance physical performance in older adults. Several modalities, in addition to traditional strengthening programs, have been designed to manage age-related functional decline more effectively. In this study, we reviewed the current relevant literatures to assess the therapeutic potential of eccentric exercises for age-related muscle atrophy (sarcopenia). Age-related changes in human skeletal muscle, and their relationship with physical performance, are discussed with reference to in vitro physiologic and human biomechanics studies. An overview of issues relevant to sarcopenia is provided in the context of the recent consensus on the diagnosis and management of the condition. A decline in mobility among the aging population is closely linked with changes in the muscle force–velocity relationship. Interventions based specifically on increasing velocity and eccentric strength can improve function more effectively compared with traditional strengthening programs. Eccentric strengthening programs are introduced as a specific method for improving both muscle force and velocity. To be more effective, exercise interventions for older adults should focus on enhancing the muscle force–velocity relationship. Exercises that can be performed easily, and that utilize eccentric strength (which is relatively spared during the aging process), are needed to improve both muscle force and velocity.
Keywords: aging, eccentric exercise, exercise intervention, sarcopenia
1. Introduction
By the age of 80 years, humans generally lose ∼30–40% of their skeletal muscle fibers, particularly Type II fibers.1 The decline in muscle mass and strength with age is well documented.2, 3 In general, the muscle mass of 60–70-year-olds decreases to 70–80% of that of younger people (< 60 years old).4, 5 The main features associated with aging skeletal muscles are muscle weakness, decreased flexibility, vulnerability to certain types of injury, and impaired functional restoration, which result in the deterioration of physical performance and function.6 Lower extremity strength decreases linearly with age in both men and women, as shown by numerous cohort studies.7, 8, 9 Physical performance, with respect to balance and gait speed, for example, is closely linked with muscle mass and strength.10 Therefore, age-related changes in muscle mass and strength should be carefully monitored for signs of decline. Although functional decline with increased age is typical, the mechanisms underlying this decline, and the features that characterize it, are complex and variable. Therefore, it is important to develop specific interventions that will prevent or delay functional decline based on understanding age-related changes in the muscular systems of older adults.
Concerning exercise interventions that have therapeutic potential specifically for age-related muscle atrophy, recent studies have focused on evidence-based interventions aimed at preventing mobility decline or enhancing physical performance in older adults.11, 12, 13 Several different treatment modalities, other than traditional strengthening programs, have been designed to manage age-related functional decline more effectively.12, 14 In this study, we reviewed the literature to delineate the role of physical function in disability and falls in older adults, and to assess the therapeutic potential of eccentric exercises for age-related muscle atrophy.
2. Sarcopenia: Definition and diagnosis
Sarcopenia is defined as the loss of skeletal muscle mass and strength with increased age.15, 16, 17 This results in weakness, limited mobility, and increased susceptibility to injury. Several operational definitions have been used to diagnose sarcopenia, of which the most prevalent is an appendicular skeletal muscle mass (ASM) value ≥ 2 standard deviations below the ASM of young adults, divided by height squared (kg/m2) or weight. As a rule, the relationship between mass and strength is linear, as is that between muscle strength and performance. However, there is no linear correlation between muscle mass and function. We found that muscle mass was not associated with physical performance in weak older adults, suggesting that measures of muscle strength may be of greater clinical importance in this population than muscle mass per se. The correlation between muscle mass and functional performance was significant in the higher strength group, but not in the weaker group.18 This is because fatty or connective tissue infiltration into muscle tissues are included in muscle mass in those patients such that we cannot distinguish abnormal intramuscular changes by evaluating muscle mass alone, particularly in patients with mobility impairments. Therefore, the functional status of intramuscular tissues should be included in any assessment of sarcopenia.8, 18
