Graphical abstract
Keywords: anovulation, amenorrhea, clomiphene citrate, estrus cycle
Abstract
Background
To investigate the effect of the combination of hydroalcoholic extract of Withania somnifera (WS) and Tribulus terrestris (TT) on letrozole induced polycystic ovarian syndrome (PCOS) in rat.
Methods
Twenty four female Wistar rats of regular estrus cycle were divided into four groups of six animals each. The negative control group received 1 mL of 0.5% carboxy methyl cellulose. The animals of the other groups were treated with letrozole (1 mg/kg) for 21 days for induction of PCOS. The animals of the positive control group were sacrificed on the 22nd day. In the test and standard groups, the treatment was started from the 22nd day and continued for a further 28 days. The test group was treated with hydroalcoholic extract of the combination of WS and TT (198 mg/kg) and the standard group with clomiphene citrate (1 mg/kg). Throughout the study, vaginal smears were collected daily from each animal for the determination of different phases of the estrus cycle. After completion of the treatment schedule all the animals of each group were sacrificed; analysis of hormones, total cholesterol, blood glucose, ovarian and uterine weight, and histopathological study of the ovary were carried out.
Results
The combination of the test drugs showed significant effects in normalizing the estrus cycle after being altered by letrozole. In the positive control group follicle-stimulating hormone level was decreased although luteinizing hormone, estradiole, and testosterone levels were increased (p < 0.05), however, after treatment the reverse effect was observed in the level of these hormones. Significant reduction in serum total cholesterol was also observed (p < 0.05). The test drugs decreased ovarian weight, and uterine weight was also returned to normalcy. Histopathology of the ovary showed almost normal ovary.
Conclusion
The above findings indicate the effectiveness of the combination of hydroalcoholic extract of WS and TT against letrozole induced polycystic ovarian syndrome in rat. This validates the usefulness of combination in PCOS and other related disorders as mentioned by Unani physicians.
1. Introduction
Polycystic ovarian syndrome (PCOS) is a heterogeneous disorder characterized by hyperandrogenism, anovulation, and obesity in women with enlarged polycystic ovaries.1 A total of 1–5 of women suffer from PCOS and the incidence appears to be on the increase due to changes in lifestyle and stress.2 The exact pathophysiology of PCOS are uncertain, evidence suggests that an excess of ovarian androgen production, either genetically or due to extraovarian factors such as hyperinsulinemia or disturbances of the hypothalamic–pituitary–ovarian axis is the main cause in the pathogenesis of PCOS.3, 4 Research over the past few decades has established that PCOS is an important metabolic disorder, associated with an increased risk of Type II diabetes mellitus (T2DM) as well as metabolic syndrome. Increased luteinizing hormone (LH) and insulin levels mainly amplify the intrinsic abnormality of their steroidogenesis. In PCOS, excess androgen activity may alter gonadotropin induced estrogen and progesterone synthesis in the follicles.5 Although various short term symptomatic therapies are available, the best long term management strategies have not been recognized. All of these factors, if taken together, make PCOS a challenging disorder to diagnose, treat, and study.6
Many scientific studies have proven the effect of Unani drugs in PCOS, such as Aloe barbadensis.5 Tephrosia purpurea7, Mimosa pudica, Withania somnifera Dunal. and Tribulus terrestris Linn., are important drugs of the Unani system of medicine described to possess emmenagogue, aphrodisiac, uterine tonic, general tonic, galactogogue, and galterative properties, and widely used as an ingredient in many of the Unani formulations such as Majoone muqawwie reham and Majoone zanjabeel which are used for the treatment of infertility, amenorrhea, dysfunctional uterine bleeding, and other associated disorders. Withania somnifera is useful in all inflammatory conditions of organs particularly in oophritis. It is given with other drugs in the post partum period. In infertile woman if the powder of this drug is used along with milk and sugars for 21 days it aids conception. Tribulus terrestris is recommended in painful menstruation and used in diseases of the uterus. Decoction of fruits is useful in regularizing menstruation. It is also useful in infertility.8
Many scientific studies have been carried out on these drugs. Withania somnifera possesses antistress, antioxidant, anticarcinogenic, antiaging, cardioprotective, hypothyroid, immunomodulatory, hypocholesterolemic, and hypolipedemic activities.9 The phytochemical investigations show several phytoestrogens such as phytosterol, saponins, phenols, and flavanoids.10 Tribulus terrestris possesses antiurolithiatic, aphrodisiac, central nervous system, stimulatory, and cardiotonic activities.11 A study was carried out on Tribulus terrestris extract for its effect on ovarian activity of immature Wistar rats. Tribulus terrestris extract induces corpus luteum formation and growth and therefore exhibited the onset of puberty with its LH like activity.12 Withania somnifera root supports overall functions of the endocrine system, whereas the aerial part and the fruit of Tribulus terrestris promotes regular ovulation, and may reduce ovarian cyst in women with PCOS.13 These actions have been ascribed to various phytoconstituents present in the drugs. There is no study undertaken to evaluate the efficacy of these two drugs in PCOS. Therefore, there is need for scientific validation of proclaimed efficacy on PCOS of these two drugs. Keeping all important factors and need for research to be easily available, promising, economical, and safe drugs the present study was designed to evaluate the effect of hydroalcoholic extract of Withania somnifera and Tribulus terrestris on letrozole induced PCOS in female Wistar rats.
