Abstract
Tetraspanin 2 (Tspan2) is one of the less well-characterised members of the tetraspanin superfamily, and its precise function in different human tissue types remains to be explored. Initial studies have highlighted its possible association in neuroinflammation and carcinogenesis. In the central nervous system, Tspan2 may contribute to the early stages of the oligodendrocyte differentiation into myelin-forming glia. Furthermore, in human lung cancer, Tspan2 could be involved in the progression of the tumour metastasis by modulating cancer cell motility and invasion functions. In this review, we discuss the available evidence for the potential role of Tspan2 and introduce possible strategies for disease targeting.
Keywords: metastasis, oligodendrocytes, TEMs, tetraspanin 2, transmembrane proteins, Tspan2
Introduction
Cellular development and pathology are controlled through multiple biological complexes including transmembrane molecules and their associated partners. Tetraspanins, a superfamily of transmembrane proteins of 20–50 kDa, are abundantly expressed and distributed in multicellular organisms and significantly contribute to health and disease. The tetraspanin family is constituted of 33 members in mammals and retains a highly conserved pattern of amino acid (aa) residues within the family itself, and in evolution with distantly related species [1]. Since the discovery of the family in 1990, research conducted on tetraspanins has shown their wide expression in different tissue and cell types, with most cells expressing several different tetraspanin members [2].
Although interactions with conventional ligands are limited, tetraspanins characteristically interact laterally with other membrane molecules that may include lipids; such assemblies have been termed tetraspanin-enriched microdomains (TEMs) [3]. Within TEMs, it has been suggested that tetraspanin members form direct homologous and heterologous dynamic associations with one another. These associations can be extended to non-tetraspanin partners including integrins, growth factor receptors, ectoenzymes and cytoplasmic signalling molecules, giving tetraspanins crucial roles in cellular physiology [4]. The network of interactions of tetraspanins with other membrane proteins, which are often indirect, has also given rise to the concept of a dynamic compartmentalised ‘web’ on the cell surface [5]. While such associations were originally imputed from co-immunoprecipitation experiments [3], some direct interactions between tetraspanins and their partners have been verified by covalent cross-linking and the biophysical properties of TEM are being characterised by high-resolution microscopy techniques. Recent investigations on the molecular organisation of individual tetraspanins in plasma membrane sheets using dual-colour STED microscopy have, in fact, challenged the view that TEMs contain multiple different tetraspanins [6]. These studies suggested that tetraspanins are arranged in clusters of ∼120 nm containing <10 molecules of a particular tetraspanin and that while some tetraspanins partially co-localise, others appear as clusters of a single tetraspanin type. Moreover, while clusters of individual tetraspanins were relatively close or adjacent in the membrane, non-tetraspanin partner proteins were in even closer proximity [6].
While the precise molecular functions of tetraspanins are still largely conjectured, the family appears to generally be involved in regulating the membrane expression, trafficking and spatial organisation of the partner molecules they associate with in cell membranes [5]. For example, members of the TspanC8 subgroup regulate the maturation and ER exit of the ADAM10 metalloprotease [7,8], and different TspanC8 members may regulate its activity [9]. Therefore, tetraspanins have been implicated in many diverse biological pathways and functions including cell migration and adhesion, cell:cell fusion, tumour progression and metastasis, infection and immune response activation [5].
Interestingly, tetraspanins are also enriched on extracellular vesicles (EVs) such as exosomes. These vesicles are small in size (30–120 nm) and allow the transfer of biologically active molecules between cells, facilitating intercellular communication, and tetraspanins have been implicated in their formation and function [10,11].
Studies on some tetraspanin knockout mice have yielded important information. While female mice lacking CD9 show greatly reduced fertility due to a defect preventing oocyte fusion with sperm (reviewed in ref. [12]), other tetraspanin knockout mice initially showed few obvious phenotypes at the whole-organism level. Closer analysis of such animals, however, has revealed changes in cell behaviour that affect biological processes; for example, apparently healthy CD37−/− mice show impaired cellular immunity due to changes in dendritic cell migration [13].
Nevertheless, although certain tetraspanins (mainly those of the CD group) have received considerable attention, details about the biological characteristics of a relatively high proportion of these proteins are currently lacking and some members have been little characterised at the protein level. This is in part due to the lack of specific antibodies against the native form of the molecule. This review focuses on one such tetraspanin 2, Tspan2. The known properties of Tspan2 will be described along with studies that shed light on the emerging functions of this tetraspanin, with emphasis on its associations with oligodendrogenesis and tumour progression.
Tetraspanin structure
Tetraspanins cross the membrane four times through hydrophobic transmembrane (TM1–4) domains, forming two differently sized extracellular loops, one small intracellular loop (∼4 aa), and N- and C-cytosolic stretches that are typically short (4–19 aa), although some are longer. The TM regions contain conserved polar residues, which appear to be important for conformational stability and have been suggested to be involved in interactions between the TM helices [14]. Palmitoylation of several cytoplasmic proximal cysteines of the TM domains has been demonstrated, and palmitoylation appears to be important for microdomain organisation and interactions between tetraspanins and their partner proteins [15,16].
Kitadokoro et al. reported the 3D structure of the large extracellular domain 2 (EC2) of CD81, and until recently, detailed structural analysis and molecular modelling have concentrated on this region [17–19]. These studies have shown that the EC2 can be delineated into two regions: a conserved domain consisting of three alpha helices, two of which form a ‘stalk’ adjoining TM3 and TM4, and a variable subdomain, forming part of the ‘head’ region. Molecular modelling suggested that the first small extracellular loop (13–30 aa) is likely to be shaded by the mushroom-shaped EC2 (70–130 aa). The distinctive variable regions are predicted to be responsible for protein-specific interactions [19–21]. The EC2 contains 4–8 cysteines, including a conserved CCG ‘signature’ motif, which forms at least two disulphide bridges that stabilise the head region. Most monoclonal antibodies (mAbs) capable of recognising native tetraspanins bind to this region [22]. Recombinant versions of the EC2 appear to fold correctly and have been shown to exhibit biological activity in many systems [23].
Cryo-electron microscopy of tetraspanin uroplakin complexes, naturally present on bladder epithelium, indicated a rod-like structure, with the four TM helices closely packed and involved in interacting with their partner proteins, as well as the EC2s [24]. Strikingly, however, the full-length CD81 structure published very recently by Zimmerman et al. [25] indicates that the transmembrane domains occur as two quite separate paired helices, with a distinct hydrophobic cavity that can accommodate bound cholesterol. Interestingly, polar residues in TM1 and TM4 formed hydrogen bonds with cholesterol. Moreover, molecular simulations suggest that the CD81 EC2 may adopt an ‘open’ conformation above the TM regions when cholesterol is dissociated from the TM pocket and a ‘closed’ conformation, closer to the TM regions, when cholesterol is present. It is suggested that cholesterol binding may, therefore, serve to regulate tetraspanin function [25].
