Abstract
Skeletal muscle mitochondrial content and function are regulated by a number of specialized molecular pathways that remain to be fully defined. Although a number of proteins have been identified to be important for the maintenance of mitochondria in quiescent muscle, the requirement for these appears to decrease with the activation of multiple overlapping signaling events that are triggered by exercise. This makes exercise a valuable therapeutic tool for the treatment of mitochondrially based metabolic disorders. In this review, we summarize some of the traditional and more recently appreciated pathways that are involved in mitochondrial biogenesis in muscle, particularly during exercise.
Keywords: mitochondrial protein import, PGC-1alpha, skeletal muscle, unfolded protein response
1. Introduction
One of the most prominent adaptations within skeletal muscle is the change in mitochondrial content that occurs in response to chronic exercise, or as a result of muscle disuse. In the first instance, mitochondrial content can increase by 50–100% depending on the exercise program employed, thereby supplying the muscle with an enhanced capacity for energy provision to match the repeated energy demands of contractile activity. In the second case, the reduced energy requirements of muscle inactivity are met by a loss of mitochondrial content, to levels that may be 50–75% of normal, depending on the duration of the disuse. While this muscle malleability in response to activity/inactivity has been recognized for many years, the underlying molecular basis for how this occurs remains to be fully explained, and it is a very active field of research. The reason for this is that mitochondrial content, morphology, and function in muscle are important determinants of the efficiency of muscle metabolism, as well as muscle mass. Thus, identification of the signaling pathways and transcription factors involved in maintaining muscle mitochondria can have important therapeutic implications as pharmaceutical or nutritional targets, in addition to the use of exercise itself.
Many studies have employed gene knockout (KO) or knockdown models to investigate the importance of the gene product for the normal adaptive mitochondrial response to exercise. In many cases, KO of a selected gene leading to a protein defect results in diminished mitochondrial content and function within muscle under basal conditions,1, 2, 3, 4 thereby implicating the protein in the maintenance of mitochondria within muscle. However, the absence of these proteins does not, in large measure, prevent exercise from rescuing the situation. Exercise training has repeatedly been shown to restore mitochondria within muscle, despite the absence of “key” regulatory proteins. This information strongly suggests the existence of multiple proteins and signaling pathways that can be activated within muscle to provide protection against the inadvertent loss of normal signals directed toward mitochondrial biogenesis. In this review, we summarize some recent information on traditional, as well as alternative pathways that can be considered potentially important for mitochondrial biogenesis in the response of skeletal muscle to exercise (Fig. 1).
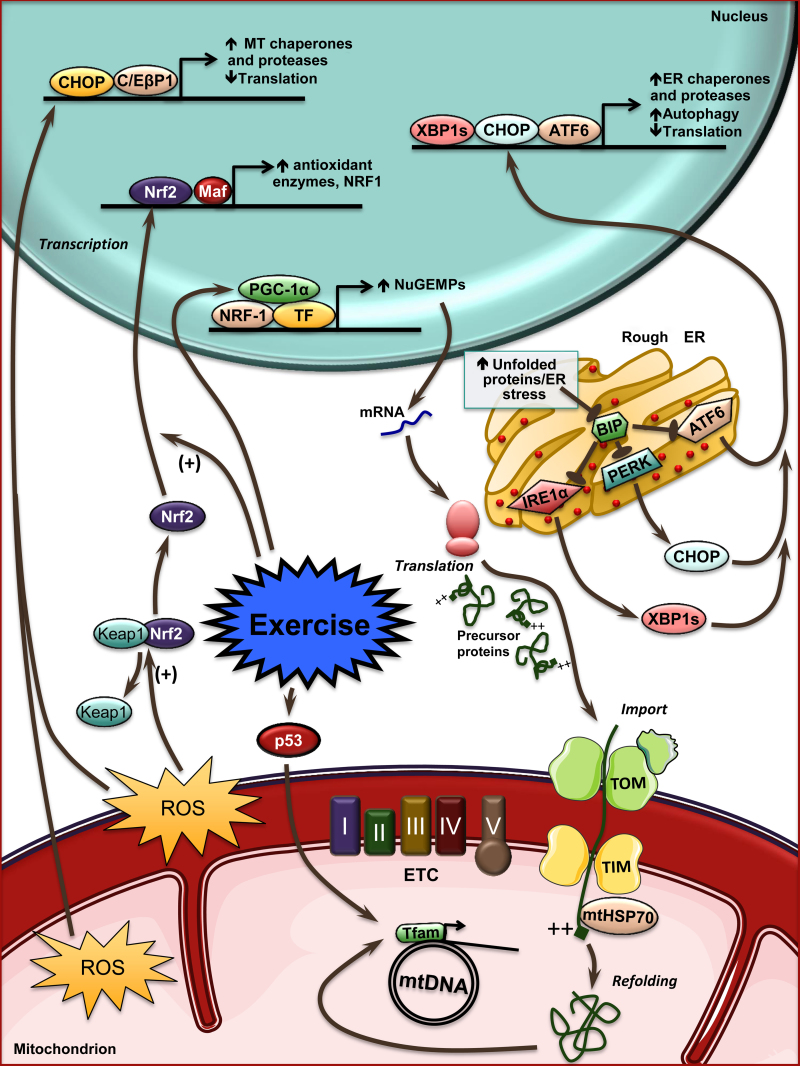
Fig. 1.
Gene expression pathways induced by acute exercise. Acute exercise generates intracellular signals that promote translocation of regulatory proteins such as PGC-1α to the nucleus. This leads to increases in the transcription of nuclear genes encoding mitochondrial proteins. The mRNAs generated are translated into proteins and then imported to the mitochondria by protein import machinery. Among these is TFAM, which enters the mitochondria, and when refolded it is able to act on mitochondrial DNA to induce its transcription. Exercise also promotes translocation of p53 to the mitochondria, to increase mtDNA transcription. However, with accumulation of proteins undergoing translation, there is a greater risk for misfolded proteins, prompting stress, accompanied by an increase in ROS. This increased stress releases BiP's inhibition of ATF6, PERK, and IRE1α, activating the UPRER. Increases in the transcriptional activation of XBP1, CHOP, and ATF6 upregulate the expression of ER chaperones and proteases, increasing autophagy and decreasing translation. In addition to misfolded proteins accumulating in the cytosol, this also occurs in the mitochondria, activating a separate UPR (UPRmt). This triggers an increase in ROS to induce the transcriptional activity of CHOP and C/EβP1, thus further reducing translation and increasing the expression of mitochondria-specific chaperones and proteases. An increase in oxidative stress with exercise releases Keap1 inhibition of Nrf2. This, along with exercise, allows for the translocation of Nrf2 into the nucleus to coactivate the transcription of several antioxidant enzymes to reduce the levels of ROS, as well as increase the transcription of Nrf-1, which contributes to mitochondrial biogenesis.