Mobility decline among the aging population is closely linked with changes in the muscle force–velocity relationship.19 Such changes have functional implications, e.g., slower walking speeds. In recent studies, gait speed, or walking velocity, was highlighted as a major indicator of mobility decline or sarcopenia in geriatric populations.20, 21, 22 Predicted years of remaining life, for both sexes, increases commensurate with gait speed regardless of age, according to a pooled analysis of nine cohort studies on walking speed and survival.23 Gait speeds of 1.0 m/s or higher were consistently associated with longer survival than would be expected based on age and sex alone. According to the consensus in European group for determining sarcopenia, gait speed should be measured initially, and mass profiles should be evaluated when gait speed is abnormal. Sarcopenia should be diagnosed when gait speed is normal only if strength is abnormal.22
3. Changes in skeletal muscle structure and function with advancing age
The mechanical properties of muscles can be assessed according to their active, contractile characteristics, and passive tension or stiffness. Intrinsic contractile characteristics related to the cross-bridge mechanics of single fibers change with aging.24 Some studies have reported that both a decrease in contractile materials and a reduced force-generating capacity per cross-bridge affect the contractile properties of aging muscles. A selective loss of Type II (fast twitch) muscle fibers is associated with the age-related decline in strength.25 Cross-sectional dissections of whole thigh muscles of older cadavers have also shown an 18% decline in total muscle area and a 25% decrease in the total number of muscle fibers, with a particular decrease in the number of Type II muscle fibers.26 These results are also supported by other cross-sectional studies that have shown that the area of Type I mean fibers is preserved with aging, whereas Type II fibers show atrophy.27 In addition to changes in muscle fiber size and number with advancing age, specific changes in the intrinsic ability of aged muscles to generate force have also been observed. In humans, decreased specific force (i.e., force normalized by cross-sectional area [CSA]) and unloaded shortening velocity in the Type I and IIa fibers of older sedentary males have been reported relative to young sedentary controls.28, 29 These changes are also consistent with those observed in rat skeletal muscle throughout life. Thompson et al 30 reported decreases in the specific force and unloaded shortening velocity of the soleus muscles of aging rats. They further noted a dissociation between loss of muscle CSA and a decline in maximal force. These human and rat data provide evidence that intrinsic changes in muscle quality may also be important for understanding age-related changes in the function of skeletal muscle. Future studies should evaluate the relationship between the intrinsic force and shortening velocity of aging skeletal muscle, and the relationship between these factors and age-related declines in whole muscle strength and peak power.
Changes in both the fiber elasticity and contractility of Type I and Type IIa fibers have been assessed in terms of instantaneous stiffness, that is, according to the ratio between force changes and corresponding length changes.24 As an active component of elasticity, instantaneous stiffness reflects the elastic characteristics of muscle fibers but has some limitations with respect to indexing passive components. Passive elastic properties are instead assessed in terms of passive stiffness, or tension, which is thought to be related to sarcolemma, connective tissues, and titin filaments.31 Passive stiffness is measured by the tensile force associated with displacement during stretching of the skeletal muscles.32 Increased stiffness, or decreased extensibility, of the muscle or muscle-tendon unit has been reported in aging skeletal muscles.33 Increased stiffness, along with decreased size and contractility, could lead to impaired muscle function and an increased vulnerability to injury.32 Stiff collagen tissue, fatty changes, and changes in muscle cells are major determinants of passive stiffness in aging muscles. At a cellular level, sarcomere proteins such as titin, nebulin, and intermediate filaments control elastic muscle functions.34 There has been some controversy regarding the increase in muscle stiffness due to aging: in a previous study, muscle stiffness remained unchanged with older age; however, it accounted for a greater proportion of total tension because of significant declines in muscle mass and contractile tension with age.32
4. Relative preservation of eccentric strength in aging
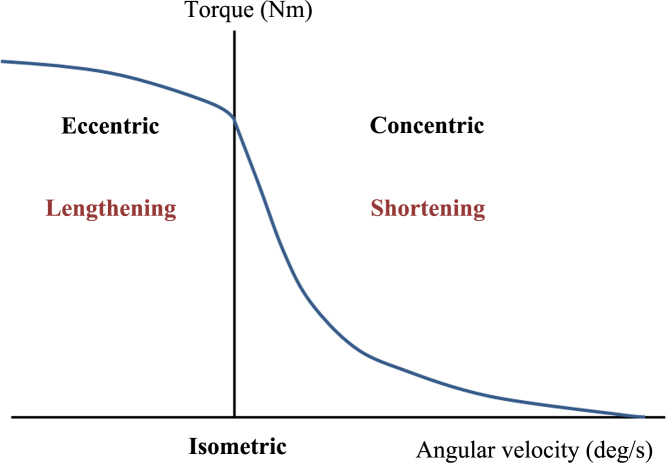