While few functions have been ascribed to the N-terminus, the intracellular C-terminal region shows considerable divergence among tetraspanins and can be involved in protein:protein interactions [26,27]. In some tetraspanins, this region contains a canonical internalisation/lysosomal targeting motif [28], which for CD63 allows interaction with adaptor protein (AP) complexes and thus clathrin-mediated trafficking. Several tetraspanins also have potential C-terminal PDZ domain-binding sites, CD63 interacts with the PDZ domain of syntenin [29] and CD81 interacts with the EBP50 and Sap97 proteins that are expressed in the developing rat retina [30]. The reported associations with the C-terminus include that of CD81 with ezrin–radixin–moesin (ERM) proteins [31] and Rac1 GTPase [32]. Mainly from co-immunoprecipitation studies, associations with intracellular signalling proteins including activated protein kinase C (PKC) [33], type-II phosphatidylinositol 4-kinase [34], an isoform of the 14-3-3 a serine-/threonine-binding protein [35] and heterotrimeric G proteins [36] have also been described.
Tetraspanins and cancer metastasis
Notably, across several types of human cancer, tetraspanins appear to play roles in tumour initiation, enhanced migration, invasion and resistance to drugs; this promotes successful metastasis [37]. This is perhaps not surprising, as TEMs may include and regulate several classes of key molecules, such as integrins and metalloproteases, which are essential in carcinogenesis and tumour progression. CD151 expression, in particular, is positively associated with various stages of cancer progression and supports de novo tumorigenesis in mouse models (Hemler [37]). The association of CD151 with various integrin partners and modulation of their activity (e.g. α6β4 integrin in squamous cell carcinoma [38] and α6β1 in hepatocellular carcinoma [39]) have been shown to be important in this regard. Matrix metalloproteinase (MMP) expression and activity, also regulated via CD151 within TEMs, may also participate in the progression of some malignancies (e.g. MMP-7 and MMP-9 in melanoma cell invasiveness [40,41]). There is also evidence that tumour-derived exosomes containing CD151 (and Tspan8) initiate active epithelial–mesenchymal transition (EMT) and metastasis progression in pancreatic carcinogenesis [42].
While some tetraspanin proteins promote tumour progression, others suppress cellular metastatic activity in the same tissue. Notably, tetraspanin CD82 has long been described as a metastasis suppressor, and expression of CD82 generally correlates with reduced cell motility and invasiveness (reviewed in ref. [43]). Evidence for the association of other tetraspanins with cancer progression is more mixed and may depend on the particular cancer and constitution of the TEMs therein. For example, expression of CD9 is associated with better prognosis in a range of cancers [44]; however, increased expression of CD9 was increased in advanced gastric cancer [45] and ovarian cancer [46]. Moreover, CD9 overexpression in human fibrosarcoma cells promoted invasiveness and secretion of MMP-9 [47], while CD9 knockdown reduced invasiveness and metastasis of human breast cancer cell models in vitro and in vivo [48]. Similarly, in glioblastoma, where high CD9 expression correlates with poor patient survival, CD9 silencing reduced behaviour associated with malignancy in glioblastoma stem cell lines in vitro and in vivo [49].
In contrast, reduced expression of the structurally related tetraspanin CD81 correlates with worse survival in hepatocellular carcinoma [50] and bladder cancer [51]. CD81 expression also correlates with a reduction in cellular activities associated with invasiveness in models of gastric cancer [52] and hepatocarcinoma [53] and promotion of cytostasis in glioma [54]. However, overexpression of CD81 promoted cell motility and increased invasiveness in melanoma cell lines through the up-regulation of metalloprotease MTI-MMP [55]. Recent evidence has also shown that primary tumour growth and metastasis are impaired in CD81 knockout mice, due to functional impairment of myeloid-derived suppressor cells and T regulatory (Treg) cells that otherwise promote tumour cell escape from anti-tumour immune responses [56].
Tspan2 discovery and general characteristics
Tspan2 was first identified in 1998 by Todd et al., who interrogated the EST database with a tetraspanin consensus sequence, resulting in the discovery of six new tetraspanins (Tspan1–Tspan6), which were subsequently sequenced from human cDNA [57]. Rat Tspan2, which has >90% homology with human Tspan2, was identified shortly afterwards from an oligodendrocyte cDNA library and shown to be strongly expressed at the mRNA level during oligodendrocyte differentiation [58].
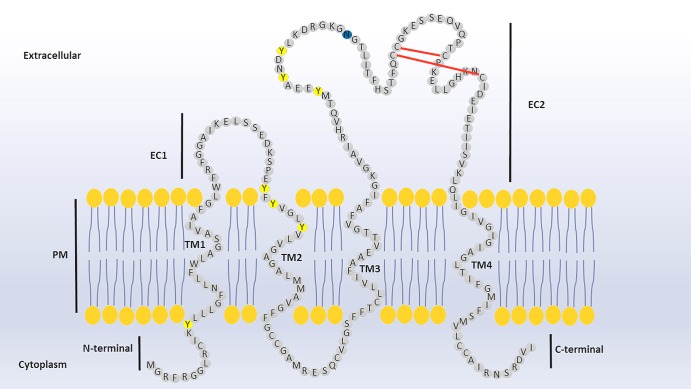
The human Tspan2 protein is encoded by the TSPAN2 gene, also known as NET3, TSN2, TSPAN-2 and FLJ12082, which is located on chromosome 1p13.2 (Ensembl gene accession ID: ENSG00000134198) [59,60]. Although four mRNA splice variants are annotated for the TSPAN2 gene in the genomic interpretation system Ensembl, three are protein-coding variants and only one of them (Ensemble transcript ID: ENST00000369516) represents the full-length Tspan2 protein [59]. According to UniProt (UniProtKB – O60636) sequence analysis and protein topology prediction, Tspan2 protein consists of 221 aa, of which 77 aa form the EC2 containing the CCG motif. The EC2 is predicted to contain two disulphide bridges between a total of four cysteine molecules (see Figure 1).
Figure 1. Prediction of Tspan2 secondary structure.
The predicted disulphide bonds between the CCG motif and the other two cysteines in the EC2 region are highlighted with red colour bonds. The seven tyrosines are illustrated with yellow colour, whereas the blue colour is the potential glycosylation site at asparagine 139. The aa arrangement and their topological hydrophobicity/hydrophilicity are extracted from the UniProt database, accession number: UniProtKB — O60636 (TSN2_HUMAN). PM: plasma membrane; EC1: extracellular domain 1; EC2: extracellular domain 2; TM: transmembrane.