ATF6, activating transcription factor 6; ER, endoplasmic reticulum; ETC, electron transport chain; Keap1, Kelch-like ECH associating protein 1; Mt, mitochondrial; mtDNA, mitochondrial DNA; mtHSP70, mitochondrial-type heat shock protein 70; Nrf-1, nuclear respiratory factor 1; Nrf2, nuclear factor erythroid 2-related factor 2; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; ROS, reactive oxygen species; TF, transcription factor; TFAM, mitochondrial transcription factor A; TIM, translocases of the inner membrane; TOM, translocases of the outer membrane; UPR, unfolded protein response; UPRER, unfolded protein response within the endoplasmic reticulum; UPRmt, unfolded protein response in the mitochondria.
2. Role of PGC-1α
The process of mitochondrial biogenesis is controlled by the coordinated transcription of nuclear as well as mitochondrial genes, regulated in large measure by the coactivator peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), its family members, and its isoforms. PGC-1α is involved in many metabolic processes, including liver gluconeogenesis, thermogenesis, and fiber-type specialization in skeletal muscle. It is often termed the “master regulator” of mitochondrial biogenesis, and it possesses the ability to mediate many exercise-induced adaptations in skeletal muscle.5 PGC-1α lacks the ability to bind DNA directly, but it acts by interacting with transcription factors such as nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2, respectively), and enhancing their activity, leading to the transcription of nuclear-encoded gene products involved in mitochondrial biogenesis.6 These include subunits of protein complexes in the electron transport chain and the factors involved in their assembly, mitochondrial DNA (mtDNA) transcription and replication machinery, and mitochondrial protein import machinery (PIM) complexes. Furthermore, PGC-1α activates NRF-1 transcription of mitochondrial transcription factor A (Tfam), which is essential for increased mtDNA expression.7 The expression of several antioxidants such as superoxide dismutases 1 and 2, and catalase are also regulated by PGC-1α.8 In addition to increasing mitochondrial content, overexpression of PGC-1α can increase the proportion of Type 1 muscle fibers, thereby contributing to the augmented endurance and resistance to fatigue.9
By contrast, whole-body ablation of PGC-1α impairs exercise performance, which is observed along with concomitant decreases in mitochondrial content. Indeed, there is a strong correlation between PGC-1α protein expression and the steady-state mitochondrial content of various tissues. PGC-1α expression responds to exercise as the muscle adapts to metabolic demands, subsequently leading to mitochondrial biogenesis.10 Expression of the coactivator is controlled by a magnitude of signaling cascades that also regulate its activity. It is well known that a single bout of exercise can increase PGC-1α mRNA and protein levels,11, 12 while repeated bouts of exercise can increase the protein expression of PGC-1α, along with NRF-1 and TFAM. This allows for further exercise-induced adaptations,12, 13 including the expression of a broad range of nuclear genes encoding mitochondrial proteins (NUGEMPs), mitochondrial PIM components, and mtDNA.14 However, there is controversy regarding whether or not PGC-1α is mandatory for exercise and training-induced adaptive gene responses.4 Studies conducted in PGC-1α KO mice found that PGC-1α is not required for training-induced increases in ALAS1, COX1, and cytochrome C expression.4 Furthermore, PGC-1α muscle-specific KO were discovered to have similar exercise capacity and exercise-induced mitochondrial biogenesis as their wild-type (WT) littermates.15 Thus, there are likely to be other factors, yet to be determined, that can mediate training-induced adaptations in skeletal muscle, and help coordinate the expression of both nuclear and mitochondrial genes.
In response to exercise, the expression of PGC-1α is regulated by increases in reactive oxygen species (ROS), the adenosine monophosphate (AMP) to adenosine triphosphate (ATP) ratio, and changes in Ca2+ concentrations. It has been shown that the activation of Ca2+/calmodulin-dependent protein kinase IV and calcineurin A help drive PGC-1α expression through the binding of cAMP response element-binding protein (CREB) and myocyte enhancer factor-2 (MEF2) to consensus binding sites.16 Furthermore, p38 mitogen-activated protein kinase activation during exercise can cause the phosphorylation of MEF2 and activating transcription factor 2 (ATF2), both of which bind to the promoter of PGC-1α and induce its expression. In addition, p38 has been shown to phosphorylate PGC-1α, resulting in PGC-1α protein stabilization.17 With exercise, the AMP to ATP ratio is increased, altering the energy status of the cell and thus activating AMP-activated protein kinase (AMPK). AMPK activation results in direct phosphorylation of PGC-1α, which appears to enhance its transcriptional activity.18 Furthermore, AMPK activation enhances Pgc-1α promoter activity,19 leading to greater PGC-1α expression. Thus, PGC-1α expression is responsive to a number of intracellular signals that are vital for contractile activity.
Recent evidence suggests the presence of several different Pgc-1α splice variants in skeletal muscle. These variants differ based on their starting exon (exon 1a, exon 1b, or exon 1b′/1c), and can be produced via alternative 3′ splicing, producing either the full-length Pgc-1α or the shorter N-truncated version.20, 21 Exon 1a-derived mRNAs are transcribed from the canonical proximal promoter. However, there is an alternative promoter approximately 14 kb upstream of the canonical promoter, which is responsible for transcribing exon 1b- and 1b′-derived mRNAs.20 Little research has been conducted regarding the isoforms of the protein that varies from these specific variants; however, it has been proposed that the isoforms respond differently to resistance and endurance exercise, and are responsible for mediating the various functions of PGC-1α. Endurance exercise enhances transcription of Exon 1a-derived mRNA and induces responses typical for angiogenesis and mitochondrial biogenesis. By contrast, resistance exercise has been shown to activate the transcription of exon 1b and b′ mRNA, leading to responses typical for muscle hypertrophy.20 A product of the truncated 1b transcript is the PGC-1α splice variant PGC-1α4. This variant is 266–amino-acid long and it undergoes downstream alternative splicing.21 PGC-1α4 expression is known to increase with exercise, and when expressed in skeletal muscle, it contributes to muscle hypertrophy by regulating IGF-1 expression and reducing levels of myostatin.21
In addition to the PGC-1α variants, PGC-1β has also been implicated as a transcriptional activator involved in mitochondrial biogenesis.23 Mice in which PGC-1β is selectively ablated in skeletal myofibers demonstrate lower mitochondrial respiration as well as impairments in exercise performance. PGC-1β has not been considered to play a role in exercise-mediated mitochondrial biogenesis, as its expression is not induced with exercise.15 However, this does not preclude the possibility that PGC-1β activity is increased with exercise via post-transcriptional modifications.15 Indeed, evidence demonstrates that deletion of both PGC-1α and PGC-1β in skeletal muscle results in a greater reduction in mitochondrial function than a deletion of either coactivator on its own.24 However, more research is required to further elucidate the role and expression of the splice variants and PGC-1α family members in exercise adaptations.