Due to physiological and structural changes, the force–velocity relationship of human muscles changes with age, and muscular strength and power are reduced at all contraction speeds.35, 36, 37, 38 However, older adults show a relatively preserved capacity for eccentric strength. The preservation of eccentric strength in older adults is a well-established phenomenon.39 Compared with concentric force, eccentric force shows relatively less decline with age (2–48%). Thus, preservation of eccentric strength in elders underpins the therapeutic potential of eccentric exercises in this population.37, 39, 40 Eccentric muscle contraction involves a lengthening contraction that has greater force than shortening contraction with less energy expended per unit of muscle force (Fig. 1). Eccentric muscle contraction is very useful when a high degree of muscle strength is required. Many functional activities require eccentric contraction, although delayed-onset muscle soreness can be problematic.39 Although the mechanisms underlying eccentric contraction remain unclear, they appear to originate in the muscle itself, where both passive and active elements may regulate muscle stiffness. Increased passive stiffness due to age-related accumulation of noncontractile material confers a mechanical advantage during eccentric contraction.33, 39 Ochala et al24 reported greater instantaneous stiffness per force unit, indicating greater active stiffness in the cross-bridge, in the fibers of older versus younger men; this may represent a compensatory mechanism against less efficient muscle contraction with aging. In older muscle, a relative increase in active stiffness in the cross-bridge may be a mechanism underlying reduced muscle contraction efficiency. In a study on skeletal muscle fiber, we measured residual force enhancement (RFE) after lengthening contraction in aged rats. RFE is a well-known phenomenon that the isometric steady-state force at the stretched length after lengthening contraction is greater than the steady-state isometric force at that same length for a purely isometric contraction.39 We found that RFE was greater in older versus younger muscle fibers (unpublished data). We concluded that preservation of eccentric strength likely contributed to the observed increase in RFE in aged skeletal muscle fibers. However, further studies are required to elucidate the mechanism underlying the preservation of eccentric strength. Although the mechanisms remain unclear, an understanding of why older adults show this relative maintenance of eccentric strength could be relevant for practical applications, such as training and rehabilitation of elderly people.39
Fig. 1.
Force–velocity relationship shows eccentric muscle contraction involves a lengthening contraction that has greater force than shortening contraction.
5. Specific interventions for the prevention of mobility decline
Many trials have aimed to understand how to prevent or delay functional decline, as well as the development of disability, in older adults. It is clear that exercise can play a key role in this respect, but issues remain regarding the specific interventions that are most effective and practically useful for functionally impaired individuals. Many types of exercise can be used to enhance the physical function of older adults. The exercise recommendations of the American Geriatric Society have been widely accepted as being applicable to older adults.41 However, although the guidelines may be effective and safe for the healthy aging population, they may be too general for the mobility-impaired aging population.
Exercise training for elderly persons involves special considerations. Aging is associated with a decrease in the proportion of Type II muscle fibers, a decrease in fiber CSA, and a change in contractile properties such as P0, P0/CSA, and V0.29, 42 Type II fibers seem to be more affected by aging than other fibers, because they show greater decreases in CSA, which in turn leads to increases in the relative muscle area occupied by Type I fibers. Several recent studies have suggested that, when the training stimulus is of an appropriate intensity (i.e., 70–90% of the one-repetition maximum [1RM]), gains in muscle strength and size in older healthy and frail individuals are comparable to the gains observed in young individuals.43 The difference in strength gains (28–227%) reported by various studies may be related to several factors, such as the participants’ initial strength, age, comorbidities, and differences in the muscles trained. Effects of resistance training on the single muscle fibers of older individuals were detected in both Type I and IIa fibers in males, but not in females.43, 44 Moreover, the contractility of single-fibers might be sensitive to changes in physical activity levels, rather than aging per se. In several studies, progressive resistance training was associated with minimal improvements in peak power; therefore, training interventions need to be designed to maximize peak power in older individuals.11, 14, 44
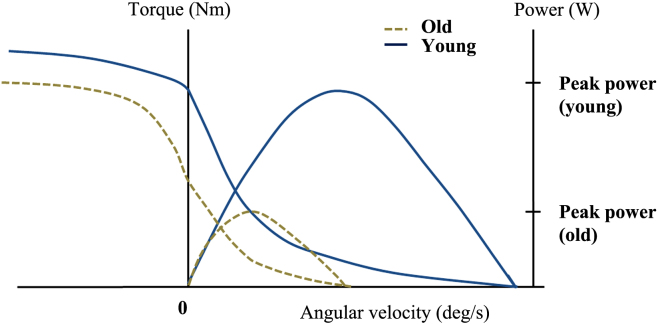
