Abstract
Background
This study explored the bioactivities and nutrient compositions of coffee (Coffea Arabica L.) pulp which was prepared in three different ways [Coffee Pulp Extracts (CPE) 1–3].
Methods
The coffee pulp was prepared in three different ways by distinct selecting and freezing processes. The nutritional values, polyphenol contents, antioxidant activity, and antibacterial properties of the coffee pulp as well as the characterization of the active ingredients by liquid chromatography-electrospray ionization-quadrupole-time-of-flight mass spectrometry (LC-ESI-Q-TOF-MS) were evaluated.
Results
The chemical profiles of three aqueous extracts were compared and characterized using LC-ESI-QTOF-MS. They showed slightly different nutrient compositions. The total phenolic content was highest in CPE1, and decreased in the following order: CPE1 > CPE2 > CPE3. Among the CPEs tested, CPE1 showed the most potent antioxidant activity with IC50 18 μg/mL and 82 μg/mL by 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and 1,1-diphenyl-2-picryl-hydrazyl assay, respectively. Chlorogenic acid and caffeine were the most prominent in CPE1 and it contained more compounds than the others. Moreover, CPE1 demonstrated antibacterial activity against both gram-positive (Staphylococcus aureus and Staphylococcus epidermidis) and gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli).
Conclusion
These findings indicated that CPE1 has powerful nutrients with antioxidant and antibacterial properties—the potency of which is impacted by the preparation process.
Keywords: antibacterial activity, antioxidant, coffee pulp, LC-ESI-QTOF, nutrient
1. Introduction
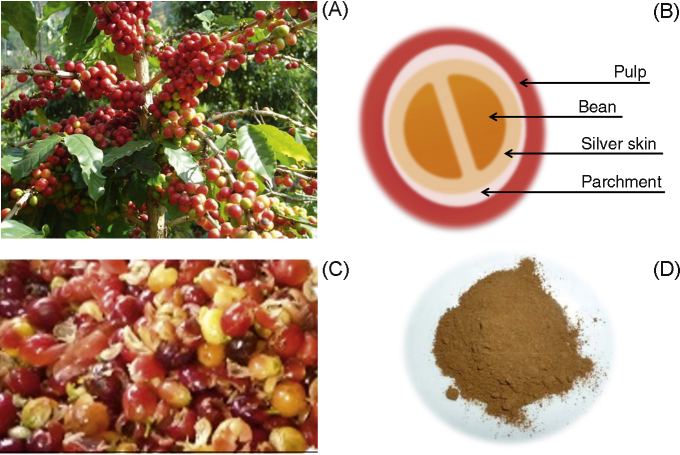
Coffee (Coffea arabica L.) belongs to the Rubiaceae family, and is a popular beverage worldwide. During coffee processing, a residue or by-product (pulp, silver skin, and parchment) is generated. Ripe coffee fruits (cherries; Fig. 1A) are harvested, and the skin and pulp are removed from the coffee beans (Fig. 1B, 1C). Every 2 tons of coffee produces 1 ton of coffee pulp (CP),1 leading to a serious environmental problem. Therefore, the development and added values of residual parts are of interest. Several studies have focused on developing and using the waste products for fermentation by cultivation of edible fungus, active ingredient extraction, animal feed, or compost2, 3, 4 due to the presence of enriched nutrients, minerals, amino acids, polyphenol, and caffeine.4, 5 Murthy and Naidu6 suggested that CP, a red color, is a source of anthocyanins for potential applications as a natural food colorant.2 Furthermore, hydroxycinnamic acids (chlorogenic, caffeic, and ferulic acid) found in CP have antioxidant properties.4 It has been noted that antioxidants neutralize excess free radicals to prevent cells against free radical damage and to contribute to disease prevention.7 In addition, CP has been revealed to have antimicrobial activity against bacteria such as Staphylococcus aureus, Enterococcus faecalis, Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli, etc.8, 9, 10 Previous studies revealed different compositions of coffee by-products,6, 11 including CP,12 in fresh and dehydrated conditions.11 However, the nutrient composition may vary depending on the processing, preparation, and storage. Even though there have been reports on the composition and utilization of CP, and antioxidant properties of different parts of coffee, there are limited reports on the nutritional values and biochemical properties of CP by different processing preparations. The aim of this study was to determine the nutritional values, polyphenol contents, antioxidant activity, and antibacterial properties of CP, as well as to characterize the active ingredients by liquid chromatography-electrospray ionization-quadrupole-time-of-flight mass spectrometry (LC-ESI-Q-TOF-MS) in three different processing preparations. A preferred processing method might thus be illuminated and contribute to the development of CP as a food additive or preservative in the food industry.
Fig. 1.