3. Mitochondrial transcription factor A
In addition to the contribution of proteins derived from the nuclear genome, expression of the genes encoded by mtDNA is required for the proper formation of the multisubunit complexes that compose the electron transport chain. A noncoding region, referred to as the D-loop, regulates mtDNA transcription. This region of DNA contains the promoters required to initiate transcription of the 13 genes encoding proteins of the electron transport chain, as well as the rRNAs and tRNAs required for their translation. Work over the past few decades has revealed that transcription of mtDNA is principally regulated by three core components, all of which are nuclear encoded. These include mitochondrial RNA polymerase, mitochondrial transcription factor B2, and mitochondrial transcription factor A (TFAM).25
TFAM is a multifunctional protein, as it plays a vital role in mtDNA replication and packaging, in addition to its role in the transcription of mtDNA. This protein belongs to the high-motility group family of proteins, which are characterized by their ability to bend, wrap, and unwind DNA. It has been shown that TFAM is capable of unwinding mtDNA promoters into a “U-turn”-like configuration,26, 27, 28 which facilitates the access of the other core components to the promoter, in turn stimulating transcription. Additionally, in concert with mtDNA polymerase γ, the mtDNA helicase TWINKLE, and an mtDNA single-stranded DNA binding protein (mtSSB), TFAM can also assist in the replication of mtDNA,29 resulting in multiple copies of the genome within the organelle network of the cell.
The necessity of TFAM for cellular function is highlighted by the result of complete ablation of TFAM in a mouse model, which is lethal to the animal in its embryonic stage.30 Further, heterozygous deletion of TFAM greatly reduces mtDNA content in multiple tissues,30 and alterations of TFAM to various levels have coincident effects on mtDNA transcription.31, 32
The role of TFAM in skeletal muscle is an area of great interest, considering the aforementioned roles of the protein in mtDNA transcription and replication, and the heavy reliance of skeletal muscle metabolism on mitochondrial function. Recently, the positive relationship between the TFAM protein expression, its localization to the mitochondrial matrix, and its ability to promote the expression of mtDNA has been highlighted in differentiating myotubes, a model of pronounced mitochondrial biogenesis.33
Exercise-induced mitochondrial biogenesis occurs in parallel with an increase in mtDNA copy number.34 which parallels oxidative capacity in exercised muscle.35 Interestingly, it appears that the Tfam gene is responsive to exercise, as an elevation in the mRNA expression of Tfam has been observed following a single session of endurance exercise.11, 13, 36 Furthermore, increases in TFAM protein content with skeletal muscle have been documented in several in vitro3, 37 and in vivo38, 39, 40 animal endurance exercise training paradigms, as well as in human skeletal muscle.41 In response to repeated bouts of exercise, a sequential series of events unfurls. First, Tfam mRNA increases, leading to the cystolic accumulation of TFAM protein. This is followed by augmented TFAM import into the matrix, and subsequently, increased TFAM-mtDNA binding and mtDNA transcription.40, 42 This process contributes substantially to the increased expression of mitochondrially encoded genes as the mitochondrial reticulum grows in response to exercise.
4. p53 and mtDNA
The transcription factor p53 has been termed the “guardian of the genome,” as it has been classically characterized as a tumor suppressor.43 It regulates the transcription of genes involved in DNA repair, autophagy, apoptosis, and cell cycle.44, 45, 46 However, in recent years, p53 has been established as an essential regulator of mitochondrial gene expression, and thus of mitochondrial content and function.47, 48 Early evidence suggesting a positive relationship between p53 and mitochondrial gene expression49 has since been corroborated by a wealth of literature demonstrating the requirement for p53 in the maintenance of basal mitochondrial content.2, 50, 51, 52, 53 Thus, p53 appears to be especially critical in mitochondrial biogenesis, since it has the unique ability to modulate gene expression through interactions with both nuclear and mitochondrial genomes.13, 54 Indeed, work in several cell types has identified that p53 is capable of transcriptionally regulating genes involved in aerobic metabolism, including the nuclear-encoded transcription factors Tfam and Nrf-1,2 as well as synthesis of cytochrome c oxidase 2 (Sco2), a cytochrome c oxidase assembly factor.52 In addition, p53 can aid in the expression of mitochondrially encoded genes, such as cytochrome c oxidase subunit I (COX I) of the COX holoenzyme13 and 16S sRNA,49 which is likely facilitated by the physical interaction between p53 and Tfam on mtDNA.13, 55 Interestingly, p53 also appears to play a role in the regulation of PGC-1α expression. Although the human Pgc-1α promoter contains a putative binding site for p53,19 work with p53 KO models has revealed that this does not impact basal Pgc-1α mRNA expression2; instead, these animals have a diminished PGC-1α protein content.53 The exact mechanism for this finding has yet to be determined.
The importance of p53 in modulating mitochondrial content and function has been further highlighted in skeletal muscle, as p53 KO mice exhibit deficits in basal mitochondrial content, and display abnormal mitochondrial ultrastructure and impairments in the assembly of the COX holoenzyme.51, 53 These changes manifest in a decrease in mitochondrial respiration and an increase in mitochondrial ROS emission,53 making these dysfunctional mitochondria more susceptible to ubiquitination and subsequent degradation through mitophagy.2 Although there is upregulation of some mitophagy-related proteins when p53 is ablated, this increase is not pronounced enough to induce notable increases in autophagy, as evidenced through the accumulation of p62 (an adaptor for autophagic markers), lack of change in LC3II, Beclin and ATG7 autophagy-inducing related proteins, and reduced lysosomal protein markers.2, 51 Functionally, these mitochondrial alterations are reflected by poor chronic endurance exercise performance53 and an elevated rate of fatigue development during repeated muscle contractions.53
Moreover, p53 plays a role in controlling mitochondrial biogenesis-associated signaling in response to an acute bout of endurance exercise. Contractile activity in both cell culture53 and human models56, 57 induces phosphorylation of p53 on its serine 15 residue to improve its stabilization and activity. This site can be targeted by kinases that are responsive to the cellular perturbations that occur during exercise, such as p38 mitogen-activated protein kinase58, 59 and AMPK.18, 60 Intriguingly, the responsiveness of these mitochondrial biogenesis-associated kinases to contractile activity is delayed when p53 is genetically deleted.2 This appears to be directly associated with an attenuation in the transcription of Pgc-1α mRNA, as well as impaired nuclear translocation of PGC-1α protein in response to acute exercise.2 While earlier reports had suggested that p53 may move to the nucleus upon cessation of an acute exercise session,61 recent evidence has also shown that translocation of p53 to the mitochondria occurs during the recovery period from an exercise bout.13 While these studies appear to conflict, this may be due to differences in the exercise paradigm employed and the muscle type analyzed. Nonetheless, this shift in p53 subcellular localization to the mitochondrion is vital for the formation of a complex between TFAM and mtDNA, and the expression of mtDNA-encoded genes following acute exercise.13
An increase in overall p53 protein content in skeletal muscle has also been shown to occur in response to various modes of chronic endurance exercise training.62 While p53 KO mice have a reduced skeletal muscle mitochondrial content, exercise training appears to be a viable means by which mitochondrial content can be restored in the animals,53 perhaps indicating that the mitochondrial adaptations to endurance exercise training are independent of p53. However, more studies are warranted to carefully delineate the necessity of p53 in endurance training-associated adaptations with respect to mitochondrial turnover processes.