Regarding the muscle force–velocity relationship, power output is derived from the force–velocity curve using Hill's Equation (Fig. 2). In an assessment of decline in older versus younger adults, there was a greater decrease in power output than in force in the former group. Power output, which is the maximum capacity to perform muscular work per unit time, is a critical variable in muscle impairment, functional limitation, and subsequent disability.44, 45, 46 Bean et al47, 48 reported that peak power in lower extremities was closely associated with functional limitations and self-reported disability in community-dwelling men and women. It was also more closely associated with gait speed than strength. Strength (i.e., force) and muscle power are both closely linked to functional performance; power output has a strong correlation with performance in a number of functional tasks, such as climbing stairs, rising from a chair, and walking, which shows that power is a better indicator of poor mobility compared with hand grip, knee extensor torque, and muscle mass. Therefore, interventions capable of enhancing or maintaining power output in older adults will be more effective. Regarding ways to improve the force–velocity relationship in old age, Raj et al19 reviewed studies on the effects of different exercises on the force–velocity relationship. Traditional resistance training (TRT), such as the slow lifting and lowering of heavy loads according to the 1RM, had a significant positive effect on strength, but only modest effects on functional measures such as gait speed; furthermore, changes in the force–velocity curve were not significant.49 In other studies, power training (PT) was more effective for improving muscular power and functional capacity in older adults, and was also associated with significant improvements in isometric and concentric strength, as well as power, compared with TRT.11, 46 Concerning power-oriented exercises, high velocity, low-resistance exercise can be used for PT. When using an exercise machine, around 40% 1RM represents a suitable external force for an effective increase in exercise velocity. Reid et al12 reported several positive effects of power-specific exercise interventions in community-dwelling older women. However, a study that compared TRT with PT found that they were equally effective for improving muscular strength.50 Another study reported changes in peak leg extensor power in response to 12 weeks of resistance training in older women; increases in strength of 22–27% were observed, in addition to a nonsignificant increase in leg extensor power.51
Fig. 2.
Power output derived from the force–velocity curve using Hill's Equation indicates a greater decrease in power output than in force in old group compared with young group.
6. Therapeutic potential of eccentric contraction and eccentrically biased training
One potential intervention to enhance force and function in aging muscles is eccentric muscle exercise. Eccentric exercise has several important therapeutic advantages for aging populations, including low metabolic cost and minimal cardiorespiratory burden. Why is the metabolic cost lower during eccentric contractions? Elastic strain energy is used to resist the external force during eccentric contractions, such that muscle exertion requires minimal energy from adenosine 5ʹ-triphosphate (ATP) breakdown; although some ATP is used during eccentric contractions, more is used during concentric and static contractions.52 Therefore, chronic eccentric exercises can be easily applied to elderly and functionally limited patients, because these exercises do not require high energy consumption. Furthermore, such individuals may be so limited in their ability to perform exercises that walking may represent the limit of, or even exceed, their aerobic capacity, thereby precluding exercises performed at intensities sufficient to prevent muscle wasting (sarcopenia).
Eccentric exercise can cause substantial biologic changes in intramuscular structure and functional capability (e.g., increased muscle fascicle length).19 Muscle fascicle length is known to decrease following immobilization, as well as with aging,53 but it can be increased by lengthening contractions.19, 54 Eccentric resistance training alone has been reported to increase vastus lateralis fascicle length in elderly people to a greater extent than TRT.54 Carlsson et al55 reported that myotilin, which plays a key role in the dynamic molecular events that mediate myofibrillar assembly, may also be involved in the development of new sarcomere structures after stretching stimulation.
Another reason for the usefulness of eccentric exercise is a less delayed onset of muscle soreness in older populations.56, 57 Age-related muscle atrophy tends to occur predominantly in Type II fibers. Choi et al58 found that resistance of the myofilament lattice to mechanical strain was reduced in Type IIa and Type IIa/IIx, but not in Type I, fibers, which indicates that aging muscle may be less vulnerable to eccentric exercise-induced muscle injury. As mentioned above, compared with the decline in concentric force that occurs with age, eccentric force is relatively unimpaired. This preservation of eccentric strength in elders underlies the therapeutic potential of eccentric exercises, which could be used to specifically target this facet of strength.