Coffea arabica L. tree. (A) Coffee cherries. (B) Cross-section of coffee cherry. (C) Coffee pulp after removal from the bean. (D) Characteristics of coffee pulp powder after drying and blending processes.
2. Methods
2.1. Chemical and reagents
Standard D-(-)-quinic acid, L-(-)-malic acid, citric acid, chlorogenic acid, Folin–Ciocalteu's phenol reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Methanol (high performance liquid chromatography grade) acetonitrile (LC-MS reagent) and water (LC-MS grade) were purchased from RCI Labscan Limited (Bangkok, Thailand). Formic acid (analytical grade) was purchased from JT Baker (Philipsburg, NJ, USA). A nylon syringe filter 0.45 μm was bought from Lubitech Technologies Ltd. (Shanghai, China). Muller-Hinton agar (MHA) and Muller-Hinton broth (MHB) were purchased from Difco Laboratories, Inc. (Difco, Franklin Lakes, NJ, USA).
2.2. Plant materials
The pulp of C. arabica L. was obtained from Chao-Thai-Pukao Factory (Chiang Mai, Thailand), that collected samples from Baan Khun Lao, Wieng Pa Pao, and Chiang Rai, Thailand. The plant was taxonomically authenticated and a voucher specimen was deposited in the herbarium of the Faculty of Biology (NU herbarium), Naresuan University, Phitsanulok, Thailand.
2.3. Sample preparation
To prepare CP, ripe coffee cherries were harvested, washed (washing process), and coffee fruits were selected by removing the floating fruit (selecting process). The coffee bean was removed from the pulp using machine separates (removing process) as shown in Fig. 1C. Pulp was frozen at –20 °C (freezing process) and then dried using far-infrared rays, and blended using blender machine as shown in Fig. 1D (drying and blending process). In the present study, CP was prepared three different ways. CP extract 1 (CPE1) was prepared as follows: washing → removing → drying and blending; CPE2: washing → selecting → removing → drying and blending; CPE3: washing → selecting → removing → freezing → drying and blending.
The dried CP powder resulting from each of these process preparations was extracted with hot water (92± 3 °C) for 2 minutes. The ratio of CP and hot water was 1:5. The filtered solution was further lyophilized to give three aqueous extracts labeled as CPE1, CPE2, and CPE3. The CP aqueous extracts were kept at –20 °C before use. For the chemical characterization, 2 mg/mL of each was dissolved in methanol and water [1:1 volume/volume (v/v)] and then further filtered through a 0.45-μm nylon syringe filter prior to injection into the LC-MS system.
2.4. ESI-Q-TOF-MS conditions
A 6540 ultrahigh definition accurate mass Q-TOF (Agilent Technologies, Palo Alto, CA, USA) was converted into an Agilent 1260 infinity high performance liquid chromatography instrument (Agilent, Waldbonn, Germany) via an ESI interface. Analysis parameters were set using both negative and positive ion modes with spectra acquired over a mass range of m/z 100–1,000 amu. The ESI-MS condition parameters were as follows: capillary voltage, +3,500 V; dry gas temperature, 350 °C; dry gas flow, 10 L/min; nebulizer pressure, 30 psig; and spectra rate, 4 Hz. Fragmentations were performed using auto MS/MS experiments with collision energies at 10 V, 20 V, and 40 V. Nitrogen was used as a collision gas.
Chromatographic separation was performed using a phenomenex Luna C-18(2) column (5 μm, 150 × 4.6 mm internal diameter) (Phenomenex Inc., Torrance, CA, USA). The mobile phase consisted of 0.1% formic acid in water v/v (Solvent A) and 0.1% formic acid in acetonitrile v/v (Solvent B). The linear gradient started from 10% to 90% of Solvent B for 30 minutes. The injection volume was 5 μL. The mobile phase flow rate was 0.5 mL/min.
2.5. Peak identification
Peak identification was performed by comparing the retention time, mass spectra, and fragmentation patterns with standard compounds, reported data, and a library search of the Mass Hunter METLIN metabolite database (Agilent Technologies).
2.6. Proximate analysis
Moisture, crude proteins, fat, fiber, and ash were analyzed using the Association of Official Analytical Chemists procedures.13 The crude protein content (N × 6.25) of CP was estimated using the macro Kjeldahl method; the crude fat was determined by extraction using Soxhlet apparatus, and the ash content was analyzed by weight before and after incineration at 600 ± 15 °C for 24 hours.
2.7. Determination of total phenolic contents
The total phenolic content of the CPEs were assessed using the Folin–Ciocalteu procedure as modified by Kahkonen and coworkers.14 Briefly, 200 μL of crude extract solution was mixed with 1 mL of Folin–Ciocalteu reagent, and then 0.8 mL sodium carbonate (7.5%) was added. The mixture was incubated at room temperature for 30 minutes, and was measured at 750 nm using a spectrophotometer. The results were expressed as mg/L of gallic acid.