5. Unfolded protein response signaling
During cellular stress, or when proteins synthesis rates increase drastically, an increase in the number of incompletely folded proteins can occur. This accumulation can trigger unfolded protein responses within the endoplasmic reticulum (UPRER) and in the mitochondria (UPRmt).63, 64, 65 These signal transduction pathways initiate transcription of proteins within the nucleus as a feedback response, in order to (1) increase protein folding efficiency, (2) degrade misfolded proteins, or (3) if proteostasis still cannot be attained, trigger apoptosis of the cell.66. Stresses that can initiate the UPR include mutations in constituent proteins, presence of damaging ROS, or harsh environmental conditions that can negatively impact protein folding.67
Upon endoplasmic reticulum (ER) stress, the ER chaperone BiP senses and binds misfolded proteins, thus releasing its inhibition on three transmembrane signal transducers ATF6, protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), and inositol-requiring enzyme 1 (IRE1). Upon BiP release, ATF6 translocates from the ER membrane to the Golgi apparatus where proteases cleave and release active ATF6 to the nucleus. There, ATF6 initiates transcription of a number of proteins, including transcription factors X-box binding protein 1 (XBP-1) and CCAAT/enhancer binding protein (C/EBP), homologous protein (CHOP), as well as quality control chaperones such as BiP and GRP94.68, 69, 70, 71, 72, 73, 74 At the same time, the PERK pathway phosphorylates eIF2α, resulting in inhibition of global protein translation. This decreases the ER folding load, but also selectively activates the translation of the transcription factor ATF4 to increase CHOP expression, a proapoptotic transcription factor partially involved in the regulation of cell fate.75, 76, 77 The third branch involves IRE1, which has a cytosolic domain with endoribonuclease activity. IRE1 splices a 26-nt segment from the full-length XBP1 transcript, to produce a frameshift in translation and create transcriptionally active XBP1 (XBP1s). XBP1s enters the nucleus to promote the transcription of genes involved in protein transport, folding, and degradation.78
The UPRmt pathway functions separately from, yet similarly to, the UPRER pathway.63, 65 Mitochondrial chaperones and proteases within the matrix and the intermembrane space (IMS) are the prime sensors of misfolded mitochondrial proteins, and they are capable of sensing, and eliciting, a compartment-specific response.79, 80, 81 Proteotoxic stress localized to the IMS induces an increase in ROS, leading to the phosphorylation of ERα and driving the expression of IMS proteases in order to degrade and remove the accumulated proteins.82 Similarly, the proteases ClpP and LonP monitor mitochondrial matrix protein status, and can help facilitate the activation of a set of pathways in response to misfolded protein aggregates. Our evolving understanding of the UPRmt and the UPRER suggests that these signaling events associated with cellular stress help align the mitochondrial and nuclear genomes at the protein level, thus helping in coordinating mitochondrial adaptations and remodeling.
Increasingly, evidence continues to mount in support of a role for the UPR in skeletal muscle in response to exercise. As exercise plays a positive role in skeletal muscle health, research has turned its attention to the potential role of the UPR in mediating these adaptations. Recently, UPR activation was observed in skeletal muscle following a single unaccustomed bout of resistance exercise.83 These results corroborated a previous report that found an association between activation of UPR transcripts in muscle subject to a single bout of resistance exercise, which was attenuated with prolonged training.84 Acute endurance exercise has also been shown to elicit the UPR, with the intensity of the exercise contributing to the magnitude of the response.66, 85, 86 The activation of the UPR during acute endurance and resistance exercise indicates a potential role of the UPR in the initial onset of muscle adaptations.87, 88 In order to provide a more complete documentation of the time course of UPR activation in response to acute exercise, and the adaptations to training, recent work in our laboratory has examined the activation of both the UPRER and the UPRmt in response to repeated bouts of exercise of contractile activity in an animal model. Our data indicate that both UPRs are activated in the early stages of the contractile activity training program, preceding the adaptive responses in mitochondrial biogenesis and autophagy.88 It should be noted that PGC-1α has been shown to be induced by, and to regulate, UPR signaling in an ATF6-dependent manner, indicating an integrated link between UPR and mitochondrial biogenesis pathways.85 Future work using selective KO models should aim to reveal important regulatory steps between these two adaptation processes.89, 90, 91
6. Protein import into the mitochondrion
The most recent MitoCarta 2.0 posits that 1158 mitochondrial proteins are encoded by nuclear DNA, and a tightly regulated mechanism is required for transporting proteins synthesized in the cytosol into the various compartments of the mitochondria.92 Mitochondrial protein import is regulated by the PIM, and this process has been well characterized in yeast; however, its mammalian counterpart remains to be sufficiently investigated, particularly under physiological conditions.93
Import is a multistep process that involves linearization of proteins to allow passage through the translocases of the outer and inner membrane (TOM and TIM, respectively) complexes.93 In the cytosol, mitochondrial proteins are recognized and actively unfolded by chaperones such as mitochondrial import stimulating factor or heat shock proteins (HSP70 and HSP90).93 Once in a linear conformation, these proteins contain presequences that target their movement into the mitochondria and are recognized by receptors on the TOM complex.93 The TOM37-TOM70 receptor complex recognizes internal hydrophobic presequences, whereas the TOM20-TOM22 receptor complex is responsible for binding positive presequences, which comprise the vast majority of mitochondrial proteins.93 Once recognized by the receptor, proteins are then passed to Tom40, the major channel of the outer membrane (OM) that allows proteins to translocate into the IMS.93 Proteins destined for the OM or IMS will be incorporated into the OM via the sorting and assembly machinery, or undergo maturation (i.e., refolding and cleavage of the presequence) by chaperones and proteases of the IMS.93 Matrix or inner membrane (IM)-destined proteins require help from small TIM8-TIM13 and TIM9-TIM10 complexes that facilitate the passage through the IMS and deliver the protein to TIM22 and TIM23.94, 95 Proteins destined for the IM are shuttled to TIM22 or OXA1 for direct insertion into the IM.94, 96 By contrast, matrix-destined proteins are passed to TIM23, the major channel of the IM, and passage through the IM is achieved through the ratchet-like action of mtHSP70 that hydrolyzes ATP to pull the protein into the matrix and prevent retrograde movements.94 Once inside the matrix, the positive presequence is cleaved by mitochondrial processing peptidase, and the mature protein is refolded by mitochondrial chaperones (HSP60 and CPN10) resulting in a functional protein.94
Beyond its role in transporting the essentials proteins required for mitochondrial expansion and maintenance, import has been found to be a dynamic process, able to respond to metabolic changes in the cell. Modifications in the rate of protein import are able to match the needs of the mitochondria through increased expression of either cystolic or PIM components. Chronic exercise induced using a contractile activity model in rodents revealed that exercise can serve as a stimulant to increase the import of nuclear-encoded proteins.42, 97, 98, 99 This occurs as a result of contractile activity-induced increases in PIM component expression, as well as changes within the cytosolic fraction that enhance import in the organelle.42 The identity of these cytosolic factors remains largely to be determined.