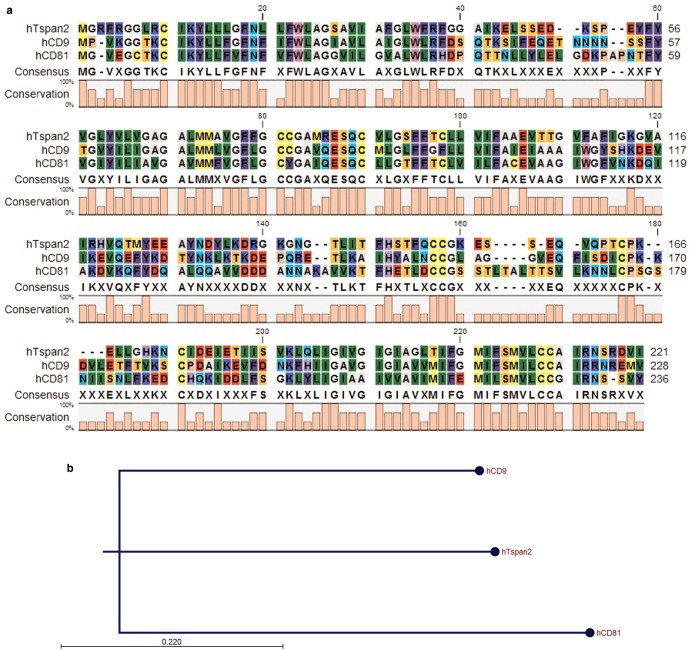
The cytoplasmic N- and C-terminal regions are predicted to be 13 and 12 aa, respectively, and the latter has a potential PDZ-binding motif class III (RDVI). As with other tetraspanins, Tspan2 is potentially palmitoylated at the membrane-proximal cysteines. Tspan2 is predicted to be N-glycosylated at asparagine 139 and shares a relatively high sequence homology with both CD9 and CD81, represented by aa identities of 46.7 and 41%, respectively. The EC2 region, however, shows greater divergence, and here Tspan2 has residue identities of 31 and 29% with the EC2s of CD9 EC2 and CD81 EC2, respectively [61–63]. Furthermore, genomic analysis of the tetraspanin orthologue groups, such as Tspan2/CD9/CD81, suggests functional relatedness between these groups (see Figure 2) [1].
Figure 2. Tspan2 protein sequence alignment and dendrogram.
(a) The protein sequence alignment of human Tspan2 along with CD9 and CD81. The bar graph represents the conservation percentages between each single aa of the concerned tetraspanins. (b) The tree phylogeny of human Tspan2, CD9 and CD81 based on their protein sequence alignment. The phylogram constructed by the RapidNJ plugin, and the Juke-Cantor evolution model used for distance calculation. Scale bar indicates the branch length. All the analysis was performed on CLC Sequence Viewer platform version 7.7.1 (QIAGEN, Aarhus, Denmark).
In the rat, Tspan2 mRNA expression was reported to be restricted to the nervous system [58]. However, Tspan2 clones were isolated from human libraries derived from the foetal heart, T cells and pregnant uterus [57]. Expression by various lymphoid and non-lymphoid carcinoma cell lines has been described at the mRNA level [64], and information from online databases also indicates a fairly wide tissue expression. Preliminary studies by our group using anti-Tspan2 monoclonal antibodies also suggest that the protein is expressed quite widely in a variety of human tissues and cell lines.
Possible functions of Tspan2
The main functions so far attributed to Tspan2 are for roles in oligodendrocyte development and cancer metastasis. As mentioned above, Tspan2 appears to be most closely related to the much better characterised CD9 and CD81 proteins, which have been implicated in biological functions related to cell:cell fusion, cell adhesion/motility and in processes that include the immune response, infection and cancer progression and metastasis.
Tspan2 in the nervous system
In the central nervous system (CNS) of invertebrates, the myelin sheath is formed by oligodendrocytes to facilitate neurone signal transmission. As mentioned above, Birling et al. reported that rat Tspan2 has a restricted expression at the mRNA level within the nervous system. The gene was predominantly expressed by cells of the oligodendrocyte lineage in the developing rat brain, although some expression was still evident in adults. Immunocytochemistry using an antibody raised against a Tspan2 EC2 peptide confirmed its presence in oligodendrocytes, where it co-localised with myelin basic protein in compact myelin, and also showed labelling of a subpopulation of neurones. Overall, the expression pattern suggested a role for Tspan2 in myelination by the myelin-forming glia and possibly in the stabilisation of the mature sheath [58]. The tetraspanin CD9 was also expressed in oligodendrocytes, but had a wider distribution pattern in rat brain and other tissues compared with Tspan2. In a study aimed at investigating the role of CD9 in oligodendrogenesis, Tspan2 and β1 integrin along with CD81 were co-immunoprecipitated with CD9 from postnatal rat cerebrum [65]. CD9-deficient mice, however, showed normal oligodendrogenesis and myelination. This suggests that although CD9 may assist in the organisation of TEMs in oligodendrocytes, their development could occur in the absence of CD9 [65].
Interestingly, Tspan2 protein expression is significantly increased in the myelin of Plp-knockout mice, a gene that codes for the proteolipid (PLP) protein which is a major membrane protein component in the CNS myelin [66]. These mice show axonal pathology similar to that observed in spastic paraplegia type-2 patients, which are deficient in PLP, but show almost normal myelination. Although not a member of the tetraspanin superfamily, PLP is a four-span membrane protein that sequesters cholesterol, and it was hypothesised that Tspan2 might be compensating for its loss in myelin in the Plp-knockout mice. However, TSPAN2 knockout mice showed normal development and biogenesis of myelin. The mice were also fertile and appeared normal with respect to motor abilities. Furthermore, mice with double knockout of TSPAN2 and Plp genes showed no further increase in demyelination and axonal degeneration. However, both single Plp and TSPAN2 knockout mice showed moderate activation of microglia and astrocytes in the white matter of the brain, and this activation was strongly increased in the double knockout animals [66]. Overall, this suggests that while Tspan2, along with PLP, may play a role in regulating neuroinflammation, it is not directly involved in myelination. Microglia belong to the mononuclear phagocyte lineage and it is interesting to note that knockdown/knockout of CD9 in murine macrophages led to increased activation in response to LPS [67]. In addition, macrophages from CD9/CD81 double knockout mice showed increased MMP production, and the mice spontaneously developed pulmonary emphysema [68]. This suggests that these related tetraspanins may generally be involved in negatively regulating mononuclear phagocyte activation, and it would be interesting to investigate Tspan2 function in other cells of this lineage.
Interestingly, Tspan3 is also expressed on oligodendrocytes and in the myelin sheath and interacts with oligodendrocyte-specific protein and β1 integrin to form a complex that may be involved in oligodendrocyte proliferation and migration [69]. The tetraspanin CD82 has also been associated with oligodendrocyte differentiation and myelination [70]. CD81 is strongly expressed on glial cells in the CNS, and the brains of CD81 knockout mice show increased numbers of astrocytes and microglial cells [71], suggesting a role for this tetraspanin in controlling their number. CD81 is up-regulated by astrocytes and microglial cells following spinal cord injury; an antibody to the tetraspanin, which blocks adhesion and proliferation of microglial cells, was shown to enhance recovery after spinal cord injury in rats [72]. As mentioned above, CD9 is also expressed in the CNS and by oligodendrocytes. CD9 is also expressed in the peripheral nervous system and studies using CD9−/− mice indicate that the tetraspanin is involved in the formation of paranodal junctions and the formation of compact myelin sheaths [73].