2.8. Determination of antioxidant activity
2.8.1. ABTS assay
The ABTS free radical cation scavenging activity was measured according to the method of Re et al15 with a slight modification. Briefly, ABTS+ was made with 7 mM ABTS and 2.45 mM potassium persulfate in 100 mM phosphate buffer solution (pH 7.4) stored in the dark for 12–16 hours at room temperature. A working solution was diluted to absorbance values 0.7 ± 0.02 at 734 nm with 100 mM phosphate buffer solution (pH 7.4). Then, 990 μL of working ABTS solution was added to different concentrations of the extracts (2 μL). After 6 minutes of incubation at room temperature, absorbance was measured at 734 nm and the percentage of inhibition was calculated as follows:
% inhibition = [(A734 control – A734 test sample)/A734 control] × 100.
2.8.2. DPPH assay
Scavenging activity on DPPH was assessed according to the method reported by Blois16 with a slight modification. Briefly, an action mixture of 10 μL of different concentrations of the extracts and 190 μL of 80 μM DPPH in methanol was shaken and incubated at room temperature in the dark for 30 minutes. The control was prepared without extract. Absorbance at 517 nm was measured with a spectrophotometer. The experiment was performed three times, each time in triplicate. The percent inhibition was calculated from control using the following equation:
% inhibition = [(A517 control – A517 test sample)/A517 control)] × 100.
2.9. Antibacterial activity
2.9.1. Inoculum preparation
Inoculums were prepared by transferring colonies of S. aureus, S. epidermidis, P. aeruginosa, and E. coli growth on MHA into individual tubes of MHB. All strains were incubated at 37 °C for 24 hours and then adjusted either growing inoculums to the turbidity of the McFarland standard number 0.5.
2.9.2. Determination of antibacterial activity
Lyophilized CPE1 was chosen to determine antibacterial activity, as CPE1 produced the highest lyophilized dried weight using the agar well diffusion method. Briefly, the inoculums [107 colony forming units (CFU)/mL] were swabbed on MHA and followed by punching the agar with a 6-mm sterile cork borer. The sample, at a concentration of 300 mg/mL, was applied to each well. Gentamicin (Oxoid, Basingstoke, UK) and sterile distilled water was used as a positive control and negative control. The plates were incubated at 37 °C for 24 hours and the diameter (mm) of the inhibition zone was measured.
2.9.3. Determination of the minimum inhibitory concentration and minimal bactericidal concentration
Minimum inhibitory concentrations (MICs) were determined using a broth microdilution method recommended by the Clinical and Laboratory Standards Institute.17 Twofold serial dilutions of the samples were carried out using MHB and then were added to a sterile 96-well plate (final concentration ranging from 0.59 mg/mL to 300 mg/mL). A bacterial suspension was subsequently added to obtain a final concentration at 5 × 105 CFU/mL. All plates were incubated at 37 °C for 24 hours and MIC was defined as the lowest concentration of CPE1 sample that inhibited the growth of bacteria. Next, the suspension in the well of MIC and higher concentrations were spotted on MHA and incubated. The lowest concentration that inhibited growth of bacteria was defined as the minimal bactericidal concentration.
2.10. Statistical analysis
Data are reported as means ± standard error. Data were analyzed by one-way analysis of variance and post hoc Duncan's multiple range test. Statistical significance was considered at a p value of < 0.05.
3. Results
In the present study, dried powder of three CPEs, prepared by different processes, was extracted with hot water (92 ± 3 °C) for 2 minutes and lyophilized. The percent yield of lyophilized dried weight of CPE1, CPE2, and CPE3 were 28.74%, 16.59%, and 13.89%, respectively. The preliminary chemical constituents in these samples were studied using thin layer chromatography with ethyl acetate:methanol:water (7.0:2.5:0.5 v/v/v) as a mobile phase. Similarities in band patterns for all extracts were found (data not shown). The identity of chemical constituents from the aqueous extracts was further investigated with high performance liquid chromatography LC-ESI-Q-TOF-MS.