At the other end of the spectrum, import can be downregulated with muscle disuse, leading to an aggravation of the metabolic disturbance. Singh and Hood100 demonstrated that import is sensitive to changes in ROS and the elevation in ROS seen with disuse promotes protein import arrest. During denervation, a commonly used model of disuse, the early decline in import kinetics is a crucial regulator of mitochondrial content, since transcriptional regulators such as Tfam are limited by their translocation into the mitochondria.7 Taken together, a strong correlation exists between mitochondrial protein import kinetics and organelle content and function.100
Adaptability of the import system has also been demonstrated in other ways. For example, in cells lacking mtDNA, protein import is increased, concomitant with changes in the expression of PIM components. In aging muscle protein, import rates are modestly elevated, to potentially compensate for reduced mitochondrial content.101, 102 In BAX/BAK double KO animals, abrogation of these two proteins canonically associated with apoptosis, caused an unexpected defect in the kinetics of import into the OM and matrix.97 However, this impairment was rescued with endurance training, which caused upregulation of PIM components and normalization of the import rate.97 Thus, adaptability of the protein import system to metabolic disturbances makes import an important topic for research as a potential therapeutic target, or a way to manipulate mitochondrial health.
7. Nuclear factor erythroid 2-related factor 2 and mitochondria
The transcription factor nuclear factor erythroid 2-related factor 2 (NFE2L2 or Nrf2) is regarded as the central regulator of the expression of antioxidants and enzymes essential for the protection of cells against oxidative damage-generated free radical species. Abrogation of Nrf2 blunts the expression of these enzymes, consequently increasing their sensitivity to the toxic effects of various drugs103 and inflammatory compounds.104 The inability of Nrf2 KO animals to initiate a response that counteracts the adverse effects of oxidative stress illustrates the importance of Nrf2 in maintaining cellular redox homeostasis.
Under basal conditions, Nrf2 is bound to its repressor Kelch-like ECH associating protein 1 (Keap1) within the cytosol. Keap1 targets Nrf2 for ubiquitin conjugation and subsequent proteasome degradation in the cytoplasm by acting as a substrate adaptor for the Cul3-based E3 ubiquitin ligase complex.105, 106 However, an increase in oxidative stress promotes the stabilization and activation of Nrf2. Mechanistically, activation of Nrf2 is thought to involve modification of the sulfhydryl groups of critical cysteine residues within Keap1, resulting in a conformational change that ultimately reduces the binding capacity of Keap1 to Nrf2.107, 108 In turn, this promotes translocation of Nrf2 to the nucleus, where it can interact and form heterodimers with small Maf proteins,109, 110 recruit transcriptional coactivators,111, 112 and bind to the antioxidant response element (ARE) found within the promoter region of target genes.113
While it is well established that Nrf2 is important for antioxidant expression, emerging evidence also suggests that Nrf2 signaling is critical for the regulation of mitochondrial content. It has been reported that, compared with WT animals, the livers of Nrf2 KO mice have a lower mitochondrial content.114 In 3T3-L1 adipocytes, supplementation of the cells with (R)-α-lipoic acid, a well-known Nrf2 activator,115, 116 promotes mitochondrial biogenesis.117 The connection between Nrf2 and regulation of mitochondrial content may be through its interaction with NRF-1, a well-known transcription factor that is essential for the expression of genes encoding subunits of the five respiratory complexes, mitochondrial translational components, and heme biosynthetic enzymes that are localized to the mitochondrial matrix.118 For instance, the work conducted by Piantadosi et al119 revealed that the Nrf-1 gene contains multiple AREs within its promoter, which become occupied by Nrf2 upon induction by ROS. Further, they showed that the Nrf2-dependent transcriptional upregulation of Nrf-1 promotes mitochondrial biogenesis and protects cardiomyocytes from the cytotoxicity of the chemotherapeutic agent doxorubicin. As noted above, one of the key regulators of mitochondrial biogenesis is the coactivator PGC-1α. At this time, the interaction between Nrf2 and PGC-1α remains to be determined.120 However, treatment of human fibroblasts with the potent Nrf2 activator sulforaphane increased mitochondrial mass and induced both PGC-1α and PGC-1β.121 While this study did not examine whether the increase in mitochondrial mass and expression of PGC-1α was Nrf2 dependent, it is possible that an Nrf2–PGC-1α interaction facilitates the expansion of the mitochondrial reticulum under the appropriate stimulus. This notion is supported by the fact that the promoter of the mouse Pgc-1α gene contains two AREs at –1723 bp and –226 bp from the transcription start site.8, 122 Indeed, it has been shown that relative to WT mice, Nrf2 KO animals experiencing septic infection or acute lung injury exhibit attenuation in the transcriptional upregulation of both Nrf-1 and Pgc-1α.123, 124 Collectively, these results suggest that the interaction between Nrf2 and the mitochondrial regulators NRF-1 and PGC-1α is complex and most prominent under conditions of stress.
Exercise is a particular stressor that is capable of inducing Nrf2 activation, nuclear translocation, and enhanced ARE binding in cardiomyocytes.125 Although there is accumulating evidence demonstrating the importance of Nrf2 for the regulation of mitochondrial content in various cell types, its contribution within skeletal muscle remains to be elucidated. Further, whether Nrf2 is required for exercise-induced adaptations is largely unknown. Research conducted in our laboratory has shown that an exhaustive exercise bout results in a 1.5-fold increase in Nrf2–ARE binding, suggesting that Nrf2 may participate in the signaling events promoting expansion of the mitochondrial reticulum with training. Indeed, we demonstrated that COX activity did not differ between Nrf2 WT and KO mice basally, but that training-induced adaptations in mitochondrial content were less pronounced in KO animals. Our results suggest that Nrf2 is a component of the transcription factor network that contributes to exercise-induced mitochondrial biogenesis in muscle.
8. Conclusion
Exercise-induced mitochondrial biogenesis in muscle involves the coordination of multiple proteins and signaling pathways, improving the health of this tissue and enhancing its metabolic capacity. As outlined above, it has become clear in recent years through the use of novel genetic and physiological models that the molecular signaling pathways regulating exercise-induced mitochondrial biogenesis are often redundant and overlapping. This also suggests that no single protein is absolutely vital for these exercise adaptations. As a result, it appears that potential therapeutic targets, for intervention by physiological, pharmacological, or nutraceutical means, are plentiful. This could be of great value when searching for alternative mechanisms of inducing mitochondrial biogenesis in conditions where exercise programs are not feasible, such as in individuals with disabilities or during prolonged muscle inactivity. Accordingly, continued research in understanding these basic molecular mechanisms regulating mitochondrial content and function is warranted.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
Research in the authors’ laboratory is funded by the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council (NSERC) of Canada. D.A.H. is the holder of a Canada Research Chair in Cell Physiology.