2. Methods
2.1. Animals
The study was conducted on healthy female Wistar rats weighing 200–250 g. The animals were obtained from registered breeders and allowed to get acclimatized for 1 week. They were housed in clean and sterilized polypropylene cages at room temperature (25 ± 2 °C), humidity at 45–55% with 12-hour light and dark cycles. Animals were provided with standard diet and water ad libitum. The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines were followed for the animal care procedures and experimental protocol. The study was started after obtaining the ethical clearance by the Institutional Animal Ethics Committee (IAEC) of National Institute of Unani Medicine (NIUM), Bengaluru (Reg. no. IAEC/11/07/IA dated 19/11/2014).
2.2. Chemicals and reagents
Analytical grade chemicals and reagents were used for the study. Letrozole tablet was procured from Sun Pharmaceutical Industry Limited, Bengaluru, India. Clomiphene citrate (CC) tablet from SVIZERA Company, Mumbai, India and carboxy methyl cellulose (CMC) sodium salt, manufactured by HIMEDA were procured from the market of Bengaluru. Formaldehyde solution was obtained from Multilab, Bengaluru and Methylene blue from Central Drug House Private Limited. Total cholesterol kit (CHOD-POD method) was obtained from EURO (Chennai, India) diagnostic system and blood glucose monitoring system (Gluco Check Company-MT Promedt Consulting GmbH, Germany) were obtained from Bengaluru (India).
2.3. Plant material
The roots of Withania somnifera (WS) and fruits of Tribulus terrestris (TT) were collected from the pharmacy at NIUM, Bengaluru (India). The drugs were identified by the Institute of Trans Disciplinary Health Science and Technology, Yelahanka, Bengaluru (FRLHT Account number is 3467 of TT and 3468 of WS). The voucher specimens were deposited in the Drug Museum/Herbarium of NIUM, Bengaluru for future reference (29/IA/Res/2015). The test drugs were cleaned from impurities and coarse powder was made with electrical grinder, 100 g of which was used for extraction in (50% distilled water and 50% ethanol) solvent for approximately 6 hours by using Soxhlet apparatus (Borosil glass works Ltd. Gujrat, India) at 60 °C. The liquid extract was cooled and filtered by filter paper (Whatman no. 40) and then evaporated on a water bath (60 °C) until it dried completely.14 The yield percentage of hydroalcoholic extract was found to be 17% for WS and 11.92% w/w for TT.
2.4. Dosage and administration
The human therapeutic dose of Withania somnifera and Tribulus terrestris are 5 g and 7 g, respectively as mentioned in the Unani literature.8 The dose for rat was calculated by dividing it by adult human weight of 60 kg and multiplying it by factor 7 to accommodate the body surface area of the animal.15 The dose of the crude drug was found to be 585 mg/kg for WS and 820 mg/kg for TT. The dose of the extract was determined with reference to the yield % of extract with the dose of crude drug and calculated as 100 mg/kg for WS and 98 mg/kg for TT. The combination of extract was 198 mg/kg which was less than the safe dose of 2 g/kg as indicated by acute toxicity study carried out by Prabu et al16 for WS and Hemlatha and Hari17 for TT. The suspensions of extract and clomiphene citrate (CC) were freshly prepared (in 1 mL of 0.5% CMC) daily before each administration.