3.1. Phytochemical characterization
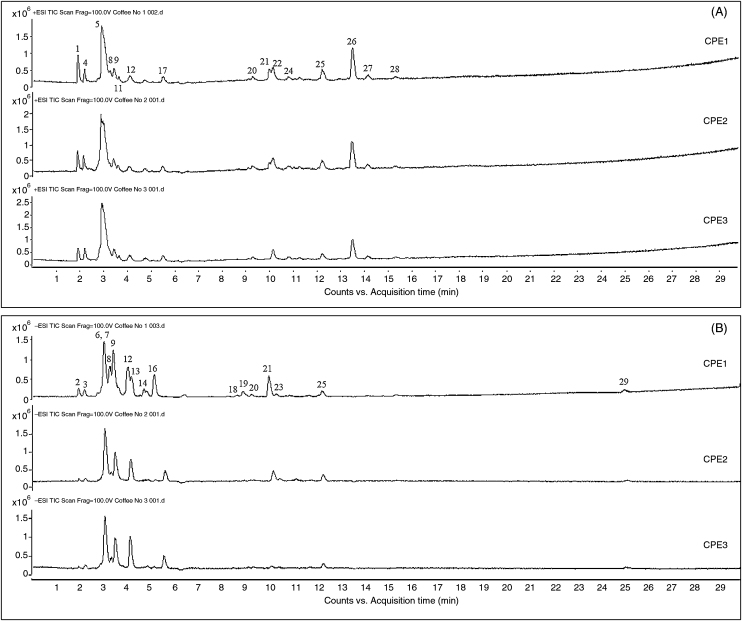
The chromatographic profiling of three aqueous CPEs were studied and identified using LC-ESI-QTOF-MS. The total ion current (TIC) chromatograms were compared in both ESI positive mode and negative modes (Fig. 2). The three extracts displayed similar chromatographic profiles; however, slightly different components were observed in two ranges–retention time (tR) at 3–6 minutes and 10 minutes. The results revealed that CPE1 contained the highest number of chemical compounds and was used for compound identification.
Fig. 2.
Total ion current (TIC) chromatograms of 2 mg/mL coffee pulp CPE1–3. Monitored in (A) electrospray ionization (ESI) positive mode and (B) ESI negative mode. The peak numbers and compounds identification were summarized in Table 1.
CPE, coffee pulp extract.
Twenty-nine compounds were detected and 20 compounds were tentatively identified and compared with the results of standard compounds, published data, and the pubic database. Table 1 provides a summary of the identified compounds, including retention times, ionization mode, measured mass, and MS/MS fragmentation ions. The compounds found in CP were proposed as sugar, small organic acid, alkaloid, hydroxycinnamic acid, fatty amide, and sterol.
Table 1.
Mass spectrometry (MS) data of (+/–) LC-ESI-QTOF-MS spectra and the identification of the coffee pulp aqueous extract method 1 (CPE1)
| Peak | tR (min) |
Ion mode | Measured mass | MS/MS | Molecular formula | Error (ppm) |
Compound identified |
|---|---|---|---|---|---|---|---|
| 1 | 1.87 | + | 125.9682 [M+H]+ | — | Unidentified | ||
| 2 | 1.94 | – | 174.9623 [M–H]– | — | Unidentified | ||
| 3 | 2.13 | – | 128.8650 [M–H]– | 84.9606 | Unidentified | ||
| 4 | 2.19 | + | 104.1069 [M+H]+ | 58.6055 | C5H13NO | 0.88 | 2-amino-3-methyl-1-butanol |
| 5 | 2.91 | + | 138.0555 [M+H]+ | 92.0496 | C7H7NO2 | –3.95 | Trigonelline |
| 6 | 3.01 | – | 181.0506 [M–H]– | 89.0209, 59.0160 | C6H14O6 | 23.40 | D-manitol |
| 7 | 3.06 | – | 179,0518 [M–H]– | 89.0209, 59.0109 | C6H12O6 | 24.08 | Hexose |
| 8 | 3.23 | – | 195.0577 [M–H]– | 129.0513, 75.0088 | C6H12O7 | –34.4 | Gluconic acid |
| + | 219.0470 [M+Na]+ | 132.9535, 84.9534 | 2.39 | ||||
| 9 | 3.36 | – | 191.0517 [M–H]– | 127.0351, 85.0261 | C7H12O6 | 22.97 | Quinic acid* |
| + | 215.0527 [M+Na]+ | 136.0241, 100.8086 | –0.42 | ||||
| 10 | 3.64 | + | 407.1159 [M+H]+ | 215.0506, 110.9952 | Unidentified | ||
| 11 | 3.66 | + | 167.0786 [M+H]+ | 119.0835 | Unidentified | ||
| 12 | 3.98 | – | 133.0102 [M–H]– | 114.9995, 71.0105 | C4H6O5 | 30.43 | Malic acid* |
| 13 | 4.12 | – | 193.0420 [M–H]– | 103.0038, 59.1040 | C10H10O4 | 44.72 | Ferulic acid |
| 14 | 4.67 | – | 191.0153 [M–H]– | 111.0045, 87.0053 | C6H8O7 | 23.17 | Citric acid* |
| 15 | 4.75 | + | 275.1096 [M+H]+ | — | Unidentified | ||
| 16 | 5.11 | – | 147.0356 [M–H]– | 115.0035, 71.0139 | C5H8O5 | –38.53 | Citramalic acid |
| 17 | 5.50 | + | 171.0261 [M+Na]+ | 111.0412 | Unidentified | ||
| 18 | 8.63 | – | 353.0966 [M–H]– | 191.0563, 179.0347, 135.0453 |
C16H18O9 | –24.91 | 3-Caffeoyl quinic acid |