Of note, given the distinct expression of the Tspan2 protein in the CNS, the TSPAN2 gene is suggested to be involved in migraine disease [74,75]. Analysing >2000 migraine cases through genome-wide association studies (GWAS), 12 GWAS loci associated with migraine susceptibility have been detected, including a region near TSPAN2 at 1p13 locus [74]. However, the TSPAN2 gene has not been recognised as one of the high-confidence genes attributed to migraine disorder, perhaps due to the large distance between TSPAN2 gene and its GWAS locus [75]. Another large-scale GWAS has identified 1p13.2 near TSPAN2 as a novel locus that is strongly suggested to be associated with human stroke related to large artery atherosclerosis disease [76].
Tspan2 and cancer progression
In malignancy, strong expression of Tspan2 is associated with poor prognosis in lung cancer and it has been linked to disease progression associated with p53 inactivation [77]. Mutation of the tumour suppressor p53 is often observed in invasive tumours and contributes towards cancer metastasis by modulating EMT [78]. In a study investigating the contribution of p53 to lung cancer progression, Tspan2 was identified as being highly expressed in human small airway epithelial cells (SAECs) knocked down for p53 at both the mRNA and protein level. Expression of Tspan2 mRNA was also elevated in lung cancer cell lines with p53 mutations. Knockdown of Tspan2 reduced invasiveness and motility in p53-depleted SAECs or cancer cell lines with p53 mutations, whereas overexpression of Tspan2 enhanced these attributes. Knockdown of Tspan2 was also shown to reduce lung metastasis in a mouse model [77]. Co-immunoprecipitation identified CD44 as one of the proteins interacting with Tspan2 and the role of this protein, which is known to interact with other tetraspanins, was investigated further. CD44 has been implicated in the cellular response to oxidative stress and CD44 knockdown resulted in increased reactive oxygen species (ROS) production and decreased invasiveness of SAECs . Knockdown of Tspan2 also caused increased ROS production, whereas its overexpression reduced ROS production; however, knockdown of CD44 negated this effect. The authors therefore propose that Tspan2 promotes invasiveness in p53-depleted lung cancer cells by binding to CD44 and enhancing its ROS-scavenger function [77]. It would be interesting, however, to explore further possible effects on some of the other proteins (MT1-MMP, ADAM-10 and integrins) that were also reported to interact with Tspan2 in these cells.
Proteins that may interact with Tspan2
In addition to the tetraspanins CD9 and CD81, Tspan2 is reported to interact with β1 integrin in oligodendrocytes [65] and with CD44, MT1-MMP and ADAM10 in lung cancer cell lines [77]. These interactions were identified by immunoprecipitation, albeit in the latter case using stringent detergent, so direct interactions between Tspan2 and these proteins have not yet been incontrovertibly demonstrated. Associations with these non-tetraspanin partners have also been described for CD9 and CD81, which are most closely related to Tspan2 [79–81]. CD9 and CD81 are known to be direct partners of the immunoglobulin superfamily proteins EWI-2 and EWI-F, which interact with ERM proteins and so can link the tetraspanins to the actin cytoskeleton [31]. The interactions of CD9 and CD81 with the EWI proteins may be regulated by tetraspanin palmitoylation [82,83] and involves the EC2 domains and some of the TM regions [84,85]. As mentioned previously, the EC2 region of Tspan2 shows greatest divergence from CD9 and CD81 and as far as we are aware, an interaction between Tspan2 and the EWI proteins has not been demonstrated, although EWI-2 is strongly expressed in normal human brain and on astrocytes, but down-regulated in glioblastoma [86]. Notably, immunohistochemistry staining of glioma tissue showed high expression of Tspan2 [87].
Interestingly, in a recent study aimed at identifying phosphotyrosine-dependent (pY) protein–protein interactions using yeast two-hybrid analysis, pY-dependent interactions between Tspan2 and the AP growth factor receptor-bound protein 2 (GRB2) and the regulatory phosphoinositide-3-kinase subunit 3 gamma (PIK3R3) were identified [88]. The pY dependence of these interactions was investigated by pull-down experiments in HEK293 cells. Tspan2 contains seven tyrosines (Figure 1) and mutations of these abrogated the interactions with GRB2 and PIK3R3. Tyrosine 124 appeared to be critical, as a reversal of the Y124F mutation restored binding of both proteins and a 15 aa Tspan2 peptide containing Y124-bound purified GRB2 and PIK3R3. HEK293 cells overexpressing Tspan2 formed numerous long membrane protrusions and extensive cell:cell contacts, but this phenotype was greatly reduced for cells expressing Tspan2-Y124F. The interaction between Tspan2 and GRB2 was confirmed at the plasma membrane by YFP-based complementation, whereas the interaction with PIK3R3 was mainly cytoplasmic [88].
Phosphorylation of Tspan2 has not been directly shown in vivo; however, and since Y124 is in the putative extracellular EC2 domain of Tspan2, the role for phosphorylation here in cell signalling is unclear. Nevertheless, phosphoproteomics data indicate that a large number of tetraspanins may be phosphorylated [89,90], and of interest, Tspan8 is described as being phosphorylated at position Y122 [91], which corresponds to Y124 in Tspan2 (and Y125 in CD9, which can be phosphorylated experimentally [90]). Although tyrosine phosphorylation associated with cell signalling is generally assumed to be mediated by kinases that act intracellularly, it is also worth noting that the properties of secreted tyrosine kinases that act extracellularly have recently been described [92–94].
Conclusion and future prospects
The diversity of tetraspanin biology is expanding as the field moves on, and more discoveries relating to member-specific mechanisms of functions are to be made. Tspan2, as one of the more recently discovered tetraspanins, introduces new questions about the key role it may play, especially in CNS development and carcinogenesis. In malignancy, studies on Tspan2 have revealed characteristics that may explain its involvement in tumour invasiveness and metastasis including human lung adenocarcinomas [77]. In addition, a link has been established through GWAS to a potential association of Tspan2 with a migraine in humans, although further studies are required here. Understanding the differential expression of Tspan2, particularly in the oligodendrocyte lineage, may also identify targets for future therapies in neurodegenerative diseases.
As with the other tetraspanin molecules, Tspan2 may modulate some pathophysiological conditions and has very recently been proposed to be involved in diabetes [95]. At high glucose concentrations, Tspan2 appeared to induce apoptosis of a human pancreatic β-cell line by modulating the JNK/β-catenin signalling pathway, suggesting a potential therapeutic approach against the glucotoxic apoptosis in diabetes [95]. The TSPAN2 gene locus has also recently been identified as a risk factor for ischaemic stroke [96]. Interestingly, recent unpublished data from our laboratory indicate a role for Tspan2 in the secretion of EVs by some cancer cell lines. Altogether this suggests that Tspan2 may constitute a target in various disease conditions.