The potential advantages of eccentric resistance training might be particularly important during the initial stages of resistance training interventions for older adults with poor muscle strength.59 Eccentric resistance training is generally conducted using specialized equipment, such as an isokinetic dynamometer. In a recent study, resistance exercises involving negative, eccentrically induced work were applied to older cancer survivors using a specially designed, motor-driven ergometer.60 At only low levels of exertion, these exercises can produce a relatively high muscle workload via the motor-driven eccentric exercise machine. In addition, reverse cycling of electric-powered pedals can induce biological overload through progressive increases in eccentric power. During this exercise, the patient must try to slow down the moving pedals. To date, consistent with the evidence presented above, a number of studies have reported advantages of eccentric versus concentric resistance training. However, other studies have reported little or no additional benefit of eccentric resistance training over conventional training. In addition, the traditional negative standpoint on damage induction by eccentric contraction still remains an academic barrier to its clinical application. Accordingly, further research is needed to optimize eccentric resistance training for older adults, particularly by improving the physical function domain.
7. Conclusion
To be more effective, exercise interventions for older adults should focus on enhancing the muscle force–velocity relationship. Physiological and biomechanical evidence for an effect of lengthening contraction, as well as eccentrically biased training, on skeletal muscle properties and function has accumulated. There is a need to develop exercises that can be performed easily and utilize eccentric strength, which is relatively spared during the aging process, to improve both force and velocity in people with age-related muscle atrophy.
Conflicts of interest
The author has no conflicts of interest to declare.
Acknowledgments
This work was supported by the Bio & Medical Technology Development Program of the National Research Fund (NRF) funded by the Korean Government (No. 2011–0030135) and (NRF–2010–0013261)
References
- 1.Walston J.D. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauretani F., Russo C.R., Bandinelli S., Bartali B., Cavazzini C., Di Iorio A. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Frontera W.R., Reid K.F., Phillips E.M., Krivickas L.S., Hughes V.A., Roubenoff R. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frontera W.R., Hughes V.A., Fielding R.A., Fiatarone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: a 12-year longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 6.Walston J., Fried L.P. Frailty and the older man. Med Clin North Am. 1999;83:1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 7.Abellan van Kan G., Cesari M., Gillette-Guyonnet S., Dupuy C., Nourhashemi F., Schott A.M. Sarcopenia and cognitive impairment in elderly women: results from the EPIDOS cohort. Age Ageing. 2013;42:196–202. doi: 10.1093/ageing/afs173. [DOI] [PubMed] [Google Scholar]
- 8.Auyeung T.W., Lee J.S., Leung J., Kwok T., Woo J. Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3153 older adults--an alternative measurement of sarcopenia and sarcopenic obesity. Age (Dordr) 2013;35:1377–1385. doi: 10.1007/s11357-012-9423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesari M., Pahor M., Lauretani F., Zamboni V., Bandinelli S., Bernabei R. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman A.B., Kupelian V., Visser M., Simonsick E., Goodpaster B., Nevitt M. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 11.Bean J.F., Herman S., Kiely D.K., Frey I.C., Leveille S.G., Fielding R.A. Increased velocity exercise specific to task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community-dwelling older women. J Am Geriatr Soc. 2004;52:799–804. doi: 10.1111/j.1532-5415.2004.52222.x. [DOI] [PubMed] [Google Scholar]
- 12.Reid K.F., Callahan D.M., Carabello R.J., Phillips E.M., Frontera W.R., Fielding R.A. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res. 2008;20:337–343. doi: 10.1007/bf03324865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fielding R.A., Rejeski W.J., Blair S., Church T., Espeland M.A., Gill T.M. The lifestyle interventions and independence for elders study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouellette M.M., LeBrasseur N.K., Bean J.F., Phillips E., Stein J., Frontera W.R. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35:1404–1409. doi: 10.1161/01.STR.0000127785.73065.34. [DOI] [PubMed] [Google Scholar]