| 19 | 8.87 | – | 153.0250 [M–H]– | 109.0298 | C7H6O4 | –37.04 | Protocatechuic acid |
| 20 | 9.25 | – | 415.2655 [M+HCOO]– | — | C24H34O3 | –39.74 | 3-Oxo-5β- chola-7,9(11)- |
| + | 393.2461 [M+Na]+ | 179.0814 | –15.47 | dien-24-oic Acid | |||
| 21 | 9.94 | – | 353.0826 [M–H]– | 191.0509, 85.0262 | C16H18O9 | 14.74 | Chlorogenic acid* |
| + | 355.1011 [M+H]+ | - | 3.54 | ||||
| 22 | 10.15 | + | 195.0873 [M+H]+ | 138.0663, 110.0711, 83.0607 |
C8H10N4O2 | 1.80 | Caffeine |
| 23 | 10.26 | – | 353.0962 [M–H]– | 191.0566, 173.0453, 135.0452 |
C16H18O9 | –23.77 | 4- Caffeoyl quinic acid |
| 24 | 10.82 | + | 518.8894 [M+H]+ | — | Unidentified | ||
| 25 | 12.26 | – | 497.3455 [M+HCOO]– | — | C27H48O5 | –5.75 | Cholestane-3α,7α,12α,24R, |
| + | 453.3444 [M+H]+ | 100.112, 55.0543 | 28.79 | 25-pentol | |||
| 26 | 13.51 | + | 340.2611 [M+Na]+ | 100.1120, 55.0544 | C21H35NO | –0.04 | N-methyl arachidonoyl amine |
| 27 | 14.17 | + | 453.3447 [M+Na]+ | 387.7959, 228.1591, 114.0911, 69.0702 |
C28H46O3 | 16.89 | 3β-Hydroxy-5α -cholest-7-ene-4 α -carboxylate |
| 28 | 15.32 | + | 463.3241 [M+Na]+ | — | C29H44O3 | –37.4 | 5α,8α-epidioxy-stigmasta- |
| 15.36 | – | 485.3437 [M+HCOO]– | — | –33.9 | 6,9(11),22E-trien-3β-ol | ||
| 29 | 25.00 | – | 293.1845 [M–H]– | 236.1047, 221.1549 | Unidentified |
Compared with standard compound.
LC-ESI-Q-TOF-MS, liquid chromatography-electrospray ionization-quadrupole-time-of-flight mass spectrometry.
3.2. Nutritional values of CP
The nutritional composition of the CPEs is shown in Table 2. All CPEs contained moisture, ash, fat, and protein at ∼5.28–5.93%, 8.32–8.88%, 1.40–2.51%, and 7%, respectively. Additionally, caffeine was found in CPE1, CPE2, and CPE3 at 0.69%, 0.77%, and 0.68%, respectively.
Table 2.
Nutrient, total polyphenol contents, and antioxidant activity of coffee pulp extracts
| Nutrients |
Antioxidant activity |
||||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Ash (%) | Fat (%) | Protein (%) | Total polyphenolic content (mg/L of gallic acid) | ABTS (IC50 μg/mL) | DPPH (IC50 μg/mL) | |
| CPE1 | 5.63 | 8.78 | 1.40 | 7.06 | 17.40 ± 0.74 | 18 ± 1.9 | 82 ± 7.8 |
| CPE2 | 5.28 | 8.88 | 1.76 | 7.00 | 10.47 ± 0.77 | 27 ± 0.6 | 153 ± 9.3 |
| CPE3 | 5.93 | 8.32 | 2.51 | 7.00 | 7.61 ± 0.42 | 27 ± 1.2 | 140 ± 9.2 |
Mean ± standard deviation.
ABTS, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); CPE, coffee pulp extract; DPPH, 2,2-diphenyl-1-picrylhydrazyl.
3.3. Polyphenol contents and antioxidant activity
Total phenolic content (TPC) of the three CPEs are presented in Table 2. CPE1 had the highest TPC. CPE1 and CPE2 were significantly different among groups. TPC extracts decreased in following order: CPE1 > CPE2 > CPE3, likely due to the different processing methods used. Based on the DPPH and ABTS radical scavenging assay, the antioxidant activity of the CPEs were confirmed with IC50 values (the concentration required to inhibit radical formation by 50%). CPE1 showed a highly significant difference antioxidant activity with an IC50 of 18 μg/mL and 82 μg/mL by ABTS and DPPH, respectively, compared with CPE2 and CPE3. Different antioxidant activities were observed depending on the assay.