Strategies for developing cell-specific reagents are essential to avoid the unfavourable consequences when targeting Tspan2. Monoclonal antibodies against the native form of Tspan2 are valuable tools that would introduce wide treatment options where applicable. Peptides representing the EC2 offer an alternative strategy to block or attenuate Tspan2 activity, as would strategies to knockdown Tspan2 gene expression.
Abbreviations
- aa
amino acid
- ADAM10
a disintegrin and metalloproteinase 10
- APs
adaptor proteins
- CD
cluster of differentiation
- CNS
central nervous system
- EC2
extracellular domain 2
- EMT
epithelial–mesenchymal transition
- ER
endoplasmic reticulum
- ERM
ezrin–radixin–moesin
- GRB2
growth factor receptor-bound protein 2
- GWAS
genome-wide association studies
- mAbs
monoclonal antibodies
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type 1-matrix metalloproteinase
- PIK3R3
phosphoinositide-3-kinase- regulatory subunit 3 gamma
- PKC
protein kinase C
- PLP
proteolipid
- pY
phosphotyrosine
- ROS
reactive oxygen species
- TEMs
tetraspanin-enriched microdomains
- TM
transmembrane
- Tspan2
tetraspanin 2.
Funding
This work is supported by the Higher Committee for Education Development (HCED) in Iraq as a part of the PhD scholarship programme [D-11-1771].
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Garcia-España A., Chung P.-J., Sarkar I.N., Stiner E., Sun T.-T. and Desalle R. (2008) Appearance of new tetraspanin genes during vertebrate evolution. Genomics 91, 326–334 doi: 10.1016/j.ygeno.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 2.Oren R., Takahashi S., Doss C., Levy R. and Levy S. (1990) TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol. Cell. Biol. 10, 4007–4015 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemler M.E. (2003) Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19, 397–422 doi: 10.1146/annurev.cellbio.19.111301.153609 [DOI] [PubMed] [Google Scholar]
- 4.Yáñez-Mó M., Barreiro O., Gordon-Alonso M., Sala-Valdés M. and Sánchez-Madrid F. (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 19, 434–446 doi: 10.1016/j.tcb.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 5.Charrin S., Jouannet S., Boucheix C. and Rubinstein E. (2014) Tetraspanins at a glance. J. Cell Sci. 127, 3641–3648 doi: 10.1242/jcs.154906 [DOI] [PubMed] [Google Scholar]
- 6.Zuidscherwoude M., Göttfert F., Dunlock V.M.E., Figdor C.G., van den Bogaart G. and Van Spriel A.B. (2015) The tetraspanin web revisited by super-resolution microscopy. Sci. Rep. 5, 12201 doi: 10.1038/srep12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dornier E., Coumailleau F., Ottavi J.-F., Moretti J., Boucheix C., Mauduit P. et al. (2012) Tspanc8 tetraspanins regulate ADAM10/Kuzbanian trafficking and promote Notch activation in flies and mammals. J. Cell Biol. 199, 481–496 doi: 10.1083/jcb.201201133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haining E.J., Yang J., Bailey R.L., Khan K., Collier R., Tsai S. et al. (2012) The TspanC8 subgroup of tetraspanins interacts with a disintegrin and metalloprotease 10 (ADAM10) and regulates its maturation and cell surface expression. J. Biol. Chem. 287, 39753–39765 doi: 10.1074/jbc.M112.416503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noy P.J., Yang J., Reyat J.S., Matthews A.L., Charlton A.E., Furmston J. et al. (2016) Tspanc8 tetraspanins and a disintegrin and metalloprotease 10 (ADAM10) interact via their extracellular regions: evidence for distinct binding mechanisms for different TspanC8 proteins. J. Biol. Chem. 291, 3145–3157 doi: 10.1074/jbc.M115.703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreu Z. and Yáñez-Mó M. (2014) Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 5, 442 doi: 10.3389/fimmu.2014.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlassov A.V., Magdaleno S., Setterquist R. and Conrad R. (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta, Gen. Subj. 1820, 940–948 doi: 10.1016/j.bbagen.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 12.Evans J.P. (2012) Sperm-egg interaction. Annu. Rev. Physiol. 74, 477–502 doi: 10.1146/annurev-physiol-020911-153339 [DOI] [PubMed] [Google Scholar]
- 13.Gartlan K.H., Wee J.L., Demaria M.C., Nastovska R., Chang T.M., Jones E.L. et al. (2013) Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur. J. Immunol. 43, 1208–1219 doi: 10.1002/eji.201242730 [DOI] [PubMed] [Google Scholar]
- 14.Bari R., Zhang Y.H., Zhang F., Wang N.X., Stipp C.S., Zheng J.J. et al. (2009) Transmembrane interactions are needed for KAI1/CD82-mediated suppression of cancer invasion and metastasis. Am. J. Pathol. 174, 647–660 doi: 10.2353/ajpath.2009.080685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zevian S., Winterwood N.E. and Stipp C.S. (2011) Structure-function analysis of tetraspanin CD151 reveals distinct requirements for tumor cell behaviors mediated by α3β1 versus α6β4 integrin. J. Biol. Chem. 286, 7496–7506 doi: 10.1074/jbc.M110.173583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marjon K.D., Termini C.M., Karlen K.L., Saito-Reis C., Soria C.E., Lidke K.A. et al. (2016) Tetraspanin CD82 regulates bone marrow homing of acute myeloid leukemia by modulating the molecular organization of N-cadherin. Oncogene 35, 4132–4140 doi: 10.1038/onc.2015.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitadokoro K. (2001) CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 20, 12–18 doi: 10.1093/emboj/20.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajesh S., Sridhar P., Tews B.A., Feneant L., Cocquerel L., Ward D.G. et al. (2012) Structural basis of ligand interactions of the large extracellular domain of tetraspanin CD81. J. Virol. 86, 9606–9616 doi: 10.1128/JVI.00559-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seigneuret M., Conjeaud H., Zhang H.-T. and Kong X.-P. (2013) Structural bases for tetraspanin functions In Tetraspanins (Berditchevski F. and Rubinstein E., eds), pp. 1–29, Springer Science, Dordrecht [Google Scholar]
- 20.Seigneuret M., Delaguillaumie A., Lagaudrière-Gesbert C. and Conjeaud H. (2001) Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 276, 40055–40064 doi: 10.1074/jbc.M105557200 [DOI] [PubMed] [Google Scholar]
- 21.Seigneuret M. (2006) Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: conserved and variable structural domains in the tetraspanin superfamily. Biophys. J. 90, 212–227 doi: 10.1529/biophysj.105.069666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemler M.E. (2005) Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811 doi: 10.1038/nrm1736 [DOI] [PubMed] [Google Scholar]