2.5. Letrozole induced polycystic ovarian syndrome
The method of Sasikala and Shamila18 and Jadhav et al19 was followed with slight modification in the treatment schedule. First the animals were weighed, checked for two consecutive normal estrus cycles by vaginal smear examination, microscopically,20 and divided in to four groups of six animals each. Group I served as negative control and received 1 mL of 0.5% CMC. The animals of Groups II–IV were treated with letrozole (1 mg/kg dissolved in 0.5% CMC) for 21 days for induction of PCOS. The animals of Group II served as positive control. From the 22nd day the animals of Group III were treated with hydroalcoholic extract of the combination of WS and TT in the dose of 198 mg/kg and served as the test group, whereas the animals of Group IV were treated with CC in the dose of 1 mg/kg and served as standard control.21 The hydroalcoholic extract and standard drugs were administered daily orally and continued for a further 28 days. Throughout the study, vaginal smears were collected daily and evaluated microscopically for the determination of various phases of estrus cycle. The animals of the positive control group were sacrificed after 21 days treatment of letrozole whereas the animals of the other groups were sacrificed after 24 hours of the last dose of treatment with test and standard drugs and after 18 hours of fasting.
2.6. Vaginal smear observation
During the whole period of study, every morning between 9.00 AM to 10.00 am, vaginal secretions were collected with a plastic pipette by inserting the tip in to the rat vagina, filled with 10 μL of normal saline. One drop of collected vaginal fluid was placed on glass slides. A separate glass slide and pipette tips were used for each animal. Collected vaginal fluid was fixed by placing the slides on a slide warming table and stained with methylene blue (aqueous) staining solution. After staining, slides were washed, dried, and observed through a light microscope (40×). Three types of cells were recognized: round and nucleated cells were epithelial cells; irregular cells without a nucleus were the cornified cells; and the small round cells were the leucocytes, their mutual proportion was used for the determination of different phases of the estrus cycle.20
2.7. Serum analysis
First the animals were tested for blood glucose by glucometer; thereafter they were sacrificed under theopentone anesthesia (50 mg/kg ip). Blood samples were collected by cardiac puncture and serum was separated by centrifugation at 3,000 rpm for 15 minutes.19 Total serum cholesterol was analyzed by Total Cholesterol kit using auto analyzer–Rapid diagnostic Pvt. Ltd. Mumbai, India (Star plus) Plasma testosterone, estradiole, follicle-stimulating hormone (FSH), and LH were assayed by electrochemiluminescence immunoassay. After collection of blood; ovaries and uterus were dissected out, and weighed on an electronic balance. One ovary from one animal of each group was preserved in 10% formalin for histopathological study.20
2.8. Phytochemical analysis
The hydroalcoholic extracts of WS and TT were subjected to preliminary screening of phytochemicals such as alkaloids, flavonoids, phenols, tannins, saponins, sterols, and glycosides.21
2.9. Statistical analysis
The results of different groups were analyzed by one way Analysis of variance space repeated measure with Tukey–Kramer multiple pair comparison test and Dunn comparison test. Statistical difference was considered significant at p < 0.05. Data were expressed as mean ± standard error of mean (SEM).
3. Results
3.1. Effect of combination of hydroalcoholic extract of Withania somnifera and Tribulus terrestris on duration of different phases of estrus cycle
The number of days spent in proestrus and metestrus phase by rats of the positive control group treated with letrozole were significantly (p < 0.001) reduced from that of the number of days spent by the rats of the negative control group treated with vehicle only in proestrus and in metestrus phases. Rats of the test group showed a significant (p < 0.01) increase in the number of days spent in estrus phase in comparison to the positive control. Duration of the diestrus phase in rats of the test group showed significant reduction (p < 0.01) when compared with the positive control group (Table 1).
Table 1.
Effect of Withania somnifera Dunal and Tribulus terrestris Linn on duration of different phases of estrus cycle in letrozole induced polycystic ovarian syndrome
| Groups | Treatment | Proestrus (d ± SEM) | Estrus (d ± SEM) | Metestrus (d ± SEM) | Diestrus (d ± SEM) |
|---|---|---|---|---|---|
| Negative control | 1 mL of 0.5% CMC | 4 ± 0.00 | 4.16 ± 0.16 | 3.83 ± 0.16 | 8.83 ± 0.16 |
| Positive control | Letrozole 1 mg/kg | 1.66 ± 0.21*,† | 1.33 ± 0.21*,† | 2 ± 0.00*,† | 16.00 ± 0.25*,† |
| Test group | Letrozole 1 mg/kg + WS + TT 198 mg/kg | 3 ± 0.00 | 3 ± 0.00‡,§ | 3 ± 0.00 | 11.83 ± 0.16‡,§ |
| Standard group | Letrozole 1 mg/kg + CC 1 mg/kg | 3 ± 0.00 | 2.8 ± 0.16‡,‖ | 3.16 ± 0.16‡,‖ | 11 ± 0.25‡,† |
Analyzed by Kruskal–Wallis test with Dunn's compare all pair of columns.
Data are expressed as mean ± SEM. (N = 6 animals in each group).
With respect to negative control.
p < 0.001.