References
- 1.Menzies K.J., Singh K., Saleem A., Hood D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleem A., Carter H.N., Hood D.A. p53 is necessary for the adaptive changes in the cellular milieu subsequent to an acute bout of endurance exercise. Am J Physiol Cell Physiol. 2014;306:C241–C249. doi: 10.1152/ajpcell.00270.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter H.N., Hood D.A., Contractile activity-induced mitochondrial biogenesis mTORC1., Am J. Physiol Cell Physiol. 2012;303:C540–C547. doi: 10.1152/ajpcell.00156.2012. [DOI] [PubMed] [Google Scholar]
- 4.Leick L., Wojtaszewski J., Johansen S., Kiilerich K., Comes J., Hellsten Y. PGC-1α is not mandatory for exercise-and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:463–474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- 5.Adhihetty P.J., Uguccioni G., Leick L., Hidalgo J., Pilegaard H., Hood D.A. The role of PGC-1α on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol. 2009;297:C217–C225. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- 6.Hood D.A., Uguccioni G., Vainshtein A. D'souza D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: implications for health and disease. Compr Physiol. 2011;1:1119–1134. doi: 10.1002/cphy.c100074. [DOI] [PubMed] [Google Scholar]
- 7.Tryon L.D., Crilly M.J., Hood D.A. Effect of denervation on the regulation of mitochondrial transcription factor A expression in skeletal muscle. Am J Physiol Cell Physiol. 2015;309:C228–C238. doi: 10.1152/ajpcell.00266.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jäger S. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Calvo J.A., Daniels T.G., Wang X., Paul A., Lin J., Spiegelman B.M. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 10.Adamovich Y., Shlomai A., Tsvetkov P., Umansky K.B., Reuven N., Estall J.L. The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH-NQO1. Mol Cell Biol. 2013;33:2603–2613. doi: 10.1128/MCB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. Accessed: January 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baar K., Wende A.R., Jones T.E., Marison M., Nolte L.A., Chen M. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 13.Saleem A., Hood D.A. Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. J Physiol. 2013;591(Pt 14):3625–3636. doi: 10.1113/jphysiol.2013.252791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood D.A. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 15.Rowe G.C., El-Khoury R., Patten I.S., Rustin P., Arany Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One. 2012;7:e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Marcos P., Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884–890. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Uguccioni G., Ljubicic V., Irrcher I., Iqbal S., Singh K. Multiple signaling pathways regulate contractile activity-mediated PGC-1 gene expression and activity in skeletal muscle cells. Physiol Rep. 2014;2:e12008. doi: 10.14814/phy2.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäger S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irrcher I., Ljubicic V., Kirwan A.F., Hood D.A. AMP-activated protein kinase-regulated activation of the PGC-1α promoter in skeletal muscle cells. PLoS One. 2008;3:e3614. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvennoinen M., Ahtiainen J.P., Hulmi J.J., Pekkala S., Taipale R.S., Nindl B.C. PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep. 2015;3:e12563. doi: 10.14814/phy2.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Redondo V., Pettersson A.T., Ruas J.L. The hitchhiker's guide to PGC-1α isoform structure and biological functions. Diabetologia. 2015;58:1969–1977. doi: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- 23.Rowe G.C., Patten I.S., Zsengeller Z.K., El-khoury R., Bampoh S., Koulisis N. Disconnecting mitochondrial content from respiratory chain capacity in PGC-1 deficient skeletal muscle. Cell Rep. 2013;3:1449–1456. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zechner C., Lai L., Fong J.L., Geng T., Yan Z., John W. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bestwick M.L., Shadel G.S. Accessorizing the human mitochondrial transcription machinery. Trends Biochem Sci. 2013;38:283–291. doi: 10.1016/j.tibs.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngo H.B., Kaiser J.T., Chan D.C. The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA. Nat Struct Mol Biol. 2011;18:1290–1296. doi: 10.1038/nsmb.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngo H.B., Lovely G.A., Phillips R., Chan D.C. Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation. Nat Commun. 2014;5:3077. doi: 10.1038/ncomms4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubio-Cosials A., Sidow J.F., Jiménez-Menéndez N., Fernández-Millán P., Montoya J., Jacobs H.T. Human mitochondrial transcription factor A induces a U-turn structure in the light strand promoter. Nat Struct Mol Biol. 2011;18:1281–1289. doi: 10.1038/nsmb.2160. [DOI] [PubMed] [Google Scholar]
- 29.Falkenberg M., Larsson N.G., Gustafsson C.M. DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem. 2007;76:679–699. doi: 10.1146/annurev.biochem.76.060305.152028. [DOI] [PubMed] [Google Scholar]
- 30.Larsson N.G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandowski M. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nature. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa R., Yamada Y., Matsushima Y., Goto Y.I., Harashima H. The manner in which DNA is packaged with TFAM has an impact on transcription activation and inhibition. FEBS Open Bio. 2012;2:145–150. doi: 10.1016/j.fob.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maniura-Weber K., Goffart S., Garstka H.L., Montoya J., Wiesner R.J. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 2004;32:6015–6027. doi: 10.1093/nar/gkh921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collu-Marchese M., Shuen M., Pauly M., Saleem A., Hood D.A. The regulation of mitochondrial transcription factor A (Tfam) expression during skeletal muscle cell differentiation. Biosci Rep. 2015;35:e00221. doi: 10.1042/BSR20150073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puntschart A., Claassen H., Jostarndt K., Hoppeler H., Billeter R. mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol. 1995;269(3 Pt 1):C619–C625. doi: 10.1152/ajpcell.1995.269.3.C619. [DOI] [PubMed] [Google Scholar]
- 35.Williams R.S. Mitochondrial gene expression in mammalian striated muscle. Evidence that variation in gene dosage is the major regulatory event. J Biol Chem. 1986;261:12390–12394. [PubMed] [Google Scholar]
- 36.Perry C.G.R., Lally J., Holloway G.P., Heigenhauser G.J.F., Bonen A., Spriet L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uguccioni G., Hood D.A. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab. 2011;300:E361–E371. doi: 10.1152/ajpendo.00292.2010. [DOI] [PubMed] [Google Scholar]
- 38.Lai R.Y.J., Ljubicic V., D'souza D., Hood D.A. Effect of chronic contractile activity on mRNA stability in skeletal muscle. Am J Physiol Cell Physiol. 2010;299:C155–C163. doi: 10.1152/ajpcell.00523.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastore S., Hood D.A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J Appl Physiol. 2013;114:1076–1084. doi: 10.1152/japplphysiol.01505.2012. [DOI] [PubMed] [Google Scholar]
- 40.Gordon J.W., Rungi A.A., Inagaki H., Hood D.A. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol. 2001;90:389–396. doi: 10.1152/jappl.2001.90.1.389. [DOI] [PubMed] [Google Scholar]