- 23.Hassuna N., Monk P.N., Moseley G.W. and Partridge L.J. (2009) Strategies for targeting tetraspanin proteins: potential therapeutic applications in microbial infections. BioDrugs 23, 341–359 doi: 10.2165/11315650-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min G., Wang H., Sun T.-T. and Kong X.-P. (2006) Structural basis for tetraspanin functions as revealed by the cryo-EM structure of uroplakin complexes at 6-A resolution. J. Cell Biol. 173, 975–983 doi: 10.1083/jcb.200602086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman B., Kelly B., McMillan B.J., Seegar T.C.M., Dror R.O., Kruse A.C. et al. (2016) Crystal structure of a full-Length human tetraspanin reveals a cholesterol-Binding pocket. Cell 167, 1041–1051.e11 doi: 10.1016/j.cell.2016.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stipp C.S., Kolesnikova T.V. and Hemler M.E. (2003) Functional domains in tetraspanin proteins. Trends Biochem. Sci. 28, 106–112 doi: 10.1016/S0968-0004(02)00014-2 [DOI] [PubMed] [Google Scholar]
- 27.Charrin S., le Naour F., Silvie O., Milhiet P.-E., Boucheix C. and Rubinstein E. (2009) Lateral organization of membrane proteins: tetraspanins spin their web. Biochem. J. 420, 133–154 doi: 10.1042/BJ20082422 [DOI] [PubMed] [Google Scholar]
- 28.Berditchevski F. and Odintsova E. (2007) Tetraspanins as regulators of protein trafficking. Traffic 8, 89–96 doi: 10.1111/j.1600-0854.2006.00515.x [DOI] [PubMed] [Google Scholar]
- 29.Latysheva N., Muratov G., Rajesh S., Padgett M., Hotchin N.A., Overduin M. et al. (2006) Syntenin-1 is a new component of tetraspanin-enriched microdomains: mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol. Cell. Biol. 26, 7707–7718 doi: 10.1128/MCB.00849-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Y., Brown C., Wang X. and Geisert E.E. (2007) The developmental regulation of CD81 in the rat retina. Mol. Vis. 13, 181–189 [PMC free article] [PubMed] [Google Scholar]
- 31.Sala-Valdés M., Ursa A., Charrin S., Rubinstein E., Hemler M.E., Sánchez-Madrid F. et al. (2006) EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 281, 19665–19675 doi: 10.1074/jbc.M602116200 [DOI] [PubMed] [Google Scholar]
- 32.Tejera E., Rocha-Perugini V., López-Martín S., Pérez-Hernández D., Bachir A.I., Horwitz A.R. et al. (2013) CD81 regulates cell migration through its association with Rac GTPase. Mol. Biol. Cell 24, 261–273 doi: 10.1091/mbc.E12-09-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X.A., Bontrager A.L. and Hemler M.E. (2001) Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific β1 integrins. J. Biol. Chem. 276, 25005–25013 doi: 10.1074/jbc.M102156200 [DOI] [PubMed] [Google Scholar]
- 34.Berditchevski F., Tolias K.F., Wong K., Carpenter C.L. and Hemler M.E. (1997) A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-Kinase. J. Biol. Chem. 272, 2595–2598 PMID: [DOI] [PubMed] [Google Scholar]
- 35.Clark K.L., Oelke A., Johnson M.E., Eilert K.D., Simpson P.C. and Todd S.C. (2004) CD81 associates with 14-3-3 in a redox-regulated palmitoylation-dependent manner. J. Biol. Chem. 279, 19401–19406 doi: 10.1074/jbc.M312626200 [DOI] [PubMed] [Google Scholar]
- 36.Little K.D., Hemler M.E. and Stipp C.S. (2004) Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: central role of CD81 in facilitating GPR56-Gαq/11 association. Mol. Biol. Cell 15, 2375–2387 doi: 10.1091/mbc.E03-12-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemler M.E. (2014) Tetraspanin proteins promote multiple cancer stages. Nat. Rev. Cancer 14, 49–60 doi: 10.1038/nrc3640 [DOI] [PubMed] [Google Scholar]
- 38.Li Q., Yang X.H., Xu F., Sharma C., Wang H.-X., Knoblich K. et al. (2013) Tetraspanin CD151 plays a key role in skin squamous cell carcinoma. Oncogene 32, 1772–1783 doi: 10.1038/onc.2012.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke A., Zhang P., Shen Y., Gao P., Dong Z., Zhang C. et al. (2016) Generation and characterization of a tetraspanin CD151/integrin α6β1-binding domain competitively binding monoclonal antibody for inhibition of tumor progression in HCC. Oncotarget 7, 6314–6322 doi: 10.18632/oncotarget.6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranjan A., Bane S.M. and Kalraiya R.D. (2014) Glycosylation of the laminin receptor (α3β1) regulates its association with tetraspanin CD151: Impact on cell spreading, motility, degradation and invasion of basement membrane by tumor cells. Exp. Cell Res. 322, 249–264 doi: 10.1016/j.yexcr.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 41.Hong I.-K., Jin Y.-J., Byun H.-J., Jeoung D.-I., Kim Y.-M. and Lee H. (2006) Homophilic interactions of tetraspanin CD151 up-regulate motility and matrix metalloproteinase-9 expression of human melanoma cells through adhesion-dependent c-Jun activation signaling pathways. J. Biol. Chem. 281, 24279–24292 doi: 10.1074/jbc.M601209200 [DOI] [PubMed] [Google Scholar]
- 42.Yue S., Mu W., Erb U. and Zöller M. (2015) The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget 6, 2366–2384 doi: 10.18632/oncotarget.2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng J., Huang C., Wren J.D., Wang D.-W., Yan J., Zhang J. et al. (2015) Tetraspanin CD82: a suppressor of solid tumors and a modulator of membrane heterogeneity. Cancer Metastasis Rev. 34, 619–633. doi: 10.1007/s10555-015-9585-x [DOI] [PubMed] [Google Scholar]
- 44.Romanska H.M. and Berditchevski F. (2011) Tetraspanins in human epithelial malignancies. J. Pathol. 223, 4–14 doi: 10.1002/path.2779 [DOI] [PubMed] [Google Scholar]
- 45.Hori H., Yano S., Koufuji K., Takeda J. and Shirouzu K. (2004) CD9 expression in gastric cancer and its significance. J. Surg. Res. 117, 208–215 doi: 10.1016/j.jss.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 46.Houle C.D., Ding X.-Y., Foley J.F., Afshari C.A., Barrett J.C. and Davis B.J. (2002) Loss of expression and altered localization of KAI1 and CD9 protein are associated with epithelial ovarian cancer progression. Gynecol. Oncol. 86, 69–78 doi: 10.1006/gyno.2002.6729 [DOI] [PubMed] [Google Scholar]