3.4. Antibacterial activity
Because of the highest TPC and lyophilized dried yield of CPE1, it was chosen to subsequently determine the antibacterial activity using the agar well diffusion method. The results, zone of inhibition, revealed that the CPE1 showed inhibitory action against all tested bacteria as shown in Table 3. The inhibition zone of CPE1 against gram-positive bacteria, S. aureus and S. epidermidis, was significantly more than against gram-negative bacteria, P. aeruginosa and E. coli. The results of MIC were according to those obtained in the agar diffusion method. The concentration of CPE1 inhibiting the growth of gram-positive bacteria was lower than the concentration that inhibited gram-negative bacteria. Noticeably, CPE1 was effective against S. epidermidis and had the lowest MIC, only 4.69 mg/mL. Amounts in the range of 4.69–75 mg/mL of CPE1 had the potential to inhibit the growth of gram-positive and gram-negative bacteria (5 x 105 CFU/mL). The minimal bactericidal concentration results showed that CPE1, at a concentration of 300 mg/mL, was not antibacterial. CPE1 extract may act as a bacteriostatic agent only.
Table 3.
In-vitro antibacterial activity of coffee pulp extract 1 (CPE1)
| Bacteria | Inhibition zone (mm) | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|---|
| Staphylococcus aureus | 12* | 37.5 | > 300 |
| Staphylococcus epidermidis | 16* | 4.69 | > 300 |
| Pseudomonas aeruginosa | 10† | 75 | > 300 |
| Escherichia coli | 10† | 37.5 | > 300 |
| Negative control | 6 | — | — |
Significant differences are as follows:
*p < 0.05 compared among groups (n = 3).
†p < 0.05 compared with negative control.
MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration.
4. Discussion
This study explored the bioactivities and nutrient compositions of CPs in three different preparation processes. The TIC chromatograms of three CPEs displayed similar chromatographic profiles. The m/z 181.0506 [M–H]– (Peak 6) and 179.0518 [M–H]– (Peak 7) looked like dihydrocaffeic acid and caffeic acid but the fragmentation pattern and mass accuracy are related to D-manitol and hexose.
Trigonelline (Peak 5), an alkaloid, was detected with m/z 138.0555 [M+H]+ corresponding with reported data on a compound identified in aqueous extract of pure arabica and robusta coffees.18, 19 Caffeine (Peak 22), an alkaloid was identified at tR 10.15 minutes with a positively charged molecular ion [M+H]+ at 195.0873, and producing MS2 fragment ions at m/z 138.0663, 110.0711, and 83.0607 corresponding to [M+H-CH3-N=C=O]+, –CO and –CHN, respectively.20, 21
Peak 8, Peak 9, Peak 12, Peak 13, Peak 14, and Peak 16 with m/z [M–H]– at 195.0577, 191.0517, 133.0102, 193.0420, 191.0153, and 147.0356 were tentatively identified as gluconic acid, quinic acid, malic acid, ferulic acid, citric acid, and citramalic acid, respectively, based on comparison with a database search and authenticated compounds.
Peak 18, Peak 21, and Peak 23 showed the same molecular ion [M–H]– at m/z 353. The MS2 fragmentation pattern at m/z 191 corresponded to the loss of the caffeoyl acid moiety (162 Da). With the aid of available commercial standards, it was possible to identify Peak 21 as chlorogenic acid. Other compounds (Peak 18 and Peak 23), with the same molecular ion, were identified as the isomers, 3-caffeoylquinic acid and 4-caffeoylquinic acid, by comparing the fragmentation pattern with spectrometric characteristics previously reported in literature.22, 23
A signal of dihydroxybenzoic acid; protocatechuic acid was detected and identified at tR 8.87 minutes with m/z 153.0250 [M–H]–. The fragment ion at m/z 109.0298 was observed, corresponding to loss of CO2.24
Peak 25, Peak 27, and Peak 28 were identified as sterol compounds, while Peak 26 was a fatty amide. As mentioned above, phenolic compounds such as hydroxycinnamic acid and derivatives, hydroxylbenzoic acids, were tentatively identified. Additionally, alkaloid compound, trigonelline, and caffeine, and some organic acids were identified in aqueous extract of CP. These results correspond to those previously reported.25
In addition, CPEs showed powerful of nutrients and antioxidant activities. The moisture, ash, fat, and protein content of all CPEs possessed similar levels. The antioxidant results of ABTS+ were higher than the results of DPPH free radicals. The DPPH had a stable free radical 2,2-diphenyl-1-picrylhydrazyl reaction with H-donors, while ABTS+ had poor selectivity in the reaction with H-atom donors.26 Several studies report that CP contains antioxidant compounds.4, 5, 6 Arellano-González and coworkers4 reported CP contains hydroxycinnamic acids (chlorogenic, caffeic, and ferulic acid). These compounds donate a hydrogen atom to an oxidized molecule. In support of Arellano-González et al's4 study, hydroxycinnamic acids—quinic acid, chlorogenic acid, and caffeic acid—were present in the CP in the present investigation. The results point to a relationship between antioxidant activities of CP and phenolic content. This study showed that CPE1 has the potential to be developed as a natural antioxidant supplement.