- 41.Bengtsson J., Gustafsson T., Widegren U., Jansson E., Sundberg C.J. Mitochondrial transcription factor A and respiratory complex IV increase in response to exercise training in humans. Pflugers Arch. 2001;443:61–66. doi: 10.1007/s004240100628. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi M., Chesley A., Freyssenet D., Hood D.A. Contractile activity-induced adaptations in the mitochondrial protein import system. Am J Physiol. 1998;274:C1380–C1387. doi: 10.1152/ajpcell.1998.274.5.C1380. [DOI] [PubMed] [Google Scholar]
- 43.Lane D.P. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 44.Levine A.J., Hu W., Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 45.Maiuri M.C., Tasdemir E., Criollo A., Morselli E., Vicencio J.M., Carnuccio R. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 46.Yu J., Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 47.Bartlett J.D., Close G.L., Drust B., Morton J.P. The emerging role of p53 in exercise metabolism. Sport Med. 2014;44:303–309. doi: 10.1007/s40279-013-0127-9. [DOI] [PubMed] [Google Scholar]
- 48.Saleem A., Carter H.N., Iqbal S., Hood D.A. Role of p53 within the regulatory network controlling muscle mitochondrial biogenesis. Exerc Sport Sci Rev. 2011;39:199–205. doi: 10.1097/JES.0b013e31822d71be. [DOI] [PubMed] [Google Scholar]
- 49.Donahue R.J., Razmara M., Hoek J.B., Knudsen T.B. Direct influence of the p53 tumor suppressor on mitochondrial biogenesis and function. FASEB J. 2001;15:635–644. doi: 10.1096/fj.00-0262com. [DOI] [PubMed] [Google Scholar]
- 50.Zhou S., Kachhap S., Singh K.K. Mitochondrial impairment in p53-deficient human cancer cells. Mutagenesis. 2003;18:287–292. doi: 10.1093/mutage/18.3.287. [DOI] [PubMed] [Google Scholar]
- 51.Saleem A., Iqbal S., Zhang Y., Hood D.A. Effect of p53 on mitochondrial morphology, import and assembly in skeletal muscle. Am J Physiol Cell Physiol. 2015;308:C319–C329. doi: 10.1152/ajpcell.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matoba S., Kang J.G., Patino W.D., Wragg A., Boehm M., Gavrilova O. p53 regulates mitochondrial metabolism. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 53.Saleem A., Adhihetty P.J., Hood D.A. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol Genomics. 2009;3:58–66. doi: 10.1152/physiolgenomics.90346.2008. [DOI] [PubMed] [Google Scholar]
- 54.Heyne K., Mannebach S., Wuertz E., Knaup K.X., Mahyar-Roemer M., Roemer K. Identification of a putative p53 binding sequence within the human mitochondrial genome. FEBS Lett. 2004;578:198–202. doi: 10.1016/j.febslet.2004.10.099. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida Y., Izumi H., Torigoe T., Ishiguchi H., Itoh H., Kang D. p53 physically interacts with mitochondrial transcription factor A and differentially regulates binding to damaged DNA. Cancer Res. 2003;63:3729–3734. [PubMed] [Google Scholar]
- 56.Bartlett J.D., Louhelainen J., Iqbal Z., Cochran A.J., Gibala M.J., Gregson W. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R450–R458. doi: 10.1152/ajpregu.00498.2012. [DOI] [PubMed] [Google Scholar]
- 57.Bartlett J.D., Hwa Joo C., Jeong T.S., Louhelainen J., Cochran A.J., Gibala M.J. Matched work high-intensity interval and continuous running induce similar increases in PGC-1α mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112:1135–1143. doi: 10.1152/japplphysiol.01040.2011. [DOI] [PubMed] [Google Scholar]
- 58.She Q.-B., Bode A.M., Ma W.-Y., Chen N.-Y., Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular- signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604–1610. [PubMed] [Google Scholar]
- 59.Wright D.C., Geiger P.C., Han D.H., Jones T.E., Holloszy J.O. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- 60.Jones R.G., Plas D.R., Kubek S., Buzzai M., Mu J., Xu Y. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 61.Philp A., Chen A., Lan D., Meyer G.A., Murphy A.N., Knapp A.E. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation following endurance exercise. J Biol Chem. 2011;286:30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Granata C., Oliveira R.S.F., Little J.P., Renner K., Bishop D.J. Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J. 2015;30:1–12. doi: 10.1096/fj.15-276907. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Q., Wang J., Levichkin I.V., Stasinopoulos S., Ryan M.T., Hoogenraad N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bradshaw R., Dennis E. 1st ed. Elsevier Academic Press; London: 2011. Regulation of organelle and cell compartment signaling; p. 374. 413. [Google Scholar]
- 65.Mottis A., Jovaisaite V., Auwerx J. The mitochondrial unfolded protein response in mammalian physiology. Mamm Genome. 2014;25:424–433. doi: 10.1007/s00335-014-9525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H.J., Jamart C., Deldicque L., An G., Lee Y.H., Kim C.K. Endoplasmic reticulum stress markers and ubiquitin–proteasome pathway activity in response to a 200-km run. Med Sci Sports Exerc. 2011;43:18–25. doi: 10.1249/MSS.0b013e3181e4c5d1. [DOI] [PubMed] [Google Scholar]
- 67.Haynes C.M., Ron D. The mitochondrial UPR—protecting organelle protein homeostasis. J Cell Sci. 2010;123(Pt 22):3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Shen J., Arenzana N., Tirasophon W., Kaufman R.J., Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem. 2000;275:27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- 69.Kokame K., Kato H., Miyata T. Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem. 2001;276:9199–9205. doi: 10.1074/jbc.M010486200. [DOI] [PubMed] [Google Scholar]
- 70.Schröder M., Kaufman R.J. the mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 71.Wu J., Rutkowski D.T., Dubois M., Swathirajan J., Saunders T., Wang J. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Adachi Y., Yamamoto K., Okada T., Yoshida H., Harada A., Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33:75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- 73.Chen X., Shen J., Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- 74.Ye J., Rawson R.B., Komuro R., Chen X., Dave U.P., Prywes R. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 75.B’chir W., Chaveroux C., Carraro V., Averous J., Maurin A.C., Jousse C. Dual role for CHOP in the crosstalk between autophagy and apoptosis to determine cell fate in response to amino acid deprivation. Cell Signal. 2014;26:1385–1391. doi: 10.1016/j.cellsig.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Shen J., Chen X., Hendershot L., Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 77.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 79.Baker B.M., Haynes C.M. Mitochondrial protein quality control during biogenesis and aging. Trends Biochem Sci. 2011;36:254–261. doi: 10.1016/j.tibs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Tatsuta T., Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pellegrino M.W., Nargund A.M., Haynes C.M. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta Mol Cell Res. 2013;1833:410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papa L., Germain D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J Cell Sci. 2011;124(Pt 9):1396–1402. doi: 10.1242/jcs.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogborn D.I., McKay B.R., Crane J.D., Parise G., Tarnopolsky M.A. The unfolded protein response is triggered following a single, unaccustomed resistance-exercise bout. Am J Physiol Regul Integr Comp Physiol. 2014;307:R664–R683. doi: 10.1152/ajpregu.00511.2013. [DOI] [PubMed] [Google Scholar]