- 47.Herr M.J., Kotha J., Hagedorn N., Smith B., Jennings L.K. and Weaver A.M. (2013) Tetraspanin CD9 promotes the invasive phenotype of human fibrosarcoma cells via upregulation of matrix metalloproteinase-9. PLoS ONE 8, e67766 doi: 10.1371/journal.pone.0067766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rappa G., Green T.M., Karbanová J., Corbeil D. and Lorico A. (2015) Tetraspanin CD9 determines invasiveness and tumorigenicity of human breast cancer cells. Oncotarget 6, 7970–7991 doi: 10.18632/oncotarget.3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podergajs N., Motaln H., Rajčević U., Verbovšek U., Koršič M., Obad N. et al. (2015) Transmembrane protein CD9 is glioblastoma biomarker, relevant for maintenance of glioblastoma stem cells. Oncotarget 7, 593–609 doi: 10.18632/oncotarget.5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoue G., Horiike N. and Onji M. (2001) The CD81 expression in liver in hepatocellular carcinoma. Int. J. Mol. Med. 7, 67–71 PMID: [DOI] [PubMed] [Google Scholar]
- 51.Lee M.-S., Kim J.H., Lee J.-S., Yun S.J., Kim W.-J., Ahn H. et al. (2015) Prognostic significance of CREB-binding protein and CD81 expression in primary high grade non-muscle invasive bladder cancer: identification of novel biomarkers for bladder cancer using antibody microarray. PLoS ONE 10, e0125405 doi: 10.1371/journal.pone.0125405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoo T.-H., Ryu B.-K., Lee M.-G. and Chi S.-G. (2013) CD81 is a candidate tumor suppressor gene in human gastric cancer. Cell. Oncol. 36, 141–153 doi: 10.1007/s13402-012-0119-z [DOI] [PubMed] [Google Scholar]
- 53.Mazzocca A., Liotta F. and Carloni V. (2008) Tetraspanin CD81-regulated cell motility plays a critical role in intrahepatic metastasis of hepatocellular carcinoma. Gastroenterology 135, 244–256.e1 doi: 10.1053/j.gastro.2008.03.024 [DOI] [PubMed] [Google Scholar]
- 54.Gensert J.M., Baranova O.V., Weinstein D.E. and Ratan R.R. (2007) CD81, a cell cycle regulator, is a novel target for histone deacetylase inhibition in glioma cells. Neurobiol. Dis. 26, 671–680 doi: 10.1016/j.nbd.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 55.Hong I.-K.K., Byun H.-J.J., Lee J., Jin Y.-J.J., Wang S.-J.J., Jeoung D.-I. II et al. (2014) The tetraspanin CD81 protein increases melanoma cell motility by up-regulating metalloproteinase MT1-MMP expression through the pro-oncogenic Akt-dependent Sp1 activation signaling pathways. J. Biol. Chem. 289, 15691–15704 doi: 10.1074/jbc.M113.534206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vences-Catalán F., Rajapaksa R., Srivastava M.K., Marabelle A., Kuo C.-C., Levy R. et al. (2015) Tetraspanin CD81 promotes tumor growth and metastasis by modulating the functions of T regulatory and myeloid-derived suppressor cells. Cancer Res. 75, 4517–4526 doi: 10.1158/0008-5472.CAN-15-1021 [DOI] [PubMed] [Google Scholar]
- 57.Todd S.C., Doctor V.S. and Levy S. (1998) Sequences and expression of six new members of the tetraspanin/TM4SF family. Biochim. Biophys. Acta 1399, 101–104 PMID: [DOI] [PubMed] [Google Scholar]
- 58.Birling M.C., Tait S., Hardy R.J. and Brophy P.J. (1999) A novel rat tetraspan protein in cells of the oligodendrocyte lineage. J. Neurochem. 73, 2600–2608 PMID: [DOI] [PubMed] [Google Scholar]
- 59.Cunningham F., Amode M.R., Barrell D., Beal K., Billis K., Brent S. et al. (2015) Ensembl 2015. Nucleic Acids Res. 43, D662–D669 doi: 10.1093/nar/gku1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stelzer G., Dalah I., Stein T.I., Satanower Y., Rosen N., Nativ N. et al. (2011) In-silico human genomics with GeneCards. Hum. Genomics 5, 709–717 doi: 10.1186/1479-7364-5-6-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.UniProt Consortium (2015) Uniprot: a hub for protein information. Nucleic Acids Res. 43, D204–D212 doi: 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassani S. and Cingolani L.A. (2012) Tetraspanins: interactions and interplay with integrins. Int. J. Biochem. Cell Biol. 44, 703–708 doi: 10.1016/j.biocel.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 63.Huang X. and Miller W. (1991) A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 12, 337–357 doi: 10.1016/0196-8858(91)90017-D [DOI] [Google Scholar]
- 64.Serru V., Dessen P., Boucheix C. and Rubinstein E. (2000) Sequence and expression of seven new tetraspans. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1478, 159–163 doi: 10.1016/S0167-4838(00)00022-4 [DOI] [PubMed] [Google Scholar]
- 65.Terada N., Baracskay K., Kinter M., Melrose S., Brophy P.J., Boucheix C. et al. (2002) The tetraspanin protein, CD9, is expressed by progenitor cells committed to oligodendrogenesis and is linked to β1 integrin, CD81, and Tspan-2. Glia 40, 350–359 doi: 10.1002/glia.10134 [DOI] [PubMed] [Google Scholar]
- 66.de Monasterio-Schrader P., Patzig J., Möbius W., Barrette B., Wagner T.L., Kusch K. et al. (2013) Uncoupling of neuroinflammation from axonal degeneration in mice lacking the myelin protein tetraspanin-2. Glia 61, 1832–1847 doi: 10.1002/glia.22561 [DOI] [PubMed] [Google Scholar]
- 67.Suzuki M., Tachibana I., Takeda Y., He P., Minami S., Iwasaki T. et al. (2009) Tetraspanin CD9 negatively regulates lipopolysaccharide-induced macrophage activation and lung inflammation. J. Immunol. 182, 6485–6493 doi: 10.4049/jimmunol.0802797 [DOI] [PubMed] [Google Scholar]
- 68.Takeda Y., He P., Tachibana I., Zhou B., Miyado K., Kaneko H. et al. (2008) Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease-like phenotype in mice. J. Biol. Chem. 283, 26089–26097 doi: 10.1074/jbc.M801902200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiwari-woodruff S.K., Kaplan R., Kornblum H.I. and Bronstein J.M. (2004) Developmental expression of OAP-1/Tspan- 3, a member of the tetraspanin superfamily. J. Neurosci. Res. 77, 166–173 doi: 10.1002/jnr.20141 [DOI] [PubMed] [Google Scholar]
- 70.Mela A. and Goldman J.E. (2009) The tetraspanin KAI1/CD82 is expressed by late-lineage oligodendrocyte precursors and may function to restrict precursor migration and promote oligodendrocyte differentiation and myelination. J. Neurosci. 29, 11172–11181 doi: 10.1523/JNEUROSCI.3075-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geisert E.E., Williams R.W., Geisert G.R., Fan L., Asbury A.M., Maecker H.T. et al. (2002) Increased brain size and glial cell number in CD81-null mice. J. Comp. Neurol. 453, 22–32 doi: 10.1002/cne.10364 [DOI] [PubMed] [Google Scholar]