- 15.Evans W.J. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50 doi: 10.1093/gerona/50a.special_issue.5. Spec No:5-8. [DOI] [PubMed] [Google Scholar]
- 16.Waters D.L., Baumgartner R.N., Garry P.J. Sarcopenia: current perspectives. J Nutr Health Aging. 2000;4:133–139. [PubMed] [Google Scholar]
- 17.Doherty T.J. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kim K.E., Jang S.N., Lim S., Park Y.J., Paik N.J., Kim K.W. Relationship between muscle mass and physical performance: is it the same in older adults with weak muscle strength? Age Ageing Nov. 2012;41:799–803. doi: 10.1093/ageing/afs115. [DOI] [PubMed] [Google Scholar]
- 19.Raj I.S., Bird S.R., Shield A.J. Aging and the force-velocity relationship of muscles. Exp Gerontol. 2010;45:81–90. doi: 10.1016/j.exger.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Satake S. Sarcopenia in relation to locomotive syndrome and frailty. Clin Calcium. 2012;22:67–73. [PubMed] [Google Scholar]
- 21.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Gro**up on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochala J., Frontera W.R., Dorer D.J., Van Hoecke J., Krivickas L.S. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 25.Larsson L., Grimby G., Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J. Appl. Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 26.Lexell J., Henriksson-Larsen K., Wimblod B., Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 27.Porter M.M., Vandervoort A.A., Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 28.Larsson L., Li X., Frontera W.R. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am. J. Physiol. 1997;272:C638–C649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 29.Frontera W.R., Suh D., Krivickas L.S., Hughes V.A., Goldstein R., Roubenoff R. Skeletal muscle fiber quality in older men and women. Am. J. Physiol. 2000;279:C611–C618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 30.Thompson L.V., Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J. Appl. Physiol. 1999;86:881–886. doi: 10.1152/jappl.1999.86.3.881. [DOI] [PubMed] [Google Scholar]
- 31.Linke W.A., Ivemeyer M., Olivieri N., Kolmerer B., Ruegg J.C., Labeit S. Towards a molecular understanding of the elasticity of titin. J Mol Biol. 1996;261:62–71. doi: 10.1006/jmbi.1996.0441. [DOI] [PubMed] [Google Scholar]
- 32.Brown M., Fisher J.S., Salsich G. Stiffness and muscle function with age and reduced muscle use. J Orthop Res. 1999;17:409–414. doi: 10.1002/jor.1100170317. [DOI] [PubMed] [Google Scholar]
- 33.Gajdosik R.L., Vander Linden D.W., Williams A.K. Influence of age on length and passive elastic stiffness characteristics of the calf muscle-tendon unit of women. Phys Ther. 1999;79:827–838. [PubMed] [Google Scholar]
- 34.Horowits R. Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys J. 1992;61:392–398. doi: 10.1016/S0006-3495(92)81845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochala J., Lambertz D., Pousson M., Goubel F., Hoecke J.V. Changes in mechanical properties of human plantar flexor muscles in ageing. Exp Gerontol. 2004;39:349–358. doi: 10.1016/j.exger.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Gajdosik R.L., Vander Linden D.W., Williams A.K. Concentric isokinetic torque characteristics of the calf muscles of active women aged 20 to 84 years. J Orthop Sports Phys Ther. 1999;29:181–190. doi: 10.2519/jospt.1999.29.3.181. [DOI] [PubMed] [Google Scholar]
- 37.Ochala J., Dorer D.J., Frontera W.R., Krivickas L.S. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflugers Arch. 2006;452:464–470. doi: 10.1007/s00424-006-0065-6. [DOI] [PubMed] [Google Scholar]
- 38.Vandervoort A.A. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 39.Roig M., Macintyre D.L., Eng J.J., Narici M.V., Maganaris C.N., Reid W.D. Preservation of eccentric strength in older adults: Evidence, mechanisms and implications for training and rehabilitation. Exp Gerontol. 2010;45:400–409. doi: 10.1016/j.exger.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter M.M., Vandervoort A.A., Kramer J.F. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52:B125–B131. doi: 10.1093/gerona/52a.2.b125. [DOI] [PubMed] [Google Scholar]