- 84.Gordon P.M., Liu D., Sartor M.A., IglayReger H.B., Pistilli E.E., Gutmann L. Resistance exercise training influences skeletal muscle immune activation: A microarray analysis. J Appl Physiol. 2012;112:443–453. doi: 10.1152/japplphysiol.00860.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu J., Ruas J.L., Estall J.L., Rasbach K.A., Choi J.H., Ye L. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim K., Kim Y.H., Lee S.H., Jeon M.J., Park S.Y., Doh K.O. Effect of exercise intensity on unfolded protein response in skeletal muscle of rat. Korean J Physiol Pharmacol. 2014;18:211–216. doi: 10.4196/kjpp.2014.18.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ornatsky O.I., Connor M.K., Hood D.A. Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem J. 1995;311:119–123. doi: 10.1042/bj3110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Memme J.M., Oliveira A.N., Hood D.A. The chronologyof UPR activation in skeletal muscle adaptations to chronic contractile activity. Am J Physiol Cell Physiol. 2016;310:C1024–C1036. doi: 10.1152/ajpcell.00009.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Canto C. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pirinen E., Cantó C., Jo Y.S., Morato L., Zhang H., Menzies K.J. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cerutti R., Pirinen E., Lamperti C., Marchet S., Sauve A.A., Li W. NAD+-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calvo S.E., Clauser K.R., Mootha V.K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–D1260. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neupert W., Herrmann J.M. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 94.Jensen R.E., Dunn C.D. Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim Biophys Acta Mol Cell Res. 2002;1592:25–34. doi: 10.1016/s0167-4889(02)00261-6. [DOI] [PubMed] [Google Scholar]
- 95.Bauer M.F., Hofmann S., Neupert W., Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31. doi: 10.1016/s0962-8924(99)01684-0. [DOI] [PubMed] [Google Scholar]
- 96.Krüger V., Deckers M., Hildenbeutel M., Van Der Laan M., Hellmers M., Dreker C. The mitochondrial oxidase assembly protein1 (Oxa1) insertase forms a membrane pore in lipid bilayers. J Biol Chem. 2012;287:33314–33326. doi: 10.1074/jbc.M112.387563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y., Iqbal S., O’Leary M.F.N., Menzies K.J., Saleem A., Ding S. Altered mitochondrial morphology and defective protein import reveal novel roles for Bax and/or Bak in skeletal muscle. Am J Physiol Cell Physiol. 2013;305:C502–C511. doi: 10.1152/ajpcell.00058.2013. [DOI] [PubMed] [Google Scholar]
- 98.Joseph A.M., Ljubicic V., Adhihetty P.J., Hood D.A. Biogenesis of the mitochondrial Tom40 channel in skeletal muscle from aged animals and its adaptability to chronic contractile activity. Am J Physiol Cell Physiol. 2010;298:C1308–C1314. doi: 10.1152/ajpcell.00644.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hood D.A., Tryon L.D., Vainshtein A., Memme J., Chen C., Pauly M. Exercise and the regulation of mitochondrial turnover. Prog Mol Biol Transl Sci. 2015;135:99–127. doi: 10.1016/bs.pmbts.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Singh K., Hood D.A. Effect of denervation-induced muscle disuse on mitochondrial protein import. Am J Physiol Cell Physiol. 2011;300:C138–C145. doi: 10.1152/ajpcell.00181.2010. [DOI] [PubMed] [Google Scholar]
- 101.Craig E., Hood D.A. Influence of aging on protein into cardiac mitochondria import. Am J Physiol. 1997;272:H2983–H2988. doi: 10.1152/ajpheart.1997.272.6.H2983. [DOI] [PubMed] [Google Scholar]
- 102.Joseph A.M., Rungi A.A., Robinson B.H., Hood D.A. Compensatory responses of protein import and transcription factor expression in mitochondrial DNA defects. Am J Physiol Cell Physiol. 2004;286:C867–C875. doi: 10.1152/ajpcell.00191.2003. [DOI] [PubMed] [Google Scholar]
- 103.Enomoto A., Itoh K., Nagayoshi E., Haruta J., Kimura T., Connor T.O. High Sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;177:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 104.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cullinan S., Gordan J., Jin J., Harper J., Diehl J. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase T. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi A., Kang M., Okawa H., Zenke Y., Chiba T., Igarashi K. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi M., Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 108.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 110.Katsuoka F., Motohashi H., Ishii T., Engel J.D., Yamamoto M., Aburatani H. Genetic evidence that small Maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol. 2005;25:8044–8051. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shen G., Hebbar V., Nair S., Xu C., Li W., Lin W. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 112.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nguyen T., Sherratt P.J., Nioi P., Yang C.S., Pickett C.B. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280:32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y.K.J., Wu K.C., Klaassen C.D. Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. PLoS One. 2013;8:e59122. doi: 10.1371/journal.pone.0059122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suh J.H., Shenvi S.V., Dixon B.M., Liu H., Jaiswal A.K., Liu R. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cao Z., Tsang M., Zhao H., Li Y. Induction of endogenous antioxidants and phase 2 enzymes by α-lipoic acid in rat cardiac H9C2 cells: protection against oxidative injury. Biochem Biophys Res Commun. 2003;310:979–985. doi: 10.1016/j.bbrc.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 117.Shen W., Liu K., Tian C., Yang L., Li X., Ren J. R-alpha-lipoic acid and acetyl-L-carnitine complementarily promote mitochondrial biogenesis in murine 3T3-L1 adipocytes. Diabetologia. 2008;51:165–174. doi: 10.1007/s00125-007-0852-4. [DOI] [PubMed] [Google Scholar]
- 118.Scarpulla R.C. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC 1 related coactivator. Ann N Y Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piantadosi C.A., Carraway M.S., Babiker A., Suliman H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aquilano K., Baldelli S., Pagliei B., Cannata S.M., Rotilio G., Ciriolo M.R. p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid Redox Signal. 2013;18:386–399. doi: 10.1089/ars.2012.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brose R.D., Shin G., McGuinness M.C., Schneidereith T., Purvis S., Dong G.X. Activation of the stress proteome as a mechanism for small molecule therapeutics. Hum Mol Genet. 2012;21:4237–4252. doi: 10.1093/hmg/dds247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clark J., Simon D.K. Transcribe to survive: transcriptional control of antioxidant defense programs for neuroprotection in Parkinson's disease. Antioxid Redox Signal. 2009;11:509–528. doi: 10.1089/ars.2008.2241. [DOI] [PubMed] [Google Scholar]
- 123.Piantadosi C.A., Withers C.M., Bartz R.R., MacGarvey N.C., Fu P., Sweeney T.E. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem. 2011;286:16374–16385. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Athale J., Ulrich A., MacGarvey N., Bartz R., Welty-Wolf K., Suliman H. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med. 2012;53:1584–1594. doi: 10.1016/j.freeradbiomed.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muthusamy V.R., Kannan S., Sadhaasivam K., Gounder S.S., Davidson C.J., Boeheme C. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52:366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]