- 72.Dijkstra S., Duis S., Pans I.M., Lankhorst A.J., Hamers F.P.T., Veldman H. et al. (2006) Intraspinal administration of an antibody against CD81 enhances functional recovery and tissue sparing after experimental spinal cord injury. Exp. Neurol. 202, 57–66 doi: 10.1016/j.expneurol.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 73.Ishibashi T., Ding L., Ikenaka K., Inoue Y., Miyado K., Mekada E. et al. (2004) Tetraspanin protein CD9 is a novel paranodal component regulating paranodal junctional formation. J. Neurosci. 24, 96–102 doi: 10.1523/JNEUROSCI.1484-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anttila V., Winsvold B.S., Gormley P., Kurth T., Bettella F., McMahon G. et al. (2013) Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat. Genet. 45, 912–917 doi: 10.1038/ng.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eising E., Huisman S.M.H., Mahfouz A., Vijfhuizen L.S., Anttila V., Winsvold B.S. et al. (2016) Gene co-expression analysis identifies brain regions and cell types involved in migraine pathophysiology: a GWAS-based study using the Allen Human Brain Atlas. Hum. Genet. 135, 425–439 doi: 10.1007/s00439-016-1638-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.NINDS Stroke Genetics Network (SiGN), International Stroke Genetics Consortium (ISGC) (2016) Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol. 15, 174–184 doi: 10.1016/S1474-4422(15)00338-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otsubo C., Otomo R., Miyazaki M., Matsushima-Hibiya Y., Kohno T., Iwakawa R. et al. (2014) TSPAN2 is involved in cell invasion and motility during lung cancer progression. Cell Rep. 7, 527–538 doi: 10.1016/j.celrep.2014.03.027 [DOI] [PubMed] [Google Scholar]
- 78.Bornachea O., Santos M., Martínez-Cruz A.B., García-Escudero R., Dueñas M., Costa C. et al. (2012) EMT and induction of miR-21 mediate metastasis development in Trp53-deficient tumours. Sci. Rep. 2, 434 doi: 10.1038/srep00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Naour F., André M., Greco C., Billard M., Sordat B., Emile J.-F. et al. (2006) Profiling of the tetraspanin web of human colon cancer cells. Mol. Cell. Proteomics 5, 845–857 doi: 10.1074/mcp.M500330-MCP200 [DOI] [PubMed] [Google Scholar]
- 80.Lafleur M.A., Xu D. and Hemler M.E. (2009) Tetraspanin proteins regulate membrane type-1 matrix metalloproteinase-dependent pericellular proteolysis. Mol. Biol. Cell 20, 2030–2040 doi: 10.1091/mbc.E08-11-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzocca A., Carloni V., Sciammetta S.C., Cordella C., Pantaleo P., Caldini A. et al. (2002) Expression of transmembrane 4 superfamily (TM4SF) proteins and their role in hepatic stellate cell motility and wound healing migration. J. Hepatol. 37, 322–330 PMID: [DOI] [PubMed] [Google Scholar]
- 82.Yang X.H., Kovalenko O.V., Kolesnikova T.V., Andzelm M.M., Rubinstein E., Strominger J.L. et al. (2006) Contrasting effects of EWI proteins, integrins, and protein palmitoylation on cell surface CD9 organization. J. Biol. Chem. 281, 12976–12985 doi: 10.1074/jbc.M510617200 [DOI] [PubMed] [Google Scholar]
- 83.Delandre C., Penabaz T.R., Passarelli A.L., Chapes S.K. and Clem R.J. (2009) Mutation of juxtamembrane cysteines in the tetraspanin CD81 affects palmitoylation and alters interaction with other proteins at the cell surface. Exp. Cell Res. 315, 1953–1963 doi: 10.1016/j.yexcr.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charrin S., Manié S., Billard M., Ashman L., Gerlier D., Boucheix C. et al. (2003) Multiple levels of interactions within the tetraspanin web. Biochem. Biophys. Res. Commun. 304, 107–112 doi: 10.1016/S0006-291X(03)00545-X [DOI] [PubMed] [Google Scholar]
- 85.Montpellier C., Tews B.A., Poitrimole J., Rocha-Perugini V., D'Arienzo V., Potel J. et al. (2011) Interacting regions of CD81 and two of its partners, EWI-2 and EWI-2wint, and their effect on hepatitis C virus infection. J. Biol. Chem. 286, 13954–13965 doi: 10.1074/jbc.M111.220103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolesnikova T.V., Kazarov A.R., Lemieux M.E., Lafleur M.A., Kesari S., Kung A.L. et al. (2009) Glioblastoma inhibition by cell surface immunoglobulin protein EWI-2, in vitro and in vivo. Neoplasia 11, 77–86, 4p following 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A. et al. (2015) Tissue-based map of the human proteome. Science 347, 1260419 doi: 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 88.Grossmann A., Benlasfer N., Birth P., Hegele A., Wachsmuth F., Apelt L. et al. (2015) Phospho-tyrosine dependent protein-protein interaction network. Mol. Syst. Biol. 11, 794 doi: 10.15252/msb.20145968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hornbeck P.V., Kornhauser J.M., Tkachev S., Zhang B., Skrzypek E., Murray B. et al. (2012) Phosphositeplus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40, D261–D270 doi: 10.1093/nar/gkr1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsai C.-F., Wang Y.-T., Yen H.-Y., Tsou C.-C., Ku W.-C., Lin P.-Y. et al. (2015) Large-scale determination of absolute phosphorylation stoichiometries in human cells by motif-targeting quantitative proteomics. Nat. Commun. 6, 6622 doi: 10.1038/ncomms7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moritz A., Li Y., Guo A., Villen J., Wang Y., MacNeill J. et al. (2010) Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci. Signal. 3, ra64 doi: 10.1126/scisignal.2000998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tagliabracci V.S., Pinna L.A. and Dixon J.E. (2013) Secreted protein kinases. Trends Biochem. Sci. 38, 121–130 doi: 10.1016/j.tibs.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tagliabracci V.S., Wiley S.E., Guo X., Kinch L.N., Durrant E., Wen J. et al. (2015) A single kinase generates the majority of the secreted phosphoproteome. Cell 161, 1619–1632 doi: 10.1016/j.cell.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bordoli M.R., Yum J., Breitkopf S.B., Thon J.N., Italiano J.E., Xiao J. et al. (2014) A secreted tyrosine kinase acts in the extracellular environment. Cell 158, 1033–1044 doi: 10.1016/j.cell.2014.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hwang I.-H., Park J., Kim J.M., Kim S.I., Choi J.-S., Lee K.-B. et al. (2016) Tetraspanin-2 promotes glucotoxic apoptosis by regulating the JNK/β-catenin signaling pathway in human pancreatic β cells. FASEB J. 30, 3107–3116 doi: 10.1096/fj.201600240RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chauhan G. and Debette S. (2016) Genetic risk factors for ischemic and hemorrhagic stroke. Curr. Cardiol. Rep. 18, 124 doi: 10.1007/s11886-016-0804-z [DOI] [PMC free article] [PubMed] [Google Scholar]