- 41.American Geriatrics Society Panel on E, Osteoarthritis. Exercise prescription for older adults with osteoarthritis pain: consensus practice recommendations. A supplement to the AGS Clinical Practice Guidelines on the management of chronic pain in older adults. J Am Geriatr Soc 2001;49:808–23. [DOI] [PubMed]
- 42.Earles D.R., Judge J.O., Gunnarsson O.T. Velocity training induces power-specific adaptations in highly functioning older adults. Arch Phys Med Rehabil. 2001;82:872–878. doi: 10.1053/apmr.2001.23838. [DOI] [PubMed] [Google Scholar]
- 43.Fielding R.A. The role of progressive resistance training and nutrition in the preservation of lean body mass in the elderly. J. Am. Coll. Nutr. 1995;14:587–594. doi: 10.1080/07315724.1995.10718547. [DOI] [PubMed] [Google Scholar]
- 44.Carabello R.J., Reid K.F., Clark D.J., Phillips E.M., Fielding R.A. Lower extremity strength and power asymmetry assessment in healthy and mobility-limited populations: reliability and association with physical functioning. Aging Clin Exp Res. 2010;22:324–329. doi: 10.3275/6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuoco A., Callahan D.M., Sayers S., Frontera W.R., Bean J., Fielding R.A. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 46.Fielding R.A., LeBrasseur N.K., Cuoco A., Bean J., Mizer K., Fiatarone Singh M.A. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 47.Bean J.F., Kiely D.K., LaRose S., Leveille S.G. Which impairments are most associated with high mobility performance in older adults? Implications for a rehabilitation prescription. Arch Phys Med Rehabil. 2008;89:2278–2284. doi: 10.1016/j.apmr.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 48.Bean J.F., Kiely D.K., Herman S., Leveille S.G., Mizer K., Frontera W.R. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 49.Morse C.I., Thom J.M., Mian O.S., Birch K.M., Narici M.V. Gastrocnemius specific force is increased in elderly males following a 12-month physical training programme. Eur J Appl Physiol. 2007;100:563–570. doi: 10.1007/s00421-006-0246-1. [DOI] [PubMed] [Google Scholar]
- 50.Bottaro M., Machado S.N., Nogueira W., Scales R., Veloso J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol. 2007;99:257–264. doi: 10.1007/s00421-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 51.Skelton D.A., Young A., Greig C.A., Malbut K.E. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc. 1995;43:1081–1087. doi: 10.1111/j.1532-5415.1995.tb07004.x. [DOI] [PubMed] [Google Scholar]
- 52.Lindstedt S.L., LaStayo P.C., Reich T.E. When active muscles lengthen: properties and consequences of eccentric contractions. News in physiological sciences: an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2001;16:256–261. doi: 10.1152/physiologyonline.2001.16.6.256. [DOI] [PubMed] [Google Scholar]
- 53.Suetta C., Hvid L.G., Justesen L., Christensen U., Neergaard K., Simonsen L. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 54.Reeves N.D., Maganaris C.N., Longo S., Narici M.V. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol. 2009;94:825–833. doi: 10.1113/expphysiol.2009.046599. [DOI] [PubMed] [Google Scholar]
- 55.Carlsson L., Yu J.G., Moza M., Carpen O., Thornell L.E. Myotilin: a prominent marker of myofibrillar remodelling. Neuromuscul Disord. 2007;17:61–68. doi: 10.1016/j.nmd.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Gault M.L., Willems M.E. Aging, functional capacity, and eccentric exercise training. Aging Dis. 2013;4:351–363. doi: 10.14336/AD.2013.0400351. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovering R.M., Brooks S.V. Eccentric exercise in aging and diseased skeletal muscle: good or bad? J Appl Physiol (1985). 2014;116:1439–1445. doi: 10.1152/japplphysiol.00174.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi S.J., Lim J.Y., Nibaldi E.G., Phillips E.M., Frontera W.R., Fielding R.A. Eccentric contraction-induced injury to type I, IIa, and IIa/IIx muscle fibers of elderly adults. Age (Dordr) 2012;34:215–226. doi: 10.1007/s11357-011-9228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hortobagyi T. The positives of negatives: clinical implications of eccentric resistance exercise in old adults. J Gerontol A Biol Sci Med Sci. 2003;58:M417–M418. doi: 10.1093/gerona/58.5.m417. [DOI] [PubMed] [Google Scholar]
- 60.LaStayo P.C., Marcus R.L., Dibble L.E., Smith S.B., Beck S.L. Eccentric exercise versus usual-care with older cancer survivors: the impact on muscle and mobility--an exploratory pilot study. BMC Geriatr. 2011;11:5. doi: 10.1186/1471-2318-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]