With respect to positive control.
p < 0.01.
p < 0.05.
CC, clomiphene citrate; CMC, carboxy methyl cellulose; SEM, standard error of mean; TT, Tribulus terrestris; WS, Withania somnifera.
3.2. Effect of combination of hydroalcoholic extract of Withania somnifera and Tribulus terrestris on hormonal levels
In the positive control group treated with letrozole only, FSH level was decreased whereas LH, estradiole, and testosterone levels were increased (p < 0.05), however, after treatment with combinations of hydroalcoholic extract of Withania somnifera and Tribulus terrestris, the reverse effect was observed as FSH level was increased although LH, estradiole, and testosterone level were found to be decreased, it was not statistically significant (Table 2).
Table 2.
Effect of Withania somnifera Dunal and Tribulus terrestris Linn on hormonal levels in letrozole induced polycystic ovarian syndrome
| Groups | Treatment | FSH mIU/mL |
LH mIU/mL |
Estradiole pg/mL |
Testosterone g/dL |
|---|---|---|---|---|---|
| Negative control | 1 mL of 0.5% CMC | 16.49 ± 4.07 | 18.28 ± 2.43 | 28.47 ± 8.33 | 30.1 ± 6.21 |
| Positive control | Letrozole 1 mg/kg | 9.4 ± 1.43 | 26.76 ± 3.48 | 58.816 ± 11.20*,† | 63.16 ± 10.45*,† |
| Test group | Letrozole 1 mg/kg + WS + TT 198 mg/kg | 12.87 ± 0.72 | 17.3 ± 2.36 | 41.2 ± 4.7 | 36.98 ± 4.97 |
| Standard group | Letrozole 1 mg/kg + CC 1 mg/kg | 14.67 ± 3.00 | 19.41 ± 2.67 | 34.63 ± 3.67 | 33.23 ± 5.05†,‡ |
Analyzed by Kruskal–Wallis test with Dunn's compare all pair of columns.
Data are expressed as mean ± SEM. (N = 6 animals in each group).
With respect to negative control.
p < 0.05.
With respect to positive control.
CC, clomiphene citrate; CMC, carboxy methyl cellulose; FSH, follicle-stimulating hormone; LH, luteinizing hormone; SEM, standard error of mean; TT, Tribulus terrestris; WS, Withania somnifera.
3.3. Effect of combination of hydroalcoholic extract of Withania somnifera and Tribulus terrestris on serum total cholesterol and blood glucose
The mean value of serum total cholesterol was increased significantly (p < 0.001) in the positive control group in comparison to the negative control group. In the test group after treatment with hydroalcoholic extract of Withania somnifera and Tribulus terrestris a significant reduction (p < 0.01) in cholesterol level was observed in comparison to the positive control group (Table 3).
Table 3.
Effect of Withania somnifera Dunal and Tribulus terrestris Linn on serum cholesterol and blood glucose level in letrozole induced polycystic ovarian syndrome
| Groups | Treatment | Total cholesterol |
Blood glucose |
|---|---|---|---|
| 42nd d (mg/dL) | (mg/dL) | ||
| Negative control | 1 mL of 0.5% CMC | 51.50 ± 3.19 | 67.5 ± 3.92 |
| Positive control | Letrozole 1 mg/kg | 82.64 ± 9.01*,† | 87.33 ± 3.15 |
| Test group | Letrozole1 mg/kg + WS + TT 198 mg/kg | 55 ± 3.15‡,§ | 64.16 ± 7.10 |
| Standard group | Letrozole 1 mg/kg + CC 1 mg/kg | 50.56 ± 4.21‡,§ | 63 ± 3.6 |
Analyzed by Kruskal–Wallis test with Dunn's compare all pair of columns.
Data are expressed as mean ± SEM. (N = 6 animals in each group).
With respect to negative control.
p < 0.001.
With respect to positive control.
p < 0.01.
CC, clomiphene citrate; CMC, carboxy methyl cellulose; SEM, standard error of mean; TT, Tribulus terrestris, WS, Withania somnifera.
3.4. Effect of combination of hydroalcoholic extract of Withania somnifera and Tribulus terrestris on weight of ovary and uterus
A significant increase in mean ovarian weight (p < 0.001) and decrease in mean uterine weight (p < 0.01) in the animals of the positive control group was observed in comparison to the negative control group. The test drugs decreased ovarian weight and uterine weight were also returned to normalcy but it was not statistically significant (Table 4).
Table 4.