Moreover, CPE1 revealed antibacterial activity. Gram-positive bacteria, S. aureus, and S. epidermidis, were more susceptible than gram-negative bacteria, P. aeruginosa and E. coli. The results correlate with the results of other reports.9, 10 The efficiency of CPE1 against each bacteria was different because of many factors, such as the structure of the bacterial envelope and the active compounds of the extract. Normally, hydrophobic compounds (phenols and tannins) are difficult to uptake the outer membrane of gram-negative bacteria, which is composed of phospholipids. Our data indicated that gram-positive bacteria (particularly S. epidermidis) were susceptible to CPE1, which contains phenolic compounds, more than gram-negative bacteria. These results corresponded to the results of those previously reported.9 TIC chromatograms of CPE1 illustrated quinic acid, malic acid, chlorogenic acid, and caffeine were the primary compounds present, which may indicate their effectiveness against the growth of microorganisms. A number of other reports suggest that phenolic acids, malic acid, tannin, caffeine, and hydroxycinnamic acid (particularly the hydroxyl groups on chlorogenic acid) are responsible for the antimicrobial activity.27, 28 A possible mechanism is disruption of cell membrane permeability by these compounds.27 Some studies suggested that caffeine and malanoidins respond to antibacterial activity against gram-negative bacteria.8, 29, 30 Furthermore, some authors propose that melanoidins inhibit the growth of bacteria through metal chelating mechanisms.31, 32 Consequently, crude extracts of CP, specifically CPE1, are effective as antibacterial agents due to the mixture of active compounds.
In conclusion, the nutritional value, polyphenol content, antioxidant activity, and chemical profiles of aqueous CPEs by LC-ESI-Q-TOF-MS—in three different processing preparations—were determined. CPE1 had the highest TPC and antioxidant activity. The antimicrobial activity of CPE1 was significant against many bacteria and showed that it is potential compound for further application. CPE1 is active against S. epidermidis and P. aeruginosa found as nosocomial pathogens. Inhibition of S. aureus and E. coli (responsible for food poisoning) indicated that CPE1 was also a promising extract to develop as a food additive or preservative for use in the food industry. For the comparison of chemical profiles, the three extracts showed slightly different nutrient compositions. Chlorogenic acid and caffeine were the most prominent in active aqueous extract (CPE1) and it contained numerous compounds, more than the others. Further purification and structure elucidation of marker compounds are still needed. Finally, the pulp preparation process is significant to its potency and potential viability. CP contains several ingredients which may have use in the food industry.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The present study was supported by the Industrial Research and Technology Capacity Development Program: UPSP-IRCT 57/005, University of Phayao, Thailand. I would like to thank Mr Noppadon Yosboonruang for guidance in the statistical analysis of the data.
References
- 1.Roussos S., de los Angeles Aquiáhuatl M., del Refugio Trejo-Hernández M., Gaime Perraud I., Favela E., Ramakrishna M. Biotechnological management of coffee pulp-isolation, screening, characterization, selection of caffeine-degrading fungi and natural microflora present in coffee pulp and husk. Appl Microbiol Biotechnol. 1995;32:756–762. [Google Scholar]
- 2.Murthy P.S., Manjunatha M.R., Sulochannama G., Naidu M.M. Extraction, characterization, and bioactivity of coffee anthocyanins. Eur J Biol Sci. 2012;4:13–19. [Google Scholar]
- 3.Marcel B.K.G., André K.B., Viviane Z.T., Séraphin K.C. Potential food waste and by-products of coffee in animal feed. Electron J Biol. 2011;7:74–80. [Google Scholar]
- 4.Arellano-González M.A., Amírez-Coronel A.R., Torres-Mancera T., Pérez-Morales G.G., Saucedo-Castañeda G. Antioxidant activity of fermented and nonfermented coffee (Coffea arabica) pulp extracts. Food Technol Biotechnol. 2011;49:374–378. [Google Scholar]
- 5.Ploypradub C., Cheamsuphakit B., Punbusayakul N. Antioxidant properties of different parts of arabica coffee berry and spent coffee ground. Agricultural Sci J. 2010;41:577–580. [Google Scholar]
- 6.Murthy P.S., Naidu M.M. Sustainable management of coffee industry by-products and value addition—a review. Resour Conserv Recy. 2012;66:45–58. [Google Scholar]