Effect of Withania somnifera Dunal and Tribulus terrestris Linn on weight of ovary and uterus in letrozole induced polycystic ovarian syndrome
| Groups | Treatment | Weight of ovary |
Weight of uterus |
|---|---|---|---|
| (mg/100 g of BW) | (mg/100 g of BW) | ||
| Negative control | 1 mL of 0.5%CMC | 20.74 ± 1.33 | 132.51 ± 7.96 |
| Positive control | Letrozole 1 mg/kg | 33.72 ± 0.49*,† | 80.60 ± 5.79*,‡ |
| Test group | Letrozole1 mg/kg + WS + TT 198 mg/kg | 29.62 ± 1.61 | 101.69 ± 8.23 |
| Standard group | Letrozole 1 mg/kg + CC 1 mg/kg | 24.22 ± 1.05§,† | 82.10 ± 4.55 |
Analyzed by Kruskal–Wallis test with Dunn's compare all pair of columns.
Data are expressed as mean ± SEM. (N = 6 animals in each group).
With respect to negative control.
p < 0.001.
p < 0.01.
With respect to positive control.
BW, body weight; CC, clomiphene citrate; CMC, carboxy methyl cellulose; SEM, standard error of mean; TT, Tribulus terrestris, WS, Withania somnifera.
3.5. Effect of combination of hydroalcoholic extract of Withania somnifera and Tribulus terrestris histology of ovaries
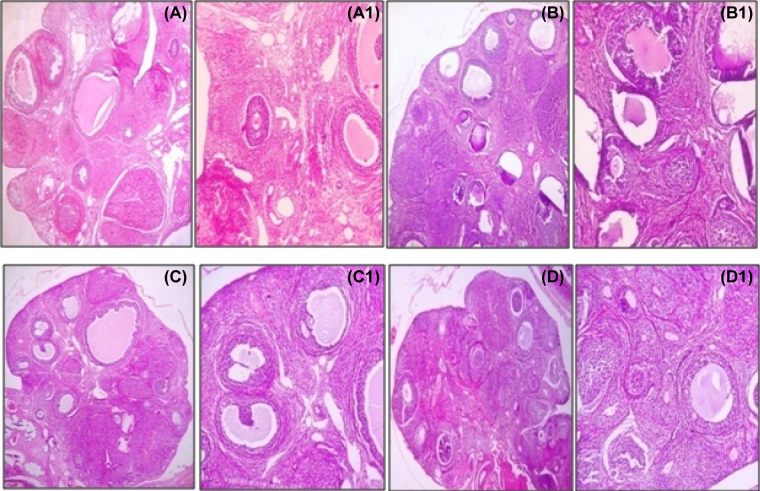
Light microscopic images (50× and 100×) of ovary sections from the negative control group (Fig. 1A and A1) showed congested vascular spaces with spindle shaped cells in the medulla. The cortex showed primary and secondary follicles with aggregation of granulosa cells and scant follicular antrum few follicles showed intact oocyte, the corpus luteum contains uniform round cells with abundant eosinophilic cytoplasm. In the positive control group (Fig. 1B and B1) the medulla and cortex showed multiple follicular cysts of varying sizes with diminished granulosa cells and increased follicular antrum, some atretic follicles and few follicles contain degenerated oocytes. The corpus luteum also showed atrophic changes. After treatment with a combination of hydroalcoholic extract of Withania somnifera and Tribulus terrestris the histopathological changes reached up to the normal which was almost similar to findings with negative control (Figs. 1C, C1, D, and D1).
Fig. 1.
Hematoxylin and Eosin stained microscopic images of ovary. (A and A1) Negative control group showed congested vascular spaces with spindle shaped cells in medulla. The cortex showed primary and secondary follicles with aggregation of granulosa cells and scant follicular antrum few follicles showed intact oocyte, the corpus luteum contains uniform round cells with abundant eosinophilic cytoplasm. (B and B1) In positive control the medulla and cortex showed multiple follicular cysts of varying sizes with diminished granulosa cells and increased follicular antrum, some atretic follicles and few follicles contain degenerated oocyte. The corpus luteum showed atrophic changes. (C, C1, D, and D1) After treatment the histopathological changes reached up to the normal which was almost similar to findings with negative control.
3.6. Phytochemical analysis
Phytochemical analysis showed that alkaloids, saponins, phenols, flavonoids, and glycosides were present in hydroalcoholic extracts of both drugs. However, Tribulus terrestris showed positive result for tannins also (Table 5).
Table 5.
Phytochemical analysis of hydroalcoholic extracts of Withania somnifera and Tribulus terrestris
| Constituents | Withania somnifera | Tribulus terrestris |
|---|---|---|
| Alkaloids | + | + |
| Saponin | + | + |
| Phenols | + | + |
| Flavonoids | + | + |
| Glycosides | + | + |
| Tannins | − | + |
4. Discussion
Throughout the estrus cycle a lot of changes occur in the ovaries.22 Duration of normal estrus cycle of rat is 4–5 days showing four phases in sequential order: proestrus, estrus, metestrus, and diestrus. All letrozole treated rats were entirely acyclic, as the number of days spent in the proestrus, estrus, and metestrus phase by rats of positive control were significantly (p < 0.001) reduced whereas the diestrus phase very significantly (p < 0.001) increased when compared with the negative control, which demonstrated irregular estrus cyclicity, as reflected by the presence of leucocytes which were predominant in their vaginal smear. Administration of hydroalcoholic extract of WS and TT for 28 days showed significant effect in normalizing the estrus cycle after being altered by letrozole which was confirmed after comparing the estrus cycle of different groups.
Letrozole induces PCOS in animals by blocking the conversion of androgens to estrogens.23 The inhibition of aromatase activity leads to increase of ovarian androgens which, in turn, leads to hyperandrogenism, a hallmark of PCOS.19, 24, 25, 26 Excess androgen feedback to the pituitary gland results in excessive LH and depressed FSH secretion. Rising estrogen levels have a negative feedback effect on FSH secretion. Anovulation is associated with an elevated estrogen level. Amongst disorders of ovulation, PCOS is unique as it is associated with normal or elevated estrogen levels.27 Therefore, letrozole in the present study might have inhibited aromatase activity and consequently conversion of androgens (notably testosterone) to estrogens with the clinical manifestation of the elevated levels of testosterone, increased levels of LH, and decreased level of FSH. This hormonal imbalance created by letrozole resulted in irregular and/or prolonged estrus cycle. The same findings were observed in other studies.5, 18, 19, 23, 24, 25
The hydroalcoholic extract of combination of WS and TT are able to normalize the hormonal level. In the positive control group FSH level was decreased whereas LH, estradiole and testosterone levels were increased but after treatment with the test drugs the reverse effect was observed though it was not statistically significant. PCOS patients are often hyperandrogenemia and associated with alterations in lipids and lipoprotein levels resulting in dislipidemia.5, 18, 19 Similar effects were observed in PCOS induced rats in the present study. Cholesterol levels were also found to be increased (p < 0.001) in PCOS induced rats which decreased significantly (p < 0.01) after treatment with test and standard drugs. The animals were also investigated for blood glucose. However, we found that letrozole does not affect insulin sensitivity. The same finding has been reported by other researchers.
Ovarian weight in PCOS-induced rats was increased (p < 0.001) which is in accordance with the previous findings.18, 19 The treatment with the test drugs prevented further increase in ovarian weight. The uterine weight was decreased (p < 0.01) in PCOS induced rats. This corresponds with the earlier findings,19 however, it returned to normalcy when rats were treated with the test combination.
The results of hormonal analysis were also supported by histopathological findings of ovaries as histopathological changes reached up to the normal level which was almost similar to findings with negative control. The result clearly demonstrated the ability of the test drugs to dissolve the cyst and normalize the function of the ovaries as the cortex showed primary and secondary follicles with aggregation of granulosa cells and scanty follicular antrum in treated groups.
The effect of the combination of WS and TT were compared with clomiphene citrate an orally active nonsteroidal agent. It binds to both estrogen receptor α (ERα) and estrogen receptor β (ERβ), and acts as a pure estrogen antagonist by stimulating Gonadotropin releasing hormone secretion.28
The phytochemical investigations of WS and TT demonstrated the presence of phytoestrogens such as phytosterol, saponins, phenols, and flavanoids. In Tribulus terrestris some saponins on hydrolysis give rise to steroidal sapogenin such as diosenin, gitogenin, chlorogenin, and ruscogenin, and an essential oil.10 Phytoestrogens have been shown to bind to two types of estrogen receptors ERα and ERβ.29 The mechanism of action of the test drugs due to presence of phytoestrogen in it is thought to be similar to the standard drug clomiphene citrate in normalization of the hormonal level and induction of ovulation. Further efficacy of antioxidant drugs in PCOS has also been noted in vitro.30 Interestingly, both the drugs possess antioxidant activity.9, 11 Withania somnifera supports overall functions of endocrine system. The aerial part and fruit of Tribulus terrestris promote regular ovulation and may reduce ovarian cyst in women with PCOS.13
According to Unani literature, altered temperament (sue mizaje barid) of the uterus and ovaries is one of the main causes of amenorrhea and obesity which disrupts the normal function of ovaries leading to oligoovulation or an ovulation. WS and TT are hot in the first and third degree respectively8 suggesting that the drugs possess such a degree of hotness which suits induction of response in amenorrhea and infertility. On treatment, the histopathological changes reached up to the normal level, which clearly indicates that the test combination stimulates the ovarian function as cortex shows primary and secondary follicles with aggregation of granulosa cells. The result clearly demonstrated that the test combination due to their hot temperament dissolves the cyst. Therefore, it can be concluded that the test combination normalizes the ovarian functions by alteration of temperament of ovaries. TT has already been reported for its effect in formation of corpus luteum when administered to immature rats for 21 days. Moreover TT has also been studied in a very low dose (5 mg/kg and 10 mg/kg) in alternative treatment of ovarian cysts induced by estradiol valerate in rat,12 which further justified the efficacy of the test drugs.