- 7.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida A.A.P., Farah A., Silva D.A., Nunan E.A., Glória M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem. 2006;54:8738–8743. doi: 10.1021/jf0617317. [DOI] [PubMed] [Google Scholar]
- 9.Runti G., Pacor S., Colomban S., Gennaro R., Navarini L., Scocchi M. Arabica coffee extract shows antibacterial activity against Staphylococcus epidermidis and Enterococcus faecalis and low toxicity towards a human cell line. LWT Food Sci Technol. 2015;62:108–114. [Google Scholar]
- 10.Monente C., Bravo J., Vitas A.I., Arbillaga L., De Peña M.P., Cid C. Coffee and spent coffee extracts protect against cell mutagens and inhibit growth of food-borne pathogen microorganisms. J Funct Foods. 2015;12:365–374. [Google Scholar]
- 11.Mazzafera P. Degradation of caffeine by microorganisms and potential use of decaffeinated coffee husk and pulp in animal feeding. Sci Agr. 2002;59:815–821. [Google Scholar]
- 12.Pandey A., Soccol C.R., Nigam P., Brand D., Mohan R., Roussos S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng J. 2000;6:153–162. doi: 10.1016/s1369-703x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 13.Association of Official Analytical Chemists (AOAC) 18th ed. AOAC; Washington DC: 2005. Official Methods of Analysis of the Association of Official Analytical Chemists. [Google Scholar]
- 14.Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J.P., Pihlaja K., Kujala T.S. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 15.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 16.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181 1199-1=200. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; second informational supplement. Document VET01-S2. Wayne, PA: CLSI; 2013.
- 18.Garrett R., Vaz B.G., Hovell A.M.C., Eberlin M.N., Rezende C.M. Arabica and robusta coffees: identification of major polar compounds and quantification of blends by direct-infusion electrospray ionization–mass spectrometry. J Agric Food Chem. 2012;60:4253–4258. doi: 10.1021/jf300388m. [DOI] [PubMed] [Google Scholar]
- 19.Perrone D., Donangelo C.M., Farah A. Fast simultaneous analysis of caffeine, trigonelline, nicotinic acid and sucrose in coffee by liquid chromatography–mass spectrometry. Food Chem. 2008;110:1030–1035. doi: 10.1016/j.foodchem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Bresciani L., Calani L., Bruni R., Brighenti F., Del Rio D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res Int. 2014;61:196–201. [Google Scholar]
- 21.Rameshkumar A., Sivasudha T., Jeyadevi R., Sangeetha B., Smilin Bell Aseervatham G., Maheshwari M. Profiling of phenolic compounds using UPLC-Q-TOF-MS/MS and nephroprotective activity of Indian green leafy vegetable Merremia emarginata (Burm. f.) Food Res Int. 2013;50:94–101. [Google Scholar]
- 22.Clifford M.N., Johnston K.L., Knight S., Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 23.Bravo L., Goya L., Lecumberri E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res Int. 2007;40:393–405. [Google Scholar]
- 24.Fang N., Yu S., Prior R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J Agric Food Chem. 2002;50:3579–3585. doi: 10.1021/jf0201327. [DOI] [PubMed] [Google Scholar]
- 25.Esquivel P., Jimenez V.M. Functional properties of coffee and coffee by-products. Food Res Int. 2012;46:488–495. [Google Scholar]
- 26.Roginsky V., Lissi E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005;92:235–254. [Google Scholar]
- 27.Lou Z., Wang H., Zhu S., Ma C., Wang Z. Antibacterial activity and mechanism of action of chlorogenic acid. J Food Sci. 2011;76:M398–M403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 28.Kabir F., Katayama S., Tanji N., Nakamura S. Antimicrobial effects of chlorogenic acid and related compounds. J Korean Soc Appl Biol Chem. 2014;57:359–365. [Google Scholar]
- 29.Wang H.-Y., Qian H., Yao W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011;128:573–584. [Google Scholar]
- 30.Almeida A., Naghetini C., Santos V., Antonio A., Farah A., Gloria M. Influence of natural coffee compounds, coffee extracts and increased levels of caffeine on the inhibition of Streptococcus mutans. Food Res Int. 2012;49:459–461. [Google Scholar]
- 31.Rufian-Henares J.A., de la Cueva S.P. Antimicrobial activity of coffee melanoidins-a study of their metal-chelating properties. J Agric Food Chem. 2009;57:432–438. doi: 10.1021/jf8027842. [DOI] [PubMed] [Google Scholar]
- 32.Stauder M., Papetti A., Mascherpa D., Schito A.M., Gazzani G., Pruzzo C. Antiadhesion and antibiofilm activities of high molecular weight coffee components against Streptococcus mutans. J Agric Food Chem. 2010;58:11662–11666. doi: 10.1021/jf1031839. [DOI] [PubMed] [Google Scholar]