Amenorrhea is the prevalent feature of the disease. The test combination has been described to possess emmenaogogue properties useful in improving the amenorrhea. Test drugs also have lenitive and resolvent properties, with these inherited properties may liquefy the viscous matter and resolve the matter forming cysts, thus correcting and normalizing the ovarian functions.31, 32, 33, 34 Therefore, the mechanism proposed by the Unani physicians appears to be comprehensive and very much in commensuration of the modern approaches of treatment.
On account of the results and discussion it can be concluded that the hydroalcoholic extract of combination WS and TT exhibited significant recovery of FSH, LH, estradiole, and testosterone levels in serum. The hydroalcoholic extract demonstrated significant antiandrogenic effects by reducing increased testosterone level and preventing ovarian dysfunction in rats. This property of the test drugs may be due to the presence of phytoestrogens in hydroalcoholic extract of the test combination. Further studies are required to explain the exact mechanism of action of the test drug combinations.
Conflicts of interest
The authors have conflicts of interest to declare.
Acknowledgments
The authors are thankful to Professor Abdul Wadud, HoD Ilmul Advia, (Pharmacology), National Institute of Unani Medicine, Bengaluru, India, for providing essential facilities for smooth proceeding of the research work and Dr. G. Sofi Reader, Department of Ilmul Advia, NIUM for his input in statistical analysis.
References
- 1.Copeland L.J., Jarrell J.F., McGregor J.A. WB Saunders Company; Philadelphia: 1993. Textbook of gynaecology; pp. 426–427. [Google Scholar]
- 2.Padubidri V.J., Daftary S.N., Howkins Bourne . 14th ed. Elsevier India Private Limited; 2008. Howkins & Bourne Shaws textbook of gynecology; p. 331. [Google Scholar]
- 3.Archer J.S., Chang R.J. Hirsutism and acne in polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004;18:737–754. doi: 10.1016/j.bpobgyn.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Goodarzi M.O., Azziz R. Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2006;20:193–205. doi: 10.1016/j.beem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Maharajan R., Nagar P.S., Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med. 2010:273–279. doi: 10.4103/0975-9476.74090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieber E.J., Sanfilippo J.S., Horowitz I.R. Churchill Livingstone; Philadelphia: 2006. Clinical gynecology; p. 893. [Google Scholar]
- 7.Jitendra P.A., Pravin T.A. Prospective use of Tephrosia purpurea in remedial treatment of PCOS, study in Wistar rat. ISCA J Biological Sci. 2012;1:1–6. [Google Scholar]
- 8.Gani N. Idara Kitabus Shifa; New Delhi: 2011. Khazaienul Advia. 258, 761, 869, 955, 1271, 367, 1260, 230-231, 1156-8. [Google Scholar]
- 9.Jain R., Kachhwaha S., Kothari L. Phytochemistry, pharmacology, and biotechnology of Withania somnifera and Withania coagulans: a review. J Med Plants Res. 2012;6:5388–5399. [Google Scholar]
- 10.The Wealth of India, Vol. II, New Delhi, National Institute of Science Cummunication and resources; 2003:283-284,581-585.
- 11.Jamil M., Akhtar A.J., Abuzar A., Javed A.A.M., Ennus Pharmacological scientific evidence for the promise of Tribulus terrestris. IRJP. 2012;3:403–406. [Google Scholar]
- 12.Esfandiari A., Dehghan A., Sharifi S., Najafi B., Vesali E. Effect of Tribulus terrestris extract on ovarian activity in immature Wistar rat: a histological evaluation. JAVA. 2011;10:883–886. [Google Scholar]
- 13.Barton D, Doula CH. How to use fertility herbs to enhance your fertility naturally. Available from http://E;/pcod/fertility Herbs Infertility Treatment Pregnancy Herbs.htm. Accessed October 1, 2013.
- 14.Ahmed A., Wadud A., Jahan N., Bilal A., Hajera S. Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol. 2013;146:411–416. doi: 10.1016/j.jep.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich E.J., Gehan E.A., Rall D.P., Schmidt Skipper H.E. Quantitative comparison of toxicity of anticancer agent in mouse, rat, dog, monkey and man. Cancer Chemotherapy Rep. 1968;50:219–244. [PubMed] [Google Scholar]
- 16.Prabu P.C., Panchapakesan S., Raj C.D. Acute and subacute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera Rrots in Wistar rats. Phytother Res. 2012;27:1169–1178. doi: 10.1002/ptr.4854. [DOI] [PubMed] [Google Scholar]
- 17.Hemlatha S., Hari R. Acute and subacute toxicity studies of the saponin rich butenol extracts of Tribulus terrestris fruits in Wistar rats. Int J Pharm Sci Rev Res. 2014;27:307–313. [Google Scholar]
- 18.Sasikala S.L., Shamila S. A novel Ayurvedic medicine Asokarishtam in the treatment of letrozole induced PCOS in rat. J Cell Tissue Res. 2009:1903–1904. [Google Scholar]
- 19.Jadhav M., Menon S., Shailajan S. Antiandrogenic effect of Symplocos racemosa Roxb. against letrozole induced polycystic ovary using rat model. J Coast Life Med. 2013;1:309–314. [Google Scholar]
- 20.Bilal A., Jahan N., Ahmed A., Bilal S., Habib S. Antifertility activity of hydro alcoholic extract of Ocimum basilicum Linn leaves on female Wistar rats. J Reprod Contraception. 2013;24:45–54. [Google Scholar]
- 21.Tabarak I.M.H., Ahmed G., Jahan N., Adeeba M. Physicochemical standardization of Tukhme karafs (seeds of Apium graveolens Linn.) IJCP. 2013;06:1–6. [Google Scholar]
- 22.Prakash A.O., Mathur R. Studies on estrus cycle of albino rats: response to Embelia ribes extracts. Planta Medica. 1979;36:131–141. doi: 10.1055/s-0028-1097253. [DOI] [PubMed] [Google Scholar]
- 23.Kafali H., Iriadam M., Ozardali I., Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35:103–108. doi: 10.1016/j.arcmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Yakubu M.T., Ibiyo B.O. Effects of aqueous extract of Cnestis ferruginea (Vahl ex DC) root on the biochemical and clinical parameters of anastrozole-induced polycystic ovarian syndrome rat model. J Endocrinol Reprod. 2013;17:99–112. [Google Scholar]
- 25.Baravalle C., Salvetti N.R., Mira G.A., Pezzone N., Hugo H., Ortega H.H. Microscopic characterization of follicular structures in letrozole-induced polycystic ovarian syndrome in the rat. Arch Med Res. 2006;37:830–839. doi: 10.1016/j.arcmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Maliqueo M. Continuous administration of a P450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. endocrinology. 2013;154:434–445. doi: 10.1210/en.2012-1693. [DOI] [PubMed] [Google Scholar]
- 27.Desai P., Malhotra N., Shah D. 3rd ed. Jaypee Brothers Medical Publishers Private Limited; New Delhi: 2008. Principles and practice of obstetrics and gynecology for postgraduates; pp. 676–683. [Google Scholar]
- 28.Tripathi K.D. 6th ed. Jaypee Brothers Publishers; New Delhi: 2010. Essentials of medical pharmacology; pp. 303–305. [Google Scholar]
- 29.Ososki A.L., Kennelly E.J. Phytoestrogens: a review of the present state of research. Phytother Res. 2003;17:845–869. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- 30.Khan N.R., Victorin E.S. Review the physiological basis of complementary and alternative medicines for polycystic ovary syndrome. AJP-Endo. 2011;301:E1–E10. doi: 10.1152/ajpendo.00667.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majoosi ABA. Kamilus Sanaa (Urdu trans. by Kantoori GH). Vol I and II. New Delhi: Idarae Kitabul Shifa; 2010, Vol I 533-34. Vol II, 128, 110-112.
- 32.Ibn Rushd. Kitabul Kulliyat. 2nd ed. New Delhi: CCRUM; 1987, p. 56, 114, 116, 226, 299.
- 33.Khan A. 1st ed. Madina Publishing Company; Karachi: 1983. Haziq; pp. 467–471. [Google Scholar]
- 34.Razi A.B.Z. Vol IX. CCRUM; New Delhi: 2001. pp. 151–168. (Al Hawi Fil Tib). [Google Scholar]